Abstract
A survey was initiated to determine the prevalence of Salmonella enterica in the environment in and around Monterey County, CA, a major agriculture region of the United States. Trypticase soy broth enrichment cultures of samples of soil/sediment (n = 617), water (n = 252), wildlife (n = 476), cattle feces (n = 795), and preharvest lettuce and spinach (n = 261) tested originally for the presence of pathogenic Escherichia coli were kept in frozen storage and later used to test for the presence of S. enterica. A multipathogen oligonucleotide microarray was employed to identify a subset of samples that might contain Salmonella in order to test various culture methods to survey a larger number of samples. Fifty-five of 2,401 (2.3%) samples yielded Salmonella, representing samples obtained from 20 different locations in Monterey and San Benito Counties. Water had the highest percentage of positives (7.1%) among sample types. Wildlife yielded 20 positive samples, the highest number among sample types, with positive samples from birds (n = 105), coyotes (n = 40), deer (n = 104), elk (n = 39), wild pig (n = 41), and skunk (n = 13). Only 16 (2.6%) of the soil/sediment samples tested positive, and none of the produce samples had detectable Salmonella. Sixteen different serotypes were identified among the isolates, including S. enterica serotypes Give, Typhimurium, Montevideo, and Infantis. Fifty-four strains were sensitive to 12 tested antibiotics; one S. Montevideo strain was resistant to streptomycin and gentamicin. Pulsed-field gel electrophoresis (PFGE) analysis of the isolates revealed over 40 different pulsotypes. Several strains were isolated from water, wildlife, or soil over a period of several months, suggesting that they were persistent in this environment.
INTRODUCTION
Salmonella enterica is the most common reported cause of bacterial food-borne illness in the United States (4, 33, 54). Salmonella strains that can persist in the environment for years, withstanding periods of stress and nutrient depletion, have been reported (35). Multiple vertebrate sources exist for this bacterium, including livestock, wildlife, poultry, and companion animals, with water functioning as a documented mode of dissemination in the environment (32, 50, 55). Fresh produce is a recognized vector for Salmonella infection, and several high-profile outbreaks and recalls have occurred in recent years (11, 24, 46). Potential routes for preharvest contamination of fresh produce with human enteric pathogens include exposure to contaminated surface or irrigation water, fecal contamination from livestock or wild animals, raw or poorly composted manure, farm equipment, human carriers, and, possibly, dust. Surveys to measure the incidence and/or amount of Salmonella contamination by some of these potential reservoirs have been reported previously (22, 53).
As part of an ongoing project to survey agricultural and wildlife environments in a leafy vegetable production region on the central California coast for the presence of enteric pathogens, we obtained enrichment cultures that resulted from separate samples of water, preharvest lettuce and spinach, cattle and wildlife feces, soil and sediment, and fecal material from several species of wildlife, for detection of Shiga toxin-producing Escherichia coli (STEC), that were preserved in frozen storage for subsequent studies. Although enrichment methods reported for Salmonella and pathogenic E. coli often are different, the same primary enrichment cultures appropriate for isolating both pathogens also have been reported (7). Indeed, tryptic soy broth (TSB) and the incubation conditions used for enrichment of E. coli potentially could also enrich for Salmonella. Recalls of Salmonella-contaminated produce grown in our sampling area have occurred (16, 17), and we wanted to determine if Salmonella was endemic to the region. Therefore, we tested a subset of this collection of frozen enrichments to assess the incidence of Salmonella in this section of California, which was not previously surveyed for Salmonella. A multipathogen oligonucleotide microarray and various Salmonella enrichment methods were tested on a subset of frozen enrichment cultures before selecting an appropriate set of media for isolation of strains. We screened 2,401 frozen enrichment cultures by a prototype method, and confirmed Salmonella isolates were analyzed subsequently for antibiotic resistance and serotype to determine the types of strains present in the region. Genomic DNA was subjected to pulsed-field gel electrophoresis (PFGE) and compared with isolates in the PulseNet database at the Centers for Disease Control and Prevention. To our knowledge, this is the first report of the incidence of Salmonella in Monterey County, CA.
MATERIALS AND METHODS
Samples.
The frozen enrichment broths we tested were from samples obtained as part of a large study to assess the incidence, reservoirs, and transport of E. coli in a specific California agriculture environment. The set of enrichments selected randomly for this study were from samples of water, wildlife, cattle, preharvest lettuce and spinach, and soil and sediment collected from May 2008 through June 2009. Most samples were taken from locations within Monterey County, with a small percentage from adjacent San Benito and San Luis Obispo Counties in California (Fig. 1 and 2). Five wildlife samples from Tuolumne and Mariposa Counties, approximately 150 to 200 miles east of Monterey County, were also included in this set of frozen enrichments. Samples included soil/sediment, water, cattle, preharvest produce, and wildlife on ranches that also contained or were close to leafy-produce-growing operations. Additionally public water sources and wildlife not located on or near ranches were sampled from the region. The numbers and types of wildlife depended on what was found on or near ranches, the trapping/hunting season ongoing at the time of harvest, or the wildlife species available as a result of predation requests in the region. Sampling was targeted to areas of leafy produce production and corresponded to the plant production and harvest cycles; thus, sampling increased during the intensive production cycles of March through October. The majority of samples tested in this study were collected from September 2008 through April 2009. Most source locations are identified by number to ensure confidentiality and to allow comparison of results for isolates obtained from the same location. Sample types are identified, i.e., cattle or wildlife feces, plant, soil, water, wildlife. Cattle samples consisted of 10 g of feces added to 90 ml of tryptic soy broth (TSB; Becton Dickinson, Franklin Lakes, NJ). For soil and sediment samples, 10 g was added to 90 ml TSB. Water samples consisted of either a grab sample of water or Moore swabs (cut cotton gauze tied to a string) placed in a watershed for 2 to 3 days and saturated with water (8). For water samples, 10 ml of 10× TSB was added to 100 ml of water. Moore swabs were suspended in 250 ml of TSB. The maps in Fig. 1 and 2 show the watershed in the sampling region. Sixty water samples were obtained from water sources with public access and included Carr Lake, San Lorenzo Creek, Natividad Creek, various locations along the Gabilan Creek, the Salinas River, Old Salinas River, Tembladero Slough, San Benito River, and other small creeks in the region. The remainder of the water samples came from private lands and were identified only by site codes as part of access agreements. All plant samples in this study were either lettuce or spinach collected preharvest from the field. For plant samples, 25 g collected from all parts of the plant was placed into 250 ml TSB. Wildlife samples were feces or colon tissue from coyotes, deer, elk, wild pigs, mice, opossums, rabbits, raccoons, skunks, and squirrels or anal/cloacal swabs from birds (crows, ducks, juncos, owls, sparrows, starlings, titmice, towhees, warblers, and woodpeckers). For wild pigs and coyotes, 10 g of feces or colon tissue was added to 100 ml TSB. Small-mammal samples were either anal swabs or 10 g of colon tissue added to 90 ml TSB; for samples <10 g, the entire sample was diluted 1:10 in TSB. Samples from birds were cloacal swabs added to 50 ml TSB. These samples had been processed originally for E. coli O157:H7, usually within 24 h of collection, as described previously (14) by an initial enrichment in TSB for 2 h at 25°C with shaking at 200 rpm, followed by 8 h at 42°C with shaking at 200 rpm and then incubation at 4°C without shaking until the following morning. Aliquots of these primary enrichment cultures were mixed with sterile glycerol to a final concentration of 14.3%, and the mixture was frozen and stored at −80°C. The frozen enrichment cultures were the starting samples for our study and were processed for Salmonella during summer 2009. The enrichment sample had been stored frozen at −80°C from 1 to 12 months prior to thawing and testing for Salmonella.
Fig. 1.
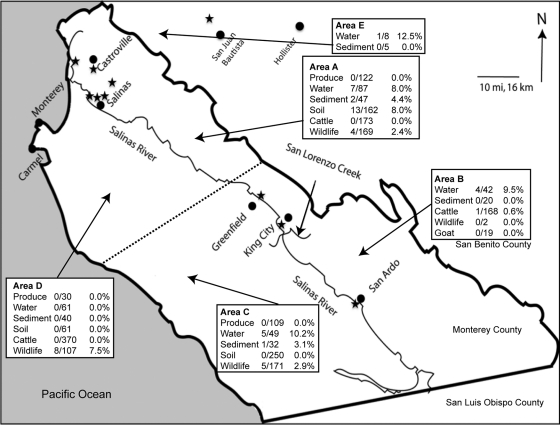
Map of Monterey County, CA, showing the Salinas River and the location of cities in the region. Areas of sampling (A to E) were assigned into sectors, with the dashed line signifying the separation between the north and south and the Salinas River being the border between the east and west in Monterey County. Some samples were taken in the northwest of San Benito County (area E). The public access sites where samples yielded positive Salmonella isolates from water collected on public lands are designated by a star. These sites are noted in Table 4. The arrows pointing to areas A to E are not specific to a particular sampling site.
Fig. 2.
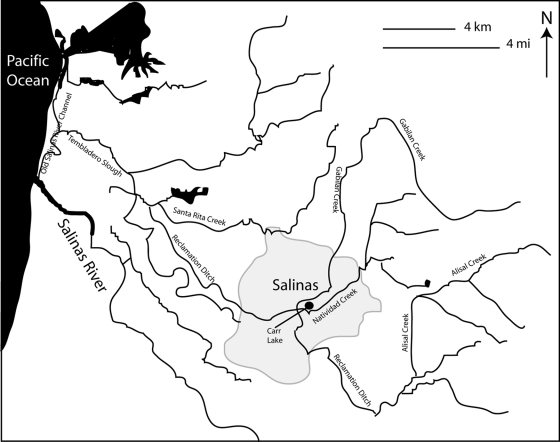
Map of the watershed surrounding Salinas, CA. Several of the creeks and lakes that were sampled are labeled.
Microarray analysis. (i) Construction of the multipathogen oligonucleotide microarray.
The 70-mer oligonucleotide probes specific for E. coli and S. enterica (see Table S1 in the supplemental material) were designed by using the Array Designer 3.0 software (Premier Biosoft International, Palo Alto, CA), with an average melting temperature of 73°C. The printing of the DNA microarray on UltraGaps glass slides (Corning Inc., Corning, NY) was described previously (39).
(ii) Genomic DNA isolation, multiplex PCR, and fluorescent labeling for microarray analysis.
Fifty-four of the primary enrichment cultures were tested on the multipathogen oligonucleotide microarray prior to freezing. To obtain DNA templates for multiplex PCR, 1 ml of each primary 24-h enrichment culture was centrifuged at 14,500 × g for 5 min. The pellet was resuspended in 1 ml of water, boiled, and extracted with phenol-chloroform (39). Alternatively, 100 μl of enrichment culture was centrifuged at 14,500 × g for 5 min, and genomic DNA was isolated by using a DNeasy blood and tissue kit (Qiagen, Valencia, CA), following the manufacturer's specifications.
Each multiplex PCR and 16S PCR consisted of 1× MasterAmp Taq PCR buffer (Epicentre, Madison, WI), 1× MasterAmp Taq enhancer, 3 mM MgCl2, 200 μM each deoxynucleoside triphosphates (New England BioLabs, Ipswich, MA), multiplex primer mix or 16S primers with forward and reverse primers at 0.1 μM each, 1 U of Taq DNA polymerase (New England BioLabs, Ipswich, MA), and approximately 50 to 200 ng of genomic DNA (final reaction volume, 50 μl). The reaction mixture was processed in an MJ Research thermocycler (Bio-Rad Laboratories, Hercules, CA) with the following settings: 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C for 30 cycles, followed by a final extension time of 5 min at 72°C. Oligonucleotides were purchased from MWG Eurofins Operon (Huntsville, AL). Aliquots of 45 μl for each multiplex PCR were combined with 1 μl of 16S PCR and purified by using a QIAquick PCR purification kit (Qiagen, Valencia, CA).
The multiplex and 16S PCR products from the enrichment cultures were labeled by mixing the combined purified reactions with 5 μl of 10× random octadeoxyribonucleotides (New England BioLabs, Ipswich, MA) and water to a final volume of 41 μl. All labeling reaction samples were heated to 95°C for 5 min, cooled for 5 min at 4°C, and then added to the remaining labeling reaction consisting of 5 μl of 10× deoxynucleoside triphosphate (dNTP) labeling mix (1.2 mM each dATP, dGTP, and dCTP and 0.5 mM dTTP in 10 mM Tris, pH 8.0; 1 mM EDTA) (Promega Corporation, Madison, WI), 3 μl of 25 nmol Amersham CyDye fluorescent nucleotides, Cy3-dUTP or Cy5-dUTP (GE Healthcare Life Sciences Corp., Piscataway, NJ), and 5 U of Klenow fragment (3′ → 5′ exo−) (New England BioLabs). The mixtures were incubated overnight at 37°C, as previously described (36). The labeled DNA was purified from unincorporated CyDye fluorescent label by using the QIAquick PCR purification kit (Qiagen, Valencia, CA) and was eluted finally in 100 μl of 10 mM Tris, pH 8.0. The CyDye-labeled DNA was combined and vacuum dried by using a Savant ISS110 speed vac concentrator (Thermo Electron Corp., Milford, MA).
(iii) Microarray hybridization.
The CyDye-labeled DNAs from the tested enrichment sample and from the reference strains were resuspended in 20 μl of Pronto! Long-Oligo/cDNA hybridization solution (Corning Inc., Corning, NY), heated to 95°C for 5 min, and centrifuged immediately at 14,500 × g for 2 min at room temperature. This hybridization mixture then was applied to each DNA microarray slide, and the slide was sealed with a coverslip (Corning). The microarray slide was placed in a hybridization chamber (Corning) and incubated at 42°C for 18 h (36). Wash buffer for the microarray slides was saline-sodium citrate buffer (SSC), and 1× SSC contained 150 mM NaCl plus 15 mM sodium citrate. Following hybridization, the slides were transferred to a microarray wash station (TeleChem International, Inc., Sunnyvale, CA) and were washed twice in 2× SSC plus 0.1% sodium dodecyl sulfate at 42°C for 5 min, washed twice in 1× SSC at room temperature for 10 min, and finally washed twice in 0.2× SSC at room temperature for 10 min. The microarray slides were transferred to a slide tray (Evergreen Scientific, Los Angeles, CA) and dried by centrifugation at 300 × g for 10 min prior to scanning. At least two replicate hybridization reactions were performed for each sample. Each replicate hybridization reaction was applied to a microarray on a different slide to account for the slide-to-slide variation.
(iv) Microarray data analysis.
DNA microarrays were scanned using an Axon GenePix 4000B microarray laser scanner (Molecular Devices Corporation, Sunnyvale, CA) at 532-nm (Cy3) and 635-nm (Cy5) excitation wavelengths with a 10-μm resolution, as previously described (36). For the analysis of the DNA microarray data, the fluorescence signal per triplicate spot for each microarray (2 replicates) with no significant differences was quantified after subtracting the local background by using GenePix 4.0 software (Molecular Devices). Spots were excluded from further analysis if they had an anomalous spot morphology or were within regions of nonspecific fluorescence. The microarray data were analyzed further with GeneSpring 7.2 software (Agilent Technologies, Santa Clara, CA) for averaging the background-corrected fluorescence signal per spot on replicate microarrays, for fluorescence signal comparisons, and for average-linkage hierarchical clustering with the standard correlation (36). Salmonella was considered present in environmental enrichment samples if fluorescence intensities of >15-fold above that of the control probes were obtained for six of 10 S. enterica serotype Typhimurium probes. To examine the detection sensitivity of the oligoarray and to validate that probes were not cross-reactive, mixed cultures containing S. Typhimurium and E. coli O157:H7 were examined. In artificial enrichment cultures with ratios of S. Typhimurium to E. coli O157:H7 of 1:10, 1:100, 1:1,000, 1:10,000, and 1:100,000, multiplex PCR followed by fluorescence labeling and array hybridization resulted in fluorescence intensities of >15-fold above the fluorescence intensities of control probes for 10/10, 9/10, 7/10, 4/10, and 2/10 S. Typhimurium probes, respectively.
Salmonella enrichment methods and indicator culture media.
The media used for testing various Salmonella enrichment methods were TSB, tetrathionate broth (TT), modified semisolid Rappaport-Vassiliadis agar (MSRV), bismuth sulfite agar (BiSA), Salmonella-Shigella agar (SS), xylose lysine deoxycholate (XLD) agar, Hektoen enteric (HE) agar, and CHROMagar Salmonella. All media were prepared from dehydrated stocks (Difco, Becton Dickinson, Franklin Lakes, NJ) except for CHROMagar Salmonella, which was purchased as prepared plates (Beckton Dickinson-BBL, Franklin Lakes, NJ), and MSRV, which was made as described previously (3).
The Salmonella enrichment methods tested were variations on standardized methods from the FDA Bacteriological Analytical Manual (BAM) method (1), USDA Food Safety Inspection Service (FSIS) method (2), and method 1682 of the U.S. Environmental Protection Agency (EPA) (3). These methods all include a nonselective enrichment overnight at 35 to 37°C followed by secondary enrichment in TT or MSRV at 42°C and then plating on a selective indicator agar (BiSA, SS, HE, XLD, or a chromogenic medium) and incubation at 35 to 37°C. In a preliminary study, TSB enrichment cultures were processed also by immunomagnetic separation (IMS) with Dynabeads anti-Salmonella (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions with the automated Dynal BeadRetriever (Invitrogen). IMS beads were collected in 100 μl of washing buffer (0.15 M NaCl, 0.01 M sodium-phosphate buffer [pH 7.4], and 0.05% Tween 20), and 50 μl was plated onto both XLD and HE agars.
To determine the most efficient method for detection of Salmonella from these frozen primary enrichment cultures, over 50 frozen enrichments, including seven indicated by microarray analysis to be positive potentially for Salmonella (by reaction with S. Typhimurium probes), were tested using various enrichment methods. A portion of a frozen TSB enrichment sample was scraped with a wooden applicator to obtain a frozen piece that corresponded to approximately 20 to 50 μl and was deposited into 10 ml of TSB and reenriched overnight at 35°C. The resulting cultures were subcultured in multiple ways. One milliliter of the reenriched culture was subcultured into 10 ml of fresh TT and grown overnight at 42°C. The nonselective enrichments were also spot inoculated onto MSRV plates (six spots of 30 μl each, deposited in a circle onto the surface of the plate) and incubated right side up at 42°C for up to 2 days. The resulting TT cultures, and the furthest edge of spreading growth (if present) on MSRV, were inoculated onto XLD and HE plates and incubated overnight at 37°C.
Three to five Salmonella presumptive (black) colonies were selected from each XLD or HE plate and streaked for isolation. PCR was performed on these isolates with primers that targeted the invA gene of Salmonella. The template for PCR was a portion of a black colony from XLD or HE added to a 25-μl PCR. The primers were INVA-1 (5′-ACA GTG CTC GTT TAC GAC CTG AAT-3′) and INVA-2 (5′-AGA CGA CTG GTA CTG ATC GAT AAT-3′) (13). PCR mixtures contained 300 nM each primer, 200 μM dNTPs, 1× PCR buffer, and 2.5 U Taq polymerase (New England BioLabs), and the protocol was 95°C for 10 min and 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, followed by 72°C for 7 min and then holding at 4°C until analysis. Those isolates that displayed an amplicon of the correct size with the invA primers (244 bp) on a 1.5% agarose gel were cultured overnight in TSB at 37°C. A 900-μl aliquot was mixed with 100 μl of 10 M glycerol and frozen at −80°C, and another aliquot (1.5 ml) was used for genomic DNA isolation using either the Wizard genomic DNA purification kit (Promega, Madison, WI) or the DNeasy blood and tissue kit (Qiagen, Valencia, CA). The 16S rRNA gene was amplified by PCR using the same ingredient concentrations as those for the invA PCR with the primers 27f (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1392r (5′-GAC GGG CGG TGT GTA C-3′) (29). Amplification conditions were described previously (21). Ten microliters of the reactions was electrophoresed on a 1% agarose gel, and those PCRs that had an amplicon of approximately 1,200 bp were purified with Exo Sap-It (USB-Affymetrix, Cleveland, OH) and sequenced using the 27f and 1392r primers. Some isolates showed no amplicon with the 27f/1392r primer set, so for those isolates the 16S rRNA gene was amplified with the primers 530f (5′-GTG CCA GCM GCC GCG G-3′) and 1525r (5′-AAG GAG GTG WTC CAR CC-3′) (29), with the same amplification conditions as those used for the 27f/1392r primer set, and sequenced using 27f and 1392r primers. DNA sequencing was done using BigDye Terminator version 3.1 chemistry (Applied Biosystems, Foster City, CA) on an ABI Prism 3730 sequencer. DNA sequences were compared to the database of 16S sequences on the Ribosomal Database Project (http://rdp.cme.msu.edu/), and genus identity was noted.
Comparison of MSRV protocol to new IMS protocol.
Nearing the end of this study, we noted a new method reported by Kalchayanand et al. (27) that is similar to the TSB-MSRV method described above, but it involved a modified IMS step that included a secondary enrichment in Rappaport-Vassiliadis soya peptone broth (RVS broth; Oxoid, Remel, Inc., Lenexa, KS). We compared the MSRV method and this new IMS method (27) with a set of unfrozen samples of water and wildlife that were part of a different study and unrelated to the samples described in the current study. These samples were enriched in TSB as described above, and one aliquot was plated on MSRV, and a second aliquot was added to Dynal anti-Salmonella beads (Invitrogen, Carlsbad, CA) and processed with a Dynal BeadRetriever (Invitrogen) as described by the manufacturer. The processed beads were placed into 3 ml of RVS broth (Oxoid, Cambridge, United Kingdom) and incubated at 42°C. Broth samples with obvious growth were plated onto XLD agar; black colonies were selected and confirmed by PCR and sequencing as described above.
Analysis of Salmonella isolates.
Those isolates confirmed as Salmonella by invA-positive PCR and 16S analysis were analyzed for serotype, antibiotic resistance, and by pulsed-field gel electrophoresis. Strains were serotyped by the USDA-APHIS, National Veterinary Services Laboratory (Ames, IA). Antibiotic resistance was determined by the Kirby-Bauer method using disks impregnated with the antibiotics (Becton-Dickinson, Franklin Lakes, NJ) deposited onto a lawn of early-logarithmic-phase bacterial culture in TSB swabbed onto Mueller-Hinton agar (Difco). The plates were incubated for 18 h at 35 to 37°C, and the zones of inhibition were measured and the manufacturer's instructions were followed to assess resistance or susceptibility. The antibiotics tested were amikacin (30 μg), amoxicillin-clavulanic acid (20 μg/10 μg), ampicillin (10 μg), ceftriaxone (30 μg), cephalothin (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), kanamycin (30 μg), streptomycin (10 μg), tetracycline (30 μg), and trimethoprim-sulfamethoxazole (1.25 μg/23.75 g).
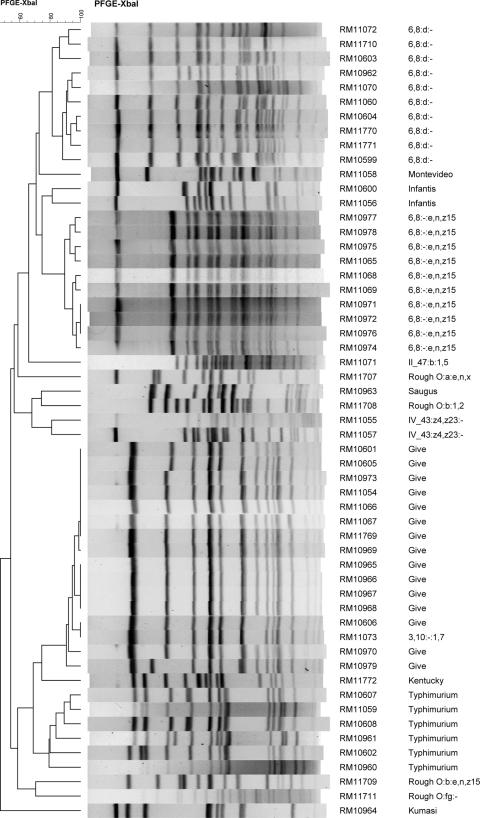
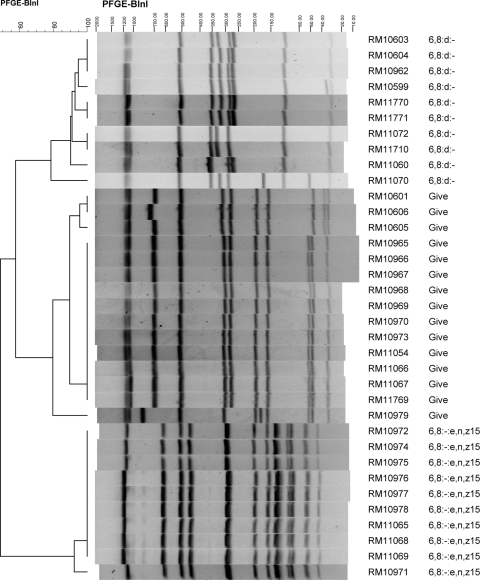
Chromosomal DNA was prepared, restricted with XbaI, and subjected to pulsed-field gel electrophoresis analysis with reference Salmonella strains as described previously (40). For strains resulting in “smearing” of DNA, thiourea was added to the running buffer (44). Images of the restriction patterns were imported into BioNumerics version 6.01 (Applied Maths, Austin, TX), and bands were assigned visually using BioNumerics band assignment. A standard isolate (S. enterica serotype Braenderup H9812) was run on three lanes per gel to allow comparisons between different gels. Banding patterns were uploaded to PulseNet (CDC). Cluster analysis based on band similarity was done with BioNumerics using Dice binary coefficients and the unweighted pair group method with arithmetic averages (UPGMA). A similarity matrix was calculated for the XbaI patterns, and strain groupings with >95% similarity were further differentiated with PFGE analysis using BlnI. The similarities between lanes or groups of lanes were determined by reading the similarity values on the dendrogram (Fig. 4).
Fig. 4.
Dendrogram of XbaI PFGE patterns of the 55 Salmonella isolates.
Analysis of recovery of Salmonella from frozen enrichment broths relative to time.
To test if length of storage time at −80°C affected the recoverability of Salmonella, serial dilutions of two Salmonella strains were frozen in three enrichment culture backgrounds that had tested negative for Salmonella previously. Soil, cattle feces, and water enrichment cultures were tested as representative of diverse background microbiota. These enrichment cultures were revived as described above and grown overnight in TSB at 37°C. The enrichment cultures were aliquoted into cryovials in 500-μl volumes. These enrichments were inoculated with dilutions of Salmonella strains that were grown overnight in TSB, diluted 1:100 into fresh medium, and grown to an A600 of 0.2. An S. enterica serovar Poona strain from our collection (RM2350) and an S. enterica serovar Infantis isolate from this study (RM10600) were used. Serial dilutions of the Salmonella cultures were made in phosphate-buffered saline (PBS; 10 mM sodium phosphate [pH 7.2], 150 mM NaCl), and 10 μl of the 100, 10−2, 10−4, and 10−6 dilutions was inoculated into the cryovials containing enrichment cultures, so the final concentrations of Salmonella in the vials were ∼1.8 × 106 CFU/ml, 1.8 × 104 CFU/ml, 1.8 × 102 CFU/ml, and 1.8 CFU/ml. Two hundred microliters of sterile 50% glycerol was added to each vial, and the vials were placed immediately in the −80°C freezer. These conditions were identical to those used to freeze the enrichments originally. The spiked enrichment cultures were tested in triplicate by the same method used to revive the frozen enrichments for sampling after 3 h (day 0), 1 week, 4 months, and 6 months of frozen storage. Enrichments that yielded spreading growth on MSRV were scored as positive for Salmonella. Unspiked negative-control enrichment cultures were also included in the experiment.
Weather.
Historical weather data for the entirety of Monterey County were retrieved from www.wunderground.com.
RESULTS
Microarray analysis.
We tested 54 environmental enrichment cultures for the presence of Salmonella by oligoarray-based bacterial detection. Seven of the 54 cultures were positive for some of the S. Typhimurium probes, and those seven are shown in Table 1. Two of these seven were culture positive for Salmonella (Table 1). Analysis of water samples W-0119 and W-0126 resulted in 9/10 and 6/10 S. Typhimurium-positive probes, respectively. Moreover, W-0119 was positive for the Salmonella wzx group C2 probe, and W-0126 was positive for the Salmonella wzx group C1 probe. The other five samples that reacted with S. Typhimurium probes on the oligoarray were SBST-29 and SBST-10 (positive for 4/10 probes and also 2 Salmonella group B probes), W-0117 and SBST-06 (positive for 3/10 probes and also 2 Salmonella group B probes), and finally W-0127 (positive for 5/10 S. Typhimurium probes). Salmonella was not recovered from these other five samples. Based on validation studies using artificial enrichment cultures (see Materials and Methods) and the culturing results, a reaction with 6/10 S. Typhimurium probes with >15-fold fluorescence above the negative-control probe fluorescence was considered the threshold indication that an enrichment culture was positive for Salmonella on the oligoarray.
Table 1.
Summary of results for some samples used to determine enrichment methoda
| Original sample identifier | No. of positive microarray probes (out of 10) | Enrichment methodb |
Salmonella isolatedc | Salmonella strain ID | ||
|---|---|---|---|---|---|---|
| TT + XLD/HE | TT + CHROMagar | MSRV + XLD/HE | ||||
| W-0117 | 3 | + | − | − | − | NA |
| W-0119 | 9 | + | + | + | + | RM10599 |
| W-0126 | 6 | + | + | + | + | RM10600 |
| W-0127 | 5 | + | − | − | − | NA |
| SBST-0006 | 3 | + | − | − | − | NA |
| SBST-0010 | 4 | + | − | − | − | NA |
| SBST-0029 | 4 | + | − | − | − | NA |
| W-0196 | ND | + | + | + | + | RM10601 |
| W-0197 | ND | + | − | + | + | RM10602 |
| W-0198 | ND | + | − | − | − | NA |
| W-0199 | ND | + | − | − | − | NA |
| S-0983 | ND | + | − | − | − | NA |
| S-0984 | ND | + | − | − | − | NA |
| S-0985 | ND | + | − | − | − | NA |
| S-0986 | ND | + | − | − | − | NA |
| S-0987 | ND | + | − | − | − | NA |
| S-0988 | ND | + | − | − | − | NA |
| S-0989 | ND | + | − | − | − | NA |
| S-0990 | ND | + | − | − | − | NA |
ND, not done (not among the 54 samples tested by microarray); NA, not applicable.
Primary cultures are in TSB followed by subculturing on TT plus XLD/HE and MSRV plus XLD/HE, which yield black colonies, and TT plus CHROMagar (mauve colonies). + and −, differentiated colonies selected for confirmation (black on XLD and/or HE, mauve on CHROMagar).
+, yes; −, no.
Testing enrichment methods and media.
TSB and universal preenrichment broth were tested as reenrichment media on a subset of samples and behaved identically (data not shown). Ultimately, we decided to reenrich the frozen cultures in TSB to avoid additional stress on the cells and because it would allow for more efficient molecular detection (21) if needed. In our experience, not all strains of Salmonella form black colonies on BiSA and SS agars, whereas HE and XLD agars were much more predictable in Salmonella differentiation across different strains (data not shown). Therefore, we focused on HE and XLD as detector media. Additionally, every nontyphoid, motile Salmonella strain we tested on MSRV at 42°C grew to form spreading colonies, indicating that this method was robust for Salmonella isolation.
To test enrichment methods for recovery of Salmonella from stored frozen TSB enrichment cultures, we tested over 50 frozen enrichment cultures, including the seven enrichments that reacted with Salmonella probes in the microarray analysis (Table 1). These enrichments were tested with variations of certified Salmonella enrichment and isolation methods as described above. A summary of the results for 19 samples, including the seven positive by microarray analysis, is shown in Table 1. All of the test samples yielded black colonies on XLD and HE agars after secondary enrichment in TT, but only four of those amplified a product with invA primers (W-0119, W-0126, W-0196, and W-0197). The rest of the black colonies resulting from TT were revealed by 16S analysis to be Citrobacter or Proteus. When the TT secondary cultures were inoculated onto CHROMagar Salmonella, the false positives were eliminated, but only three samples resulted in mauve colonies on CHROMagar (W-0117, W-0119, and W-0196). Using MSRV for secondary enrichment instead of TT, four of the samples (again, W-0119, W-0126, W-0196, and W-0197) yielded black colonies on XLD and HE agars, and all of these black colonies yielded a 244-bp amplicon after PCR with primers for invA. XLD and HE plates gave identical results in the trials; therefore, the remaining samples were processed by TSB primary enrichment followed by secondary enrichment on MSRV and plating on XLD; essentially, this is EPA method 1682 (3). IMS beads applied to primary TSB cultures revealed no enhancement of Salmonella recovery (data not shown).
Screening enrichments for Salmonella.
The 2,401 frozen enrichment cultures tested were derived from samples collected from 78 locations; 55 (2.3%) samples from 20 different locations were positive for Salmonella. Fifty-one positive samples were located in or very close to Monterey County and are indicated on the map in Fig. 1. The highest percentage of total positive samples (18 of 252; 7.1%) was from water, whereas the highest total number of positive samples (20 of 476; 4.2%) was associated with wildlife. The majority of the positive water samples (13 of 18) came from flowing public access water sources that are part of the Salinas Valley watershed. The locations of these public water sites are indicated in Fig. 1 with a star symbol. Sixteen of the 617 soil/sediment samples (2.6%) were positive. None of the 261 plant samples were positive, and only one of 795 (0.13%) fecal samples from cattle tested positive. The 20 wildlife samples positive for Salmonella were from bird (sparrow, towhee, and crow), coyote, deer, elk, opossum, wild pig, and skunk samples. Salmonella was not detected in wildlife samples taken from mouse (n = 24), rabbit (n = 57), raccoon (n = 2), and squirrel (n = 28) or bird samples from blackbirds (n = 57), geese (n = 17), mallard (n = 3), starlings (n = 4), woodpecker (n = 1), titmouse (n = 1), junco (n = 2), and warbler (n = 1). Seventeen of the Salmonella wildlife strains were isolated from samples in or very close to Monterey County and are included in the numbers shown in Fig. 1. Three additional strains from wildlife samples came from San Luis Obispo and Tuolumne Counties.
Due to access agreements for sampling on private lands, the precise locations of some sampling sites are not identified; however, we have identified the approximate locations of 51 of the positive Salmonella isolates by organizing them into five regions of Monterey County sectioned as northeast and southwest of the Salinas River and above and below a line drawn arbitrarily from the coast to the boundary for Monterey/San Benito Counties (the Gabilan Mountain Range). These are indicated in Fig. 1. The locations of four samples were in San Luis Obispo and Tuolumne Counties, which are outside the map region in Fig. 1. Salmonella was isolated from water in four of the five sectors of Monterey County and from wildlife in three of the five sectors. The soil samples that yielded Salmonella were obtained from the same site sampled on the same day. Two different serotypes were isolated from these soil samples (see below), suggesting that contamination may have occurred due to one or more contamination events.
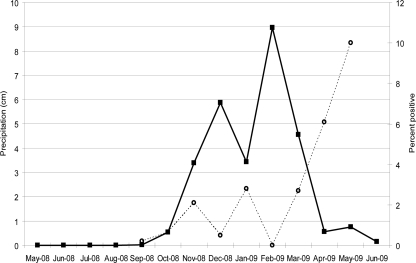
Table 2 shows the months that samples we tested were collected and the percentage of Salmonella isolated from these samples by month. The percentage of positive samples increased in the second half of the sampling period (January to May 2009), indicating that there may be a seasonal effect on Salmonella presence. Figure 3 shows the cumulative precipitation by month during the sampling year compared to the percentage of samples positive for Salmonella by month. The results indicate increases in the percentage of positive samples (November 2008, January 2009, March and April 2009) in the months following large increases in precipitation. Although the percentage of positive samples did not peak in direct relation to increases in precipitation, more positive samples appeared in the weeks following large relative amounts of precipitation. The wettest months were from November 2008 through March 2009, and Salmonella peaks were from March 2009 through May 2009. The wettest month was February 2009, when no positive Salmonella samples were recovered. April 2009, the month with the highest percentage of Salmonella-positive samples, had little precipitation but followed 2 months of the highest rainfall. There were also examples of positive and negative Salmonella samples collected during, or days after, heavy rains and also during dry periods, indicating that rainfall might play some role in Salmonella dissemination, but it is not the only factor.
Table 2.
Times of sample collections and Salmonella detection in the region
| Month | No. of samples tested | No. of samples positive for Salmonella | % positive |
|---|---|---|---|
| May 2008 | 20 | 0 | 0 |
| August 2008 | 9 | 0 | 0 |
| September 2008 | 500 | 1 | 0.2 |
| October 2008 | 154 | 1 | 0.65 |
| November 2008 | 95 | 2 | 2.1 |
| December 2008 | 420 | 2 | 0.5 |
| January 2009 | 177 | 5 | 2.8 |
| February 2009 | 124 | 0 | 0 |
| March 2009 | 402 | 11 | 2.7 |
| April 2009 | 429 | 26 | 6.1 |
| May 2009 | 70 | 7 | 10.0 |
| June 2009 | 1 | 0 | 0 |
Fig. 3.
Relationship between incidence of Salmonella and precipitation in the region. The sample months are on the x axis. The solid line with the squares indicates the amount of precipitation during the month, and the dashed line represents the percentage of samples collected during that month that were positive for Salmonella. Few or no samples were collected in the period of May 2008 through August 2008 and in June 2009; therefore, no values are shown on the “percent positive” axis.
Recovery of Salmonella from frozen enrichment cultures over time.
We were concerned about the recoverability of Salmonella from these frozen enrichments, some of which were frozen for 12 months before initiation of our study. To address this issue, we spiked Salmonella-negative enrichment cultures representing different types of microbiota with two different Salmonella strains at various concentrations and froze them for up to 6 months at −80°C. These cultures yielded Salmonella even from initial storage concentrations of 1.8 × 102 CFU/ml and in some cases from 1.8 × 100 CFU/ml (Table 3), indicating minimal loss of viability of Salmonella in frozen enrichment broths.
Table 3.
Percent recovery of Salmonella on day 0 and after 6 months of storage at −80°C, expressed as percent of replicates testing positive for Salmonella
| Starting concn in frozen sample (CFU/ml) | % recovery |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cattle fecal background |
Soil background |
Water background |
||||||||||
|
S. Poona |
S. Infantis |
S. Poona |
S. Infantis |
S. Poona |
S. Infantis |
|||||||
| Day 0 | 6 mo | Day 0 | 6 mo | Day 0 | 6 mo | Day 0 | 6 mo | Day 0 | 6 mo | Day 0 | 6 mo | |
| 1.8 × 106 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1.8 × 104 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1.8 × 102 | 66 | 66 | 66 | 100 | 100 | 100 | 66 | 66 | 66 | 100 | 100 | 66 |
| 1.8 × 100 | 0 | 0 | 0 | 33 | 33 | 0 | 0 | 33 | 0 | 66 | 66 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
PCR confirmation, PFGE, and serotyping of Salmonella isolates.
Black colonies selected from XLD agar were tested for invA by PCR and confirmed as Salmonella by 16S analysis. Isolates positive by both methods were subjected to pulsed-field gel electrophoresis, and the resulting patterns were compared. Generally, the pulsed-field patterns of individual isolates from the same sample were extremely close (1 band difference) or indistinguishable, so one isolate from a sample enrichment was sent for serotype analysis and tested for antibiotic sensitivity.
Table 4 summarizes the source, serotype, and antibiotic results of all of the Salmonella isolates. Some strains did not correspond to known serotypes, because they did not display both phases of flagellar antigens. Four strains were not typeable because they had rough surfaces and did not react with any O-typing antisera. Serotype analysis confirmed that 50 strains were S. enterica subsp. enterica. Strains RM11055 and RM11057 were S. enterica subsp. houtenae, and RM11071 was S. enterica subsp. salamae. The four rough strains were of indeterminate subspecies. S. enterica serovars Give, Typhimurium, and Infantis were isolated from 15, 6, and 2 of the samples, respectively; S. enterica serovars Montevideo, Kumasi, and Kentucky were each isolated from a single sample (Table 4). The only antibiotic-resistant strain, S. Montevideo strain RM11058, which originated from a skunk sample, was resistant to streptomycin and gentamicin. Some strains showed intermediate results with streptomycin and tetracycline, but the majority of the strains were sensitive to all 12 antibiotics.
Table 4.
Information on Salmonella strains isolated in this study
| Strain identifier | Source | Date of collection of original sample (mo/day/yr) | S. enterica serotype | Site code or locationa |
|---|---|---|---|---|
| RM10601 | Water, stream | 1/22/2009 | Give | Carr Lake, Salinas, CA |
| RM10606 | Opossum fecal swab | 3/4/2009 | Give | 2 |
| RM10605 | Water | 3/9/2009 | Give | Tembladero Slough, Castroville, CA |
| RM10965 | Soil | 4/29/2009 | Give | 3 |
| RM10966 | Soil | 4/29/2009 | Give | 3 |
| RM10967 | Soil | 4/29/2009 | Give | 3 |
| RM10968 | Soil | 4/29/2009 | Give | 3 |
| RM10969 | Soil | 4/29/2009 | Give | 3 |
| RM10970 | Soil | 4/29/2009 | Give | 3 |
| RM10973 | Soil | 4/29/2009 | Give | 3 |
| RM11054 | Soil | 4/29/2009 | Give | 3 |
| RM11769 | Soil | 4/29/2009 | Give | 3 |
| RM11066 | Crow (bird) feces | 4/29/2009 | Give | 10 |
| RM11067 | Crow (bird) feces | 4/29/2009 | Give | 10 |
| RM10979 | Opossum feces | 4/29/2009 | Give | 2 |
| RM11073 | Water | 5/5/2009 | 3,10:−:1,7 | Salinas River, San Ardo, CA |
| RM10600 | Water, trough well | 11/17/2008 | Infantis | 8 |
| RM11056 | Skunk fecal swab | 4/28/2009 | Infantis | 10 |
| RM11722 | Wild pig feces | 5/12/2009 | Kentucky | Tuolumne County, CA |
| RM10964 | Skunk fecal swab | 4/22/2009 | Kumasi | 10 |
| RM11058 | Skunk fecal swab | 5/7/2009 | Montevideob | 2 |
| RM10963 | Deer feces | 10/4/2008 | Saugus | Monterey County, CA |
| RM11059 | Water, stream | 1/6/2009 | Typhimurium | Gabilan Creek, Salinas, CA |
| RM10602 | Water, pond | 1/22/2009 | Typhimurium | Carr Lake, Salinas, CA |
| RM10961 | Water, stream | 1/22/2009 | Typhimurium | Gabilan Creek, Salinas, CA |
| RM10608 | Spotted towhee (bird) cloacal swab | 3/8/2009 | Typhimurium | San Luis Obispo County, CA |
| RM10607 | White crowned sparrow (bird) cloacal swab | 3/8/2009 | Typhimurium | San Luis Obispo County, CA |
| RM10960 | Water | 4/1/2009 | Typhimurium | Old Salinas River, Castroville, CA |
| RM10599 | Water, standing water | 11/12/2008 | 6,8:d:− | 6 |
| RM10603 | Water | 3/9/2009 | 6,8:d:− | 6 |
| RM11771 | Water | 3/9/2009 | 6,8:d:− | 6 |
| RM10604 | Water | 3/9/2009 | 6,8:d:− | Salinas River, Greenfield, CA |
| RM11060 | Water | 3/9/2009 | 6,8:d:− | San Lorenzo Creek, King City, CA |
| RM11770 | Water | 3/9/2009 | 6,8:d:− | Salinas River, San Ardo, CA |
| RM10962 | Water, stream | 3/31/2009 | 6,8:d:− | 6 |
| RM11710 | Stream sediment | 3/31/2009 | 6,8:d:− | 6 |
| RM11070 | Water, stream | 5/5/2009 | 6,8:d:− | San Benito River, San Juan Bautista, CA, San Benito County |
| RM11072 | Water, stream | 5/5/2009 | 6,8:d:− | Gabilan Creek, Salinas, CA |
| RM10978 | Water, field irrigation | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM10971 | Soil | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM10972 | Soil | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM10974 | Soil | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM10975 | Soil | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM10976 | Sediment, irrigation ditch | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM10977 | Sediment, irrigation ditch | 4/29/2009 | 6,8:−:e,n,z15 | 3 |
| RM11065 | Coyote feces | 4/29/2009 | 6,8:−:e,n,z15 | 4 |
| RM11068 | Crow (bird) feces | 4/29/2009 | 6,8:−:e,n,z15 | 10 |
| RM11069 | Crow (bird) feces | 4/29/2009 | 6,8:−:e,n,z15 | 10 |
| RM11071 | Coyote feces | 5/6/2009 | II_47:b:1,5 | 4 |
| RM11057 | Skunk fecal swab | 4/28/2009 | IV_43:z4,z23:− | 10 |
| RM11055 | Crow (bird) feces | 5/7/2009 | IV_43:z4,z23:− | 10 |
| RM11709 | Deer feces | 9/13/2008 | Rough O:b:e,n,z15 | Monterey County, CA |
| RM11708 | Cattle feces | 12/3/2008 | Rough O:b:1,2 | 5 |
| RM11707 | Elk feces | 12/29/2008 | Rough O:a:e,n,x | Monterey County, CA |
| RM11711 | Coyote feces | 1/7/2009 | Rough O:fg:− | 7 |
Public watersheds are identified and approximate locations are designated with stars in Fig. 1. The locations of sites designated by a number are not shown due to restrictions in confidentiality agreements.
Resistant to streptomycin, gentamicin.
Wildlife samples were the source of the greatest diversity of serotypes (Table 4). These included S. Give, S. Typhimurium, S. Saugus, S. Kumasi, S. Infantis, S. Montevideo, S. Kentucky, the subtype II and IV strains, as well as all of the rough O-antigen isolates. S. Typhimurium was isolated from songbirds, whereas S. Give, the monophasic type 6,8:−:e,n,z15, and subtype IV were isolated from crows. Skunks yielded S. Kumasi, S. Infantis, S. Montevideo, and a subtype IV strain. The serotypes isolated from water samples were S. Infantis, S. Give, S. Typhimurium, and the monophasic types 6,8:d:− and 6,8:−:e,n,z15. Soil and sediment samples yielded serotypes Give, 6,8:d:−, and 6,8:−:e,n,z15.
A dendrogram of the PFGE patterns for these strains is shown in Fig. 4. The 55 isolates resulted in 40 different XbaI PFGE patterns. A comparison of the pulsotypes of all of the isolates in this study indicated that the single S. Kumasi strain (RM10964) was the most different, sharing a similarity of 47% with the other 54 isolates, and those 54 isolates shared a 54% similarity with each other. The dendrogram shows that the PFGE patterns differentiated into groupings that correlated with serotype. The XbaI PFGE patterns of numerous isolates were very similar or indistinguishable, suggesting that they represent the same strain isolated from different samples.
The 10 isolates of serotype 6:8:d:−, 10 isolates of serotype 6,8:−:e,n,z15, and 15 isolates of S. Give had an 81.5%, 90%, and 91% similarity, respectively, in their XbaI PFGE patterns within serotype (Fig. 4). Included among the S. Give isolates in a cluster of the dendrogram indicating relatedness was an isolate of serotype 3,10:−:1,7 (RM11073); this isolate, indistinguishable from S. Give strain RM10606, shares the same O antigens as S. Give but was monophasic for the flagellar antigen. All of the isolates of S. Give, 6,8:d:−, and 6,8:−:e,n,z15 were further analyzed by PFGE using the restriction enzyme BlnI (Fig. 5). The XbaI and BlnI profiles for these strains were consistent in that all of the strains within a serotype cluster appeared to be closely related. The six S. Typhimurium isolates also clustered together and shared a similarity of 79% in their XbaI patterns, suggesting more diversity in this set of strains than in those of serotypes Give, 6,8:d:−, or 6,8:−:e,n,z15.
Fig. 5.
BlnI PFGE patterns of Salmonella strains of selected serovars isolated in this study.
The cluster of 10 strains with serotype 6,8:−:e,n,z15 shared a 90% and 92% similarity in their XbaI and BlnI PFGE patterns, respectively. It is noteworthy that these strains were isolated from samples collected on the same date, 29 April 2009, but from three different locations in Monterey County. Weather data for April 2009 indicates approximately 0.56 cm of precipitation for April and approximately 0.30 cm on April 23 in Northern Monterey County locations (e.g., North Salinas, Castroville). The similarities in PFGE patterns indicate possible dissemination of a strain through the region. Strains RM10972 (from soil) and RM10976 (from irrigation ditch sediment) had indistinguishable XbaI and BlnI PFGE patterns that were extremely similar to the patterns for RM10974 (soil). These three strains came from samples taken on the same day and location (site code 3, within area A in Fig. 1) and probably represent independent isolations of the same strain (Table 4). RM10972 and RM10976 also shared 100% similarity in their XbaI pattern but only 92% identity in their BlnI patterns to RM10971 (from soil), also isolated from a sample taken the same day and location (site code 3, area A in Fig. 1). The three strains (RM10972, RM10976, and RM10974) also were very similar to RM11068 and RM11069, which shared 97% and 100% similarity to each other in their XbaI and BlnI PFGE patterns and came from samples taken from two separate crows at site code 10. Four other strains of 6,8:−:e,n,z15 that shared an XbaI similarity greater than 95% were RM10977 (irrigation ditch sediment), RM10978 (irrigation water in a nearby field), and RM10975 (soil), all from site code 3, and RM11065 (coyote) from site code 4 (Table 4).
The strains of S. Give all were very similar, and many were isolated from samples taken on the same day (29 April 2009) as all of the 6,8:−:e,n,z15 strains. It is noteworthy that different strains of S. Give were capable of persisting in Monterey County over several months and were isolated from water, leafy vegetable field soil, and wildlife samples collected miles apart (Table 4). One of the XbaI dendrogram clusters of S. Give strains included strains RM10973 (soil), RM11054 (soil), RM11066 (crow), RM11067 (crow), RM11769 (soil), and RM10969 (soil), all of which shared a similarity greater than 99% in their XbaI PFGE patterns and 100% similarity in their BlnI patterns. These strains were isolated from samples taken on 29 April 2009 from two different locations approximately 10 miles apart, indicating dissemination of this strain from one or more point sources in the region, possibly by transport through wildlife (e.g., crows) or runoff due to prior rainfall. These two sites also yielded strains of serotype 6,8:−:e,n,z15 from samples taken on the same day, possibly indicating two discrete contamination events within this region that affected wildlife and soil. Two other S. Give strains with nearly indistinguishable XbaI patterns from the six noted above were RM10601, isolated from Carr Lake water sampled in January 2009, and RM10605, isolated from Tembladero Slough water sampled in March 2009 (Fig. 2). These strains shared XbaI and BlnI patterns that were 99% and 96% similar to each other, respectively. The Tembladero Slough sampling site is near Castroville and downstream of Carr Lake in Salinas, suggesting that this strain may have been transported by water in this continuous watershed (Table 4, Fig. 1). Another set of S. Give isolates with indistinguishable XbaI and BlnI pulsotypes were RM10965, RM10966, RM10967, and RM10968; these strains were isolated from leafy green field soil samples collected on the same day (29 April 2009) from the same location (site code 3, area A in Fig. 1) and probably represent independent isolations of the same strain. S. Give strain RM10606 (opossum, 4 March 2009) and RM11073, a Salmonella strain of serotype 3,10:−:1,7 (Salinas River, 5 May 2009), had indistinguishable XbaI PFGE patterns but were isolated 2 months apart from samples taken at different locations. The 3,10:−:1,7 serotype corresponds to a monophasic S. Give strain, indicating again possible transport of a strain between wildlife and water and a subtle change in the serotype-related molecules. RM10979 (opossum) was the most dissimilar of the S. Give strains, with 91% and 80% similarities in XbaI and BlnI patterns, respectively, to the rest of the S. Give strains. This wildlife sample was collected on 29 April 2009, the same collection day as several other samples, but from a location in San Benito County, different from all the other S. Give strains.
Ten of the isolates were the monophasic serotype 6,8:d:− strain. The first isolation of this serotype was RM10599 from a standing water sample collected in November 2008 from an undisclosed location in Monterey County (site code 6). This location was sampled again 9 March 2009 and resulted in the isolation, also from standing water, of RM11771, which had an 88% XbaI similarity to RM10599, indicating that they were different strains. However, RM11771 shared high XbaI (93%) and BlnI (93%) similarities with RM10604 and RM11770; these strains were isolated from samples collected on 9 March 2009 at sites on the Salinas River, at Greenfield, CA, and San Ardo, CA, respectively, which are approximately 32 miles apart. We speculate that they are related strains that were transported from one or more point sources by a dissemination event prior to March 2009. Although the remaining 6,8:d:− strains share less than 95% and 91% similarities in their XbaI and BlnI patterns, respectively, these results reveal some intriguing spatial and temporal relationships. Samples from an undisclosed location (site code 6) in March 2009 resulted in 6,8:d:− strains RM11710 (sediment), RM10603 (water), and RM10962 (water). Also in March 2009, 6,8:d:− strain RM11060 was isolated from a sample from San Lorenzo Creek in King City, CA, and 6,8:d:− strain RM11072 was isolated from a sample taken on 5 May 2009 from the Gabilan Creek, which feeds eventually into the Tembladero Slough and the Old Salinas River near Salinas, CA. Serotype 6,8:d:− strain RM11070 was isolated from a 5 May 2009 San Benito River sample and at present represents the last date for a sample yielding this serotype. The Salinas River and San Benito River are unlinked.
S. Typhimurium strains RM10607 and RM10608 were isolated from two different bird samples collected on the same date and site (March 2009, location 9). Their XbaI PFGE patterns were very similar, but they have differences, corresponding to a similarity of 89%. The remaining S. Typhimurium isolates were from water samples obtained in Monterey County within 10 miles of the city of Salinas (the Gabilan Creek, Carr Lake, and the Old Salinas River Channel), from January to April 2009. These strains have PFGE patterns that are distinguishable, and hence they are not independent isolations of the same strain.
PFGE results were submitted to CDC PulseNet. Most of the pulsotypes did not match patterns in the database corresponding to known outbreak strains; however, S. Typhimurium strain RM10602 and S. Infantis strain RM11056 had XbaI profiles that matched those of multistate outbreaks or clusters reported in 2009 and 2010 (see Discussion).
Comparison of MSRV with IMS plus secondary enrichment.
Nearing the end of the analysis of these strains, we became aware of a modified enrichment method using IMS and a secondary enrichment in RVS broth (27). We compared in a parallel study the MSRV method with this modified IMS method with 621 samples of wildlife and water from locations in California different from those sampled for the 2,401 samples described in this study. Forty-seven of the 621 samples (7.6%) were positive with the IMS plus secondary enrichment in RVS, whereas only three of 621 (0.5%) were positive with the MSRV method. Only one strain isolated with the MSRV method was not isolated with the revised IMS method. These results stimulated us to retest with the IMS method approximately 500 Monterey County samples from this study that were negative previously with the MSRV method; all of them were again negative for Salmonella.
DISCUSSION
Salmonella and E. coli O157:H7 are major food-borne pathogens, and their ecologies in agricultural regions deserve study. Many of the standardized enrichment methods for them differ, so a survey for both organisms requires setting up completely different primary enrichment cultures. A method for concurrent recovery of both organisms from bovine carcass, hide, and fecal samples was developed involving primary enrichment in TSB (7) and proved effective in a large survey of feedlot cattle (48). As part of an ongoing survey for Shiga toxin-producing E. coli (STEC) in the California agricultural environment, we tested aliquots of stored, frozen primary enrichment cultures in TSB for Salmonella. We compared a number of different enrichment methods before selecting the EPA Salmonella enrichment method for our study (3); it gave few false positives and was simple enough for screening thousands of samples. However, it should be noted that MSRV semisolid medium as a secondary enrichment medium measures motility as an indicator for Salmonella, so nonmotile Salmonella, such as S. Gallinarum, will be missed by this method. Additionally the inhibitors in MSRV preclude the isolation of S. Typhi and S. Paratyphi A (5, 15).
The performance of the oligonucleotide array showed that a positive result of at least 6/10 probes was sufficient for labeling a sample as Salmonella positive. Ultimately, we isolated Salmonella from those samples that scored a 6/10 or higher on the array (Table 1). Samples W-0119 and W-0126 reacted positively to the probes for serogroups C2 and C1, respectively, and the strains isolated from those samples (RM10599 [6,8:d:−] and RM10600 [S. Infantis]) belonged to those serogroups. However, use of the array would be costly in a large-scale study.
Based on previous surveys for Salmonella (10, 22, 26, 27, 37, 48), we anticipated identifying more than 2.3% positive samples, even though use of the array on 54 samples predicted 3.7% (2/54) positive samples. This raised concern that recovery might be decreased by prolonged storage of the enrichment cultures at −80°C. However, our efficient isolation of Salmonella from spiked enrichment broths representing different background microflora (including fecal flora) even after 6 months of storage indicated no major decrease in the viability of cells during storage. This result supported frozen TSB enrichments as a valuable resource for future analysis by new methods or as time allows.
It is worth noting that only one cattle fecal sample yielded Salmonella, especially considering that MSRV has been reported to be an efficient medium for recovery of Salmonella from feces (5, 15). Other surveys of cattle for Salmonella reported various prevalence rates, from 7 to 100%, but these surveys were from locations within the United States outside California and were of feedlot cattle (10, 27, 48). A very low incidence of Salmonella (0.46%) was reported in the feces of cull dairy cattle in Washington (19). The low incidence we obtained for cattle in Monterey County may reflect different geographic environments and/or the fact that the beef cattle we tested were on rangeland and not confined. Nevertheless, the low recovery from cattle feces enrichment broths prompted us to test shorter incubation times for Salmonella revival to allow less time for overgrowth by competing bacteria (7). However, 100 negative cattle samples retested on MSRV after only 4 to 5 h of incubation of a sample of the frozen enrichment broth in TSB also tested negative (data not shown). The cattle samples we tested represented animals sampled at multiple ranches in Monterey County, so we speculate that the incidence of Salmonella was low at the time period sampled. A longer period of study at additional sites would be required to determine whether the low incidence seen here reflects geographic differences or only a spatial and temporal snapshot of the region. These studies are important for identifying reservoirs and point sources in this region that are relevant to occasional recalls of leafy greens identified by FDA testing programs (16, 17).
IMS has been used by others to enhance Salmonella detection from cattle samples (7), but we detected no increased isolation of Salmonella with IMS using the manufacturer's instructions included with the anti-Salmonella magnetic beads. However, preliminary results in a different, ongoing study with a revised IMS protocol that includes a secondary enrichment step (27) resulted in a higher frequency of Salmonella isolation in a side-by-side comparison with the MSRV protocol used in the current study. The samples in this ongoing study are from a different region of California, collected subsequent to those reported above, and from different sources and types of water and wildlife, and the nature of the strains is under study currently. Therefore, details will be presented in a subsequent report. With this revised method, retesting of approximately 500 of the negative Monterey County samples reported in this study did not result in additional positives; thus, we conclude that the incidence data by the MSRV enrichment method are accurate. However, there is the possibility that the number of positives would be higher if all samples were retested with the revised IMS method. The nature of the strains that were detected only with IMS will be characterized, and additional fecal samples will be tested in parallel with the current and revised methods in future studies.
The serotypes isolated from water in the current study were present also in wildlife, including S. Infantis (skunk), S. Give (opossum, crow), S. Typhimurium (sparrow, towhee), and 6,8:−:e,n,z15 (crows, coyote). These results are relevant to potential contamination of surface waters by transport and shedding of Salmonella by livestock and wildlife as reported by Ferguson et al. (18). Wild birds are known to be carriers of S. Typhimurium, which can cause avian mortality (23, 28, 38), and poultry also are a source (6). However, all of the wildlife sample isolates were distinguishable from water sample isolates when both XbaI and BlnI PFGE patterns were compared (Fig. 4 and 5). Nevertheless, some near matches suggested potential sources involved in contamination of water or, alternatively, colonization of wildlife. For example, S. Give strains RM10601 and RM10605 (water) were highly related to S. Give strains RM11066 and RM11067 (crows) by XbaI and related by BlnI (although distinguishable); and serotype 6,8:−:e,n,z15 strains RM10977 and RM10978 (irrigation ditch water) appeared to be related to serotype 6,8:−:e,n,z15 strains RM11065, RM11068, and RM11069 (coyote and crows) by XbaI and highly related by BlnI (Fig. 4 and 5). These results suggest that microbial source tracking of Salmonella in this environment may be possible, but higher-resolution genotyping methods are needed to determine if strains matching by PFGE are related, since some serovars are very clonal.
Two Salmonella strains from this study matched XbaI patterns submitted to CDC PulseNet as human illness cases. S. Typhimurium strain RM10602, isolated from water from a small lake in central Salinas, had an XbaI pattern indistinguishable from that of a strain causing multistate outbreaks of human cases of salmonellosis in September/October 2010 (CDC outbreak designation 1010MLJPX-1), July/August 2009 (CDC outbreak designation 0903MLJPX-1), and September/October 2009 (CDC outbreak designation 0907MLJPX-1). S. Infantis strain RM11056, isolated from a skunk, matched, by XbaI, a strain causing a cluster of human cases reported in August/September 2010 (CDC outbreak designation 1009MDJFX-1). Although these matches are intriguing, much-higher-resolution genotyping will be necessary to determine the phylogenetic relationship of related strains from the environment and causing human illness. S. Typhimurium strain RM10602 had the same second-enzyme PFGE (BlnI) profile as the human outbreak strains. We are in the process of analyzing and comparing strains also by multilocus sequence typing.
Water samples yielded the highest percentage of samples positive for Salmonella (18 of 252 [7.1%]). Some of the positive water samples were obtained from standing water and irrigation ditches; however, most were from streams, rivers, and creeks in the region. A survey of surface waters in a region of Georgia associated with a high incidence of salmonellosis cases had a corresponding high incidence of Salmonella in the watershed (57 of 72 [79%]). The authors suggested that seasonal differences in levels and serotype diversity might be influenced by temperature and precipitation (22). A similar survey of surface waters in North Carolina resulted in identification of multiple serotypes among 47 of 86 samples positive (54.7%) for Salmonella (37), and a survey of surface waters in an area of southern Alberta, Canada, with a high incidence of enteric disease determined an incidence of Salmonella of 6.2% (88 of 1,429) (26). The incidence of Salmonella in water in our limited study (7.1%) might be relevant to recalls of Salmonella-contaminated produce grown in Monterey County (16, 17); however, there is minimal evidence that the region has a higher-than-normal number of Salmonella illnesses. Furthermore, no outbreaks of Salmonella associated with leafy greens from this region have been reported.
One factor that might explain the differences in incidence results is climate. The climate of central coastal California is conducive to leafy vegetable production and, thus, is quite different from the southeastern United States. The majority of the precipitation in California occurs in the winter and early spring, and the highest temperatures occur in the dry summer months. Following a previous 19-month survey of the incidence of E. coli O157:H7 in some of the same watersheds in the Monterey County region surveyed in this study, Cooley et al. reported that the incidence of generic E. coli and, in some locations, E. coli O157:H7 increased after rain events in winter months and correlated with significant increases in the flow rate of rivers and streams (14). In the present study, many of the positive samples were collected during months with precipitation. Exceptions are February 2009, the wettest month of this study, which yielded no positive samples, and April 2009, when the highest percentage of positive samples was collected despite it being a relatively dry month. Although it is difficult to compare Salmonella incidence by month due to differences in the number of samples, different locations, and different sources, a trend was evident to a higher incidence of Salmonella 30 to 60 days subsequent to heavy precipitation and a decreased incidence in dry summer months (Fig. 3). An expanded survey is required to determine whether these trends are significant statistically.
The greatest diversity in serotypes was associated with Salmonella isolated from wildlife (Table 4). This might relate to some serotypes being less fit than others outside animal hosts for extended periods of time, and some serotypes surviving well in multiple environments. For example, S. Give, S. Infantis, S. Typhimurium, and the monophasic 6,8:−:e,n,z15 were isolated from both water and wildlife samples, indicating that these strains can survive in, and might be transported between, the two environments.
Several of the serovars isolated in this study, including S. Typhimurium, S. Infantis, and S. Montevideo, have been implicated frequently in outbreaks or sporadic cases of human illness in the United States (12). Cases associated with S. Saugus and S. Kumasi are more rare but have been reported to cause human illness outside the United States. S. Kentucky, a serovar associated with a recall of romaine lettuce from California (17), is isolated often in surveys of domestic food animals but is associated rarely with human illness. S. Give is isolated most often from animals (34, 42, 43) but occasionally can cause human illness (20). Although we isolated multiple serotypes of Salmonella, it is possible that other strains and serovars of Salmonella remained undetected due to culture bias and differential fitness among Salmonella strains in competition with different background microbiota present in different sample source types (41, 45, 52). There is an urgent need for improved methods for robust isolation of all Salmonella serovars to facilitate accurate incidence, source tracking, and epidemiology. The multipathogen microarray we used was useful in identifying samples worth pursuing for cultural isolation of Salmonella but remains a costly step for high-throughput studies.
An important issue in public health is the emergence of multidrug-resistant strains of Salmonella. Only one isolate in the present study, S. Montevideo from a skunk, had antibiotic resistance. These results are different from the results from other surveys characterizing Salmonella antibiotic resistance, possibly because these other surveys targeted regions with reported high incidences of Salmonella, areas affected by animal agriculture, or feedlots and diseased animals (25, 26, 30, 31, 37, 49, 51). In the 1980s, human illness due to a chloramphenicol-resistant strain of S. Newport was traced to California dairy farms (9, 47). Indeed, multidrug-resistant S. Newport is a reemerging pathogen on California's central valley dairies (9). However, the region of Monterey County we surveyed resulted in only one of 795 cattle fecal samples testing positive for Salmonella (0.13%). This low incidence may be related to the fact that beef cattle production in this region is primarily cow-calf operations on rangeland, but it is noteworthy that this low incidence is about 30-fold less (0.13% versus 4.2%) than the incidence in wildlife samples from this same region. We are not aware of any other surveys or reports of Salmonella incidence or antibiotic resistance associated with our study region.
PFGE analysis indicated that some of the Salmonella isolates are indistinguishable and/or highly related (Fig. 4 and 5). For example, it appears that the same strain was isolated from multiple samples from the same location on the same date; however, there are instances of the same strain isolated from different locations on different dates. PFGE analysis also revealed that matching or related strains were isolated from water and wildlife samples, suggesting that these two reservoirs are sources of dissemination in the region. This also suggests that some strains can persist in the environment long enough to be transported by some process to different locations in the watershed. Salmonella has been reported to persist in the environment for several months to more than a year (35, 50, 55). Alternatively, there may be one or more sources that maintain the pathogen (e.g., colonization) and disseminate the organism continuously into the environment. Monitoring Salmonella in this region could facilitate assessments of the temporal and spatial factors relevant to Salmonella incidence, source tracking, higher-resolution subtyping, and comparisons to similar data from other agricultural regions in the United States. Identifying reservoirs and transport of Salmonella in this important leafy green production region and other important produce regions in the United States is critical to improving food safety and public health.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Feng and A. Tse for technical assistance and D. Carychao, R. Danielson, S. Fontanoz, G. Cavanaugh-Baird, S. Hee, K. Nguyen, and D. Brichta-Harhay for providing samples and/or advice. We thank S. Chandler and D. Orthmeyer, U.S. Department of Agriculture, APHIS-Wildlife Services, and C. Addison and A. Gwinn, California Department of Fish and Game, for providing wildlife samples and L. Benjamin, UC Davis WIFSS, for providing spatial information.
This research was funded by the U.S. Department of Agriculture, Agricultural Research Service, CRIS project number 5325-42000-044-00D, by National Research Initiative Competitive Grant numbers 2006-55212-16927 and 2007-35212-18239 from the USDA National Institute of Food and Agriculture, by U.S. Food and Drug Administration project U01-003-572, and by the Center for Produce Safety at the University of California, Davis, through California Department of Food & Agriculture Specialty Crop Block Grant SCB09055. This research also supports collaboration between the United States and the European Commission in the Sixth Framework Programme, Food CT-2006-36241, ProSafeBeef.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 4 March 2011.
ADDENDUM IN PROOF
During the review of this manuscript, we became aware of a published study of Salmonella incidence in California central coastal water bodies (S. P. Walters, A. L. Thebo, and A. B. Boehm, Water Res. 45:1752–1762, 2011), a few of which are located near sites evaluated in our study. Samples collected during 2008 and 2009 corresponded to an overall incidence of Salmonella in 30.7% of all samples (<0.75 to 7.25 MPN/liter) and incidences of 33.3% (6/18 samples), 12.5% (1/8 samples), and 41.2% (7/17 samples) in samples from sites at Moss Landing, Pajaro River, and Salinas River, respectively, which are in the vicinity of some sites we tested. The authors noted also that concentrations of Salmonella were significantly higher in samples obtained after rainfall within the 7 days prior to sampling. These incidences in the study by Walters et al. are higher than, but somewhat consistent with, the incidences we obtained for water in the areas nearest (areas A and E) to those noted above.
REFERENCES
- 1. Andrews W. H., Hammack T. S. 2007. Salmonella. In Bacteriological analytical manual online. Food and Drug Administration, Silver Spring, MD: http://www.cfsan.fda.gov/∼ebam/bam-5.html [Google Scholar]
- 2. Anonymous. 2008. Isolation and identification of Salmonella from meat, poultry, pasteurized egg and catfish products. U.S. Department of Agriculture, Washington, DC: http://www.fsis.usda.gov/PDF/MLG_4_05.pdf [Google Scholar]
- 3. Anonymous. 2006. Method 1682: Salmonella in sewage sludge (biosolids) by modified semisolid Rappaport-Vassiliadis (MSRV) medium. U.S. Environmental Protection Agency, Washington, DC: http://www.epa.gov/waterscience/methods/method/biological/1682.pdf [Google Scholar]
- 4. Anonymous. 2010. Surveillance for foodborne disease outbreaks—United States, 2007. Morb. Mortal.Wkly. Rep. 59:973–980 [PubMed] [Google Scholar]
- 5. Aspinall S. T., Hindle M. A., Hutchinson D. N. 1992. Improved isolation of salmonellae from faeces using a semisolid Rappaport-Vassiliadis medium. Eur. J. Clin. Microbiol. Infect. Dis. 11:936–939 [DOI] [PubMed] [Google Scholar]
- 6. Baggesen D. L., Sandvang D., Aarestrup F. M. 2000. Characterization of Salmonella enterica serovar Typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J. Clin. Microbiol. 38:1581–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barkocy-Gallagher G. A., et al. 2002. Development of methods for the recovery of Escherichia coli O157:H7 and Salmonella from beef carcass sponge samples and bovine fecal and hide samples. J. Food Prot. 65:1527–1534 [DOI] [PubMed] [Google Scholar]
- 8. Barrett T. J., et al. 1980. Use of Moore swabs for isolating Vibrio cholerae from sewage. J. Clin. Microbiol. 11:385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berge A. C. B., Adaska J. M., Sischo W. M. 2004. Use of antibiotic susceptibility patterns and pulsed-field gel electrophoresis to compare historic and contemporary isolates of multidrug-resistant Salmonella enterica subsp. enterica serovar Newport. Appl. Environ. Microbiol. 70:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brichta-Harhay D. M., et al. 2008. Salmonella and Escherichia coli O157:H7 contamination on hides and carcasses of cull cattle presented for slaughter in the United States: an evaluation of prevalence and bacterial loads by immunomagnetic separation and direct plating methods. Appl. Environ. Microbiol. 74:6289–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CDC 2008. Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items—United States, 2008. Morb. Mortal. Wkly. Rep. 57:929–934 [PubMed] [Google Scholar]
- 12. CDC 2008. Salmonella surveillance: annual summary, 2006. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ncidod/dbmd/phlisdata/salmonella.htm [Google Scholar]
- 13. Chiu C.-H., Ou J. T. 1996. Rapid identification of Salmonella serovars in feces by specific detection of virulence genes, invA and spvC, by an enrichment broth culture-multiplex PCR combination assay. J. Clin. Microbiol. 34:2619–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooley M., et al. 2007. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS One 2:e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dusch H., Altwegg M. 1995. Evaluation of five new plating media for isolation of Salmonella species. J. Clin. Microbiol. 33:802–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. FDA 2010. Fresh Express recalls romaine-based salads with use-by dates of May 13-16th due to possible health risk. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Safety/Recalls/ucm213247.htm [Google Scholar]
- 17. FDA 2009. Tanimura & Antle voluntarily recalls one lot of romaine lettuce because of possible health risk. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Safety/Recalls/ArchiveRecalls/2009/ucm173185.htm [Google Scholar]
- 18. Ferguson C., de Roda Husman A. M., Altavilla N., Deere D., Ashbolt N. 2003. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33:299–361 [Google Scholar]
- 19. Gay J. M., Rice D. H., Steiger J. H. 1994. Prevalence of fecal Salmonella shedding by cull dairy cattle marketed in Washington state. J. Food Prot. 57:195–197 [DOI] [PubMed] [Google Scholar]
- 20. Girardin F., Mezger N., Hächler H., Bovier P. A. 2006. Salmonella serovar Give: an unusual pathogen causing splenic abscess. Eur. J. Clin. Microbiol. Infect. Dis. 25:272–274 [DOI] [PubMed] [Google Scholar]
- 21. Gorski L., Liang A. S. 2010. Effect of enrichment medium on real-time detection of Salmonella enterica from lettuce and tomato enrichment cultures. J. Food Prot. 73:1047–1056 [DOI] [PubMed] [Google Scholar]
- 22. Haley B. J., Cole D. J., Lipp E. K. 2009. Distribution, diversity, and seasonality of waterborne Salmonellae in a rural watershed. Appl. Environ. Microbiol. 75:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall A. J., Saito E. K. 2008. Avian wildlife mortality events due to salmonellosis in the United States, 1985-2004. J. Wild. Dis. 44:585–593 [DOI] [PubMed] [Google Scholar]
- 24. Hanning I. B., Nutt J. D., Ricke S. C. 2009. Salmonellosis outbreaks in the United States due to fresh produce: sources and potential intervention measures. Foodborne Pathog. Dis. 6:635–648 [DOI] [PubMed] [Google Scholar]
- 25. Hoelzer K., et al. 2010. The prevalence of multidrug resistance is higher among bovine than human Salmonella enterica serotype Newport, Typhimurium, and 4,5,12:i isolates in the United States but differs by serotype and geographic region. Appl. Environ. Microbiol. 76:5947–5959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson J. Y. M., et al. 2003. Prevalence of Escherichia coli O157:H7 and Salmonella spp. in surface waters of southern Alberta and its relation to manure sources. Can. J. Microbiol. 49:326–335 [DOI] [PubMed] [Google Scholar]
- 27. Kalchayanand N., et al. 2009. Prevalence rates of Escherichia coli O157:H7 and Salmonella at different sampling sites on cattle hides at a feedlot and processing plant. J. Food Prot. 72:1267–1271 [DOI] [PubMed] [Google Scholar]
- 28. Kirk J. H., Holmberg C. A., Jeffrey J. S. 2002. Prevalence of Salmonella spp. in selected birds captured on California dairies. J. Am. Vet. Med. Assoc. 220:359–362 [DOI] [PubMed] [Google Scholar]
- 29. Lane D. J. 1991. 16S/23S rRNA sequencing, p. 115–175 In Stackebrandt E., Goodfellow M. (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons Ltd., New York, NY [Google Scholar]
- 30. Lanzas C., et al. 2010. Transmission dynamics of a multidrug-resistant Salmonella Typhimurium outbreak in a dairy farm. Foodborne Pathog. Dis. 7:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim S.-K., et al. 2009. Antimicrobial resistance and phage types of Salmonella isolates from healthy and diarrheic pigs in Korea. Foodborne Pathog. Dis. 6:981–987 [DOI] [PubMed] [Google Scholar]
- 32. Mandrell R. E. 2009. Enteric human pathogens associated with fresh produce: sources, transport and ecology, p. 3–42 In Fan X., Niemira B., Doona C. J., Feeherry F., Gravani R. B. (ed.), Microbial safety of fresh produce. IFT/Blackwell Publishing, Oxford, United Kingdom [Google Scholar]
- 33. Mead P. S., et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oloya J., Doetkott D., Khaitsa M. L. 2009. Antimicrobial drug resistance and molecular characterization of Salmonella isolated from domestic animals, humans, and meat products. Foodborne Pathog. Dis. 6:273–284 [DOI] [PubMed] [Google Scholar]
- 35. Parker C. T., Huynh S., Quiñones B., Harris L. J., Mandrell R. E. 2010. Comparison of genotypes of Salmonella enterica serovar Enteritidis phage type 30 and 9c strains isolated during three outbreaks associated with raw almonds. Appl. Environ. Microbiol. 76:3723–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parker C. T., Quiñones B., Miller W. G., Horn S. T., Mandrell R. E. 2006. Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J. Clin. Microbiol. 44:4125–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patchanee P., Molla B., White N., Line D. E., Gebreyes W. A. 2010. Tracking Salmonella contamination in various watersheds and phenotypic and genotypic diversity. Foodborne Pathog. Dis. 7:1113–1120 [DOI] [PubMed] [Google Scholar]
- 38. Pennycott T. W., Park A., Mather H. A. 2006. Isolation of different serovars of Salmonella enterica from wild birds in Great Britain between 1995 and 2003. Vet. Rec. 158:817–820 [DOI] [PubMed] [Google Scholar]
- 39. Quiñones B., Parker C. T., Janda J. M., Jr., Miller W. G., Mandrell R. E. 2007. Detection and genotyping of Arcobacter and Campylobacter isolates from retail chicken samples by use of DNA oligonucleotide arrays. Appl. Environ. Microbiol. 73:3645–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ribot E. M., et al. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59–67 [DOI] [PubMed] [Google Scholar]
- 41. Rostagno M. H., Gailey J. K., Hurd H. S., Mckean J. D., Leite R. C. 2005. Culture methods differ on the isolation of Salmonella enterica serotypes from naturally contaminated swine fecal samples. J. Vet. Diagn. Invest. 17:80–83 [DOI] [PubMed] [Google Scholar]
- 42. Roy R., Higgins R., Fortin M., Tardif S. 2001. Salmonella Give infection in 2 dairy herds. Can. Vet. J. 42:468–470 [PMC free article] [PubMed] [Google Scholar]
- 43. Schotte U., Borchers D., Wulff C., Geue L. 2007. Salmonella Montevideo outbreak in military kennel dogs caused by contaminated commercial feed, which was only recognized through monitoring. Vet. Microbiol. 119:316–323 [DOI] [PubMed] [Google Scholar]
- 44. Silbert S., Boyken L., Hollis R. J., Pfaller M. A. 2003. Improving typeability of multiple bacterial species using pulsed-field gel electrophoresis and thiourea. Diagn. Microbiol. Infect. Dis. 47:619–621 [DOI] [PubMed] [Google Scholar]
- 45. Singer R. S., Mayer A. E., Hanson T. E., Isaacson R. E. 2009. Do microbial interactions and cultivation media decrease the accuracy of Salmonella surveillance systems and outbreak investigations? J. Food Prot. 72:707–713 [DOI] [PubMed] [Google Scholar]
- 46. Sivapalasingam S., Friedman C. R., Cohen L., Tauxe R. V. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67:2342–2353 [DOI] [PubMed] [Google Scholar]
- 47. Spika J. S., Waterman S. H., Hoo G. W. S., St. Louis M. E., et al. 1987. Chloramphenicol-resistant Salmonella newport traced through hamburger to dairy farms. N. Engl. J. Med. 316:565–570 [DOI] [PubMed] [Google Scholar]
- 48. Stephens T. P., et al. 2007. Distribution of Escherichia coli O157 and Salmonella on hide surfaces, the oral cavity, and in feces of feedlot cattle. J. Food Prot. 70:1346–1349 [DOI] [PubMed] [Google Scholar]
- 49. Targant H., et al. 2010. Characterization of resistance genes in multidrug-resistant Salmonella enterica serotype Typhimurium isolated from diseased cattle in France (2002 to 2007). Foodborne Pathog. Dis. 7:419–425 [DOI] [PubMed] [Google Scholar]
- 50. Thomason B. M., Biddle J. W., Cherry W. B. 1975. Detection of salmonellae in the environment. Appl. Microbiol. 30:764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vigo G. B., et al. 2009. Salmonella enterica subclinical infection: bacteriological, serological, pulsed-field gel electrophoresis, and antimicrobial resistance profiles—longitudinal study in a three-site farrow-to-finish farm. Foodborne Path. Dis. 6:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Voogt N., Nagelkerke N. J. D., W. van de Giessen A., Henken A. M. 2002. Differences between reference laboratories of the European community in their ability to detect Salmonella species. Eur. J. Clin. Microbiol. Infect. Dis. 21:449–454 [DOI] [PubMed] [Google Scholar]
- 53. Wacheck S., Fredriksson-Ahomaa M., König M., Stolle A., Stephan R. 2010. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathog. Dis. 7:307–312 [DOI] [PubMed] [Google Scholar]
- 54. Walderhaug M. 2009. Salmonella. U. S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Food/FoodSafety/FoodborneIllness/FoodborneIllnessFoodbornePathogensNaturalToxins/BadBugBook/ucm069966.htm [Google Scholar]
- 55. Winfield M. D., Groisman E. A. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69:3687–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.