Abstract
Selenomonas ruminantium produces a tuft of flagella near the midpoint of the cell body and swims by rotating the cell body along the cell's long axis. The flagellum is composed of a single kind of flagellin, which is heavily glycosylated. The hook length of S. ruminantium is almost double that of Salmonella.
Selenomonas ruminantium is a Gram-negative anaerobic bacterium, often found in the rumens of domestic animals (8, 10). According to Leifson's Atlas of Bacterial Flagellation (13), S. ruminantium flagella originate “as a tuft from the concave side of the organism.” In bacteria in general, there are three flagellar families, classified according to the flagellar location on a cell: peritrichous, polar, and lateral (2, 7, 13). Thus, S. ruminantium cells are described as having several lateral flagella. Other species that have lateral (or subpolar) flagella include Vibrio alginolyticus (12), Vibrio parahaemolyticus (15, 16), Bradyrhizobium japonicum (9), Photobacterium profundum (6), and Rhodobacter sphaeroides (3, 18). In fact, these bacterial species have two flagellar systems, which can produce either polar or lateral flagella, and the bacterial cells can express either of these flagellum types or both of them, depending on growth conditions (16). Here, we report the structural analysis of the lateral flagella of S. ruminantium subsp. lactilytica TAM6421 (NBRC 103574). This is the first analysis of flagellar proteins in a solely laterally flagellate bacterium.
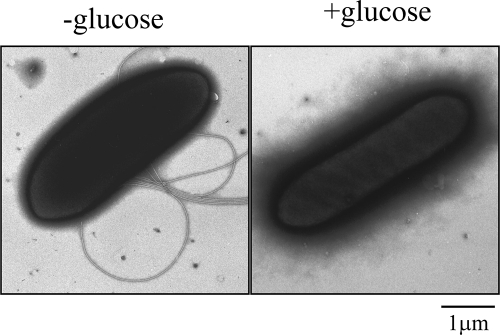
Flagellation is suppressed in the presence of glucose.
Cells were anaerobically grown at 37°C overnight as previously described (27) and observed with a phase-contrast microscope. When grown in tryptone-yeast extract-lactate (TYL) medium, which contains lactate as the sole carbon source, cells were actively motile, whereas cells grown in tryptone-yeast extract-glucose (TYG) medium, which contains glucose, were immotile. Cells grown in TYL medium supplemented with glucose or TYG medium supplemented with lactate were also immotile (data not shown). Moreover, electron microscopic observation, as previously described (7), of negatively stained cells from these cultures showed that motile cells had several flagella but that immotile cells had none (Fig. 1). These data suggest that flagellar gene expression in S. ruminantium cells might be under catabolite repression by glucose, as is known to be the case for other bacteria (1, 21, 23). In the bovine rumen, cellulose is degraded by various rumen bacteria into sugars, which are subsequently turned to lactate by other bacteria, including Streptococcus bovis (4, 19). Thus, S. ruminantium cells in the rumen likely live under conditions similar to those in TYL medium and may be motile.
Fig. 1.
Electron microscopic images of Selenomonas ruminantium. Cells were grown in the absence of glucose (−glucose, left) or in the presence of glucose (+glucose, right).
Swimming patterns and speeds.
S. ruminantium cells appeared to swim shakily by rotating the cell body along the cell's long axis, in contrast with R. sphaeroides (a bacterium with subpolar flagella [18] that is the nearest appropriate comparison), which swims by rotating in the direction perpendicular to the cell's long axis (3). Swimming speeds of cells were measured by using recorded images as previously described (14). The average speed of actively swimming cells was about 16 μm/s, which is much slower than the 29 μm/s for Salmonella enterica serovar Typhimurium cells of a similar size (Table 1).
Table 1.
Summary of physical properties of S. ruminantium flagella
| Parameter | Measured value ± SD for flagella of: |
Reference | |
|---|---|---|---|
| S. ruminantium | S. Typhimurium | ||
| Avg swimming speed | 16 ± 6 μm/s (n = 19 cells) | 29 ± 5 μm/s | This study |
| Avg no. of flagella/cell | 6 ± 1.4 (n = 20 cells) | This study | |
| Avg hook length | 105 ± 12 nm (n = 319 hooks) | 55 ± 6 nm | 20 |
| Size of FliK | 817 aa | 405 aa | 20 |
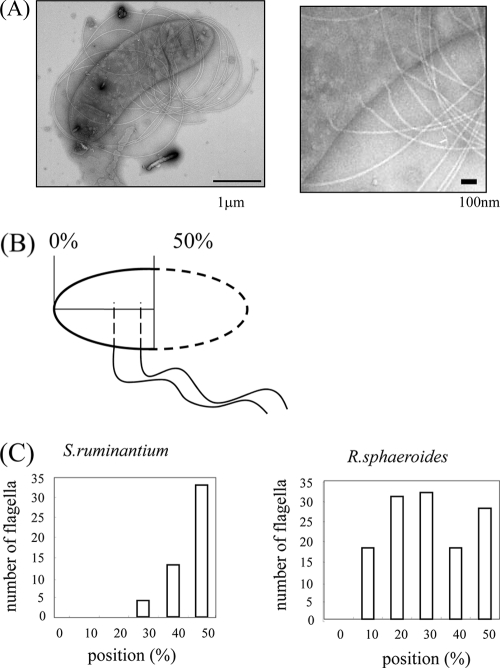
Number and position of flagella on a cell.
The number of flagella on cells of S. ruminantium was measured by electron microscopy (Fig. 1). The average flagellar number per cell for 20 cells was about six (Table 1). Sites of flagella on the cell surfaces were measured using electron microscopic images of cells that were osmotically shocked to reveal the flagellar bases (Fig. 2A). The position is expressed as a percentage of the cell length, by setting the distance from a pole to the midpoint of the cell body as 50% (Fig. 2B). As seen in the histogram (Fig. 2C, left), S. ruminantium flagella protruded from points all clustered around the midpoint. In comparison, each single Rhodobacter sphaeroides WS8 flagellum protruded randomly on the side of the cell body (Fig. 2C, right). These data indicate that the R. sphaeroides flagella, albeit known to be subpolar (3), might actually be single flagella with a random arrangement, such as individual examples of peritrichous flagella, and that S. ruminantium flagella might be authentic lateral flagella; these could be accurately regulated to grow at certain positions on the side of the cell body in a manner similar to that of polar flagella, which grow from poles of the cell body. It has been known that two genes, the flhF and flhG genes, are essential for controlling the position and number of polar flagella for P. aeruginosa (5), V. alginolyticus (12), and V. parahaemolyticus (16). Interestingly, both genes are also found in S. ruminantium but are not found in R. sphaeroides. How can lateral flagella be distinguished from peritrichous flagella? Peritrichous flagella connote flagella growing randomly around the cell body. Judging from the scattered distribution of flagellar positions, so-called lateral flagella, such as those of V. alginolyticus, may be peritrichous flagella (L. L. McCarter, private communication). For both flagellar systems, no extra genes corresponding to the flhF and flhG genes have been found.
Fig. 2.
Distribution of flagella on a cell. (A) Electron microscopic image of a cell that was osmotically shocked and stained with 1% phosphotungstic acid (PTA). The flagellar form is coiled (left). Enlarged image of the left panel showing flagellar basal bodies in the membranes (right). (B) Schematic presentation showing how the positions of flagella on a cell were measured. (C) Diagram of the distributions of sites of flagellar protrusion on a cell for S. ruminantium (left) and R. sphaeroides (right). The total numbers of flagella counted were 50 and 127, respectively.
Polymorphism of flagellar shapes.
By electron microscopy, S. ruminantium flagella at a neutral pH appeared in a coiled form, wrapping the cell body (Fig. 1). To understand how cells with coiled flagella are able to swim, we examined the polymorphic transition of isolated flagella under a dark-field microscope as previously described (7). To characterize polymorphic forms more accurately, we measured the helical parameters: the helical pitch (P), the diameter (D), and the handedness. At pHs between 5 and 8 in the absence of salt, the coiled form (∼0-μm P, 1.93-μm D, left-handed) was predominant (Fig. 3A, middle). At pHs higher than 8, the left-handed normal helical form (4.7-μm P, 1.93-μm D, left-handed) appeared at all salt concentrations (Fig. 3A, right). At pHs lower than 5, there appeared a right-handed form, which has a set of P and D similar to that of the normal form helix (Fig. 3A, left). We named this form large curly (4.84-μm P, 1.86-μm D, right-handed) according to a convention used in previous flagellar studies (7). At higher salt concentrations in the neutral pH range (5.0 to 8.0), a mixture of the large curly and coiled forms appeared (Fig. 3B). At pHs lower than 3, flagella were depolymerized and disappeared. These helical parameters suggest that S. ruminantium flagella belong to an exceptional group, which is so far dominated by Alphaproteobacteria (7). It should be noted that S. ruminantium belongs not to the Proteobacteria but rather to the Bacteroidetes. In the rumen, where the pH is between 5.5 and 7.0, flagella would take on the coiled form. The coiled form might be converted to the normal form when rotational force is applied to the flagella, similar to the change seen for the flagella of R. sphaeroides (3).
Fig. 3.
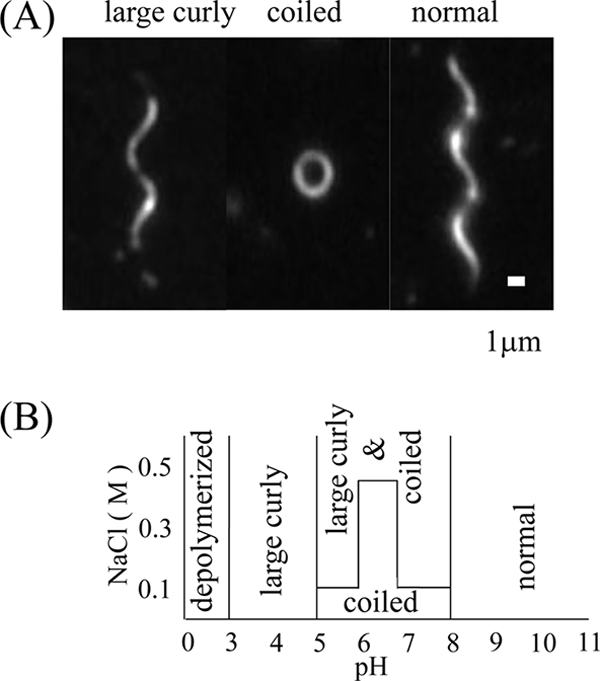
Flagellar polymorphic transition. (A) Dark-field microscopic images of polymorphs observed under various pH and salt conditions. The upper side of the helix was focused to show the handedness. (B) Schematic phase diagram of polymorphs observed at different pHs and NaCl concentrations.
Flagellin.
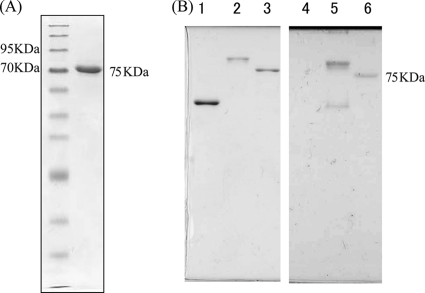
Flagellar filaments were sheared off cells by vigorous pipetting, purified by differential centrifugations, and analyzed by SDS-12.5% polyacrylamide gel electrophoresis (Fig. 4A). The filament was found to be composed of a single flagellin with an apparent molecular size of 75 kDa. The N-terminal 10-amino-acid (aa) sequence of S. ruminantium FliC1 was analyzed as previously described (9). The sequence was AMVVXNNMSA. It should be noted that the first methionine was cleaved, as is often the case for flagellar proteins. The 5th residue (X) was not read, probably because of a chemical modification of lysine (see below). We also analyzed tryptic fragments by nanoscale liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS; Matrix Science K.K., Tokyo, Japan) and obtained a fragment that read VAEGAVSSTVDILK. Both sequences agreed with that of FliC1 deduced from the fliC1 gene (chromosome 2577; 441 aa) annotated as one of the 11 flagellin genes on the S. ruminantium genome (Fig. 5). The other flagellins, FliC2 to FliC11, vary in size from 285 aa to 954 aa. Although the terminal regions of these flagellins showed a typical conserved pattern for flagellin, the central region was as diverse as that of S. Typhimurium. Since flagellins other than FliC1 were not detected in mature filaments, they may be minor components in the filament below the detection level of SDS-PAGE or may not be expressed at all.
Fig. 4.
The S. ruminantium flagellin is glycosylated. (A) SDS gel pattern of an S. ruminantium flagellin. First lane, molecular size marker; second lane, single band of flagellin. (B) Detection of glycosylation of an S. ruminantium flagellin (lanes 3 and 6). Salmonella SJW1103 flagellin was used as a negative control (lanes 1 and 4), and Azospirillum flagellin was used as a positive control (lanes 2 and 5). Coomassie brilliant blue (CBB) staining (left) and PAS staining (right) of the purified flagellins are shown.
Fig. 5.
Amino acid sequence deduced from the S. ruminantium fliC1 gene. The underlined sequences were obtained by an analysis of purified FliC.
The predicted molecular size of FliC1 is about 46 kDa. On the other hand, the apparent molecular size of FliC1 when examined by SDS-PAGE was 71 kDa, which would make it one of the largest flagellins studied so far (most are ≤60 kDa) (2). This unusually large difference in molecular sizes between predicted and observed flagellin might be derived from chemical modification of the protein. There are several flagellins known to be glycosylated in other species (24). We have tested this possibility for S. ruminantium flagellin by the periodic acid-Schiff (PAS) staining method with SDS-PAGE followed by blotting onto polyvinylidene difluoride paper. The flagellin band of S. ruminantium was stained pink as strongly as that of Azospirillum brasilense (a kind gift from Gladys Alexandre, University of Tennessee), which was chosen as a positive control (17), while the S. Typhimurium flagellin was not stained, as would be expected (Fig. 4B). Thus, the S. ruminantium flagellin is glycosylated.
Flagellar basal structure.
Intact flagella were purified and observed by electron microscopy (Fig. 6A). In the basal body, there were 4 rings, as is typical for Gram-negative species (2). Although S. ruminantium was previously categorized as a Gram-positive bacterium by analysis of 16S rRNA (22), our observation of the flagellar basal structure confirms that S. ruminantium has an outer membrane, a characteristic of Gram-negative bacteria (8, 26). Gram-positive outer membranes or mycomembranes were recently reported for the Gram-positive general Corynebacterium and Mycobacterium (28), but none of them have flagella.
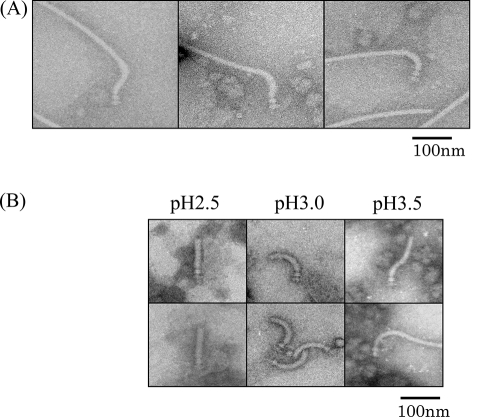
Fig. 6.
Shape and length of the hook. (A) Electron microscopic images of the hook basal body isolated from S. ruminantium. The desalted samples were stained with 2% PTA at pH 7.0. (B) Polymorphs of the hook under different pH conditions.
Hook shape and length.
The flagellar hook looked unusual, often showing an S shape by electron microscopy (Fig. 6A). Under different pH conditions (using Tris-glycine buffer), the hook underwent the following polymorphic transition (2): an S shape at and above pH 3.5, a curved shape (ordinary hook shape) at pH 3.0, and a straight form at pH 2.5 (Fig. 6B). Thus, under physiological conditions, the hook may take on an S shape rather than a simple curve. The average hook length was about 105 nm (Table 1), which is much longer than the 55-nm Salmonella hooks or the 74-nm R. sphaeroides hooks (11) and is probably the longest among naturally occurring hooks studied so far (20). The molecular size of FliK deduced from the fliK gene (chromosome 690), the hook-length control gene, was 817 amino acids, whereas that of S. Typhimurium FliK was 405 aa. This length of the hook regulator protein FliK may explain the lengthy hooks of S. ruminantium.
In peritrichous flagella of Pseudomonas syringae, glycosylation of flagellin was necessary for smooth swimming and for proper conversion of flagellar shapes (25). Heavy chemical modification of S. ruminantium flagellin, together with long hooks, might also be necessary for the smooth rotation of flagella in a bundle at the side of the concave cell body. These assumptions will be tested by making knockout mutants of related genes and point mutations of potential flagellin modification sites in the near future.
Acknowledgments
We are grateful to the National Institute of Technology and Evaluation (NITE), which kindly allowed us to use a portion of the genomic sequences prior to publication.
We thank Liz Sockett for invaluable suggestions in revising the manuscript. We also thank Satoshi Inaba and Takao Miyazaki for their technical help.
Footnotes
Published ahead of print on 18 February 2011.
REFERENCES
- 1. Adler J., Templeton B. 1967. The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 46:175–184 [DOI] [PubMed] [Google Scholar]
- 2. Aizawa S.-I. 2009. Flagella, p. 393–403 In Schaechter M. (ed.), Encyclopedia of microbiology. Elsevier, Oxford, United Kingdom [Google Scholar]
- 3. Armitage J. P., Macnab R. M. 1987. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J. Bacteriol. 169:514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotta M. 1988. Amylolytic activity of selected species of rumen bacteria. Appl. Environ. Microbiol. 54:772–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dasgupta N., Arora S. K., Ramphal R. 2000. fleN, a gene that regulates flagellar number in Pseudomonas aeruginosa. J. Bacteriol. 182:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eloe E. A., Lauro F. M., Vogel R. F., Bartlett D. H. 2008. The deep-sea bacterium Photobacterium profundum SS9 utilizes separate flagellar systems for swimming and swarming under high-pressure conditions. Appl. Environ. Microbiol. 74:6298–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujii M., Shibata S., Aizawa S.-I. 2008. Polar, peritrichous, and lateral flagella belong to three distinguishable flagellar families. J. Mol. Biol. 379:273–283 [DOI] [PubMed] [Google Scholar]
- 8. Kamio Y., Takahashi H. 1980. Isolation and characterization of outer and inner membrane of Selenomonas ruminantium: lipid compositions. J. Bacteriol. 141:888–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanbe M., Yagasaki J., Zehner S., Göttfert M., Aizawa S.-I. 2007. Characterization of two sets of sub-polar flagella in Bradyrhizobium japonicum. J. Bacteriol. 189:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanegasaki S., Takahashi H. 1967. Function of growth factors for rumen microorganisms. I. Nutritional characteristics of Selenomonas ruminantium. J. Bacteriol. 93:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi K., et al. 2003. Purification and characterization of the flagellar basal body of Rhodobacter sphaeroides. J. Bacteriol. 185:5295–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kusumoto A., et al. 2006. Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. J. Biochem. 139:113–121 [DOI] [PubMed] [Google Scholar]
- 13. Leifson E. 1960. Atlas of bacterial flagellation. Academic Press, New York, NY [Google Scholar]
- 14. Mashimo T., Hashimoto M., Yamaguchi S., Aizawa S.-I. 2007. Temperature-hypersensitive sites of the flagellar switch component FliG in Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:5153–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCarter L. L. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCarter L. L. 2004. Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 7:18–29 [DOI] [PubMed] [Google Scholar]
- 17. Moens S., Michiels K., Vanderleyden J. 1995. Glycosylation of the flagellin of the polar flagellum of Azospirillum brasilense, a Gram-negative nitrogen-fixing bacterium. Microbiology 141:2651–2657 [Google Scholar]
- 18. Poggio S., et al. 2007. A complete set of flagellar genes acquired by horizontal transfer coexists with the endogenous flagellar system in Rhodobacter sphaeroides. J. Bacteriol. 189:3208–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russell J. B., Hino T. 1985. Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis. J. Dairy Sci. 68:1712–1721 [DOI] [PubMed] [Google Scholar]
- 20. Shibata S., et al. 2007. FliK regulates flagellar hook length as an internal ruler. Mol. Microbiol. 64:1404–1415 [DOI] [PubMed] [Google Scholar]
- 21. Silverman M., Simon M. 1974. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J. Bacteriol. 120:1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stackebrandt E., Pohla H., Kroppenstedt R., Hans H., Woese C. R. 1985. 16S rRNA analysis of Sporomusa, Selenomonas, and Megasphaera: on the phylogenetic origin of Gram-positive Eubacteria. Arch. Microbiol. 143:270–276 [Google Scholar]
- 23. Strobel H. J. 1993. Evidence for catabolite inhibition in regulation of pentose utilization and transport in the ruminal bacterium Selenomonas ruminantium. Appl. Environ. Microbiol. 59:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taguchi F., et al. 2006. Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci. Cell. Microbiol. 8:923–938 [DOI] [PubMed] [Google Scholar]
- 25. Taguchi F., et al. 2008. Effects of glycosylation on swimming ability and flagellar polymorphic transformation of Pseudomonas syringae pv. tabaci 6605. J. Bacteriol. 190:764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takatsuka Y., Yamaguchi Y., Ono M., Kamio Y. 2000. Gene cloning and molecular characterization of lysine decarboxylase from Selenomonas ruminantium delineate its evolutionary relationship to ornithine decarboxylases from eukaryotes. J. Bacteriol. 182:6732–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takatsuka Y., et al. 1999. Novel characteristics of Selenomonas ruminantium lysine decarboxylase capable of decarboxylating both l-lysine and l-ornithine. Biosci. Biotechnol. Biochem. 63:1063–1069 [DOI] [PubMed] [Google Scholar]
- 28. Zuber B., et al. 2008. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190:5672–5680 [DOI] [PMC free article] [PubMed] [Google Scholar]