Abstract
Acetoin reductase (ACR) catalyzes the conversion of acetoin to 2,3-butanediol. Under certain conditions, Clostridium acetobutylicum ATCC 824 (and strains derived from it) generates both d- and l-stereoisomers of acetoin, but because of the absence of an ACR enzyme, it does not produce 2,3-butanediol. A gene encoding ACR from Clostridium beijerinckii NCIMB 8052 was functionally expressed in C. acetobutylicum under the control of two strong promoters, the constitutive thl promoter and the late exponential adc promoter. Both ACR-overproducing strains were grown in batch cultures, during which 89 to 90% of the natively produced acetoin was converted to 20 to 22 mM d-2,3-butanediol. The addition of a racemic mixture of acetoin led to the production of both d-2,3-butanediol and meso-2,3-butanediol. A metabolic network that is in agreement with the experimental data is proposed. Native 2,3-butanediol production is a first step toward a potential homofermentative 2-butanol-producing strain of C. acetobutylicum.
INTRODUCTION
To meet our future energy needs, it is necessary to develop sustainable and carbon-neutral energy sources. Liquid biofuels are attractive candidates, since little or no change is needed to the current petroleum-based fuel technologies (3). For this purpose, biological production of several alcohols is under investigation, including ethanol and butanol (4, 10).
Several clostridial species are able to ferment carbohydrates to acetone, 1-butanol, and ethanol (ABE). Industrial application of this process, also known as ABE fermentation, has a long history, but the process economics after 1960 became unfavorable compared to the petrochemical process, and its commercial exploitation was gradually abandoned (14). The inefficiency of the fermentation still hampers commercial reintroduction of this renewable butanol production process. Improving the yields and productivities of the solvent products is key to its successful reintroduction.
One of the factors reducing the fermentation efficiency is the toxic effect that 1-butanol has on the culture. Butanol has membrane-distorting properties, due to its hydrophobic chain and polar group, which cause severe cell damage (7, 28). Many efforts have been made in the past to obtain clostridial strains with increased 1-butanol tolerance but have had limited success (1, 5, 6, 12, 15, 24).
As an alternative to increasing 1-butanol tolerance, we propose replacing the production of 1-butanol with production of a compound that has similar physical and chemical properties (heat of combustion, heat of vaporization, and energy density) but that is less toxic to the cell, making higher titers possible. 2-Butanol matches these criteria and has a lower log Pow value (octanol-water coefficient) than 1-butanol. The log Pow value is a good indicator for the strength of membrane-perturbing effects (27). Generally, the lower the log Pow value, the less toxic the compound is to the membrane. However, Clostridium acetobutylicum is not known to produce 2-butanol nor its potential precursor, 2,3-butanediol (2,3-BD) (9). Nevertheless, it is known to produce acetoin as a minor fermentation product (14).
The 2,3-butanediol biosynthesis route proceeds via pyruvate, acetolactate, and acetoin to 2,3-butanediol. Acetolactate is formed in vivo by coupling two molecules of pyruvate with the concomitant release of carbon dioxide, catalyzed by acetolactate synthase. Decarboxylation by acetolactate decarboxylase yields acetoin (30), which can be reduced by an acetoin reductase (ACR) to 2,3-butanediol.
Our aim is to construct a 2,3-BD-producing C. acetobutylicum strain as a first step toward biological 2-butanol production. In this study, the cloning and functional expression of an acetoin reductase-encoding gene from Clostridium beijerinckii NCIMB 8052 in C. acetobutylicum, resulting in the production of d-2,3-butanediol, is described.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All bacterial strains and plasmids used during this study are listed in Table 1. Escherichia coli stocks were stored in 20% (vol/vol) glycerol at −80°C. Stock cultures of Clostridium acetobutylicum strains and Clostridium beijerinckii NCIMB 8052 were maintained as spore suspensions in 15% (vol/vol) glycerol at −20 or −80°C. Chemically competent, E. coli NEB 5-alpha F′ lacIq cells were used for cloning and vector maintenance. Electrocompetent E. coli DH10B(pAN1) cells were used to methylate plasmid DNA before transformation into C. acetobutylicum (18).
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant genotypea | Remark | Source |
|---|---|---|---|
| Bacterial strains | |||
| C. acetobutylicum WUR | WT | Originally obtained as ATCC 824, but shown to deviate from type strain behavior | Laboratory stock |
| C. acetobutylicum ATCC 824 | WT | Type strain | H. Bahl, Rostock, Germany |
| C. beijerinckii NCIMB 8052 | WT | Laboratory stock | |
| E. coli DH10B(pAN1) | Δ(mrr-hsdRMS-mcrBC) | Methylation strain | Laboratory stock |
| Plasmids | |||
| pAN1 | p15A ori; Cmr Φ3tI | Plasmid that expresses the phage Φ3tI methylase gene (18) | Laboratory stock |
| pMTL500E | ColE1 ori; pAMβ1 ori; MLSr Apr | Clostridial/E. coli shuttle vector (22) | Laboratory stock |
| pWUR459 | ColE1 ori; pAMβ1 ori; MLSr Apr; Padc-Cb-acr | Plasmid that expresses the Cb-acr gene under the control of the C. acetobutylicum acetoacetate decarboxylase promoter | This study |
| pWUR460 | ColE1 ori; pAMβ1 ori; MLSr Apr; Pthl-Cb-acr | Plasmid that expresses the Cb-acr gene under the control of the C. acetobutylicum thiolase promoter | This study |
Abbreviations: WT, wild type; ori, origin; Cmr, chloramphenicol resistance; MLSr, macrolide-lincosamide-streptogramin B resistance; Apr, ampicillin resistance; Cb-acr, C. beijerinckii acr (Cb-acr) gene.
Media and growth conditions.
E. coli strains were cultured in lysogeny broth (LB) medium at 37°C and 200 rpm. Sporulation plates were based on the media used by Nimcevic et al. (21) but also contained 15 g liter−1 agar. Prior to inoculation of clostridial precultures, spore suspensions were heat shocked for 10 min at 70 or 80°C. C. acetobutylicum strains were grown in MG medium or modified CGM (mCGM) medium as indicated below.
MG medium was based on the semisynthetic medium described by Nimcevic et al. (21) and contained the following (per liter of water): yeast extract, 2.5 g; KH2PO4, 1.0 g; K2HPO4, 0.76 g; ammonium acetate, 3.0 g; p-aminobenzoic acid, 0.10 g; MgSO4·7H2O, 1.0 g; FeSO4·7H2O, 0.01 g; and glucose, 60 g.
mCGM medium contained the following (per liter of water): yeast extract, 5.0 g; KH2PO4, 0.75 g; K2HPO4, 0.75 g; MgSO4·7H2O, 0.4 g; MnSO4·H2O, 0.01 g; FeSO4·7H2O, 0.01 g; NaCl, 1.0 g; asparagine, 2.0 g; (NH4)2SO4, 2.0 g; cysteine, 0.125 g; and glucose, 12.5 g.
Medium for fermentation was made anaerobic by sparging with nitrogen gas. Serum flasks (250 ml) containing 100 ml MG medium were inoculated with 2% (vol/vol) overnight precultures. Clostridial culture experiments were performed at 37°C, without shaking, and anaerobically in (i) an anaerobic chamber; or (ii) in glass serum vials as described previously (16).
Culture media were supplemented with ampicillin (100 μg ml−1), chloramphenicol (30 μg ml−1), erythromycin (40 μg ml−1 for liquid cultures and plates; 25 μg ml−1 for transformant isolation), kanamycin (50 μg ml−1), isopropyl-β-d-thiogalactopyranoside (IPTG) (50 μg ml−1), and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (40 μg ml−1) when appropriate. For challenge experiments, acetoin or 2,3-butanediol (2,3-BD) was added to the medium prior to inoculation with the preculture.
The growth of clostridial cultures was monitored spectrophotometrically at 600 nm (Pharmacia Biotech Ultrospec 2000).
DNA isolation, transformation, and manipulation.
Standard molecular work was performed according to established protocols (23). Genomic DNA from C. acetobutylicum or from C. beijerinckii was isolated using the GenElute bacterial genomic DNA kit (Sigma-Aldrich). Plasmid DNA from E. coli was isolated by the GenElute plasmid miniprep kit (Sigma-Aldrich). PCR amplification of clostridial DNA was done using Pfu polymerase (Stratagene). E. coli colony PCRs were carried out using REDTaq (Sigma-Aldrich).
Methylated plasmids were electroporated into C. acetobutylicum by the method of Oultram et al. (22). Correct methylation was checked by restriction analysis using Fnu4HI (18).
Cloning of the C. beijerinckii acr (Cb-acr) gene into C. acetobutylicum.
The clostridial expression plasmids pWUR459 and pWUR460 were constructed as detailed in the supplemental material. These plasmids and the control plasmid pMTL500E were used to transform C. acetobutylicum. Each transformation resulted in multiple erythromycin-resistant colonies. After restreaking, selected colonies were used to prepare spore suspensions for further experiments.
Acetoin reductase (ACR) enzyme assays.
C. acetobutylicum cells were harvested from cultures with an optical density (OD) of 5 by centrifugation (4,816 × g, 15 min, 4°C) and resuspended in 20 mM Tris-HCl buffer (pH 7.5) containing 1 mM tris(2-carboxyethyl)phosphine (TCEP) as reducing agent, because 2-mercaptoethanol was shown to inhibit enzyme activity. Crude cell extracts were prepared by French press homogenization (two passes at 16,000 lb/in2) and immediately assayed for enzyme activity. Assays were carried out at 37°C in 100 mM phosphate buffer (pH 6.5) containing 1 mM TCEP, 50 mM d/l-acetoin, and 0.28 mM NADPH. The reaction was started by the addition of acetoin. The decrease in absorbance at 340 nm due to NADPH oxidation was monitored on a Hitachi U2010 spectrophotometer with correction for background NADPH oxidation. One unit of enzyme activity was defined as the amount of enzyme required for the oxidation of 1 μmol of NADPH per minute. Total protein in crude extracts was determined using Roti-Nanoquant (Carl Roth, Karlsruhe, Germany) with bovine serum albumin (BSA) as a standard.
High-performance liquid chromatography (HPLC) analysis of glucose and metabolites.
Fermentation samples were centrifuged (20,800 × g, 5 min), and the supernatants were stored at −20°C. After the supernatants were thawed, an equal volume of internal standard solution (either 100 mM valeric acid [Sigma-Aldrich] in 1 M H2SO4 or 30 mM 4-methyl valeric acid [Sigma-Aldrich] in 0.5 M H2SO4) was added to the supernatant sample and then filtered (0.2 μm; Whatman). Separation of a 10-μl sample was achieved using a Shodex Ionpack KC-811 column, equipped with a refractive index detector (Waters 2414) and a UV detector (Waters 2487) operating at 210 nm, with 3 mM H2SO4 as eluent (flow, 1 ml min−1; column temperature, 85°C). The order of elution was glucose, lactic acid, acetic acid, acetoin, meso-2,3-BD, d/l-2,3-BD, butyric acid, acetone, ethanol, valeric acid, 4-methyl valeric acid, and 1-butanol.
Chiral GC-MS analysis.
To determine the enantiomeric distribution of the 2,3-BD and acetoin produced, the fermentation samples were treated like the HPLC samples. However, after thawing, the samples were additionally saturated with sodium chloride and extracted once with an equal volume of ethyl acetate. To prevent coextraction of acids, 10 M sodium hydroxide was added, since these compounds interfered with chromatographic analysis. Samples for acetoin analysis were not treated with sodium hydroxide to prevent potential racemization. The extract was then analyzed on a Finnigan Trace DSQ (dual-stage quadrupole) gas chromatography (GC)-mass spectrometry (MS) system (Thermo Electron Corporation) equipped with a CP-Chirasil-Dex CB (Varian) fused silica capillary column (25 m by 0.25 mm by 0.25 μm) with helium as the carrier gas. The injection port temperature was set at 250°C, with a split ratio of 1:10. The oven temperature program was as follows: 80°C (10 min), increased to 120°C at 10°C min−1 followed by a ramp of 40°C min−1 to 200°C (4 min). Samples (1 or 5 μl) were injected using an AS3000 autosampler (Thermo). The ion source (electron ionization [EI]) temperature was set at 200°C. Compound identification by column retention time was confirmed by analysis of the mass spectrum. The retention times of chiral standards of d-(−)-(2R,3R)- and l-(+)-(2S,3S)-2,3-BD (Sigma-Aldrich) were used for peak identification. The elution order of the acetoin enantiomers was inferred from the stereochemistry of the d-(2R,3R)-2,3-BD product (11). The order of elution was (3R)-acetoin, (3S)-acetoin, l-(2S,3S)-2,3-BD, d-(2R,3R)-2,3-BD, and meso-2,3-BD.
RESULTS
Characterization of C. acetobutylicum transformants.
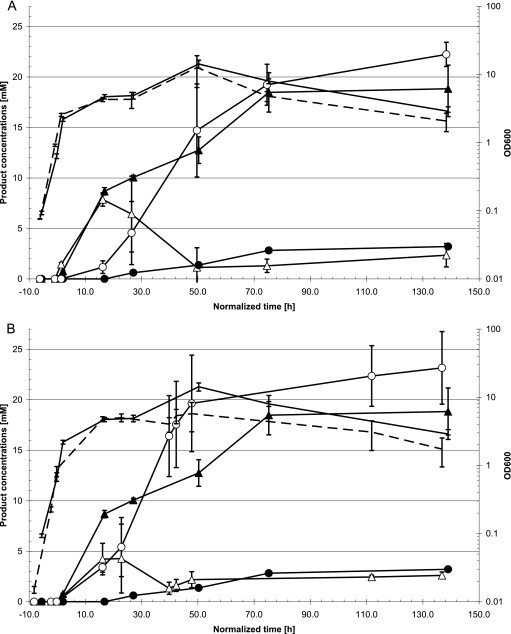
Wild-type C. acetobutylicum ATCC 824 is known to produce significant levels of acetoin, but no 2,3-butanediol (2,3-BD) (14). Recently, a gene from C. beijerinckii NCIMB 8052, Cbe_1464 annotated as an alcohol dehydrogenase, was functionally expressed in E. coli and demonstrated to possess acetoin reductase (ACR) activity (M. A. J. Siemerink and S. W. M. Kengen, unpublished results). Introduction of this acetoin reductase (ACR)-encoding gene might enable conversion of acetoin to 2,3-BD. Therefore, C. acetobutylicum transformants, containing the C. beijerinckii acr (Cb-acr) gene under the control of either the thl promoter (pWUR459) or the adc promoter (pWUR460), were constructed, and their fermentation pattern was analyzed. Both types of transformants were found to produce d-2,3-BD (Table 2). No d-2,3-BD was produced by the control strain containing the empty vector. Acetoin was found to accumulate transiently at the end of the exponential growth phase of both transformant strains, with levels reaching 4 ± 1.6, 8 ± 0.7, and 9 ± 0.3 mM for pWUR460 (thl promoter), pWUR459 (adc promoter), and pMTL500E, respectively (Fig. 1). These data suggest that the conversion of acetoin to 2,3-BD is limiting, especially in the case of the thl promoter construct, which should result in constitutive expression. The final 2,3-BD concentrations and conversion levels for both Cb-acr strains did not differ significantly from one another: 22 mM and 90% acetoin conversion for the Pthl-Cb-acr strain, respectively, and 20 mM and 89% acetoin conversion, respectively, for the Padc-Cb-acr strain. Acetoin levels of the control fermentation reached 19 mM. However, in fermentations of C. beijerinckii acetoin reductase (Cb-ACR)-expressing strains, acetoin was still detected at levels of 2 to 3 mM at the end of the fermentation (Fig. 1 and Table 2).
Table 2.
Concentrations of substrate and products of 100-ml batch fermentations of C. acetobutylicum harboring pMTL500E, pWUR459, or pWUR460 on MG medium after 145 h
| Substrate or product concn or yielda | Value for parameter for C. acetobutylicum carrying the following plasmidb: |
||
|---|---|---|---|
| pMTL500E (control) | pWUR459 (Padc-Cb-acr) | pWUR460 (Pthl-Cb-acr) | |
| Substrate concns (mM) | |||
| Consumed glucose | 318 ± 15 | 307 ± 29 | 320 ± 18 |
| Initial acetic acidc | 37 ± 0.2 | 38 ± 0.4 | 36 ± 0.8 |
| Final acetic acid | 14 ± 0.7 | 20 ± 6 | 19 ± 2 |
| Concns of 2,3-BD pathway products (mM) | |||
| Acetoin | 19 ± 2 | 2 ± 1 | 3 ± 0.3 |
| meso-2,3-Butanediol | 3 ± 0.1 | 2 ± 0.1 | 2 ± 0.3 |
| d-2,3-Butanediol | 0 | 20 ± 1 | 22 ± 3 |
| Acetoin + 2,3-BD | 22 ± 2 | 25 ± 2 | 26 ± 4 |
| % yield of acetoin + 2,3-BD per glucose (mM/mM) | 7 ± 0.8 | 8 ± 0.9 | 8 ± 1 |
| Concns of other fermentation products (mM) | |||
| Butyric acid | 2 ± 1 | 5 ± 3 | 6 ± 3 |
| Lactic acid | 3 ± 0.7 | 6 ± 1 | 4 ± 0.4 |
| Acetone | 80 ± 9 | 78 ± 12 | 86 ± 6 |
| Butanol | 161 ± 6 | 157 ± 15 | 166 ± 6 |
| Ethanol | 40 ± 2 | 34 ± 5 | 56 ± 14 |
Abbreviation: 2,3-BD, 2,3-butanediol.
Data are given as the means ± standard deviations of three replicate fermentations.
Acetate is present at the beginning (time zero) as a medium component and is consumed during the fermentation.
Fig. 1.
Production of acetoin (triangles) and 2,3-BD (circles) during batch fermentations of glucose by transformant strains of C. acetobutylicum. Strain codes: EV (closed symbols in panels A and B), C. acetobutylicum carrying pMTL500E, the empty vector control strain; ADC (open symbols in panel A), C. acetobutylicum carrying pWUR459; THL (open symbols in panel B), C. acetobutylicum carrying pWUR460. Optical densities of the cultures are shown by a solid line (EV) and a broken line (ADC and THL). On the horizontal axis, normalized time is plotted such that 0 h corresponds to an OD600 of 1.0. The control strain did not produce any d-2,3-BD, only meso-2,3-BD. Data represent the mean of triplicate fermentations averaged for each time interval. Error bars indicate standard deviations. OD600, optical density at 600 nm.
Analysis of medium samples of the control strain harboring pMTL500E, showed a ratio of approximately 12:1 (d:l) in the concentrations of the two acetoin enantiomers (data not shown).
The acid and solvent production patterns of the transformants expressing the Cb-acr gene were similar to those of the control strain harboring the empty vector (Table 2). Remarkably, all three transformants produced small but nonetheless detectable amounts of meso-2,3-BD (Table 2). To confirm this finding, we also looked at fermentations by our wild-type C. acetobutylicum strain, in the same (MG medium) and other media (mCGM and CGM media). At the end of all fermentations, small amounts of 1 to 3 mM meso-2,3-BD were found (Fig. 2 and data not shown). In all these fermentations, no d- or l-2,3-BD was detected.
Fig. 2.
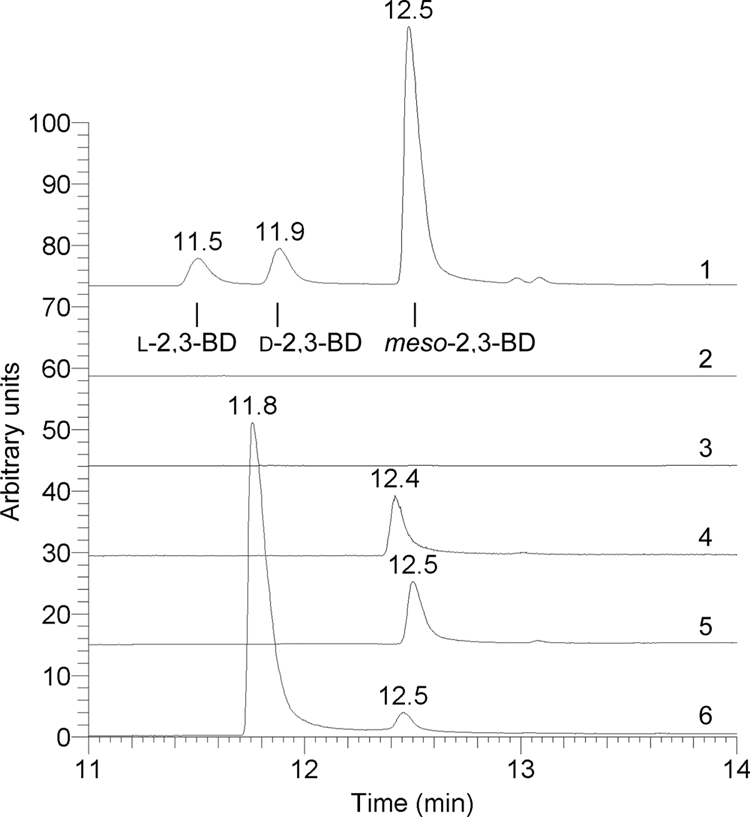
GC-MS chromatograms (single ion mode; m/z = 45) of extracts of a standard, GM medium, and samples taken at the end of the fermentation of wild-type and transformant cultures. Chromatogram 1, mixture of all three 2,3-BD stereoisomers; chromatogram 2, GM medium blank; chromatogram 3, fermentation of C. acetobutylicum ATCC 824; chromatogram 4, fermentation of C. acetobutylicum WUR; chromatogram 5, fermentation of C. acetobutylicum WUR harboring the empty vector (pMTL500E); chromatogram 6, fermentation of C. acetobutylicum WUR harboring the pWUR460 construct containing the acr gene. The retention time of the d-2,3-BD peak in chromatogram 6 is somewhat different due to the high concentration. Spiking experiments confirmed that it is indeed the d-stereoisomer.
Cell homogenates of wild-type and transformed C. acetobutylicum strains were assayed for acetoin reductase activity. A low, but significant, activity level of 0.042 ± 0.0035 U mg−1 and 0.042 ± 0.0044 U mg−1 could be detected in cell extracts for strains transformed with pWUR459 and pWUR460, respectively. The background levels were 0.018 ± 0.0015 U mg−1 and 0.024 ± 0.0021 U mg−1 for the C. acetobutylicum WUR strain and strain transformed with pMLT500E vector control, respectively. Detection was complicated by the rapid loss of activity also seen with purified enzyme isolated from E. coli extracts (Siemerink and Kengen, unpublished).
Product stereochemistry.
There are three stereoisomeric forms of 2,3-BD. The main stereoisomer produced by fermentation in MG medium was identified as d-(2R,3R)-2,3-BD. However, low levels of meso-2,3-BD were also detected in transformant strains, as well as in wild-type fermentations by both HPLC and GC-MS analysis. In all fermentations, l-(2S,3S)-2,3-BD was below our detection threshold. Figure 2 shows the gas chromatographic analysis of extracts of standard and medium samples of cultures of the various C. acetobutylicum strains.
The observation that both the plasmid vector control strain as well as our wild-type strain produced meso-2,3-BD is a new observation for C. acetobutylicum and prompted us to investigate this further. An independently obtained C. acetobutylicum ATCC 824 type strain sample that was grown under identical conditions did not produce meso-2,3-BD. This suggests that our ATCC 824 lab strain has diverged from the type strain. We therefore refer to our lab strain as C. acetobutylicum WUR (Table 1).
Acetoin- and 2,3-BD-challenged batch fermentations.
To determine possible inhibitory effects of 2,3-BD on the cultures of C. acetobutylicum transformants expressing the Cb-acr gene, fermentations in media supplemented with 20 mM d-(2R,3R) or 20 mM meso-2,3-BD were performed. In cultures challenged with d-2,3-BD, both transformants containing the Cb-acr gene produced additional d-2,3-BD in similar amounts (12 mM for strain pWUR459 and 20 mM for strain pWUR460) compared to their nonchallenged controls (15 mM and 17 mM, respectively). The use of meso-2,3-BD resulted in similar behavior (Table 3).
Table 3.
Net change of extracellular acetoin and 2,3-butanediol concentrations in challenged batch cultures of C. acetobutylicum transformants harboring pMTL500E, pWUR459, or pWUR460, after 72 h of fermentation, compared to their inoculation levels
| Plasmid | Challengea | Net change in extracellular concnb (mM) of: |
|||
|---|---|---|---|---|---|
| Acetoin | d-2,3-BDf | meso-2,3-BD | Acetoin + 2,3-BD | ||
| pMTL500E (control) | No challenge | 15 ± 1 | 0 | 2 ± 0.4 | 17 ± 1 |
| Acetoinc | 13 ± 2 | 0 | 4 ± 0.2 | 17 ± 2 | |
| d-2,3-BDd | 12 ± 2 | −0.7 ± 1 | 1 ± 0.05 | 13 ± 2 | |
| meso-2,3-BDe | 15 ± 0.2 | 0.3 ± 0.6 | 2 ± 1 | 18 ± 1 | |
| pWUR459 (Padc-Cb-acr) | No challenge | 0.2 ± 0.4 | 15 ± 1 | 1 ± 0.2 | 16 ± 1 |
| Acetoin | −20 ± 0.4g | 24 ± 1 | 11 ± 0.5 | 15 ± 1 | |
| d-2,3-BD | 0.1 ± 0.2 | 12 ± 1 | 0.9 ± 0.1 | 13 ± 1 | |
| meso-2,3-BD | 0.2 ± 0.4 | 17 ± 2 | −0.5 ± 0.2 | 17 ± 2 | |
| pWUR460 (Pthl-Cb-acr) | No challenge | 0 ± 0.3 | 17 ± 1 | 0.8 ± 0.1 | 18 ± 1 |
| Acetoin | −21 ± 0.7 | 29 ± 4 | 11 ± 0.2 | 20 ± 4 | |
| d-2,3-BD | 0.2 ± 0.3 | 20 ± 3 | 0.9 ± 0.1 | 21 ± 3 | |
| meso-2,3-BD | 0.4 ± 0.4 | 21 ± 0.5 | 0.1 ± 1 | 22 ± 1 | |
Racemic acetoin, d-2,3-BD, or meso-2,3-BD was added to the medium before inoculation at a concentration of 20 mM.
Data are given as the means ± standard deviations for three replicate fermentations and calculated by subtracting the concentration after 72 h from the concentration at the time of inoculation. For example, for the d-2,3-BD challenge of C. acetobutylicum carrying pWUR460, the initial d-2,3-BD concentration of 20 mM was subtracted from the final concentration of 40 mM, resulting in a net production of 20 mM d-2,3-BD.
The medium was supplemented with racemic acetoin.
The medium was supplemented with d-(2R,3R)-2,3-BD.
The medium was supplemented with meso-2,3-BD, which also contained approximately 10% racemic d/l-2,3-BD.
Analysis was done by nonchiral HPLC, so no separation of enantiomers was possible; however, on the basis of previous results, the d-enantiomer is expected to have been formed.
Negative values indicate consumption of acetoin relative to inoculation conditions.
We also supplemented media with racemic acetoin (20 mM) to check whether the amount of produced acetoin was limiting for the production of 2,3-BD. Supplemented cultures of ACR-expressing strains converted both d- and l-enantiomers into d- and meso-2,3-BD (Table 3). This demonstrates that the Cb-ACR enzyme is able to convert both acetoin enantiomers and is therefore not stereoselective for the configuration at the C-3 position. The total amount of both 2,3-BD diastereomers produced in the challenged cultures, 36 mM (pWUR459) and 41 mM (pWUR460), corresponds with the total amount of acetoin consumed.
Interestingly, the plasmid control strain produced more meso-2,3-BD in the acetoin-challenged cultures. It increased significantly from 2 ± 0.4 mM in the nonchallenged control to 4 ± 0.2 mM in the racemic-acetoin-challenged culture. However, still no d- or l-2,3-BD was observed.
DISCUSSION
Acetoin reductase (ACR) is an enzyme that catalyzes the reduction of acetoin to 2,3-butanediol (2,3-BD). Although Clostridium beijerinckii NCIMB 8052 contains a homologue (Cbe_1464) of a Bacillus cereus 2,3-butanediol dehydrogenase gene in its genome (13), we did not find any report in the scientific literature mentioning the production of either the ACR substrate acetoin or its product, 2,3-butanediol, by C. beijerinckii. In this study, we showed that when the C. beijerinckii acr (Cb-acr) gene is expressed in C. acetobutylicum, natively produced acetoin is reduced to d-(2R,3R)-2,3-BD (Table 2). This conversion is in agreement with the proposed acetoin reductase function of the cloned C. beijerinckii gene based on functional expression in E. coli and with a recent publication on an acetoin reductase (BdhA) of Bacillus subtilis (20). The amino acid sequence of this enzyme is very similar to the C. beijerinckii ACR with 51% identical residues and 66% similar residues.
Clostridium acetobutylicum ATCC 824 is known to produce acetoin as a minor fermentation product, but it has never been reported to produce meso-2,3-BD (8, 29). Our analysis of fermentations of an independently obtained ATCC 824 type strain confirms this. In the course of this study, however, we found that both the wild type and the plasmid control of the C. acetobutylicum WUR strain does produce meso-2,3-BD. Apparently our laboratory stock, originally acquired as the ATCC 824 type strain, evolved a divergent phenotype. Despite this, we continued to investigate the fermentative behavior of our transformant strains, as they showed interesting properties.
An earlier report describing an attempt to engineer C. acetobutylicum to produce 2,3-butanediol by heterologous expression of a Klebsiella pneumoniae ACR was unsuccessful (29). In contrast to the reported approach, we decided to express the C. beijerinckii acr gene, which has a GC content (35%) which is comparable to that of the DNA of the C. acetobutylicum host (31%), and a similar codon usage (Codon Usage Database [http://www.kazusa.or.jp/codon/]). We also used a different shuttle vector (pMTL500E) with another origin of replication in an attempt to increase the gene dosage. The pMLT500E plasmid (pAMβ1 origin) has a higher copy number in C. acetobutylicum (19, 22) than the plasmids derived from low-copy-number pSOS84 (pIM13 origin) used by Wardwell et al. (29). Combined with strong promoters (either Padc or Pthl), our approach resulted in levels of expression of the Cb-acr gene by C. acetobutylicum high enough to lead to 2,3-BD production, despite the fact that the detected specific activity is relatively low.
Two different expression constructs, pWUR459 (Padc-Cb-acr) and pWUR460 (Pthl-Cb-acr), were transformed into C. acetobutylicum to test the influence of the two different expression profiles of the promoters on fermentation. Both transformant strains were able to convert approximately 90% of the natively produced acetoin into d-2,3-BD, with values reaching 22 to 23 mM. Compared to the industrial strain Klebsiella pneumoniae SDM, which reaches concentrations up to 1,664 mM, this is relatively low (17). However, for this species, 2,3-BD is the main fermentation product, whereas for our strain, 2,3-BD is only a side product next to the solvents butanol and acetone whose levels remained unaltered.
During the acidogenic phase, the pWUR460 (thl-controlled) fermentation showed a significantly lower acetoin level compared to the pWUR459 (adc-controlled) culture or the empty vector control. The level of 2,3-BD was concomitantly higher in the thl-controlled culture. This difference in acetoin and 2,3-BD levels between the adc- and thl-driven acr expression is in good agreement with the differences in promoter activity (2, 25). Later during the fermentation, this difference leveled out. Despite constitutive expression of Cb-acr by the strain containing pWUR460, accumulation of acetoin in the medium was still observed, which suggests that the acetoin production flux under these conditions is higher than the flux from acetoin to 2,3-BD can accommodate.
Identification of bottlenecks for 2,3-BD production.
In our experiments, transformant cultures were challenged by the addition of 20 mM racemic acetoin, resembling the levels observed in the final stages of normal growth. The observation that similar amounts of acetoin were produced by the control strain in both unchallenged and acetoin-challenged fermentations (Table 3) indicates that acetoin production is not affected by its extracellular concentration at the concentrations tested. This is in agreement with the fact that in nonchallenged fermentations of Cb-acr-expressing transformants, the combined levels of acetoin and 2,3-BD do not significantly exceed those of the acetoin levels of the control strain fermentation. In racemic-acetoin-supplemented fermentations with Cb-ACR-expressing strains, all of the additional acetoin was converted into d-2,3-BD and meso-2,3-BD. This lack of selectivity for the stereochemistry of the substrate is not uncommon among acetoin reductases (26).
When exogenously added d-2,3-BD or meso-2,3-BD (20 mM) is present during fermentation, the amount of converted acetoin is not affected, resulting in final d-2,3-BD levels of 40 mM. This shows that at the levels tested, the amount of 2,3-BD is not inhibitory to the reaction or its production. On the basis of these results, we conclude that the formation of acetoin, for the fermentation as a whole, is the limiting factor for the production of 2,3-BD, even though, initially, acetoin accumulates in the medium (Fig. 1).
2,3-BD production model.
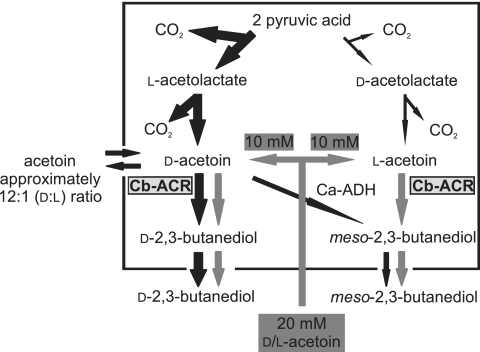
On the basis of the combined results of the wild-type strain, strain carrying the plasmid control, and strains expressing the Cb-acr gene, in normal and challenged cultures, we propose the model shown in Fig. 3 for acetoin and 2,3-BD production in our C. acetobutylicum strain. The introduced ACR enzyme can convert both d- and l-acetoin enantiomers into d-2,3-BD and meso-2,3-butanediol, respectively. The wild type and the plasmid control strain produced small amounts of meso-2,3-BD and no detectable levels of d- or l-2,3-BD, as confirmed by GC-MS analysis. In the acetoin-challenged cultures, the meso-2,3-BD formation by the control strain (pMTL500E) doubled from 2 mM to 4 mM. Most likely, one or more of the dehydrogenases that are present in C. acetobutylicum WUR do, to some extent, accept acetoin as a substrate. This would suggest that it is d-acetoin that is the source of the endogenous meso-2,3-BD production as the native acetoin enantiomer ratio is 12:1 (d:l). Thus, in the acetoin challenge experiment, the levels of d-acetoin are increased approximately 1.7-fold (from 14 mM to 24 mM), while the l-acetoin concentration increased more than 10-fold (from 1 mM to 11 mM). If l-acetoin were the source of the endogenous 2,3-BD production, then a more-substantial increase of meso-2,3-BD production would be expected.
Fig. 3.
Proposed 2,3-BD biosynthesis pathway in the ACR-expressing C. acetobutylicum transformants of this study. The boxed area indicates the intracellular space. The size of the arrows indicates the relative flux toward d- and l-acetoin from pyruvic acid. The gray arrows illustrate the impact of supplementing the medium with 20 mM racemic d/l-acetoin. The C. beijerinckii acetoin reductase (Cb-ACR) enzyme is indicated in bold type on a gray background. The proposed conversion of d-acetoin to meso-2,3-BD by an endogenous alcohol dehydrogenase (C. acetobutylicum alcohol dehydrogenase [Ca-ADH]) is indicated. In the control strain fermentation, the ratio of the two acetoin enantiomers is 12:1 (d:l).
Future perspective.
If a pathway could be established in which 2,3-BD is dehydrated to 2-butanone and then further reduced to 2-butanol, then potentially a redox balanced fermentation of glucose to 2-butanol and carbon dioxide could be established in this organism. The less-toxic nature of 2-butanol compared to 1-butanol (27) would make it an alternative approach to circumvent the limited butanol yield of the classic ABE fermentation.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Simon Extremera for performing the challenged culture experiments.
This work was financially supported by the Netherlands Ministry of Economic Affairs and the B-Basic partner organizations (www.b-basic.nl) through B-Basic, a public-private NWO-ACTS program (ACTS stands for Advanced Chemical Technologies for Sustainability).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 18 February 2011.
REFERENCES
- 1. Allcock E. R., Reid S. J., Jones D. T., Woods D. R. 1982. Clostridium acetobutylicum protoplast formation and regeneration. Appl. Environ. Microbiol. 43:719–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alsaker K. V., Papoutsakis E. T. 2005. Transcriptional program of early sporulation and stationary-phase events in Clostridium acetobutylicum. J. Bacteriol. 187:7103–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antoni D., Zverlov V. V., Schwarz W. H. 2007. Biofuels from microbes. Appl. Microbiol. Biotechnol. 77:23–35 [DOI] [PubMed] [Google Scholar]
- 4. Atsumi S., Hanai T., Liao J. C. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 [DOI] [PubMed] [Google Scholar]
- 5. Baer S. H., Blaschek H. P., Smith T. L. 1987. Effect of butanol challenge and temperature on lipid composition and membrane fluidity of butanol-tolerant Clostridium acetobutylicum. Appl. Environ. Microbiol. 53:2854–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borden J. R., Papoutsakis E. T. 2007. Dynamics of genomic-library enrichment and identification of solvent tolerance genes for Clostridium acetobutylicum. Appl. Environ. Microbiol. 73:3061–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowles L. K., Ellefson W. L. 1985. Effects of butanol on Clostridium acetobutylicum. Appl. Environ. Microbiol. 50:1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doremus M. G., Linden J. C., Moreira A. R. 1985. Agitation and pressure effects on acetone-butanol fermentation. Biotechnol. Bioeng. 27:852–860 [DOI] [PubMed] [Google Scholar]
- 9. Dürre P. (ed.). 2005. Handbook on clostridia. CRC Press, Boca Raton, FL [Google Scholar]
- 10. Fischer C. R., Klein-Marcuschamer D., Stephanopoulos G. 2008. Selection and optimization of microbial hosts for biofuels production. Metab. Eng. 10:295–304 [DOI] [PubMed] [Google Scholar]
- 11. González E., et al. 2000. Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product. Disruption and induction of the gene. J. Biol. Chem. 275:35876–35885 [DOI] [PubMed] [Google Scholar]
- 12. Hermann M., et al. 1985. Isolation and characterization of butanol-resistant mutants of Clostridium acetobutylicum. Appl. Environ. Microbiol. 50:1238–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hosaka T., et al. 2001. Characterization of the NADH-linked acetylacetoin reductase/2,3-butanediol dehydrogenase gene from Bacillus cereus YUF-4. J. Biosci. Bioeng. 91:539–544 [DOI] [PubMed] [Google Scholar]
- 14. Jones D. T., Woods D. R. 1986. Acetone-butanol fermentation revisited. Microbiol. Rev. 50:484–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Y.-L., Blaschek H. P. 1983. Butanol production by a butanol-tolerant strain of Clostridium acetobutylicum in extruded corn broth. Appl. Environ. Microbiol. 45:966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López-Contreras A. M., Claassen P. A., Mooibroek H., de Vos W. M. 2000. Utilisation of saccharides in extruded domestic organic waste by Clostridium acetobutylicum ATCC 824 for production of acetone, butanol and ethanol. Appl. Microbiol. Biotechnol. 54:162–167 [DOI] [PubMed] [Google Scholar]
- 17. Ma C., et al. 2009. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 82:49–57 [DOI] [PubMed] [Google Scholar]
- 18. Mermelstein L. D., Papoutsakis E. T. 1993. In vivo methylation in Escherichia coli by the Bacillus subtilis phage φ3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minton N. P., Oultram J. D. 1988. Host: vector systems for gene cloning in Clostridium. Microbiol. Sci. 5:310–315 [PubMed] [Google Scholar]
- 20. Nicholson W. L. 2008. The Bacillus subtilis ydjL (bdhA) gene encodes acetoin reductase/2,3-butanediol dehydrogenase. Appl. Environ. Microbiol. 74:6832–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nimcevic D., Schuster M., Gapes J. R. 1998. Solvent production by Clostridium beijerinckii NRRL B592 growing on different potato media. Appl. Microbiol. Biotechnol. 50:426–428 [DOI] [PubMed] [Google Scholar]
- 22. Oultram J. D., et al. 1988. Introduction of plasmids into whole cells of Clostridium acetobutylicum by electroporation. FEMS Microbiol. Lett. 56(1):83–88 [Google Scholar]
- 23. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 24. Tomas C. A., Welker N. E., Papoutsakis E. T. 2003. Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell's transcriptional program. Appl. Environ. Microbiol. 69:4951–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tummala S. B., Welker N. E., Papoutsakis E. T. 1999. Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 65:3793–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ui S., Masuda T., Masuda H., Muraki H. 1986. Mechanism for the formation of 2,3-butanediol stereoisomers in Bacillus polymyxa. J. Ferment. Technol. 64:481–486 [Google Scholar]
- 27. Vermuë M., Sikkema J., Verheul A., Bakker R., Tramper J. 1993. Toxicity of homologous series of organic solvents for the Gram-positive bacteria Arthrobacter and Nocardia sp. and the Gram-negative bacteria Acinetobacter and Pseudomonas sp. Biotechnol. Bioeng. 42:747–758 [DOI] [PubMed] [Google Scholar]
- 28. Vollherbst-Schneck K., Sands J. A., Montenecourt B. S. 1984. Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 47:193–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wardwell S. A., et al. 2001. Expression of the Klebsiella pneumoniae CG21 acetoin reductase gene in Clostridium acetobutylicum ATCC 824. J. Ind. Microbiol. Biotechnol. 27:220–227 [DOI] [PubMed] [Google Scholar]
- 30. Xiao Z., Xu P. 2007. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 33:127–140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.