Abstract
Cereulide and valinomycin are highly similar cyclic dodecadepsipeptides with potassium ionophoric properties. Cereulide, produced by members of the Bacillus cereus group, is known mostly as emetic toxin, and no ecological function has been assigned. A comparative analysis of the antimicrobial activity of valinomycin produced by Streptomyces spp. and cereulide was performed at a pH range of pH 5.5 to pH 9.5, under anaerobic and aerobic conditions. Both compounds display pH-dependent activity against selected Gram-positive bacteria, including Staphylococcus aureus, Listeria innocua, Listeria monocytogenes, Bacillus subtilis, and Bacillus cereus ATCC 10987. Notably, B. cereus strain ATCC 14579 and the emetic B. cereus strains F4810/72 and A529 showed reduced sensitivity to both compounds, with the latter two strains displaying full resistance to cereulide. Both compounds showed no activity against the selected Gram-negative bacteria. Antimicrobial activity against Gram-positive bacteria was highest at alkaline pH values, where the membrane potential (ΔΨ) is the main component of the proton motive force (PMF). Furthermore, inhibition of growth was observed in both aerobic and anaerobic conditions. Determination of the ΔΨ, using the membrane potential probe DiOC2(3) (in the presence of 50 mM KCl) in combination with flow cytometry, demonstrated for the first time the ability of cereulide to dissipate the ΔΨ in sensitive Gram-positive bacteria. The putative role of cereulide production in the ecology of emetic B. cereus is discussed.
INTRODUCTION
Members of the Bacillus cereus group, also known as Bacillus cereus sensu lato, are Gram-positive, rod-shaped, facultative anaerobic, spore-forming bacteria (30, 31). This group consists of the species B. cereus, B. weihenstephanensis, B. mycoides, B. pseudomycoides, B. anthracis, and B. thuringiensis (16, 30, 31, 70). B. cereus is found predominantly in the soil (3, 30, 70, 75) and has been isolated from the rhizosphere (3, 5), the air (70), the gut of invertebrates (30, 39), and a wide range of foods (16, 70). It is a notorious food spoilage organism (4, 70) and the causative agent of two types of food-borne disease (16, 22, 70), the emetic and diarrheal syndromes. The emetic toxin cereulide is produced by emetic B. cereus (1, 2, 22, 70) and some psychrotolerant B. weihenstephanensis strains (72, 73). This food intoxication, expressed by vomiting, is usually mild but occasionally results in fatalities (12, 42, 70). The toxin was found to be proteolytic resistant and highly heat and pH stable (57, 67, 68, 74). Cereulide is a so-called cyclic dodecadepsipeptide containing a three-repeat sequence [cyclo(l-O-Val–l-Val–d-O-Leu–d-Ala–)3], and its K+ ionophoric properties have been established in black-lipid membranes and by assessing its impact on mitochondrial swelling and function (1, 32, 45, 68). Recent research focused mainly on the genetic factors involved in the production of cereulide, the identification of transcriptional regulators, detection methods, and the impact of environmental conditions on cereulide levels reached, including a variety of (model) foods (13, 15, 17, 38, 69, 71, 78). Yet, besides its role in food poisoning and its effect on mammalian cells, the biological relevance of cereulide production in the environment, including its impact on other bacteria, has not been addressed. Production of ionophores has been described for a range of microbial species, including Streptomyces, Streptoverticillium, Nocardiopsis, Nocardia, Actinomadura, Bacillus, and several fungi (10, 21, 32, 52). These compounds can be divided into three major classes on the basis of their mode of transport: the neutral ionophores, carboxylic ionophores, and the channel-forming quasi-ionophores (10, 53). Notably, some information is available on the antimicrobial activity of the neutral ionophore valinomycin, a well-known peptide antibiotic that is produced by members of the soil-dwelling Gram-positive genus Streptomyces. Valinomycin shows high similarity with cereulide, being a cyclic dodecadepsipeptide also but containing the three-repeat sequence cyclo(l-Val–d-HyIva–d-Val–l-Lac–)3, where HyIva is α-hydroxyisovaleric acid and Lac is lactic acid (8, 41, 54). This peptide, like cereulide, has a central hydrophilic cavity in which a K+ ion can be accommodated (18, 55, 56). Hydrophobic side chains of valine and hydroxyisovaleric acid allow for its diffusion through the hydrophobic interior of cell membranes, a process driven by the existing K+ and/or charge gradients (33). A wide range of biological activities has been reported for valinomycin, including insecticidal, nematicidal, antiviral, and apoptosis-inducing effects in human cells (11, 62). Valinomycin has become a useful tool in the study of ion transport in biological systems, and its inhibitory effect on intact cells and the growth of bacteria and yeast has been studied, although to a limited extent (25, 26, 61, 64). The function of cereulide in B. cereus growth and ecology is unknown. Optimal conditions (20) and oxygen dependency (29) for cereulide synthesis suggest that it may be produced in a range of environments outside the human host, where it may play a role in competition of emetic B. cereus with other microorganisms for nutrients. To maximize chances of survival, microorganisms have developed strategies to outgrow or eliminate competition, and one of these strategies embraces the production of antimicrobial compounds. Antibiotics produced by bacteria can be specific, effective only against closely related species, or broad spectrum, depending on their mode of action (59). These compounds are usually synthesized during post-logarithmic growth, when nutrients and space for microbial multiplication become limited (43), which is accordingly observed for the synthesis of cereulide (20, 23, 72).
Therefore, this study was designed to assess the antimicrobial activity of cereulide and valinomycin using a selection of Gram-negative and Gram-positive bacteria at a range of pH 5.5 to pH 9.5 and under anaerobic and aerobic conditions. Moreover, combining fluorescent probes with flow cytometry allowed for the determination of the impact of these ionophores on the membrane potential (ΔΨ) in selected target organisms and in the pH range indicated. The impact of our findings on emetic B. cereus ecology is discussed.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A selection of facultative anaerobic bacteria was used, including Gram-negative Escherichia coli K-12 and Salmonella enterica subsp. enterica serovar Typhimurium II 505, and Gram-positive Staphylococcus aureus ATCC 25923, Listeria innocua, Listeria monocytogenes EGD-e, Bacillus subtilis 168, enterotoxic Bacillus cereus ATCC 10987 and ATCC 14579, and emetic Bacillus cereus F4810/72 and A529. All strains were grown in brain heart infusion (BHI) broth (Becton Dickinson, France) incubated at 30°C with shaking at 200 rpm (Innova 4335, New Brunswick Scientific, Netherlands) for 17 h unless stated otherwise. Cell cultures were maintained at −80°C in 20% glycerol as a cryoprotectant. Absorbance at 600 nm was measured using a Novaspec II spectrophotometer (Pharmacia Biotech, United Kingdom).
Chemicals.
A valinomycin ready-made solution (1 mg/ml dimethyl sulfoxide [DMSO]) and nigericin were obtained from Sigma-Aldrich. DMSO was obtained from Merck. Cereulide was synthesized as described by Biesta-Peters et al. (6). The final concentration of valinomycin and cereulide in the exposure experiments was 9 μM in the presence of 1% (vol/vol) DMSO. At this concentration, DMSO neither affected the growth nor the survival of the selected strains in this study. The concentrations of valinomycin and cereulide used in this study are within the range of values reported in the literature, including environmental and (model) food samples (23, 29, 64, 65).
Drop dilution plate assay.
To determine the effect of cereulide and valinomycin on the growth of the different test strains, BHI pour plates (0.5% [wt/vol] agar) adjusted to the appropriate pH, containing approximately 1 × 105 CFU ml−1, were prepared. After solidification, 5 μl of valinomycin (179.97 μM) or cereulide (167.84 μM), both containing 4% DMSO, was spotted directly on the agar surface. As a control, 5 μl of 4% DMSO was spotted. After the drops dried, plates were incubated overnight at 30°C, after which the inhibition zones were measured. All experiments have been performed using two independent biological duplicates.
Microtiter plate assay.
To determine the effect of valinomycin and cereulide on the growth performance of the selected strains, spectrophotometric growth curves were obtained using a 96-well microtiter plate reader (SpectraMax Plus384; Molecular Devices, United Kingdom). The optical density at 600 nm (OD600) of each well was automatically measured every 5 min for 24 h, with shaking for 30 s before each read. Inocula were prepared by resuspending overnight culture cells in 0.01 M phosphate buffer (PPi) to an OD600 of 2, reaching a final start OD600 of 0.1 in a 96-well microtiter plate (Greiner Bio-One, Germany). Plates were prepared by dispensing 180 μl of BHI broth, using the appropriate pH, into each well, supplemented with 10 μl of the proper inoculum, which was finalized by the addition of the control or test compound. Growth performance was tested at a range of pH values, namely, pH 5.5, 6.5, 7.5, 8.5, and 9.5, in aerobic and anaerobic conditions. Anaerobic conditions were created by covering the 96-well plate with optical adhesive film (MicroAmp). Each condition was tested using biological triplicates. The generated data were corrected for growth medium background signals. Log10-transformed growth curves were fitted according to the Zwietering growth model (79) using the solver from Microsoft Office Excel 2003. For each growth curve generated, the specific growth rate (μ) and duration of lag phase (λ) were determined and used to calculate the average μ and λ for each condition, as well as their standard errors. Statistical significance between two conditions was established by performing Student's t tests (two sided) and was considered significant when P values were <0.05.
Survival and recovery assay.
To study the effects of exposure to valinomycin and cereulide on survival and growth, pH-adjusted BHI broth (50 ml in 250-ml Erlenmeyer flasks) containing approximately 1 × 107 CFU ml−1 (absorbance at 600 nm, 0.1) was supplemented with cereulide or valinomycin to a final concentration of 9 μM and 1% (vol/vol) DMSO. As a control, cultures were exposed to 1% (vol/vol) DMSO. Cultures were grown aerobically at 30°C and 200 rpm. At regular intervals, appropriately diluted 50-μl aliquots of cells were surface plated in duplicate on BHI agar plates (Eddy Jet; IUL Instruments, Spain). Plates were incubated for 24 h at 30°C, and cell counts were expressed in log10 CFU ml−1. Each experiment was repeated twice, using two independent biological duplicates.
Detection and measurement of the membrane potential.
The ΔΨ was detected using the BacLight bacterial membrane potential kit (Invitrogen) in combination with flow cytometry. Briefly, cells were diluted to approximately 1 × 106 CFU ml−1 in phosphate-buffered saline (pH 5.5, 7, or 8.5) containing 50 mM KCl and 100 mM NaCl. Cells were complemented and incubated for 5 min with 10 μl (per ml cells) 100% DMSO, 500 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) in DMSO, 1 mg/ml valinomycin in DMSO, 1 mg/ml cereulide in DMSO, or 100 μM nigericin in DMSO. Cell suspensions (1 ml) were stained with 10 μl of 3 mM DiOC2(3) for 30 min at 30°C. The stained cells were analyzed by flow cytometry using a FACSCaliber (BD), with 488-nm excitation and detection through a 525- to 530-nm and 610-nm band-pass (∼20-nm bandwidth) filter. A ratiometric method was used to obtain cell size-independent values as previously described by Novo et al. (49). Each experiment was performed using three independent biological duplicates.
RESULTS
Antibacterial activity of valinomycin and its dependency on pH.
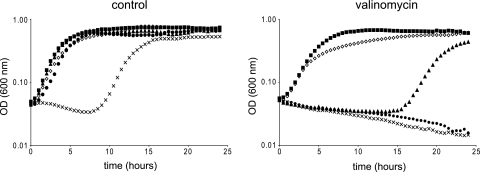
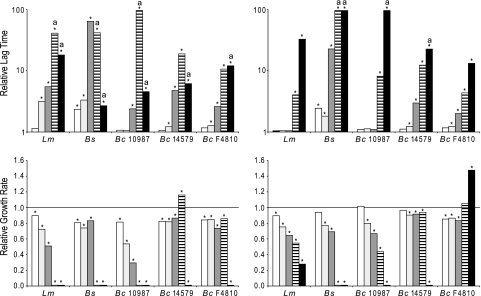
First, the impact of pH on the activity of valinomycin was assessed. Notably, the two components contributing to the proton motive force (PMF), namely, the membrane potential (ΔΨ) and the transmembrane pH gradient (ΔpH; signifying the difference between the intracellular and extracellular pH values), are closely interlinked, influencing the ability of active and passive membrane transport and ATP synthesis (7, 46–48). At acidic pH values, the ΔpH is the main component of the PMF, whereas at alkaline pH, the ΔΨ is the main component. This interlinkage suggests that the antibacterial activity of K+ ionophores could also depend on the pH of the medium and the ability of a microorganism to compensate and maintain its ion gradients and PMF. In this experiment, we investigated the effect of exposure to valinomycin on the growth of a selection of Gram-negative and Gram-positive bacteria at a broad pH range. Growth (OD600) in BHI was monitored for 24 h in a 96-well-microtiter-plate format during aerobic conditions. Figure 1 illustrates the effect of increasing the extracellular pH and the addition of valinomycin on aerobic growth of B. subtilis 168. These results were representative for all Gram-positive strains tested in this study, albeit the degree of inhibition varied. Although lag times and growth rates changed as a result of divergence from the optimal pH range for growth, the presence of valinomycin clearly decreased growth speed and/or caused increasing bacteriostatic activity as pH values became more alkaline. The left panel of Fig. 2 depicts the relative lag time and relative growth rate of a selection of Gram-positive bacteria in aerobic conditions. The growth rates of L. monocytogenes, B. cereus ATCC 10987 (Fig. 2), L. innocua, and S. aureus (data not shown) when exposed to valinomycin decreased with increasing pH values, whereas growth rates of B. subtilis, B. cereus ATCC 14579, and B. cereus F4810 remained largely unaffected up to pH 7.5 for B. subtilis and pH 8.5 for the latter two strains. In all cases, the lag phase appeared to be affected significantly, showing the largest effects for L. monocytogenes, B. subtilis, and B. cereus ATCC 10987 at alkaline pH. Notably, B. cereus ATCC 14579 and both emetic Bacillus strains were less sensitive to valinomycin, exhibiting outgrowth in 24 h at pH values up to pH 8.5 (Fig. 2 and data not shown). Gram-negative strains tested were found to be insensitive under all conditions tested, which confirms and expands upon previous reports (9, 64, 66).
Fig. 1.
Effect of pH and valinomycin on growth performance of Bacillus subtilis. Cells were grown in BHI at 30°C without (left) or with (right) 9 μM valinomycin at different pH values: pH 5.5 (open triangles), pH 6.5 (closed squares), pH 7.5 (closed triangles), pH 8.5 (closed circles), and pH 9.5 (crosses).
Fig. 2.
Impact of oxygen and pH on relative lag time and bacterial growth rate upon exposure to valinomycin. Listeria monocytogenes (Lm), Bacillus subtilis 168 (Bs), Bacillus cereus ATCC 10987, Bacillus cereus ATCC 14579, and the emetic toxin-producing strain Bacillus cereus F4810/72 were grown in BHI in the presence and absence of 9 μM valinomycin at different pH values: pH 5.5 (white), pH 6.5 (light gray), pH 7.5 (dark gray), pH 8.5 (striped), and pH 9.5 (black). Left and right panels show growth in the presence and absence of oxygen, respectively. Due to the variations in lag phases and growth rates in control experiments with the different bacteria and pH values tested, relative lag times (top) and relative growth rates (bottom) have been obtained by the deviation of valinomycin over control results. Lag times acquired from valinomycin-exposed samples equaling the experimental upper limit of 24 h are marked with an “a.” When significantly different (P < 0.05), bars are marked with an asterisk. A relative growth rate of 1 (line) depicts no difference in growth rates between the absence (control) and presence of valinomycin.
Sensitivity to valinomycin is reduced under anaerobic conditions.
To determine the effect of oxygen on the antibacterial activity of valinomycin, cells were grown in BHI at different pH values in the presence and absence of oxygen with or without 9 μM valinomycin (Fig. 2). Valinomycin extended the lag phase in close correlation with increasing pH values during aerobic and anaerobic conditions. Yet the lengths of the respective valinomycin-induced lag phases were generally shorter in the absence of oxygen. Although the sensitivities of the individual strains varied, this tendency was found for all tested Gram-positive strains. Valinomycin also reduced the relative growth rates of selected L. monocytogenes, B. subtilis, and B. cereus ATCC 10987 strains, with the largest effect again observed at alkaline pH (Fig. 2). Similar to aerobic conditions, the addition of valinomycin did not affect the growth of Gram-negative bacteria in anaerobic conditions (data not shown).
Cereulide affects growth kinetics of Gram-positive bacteria.
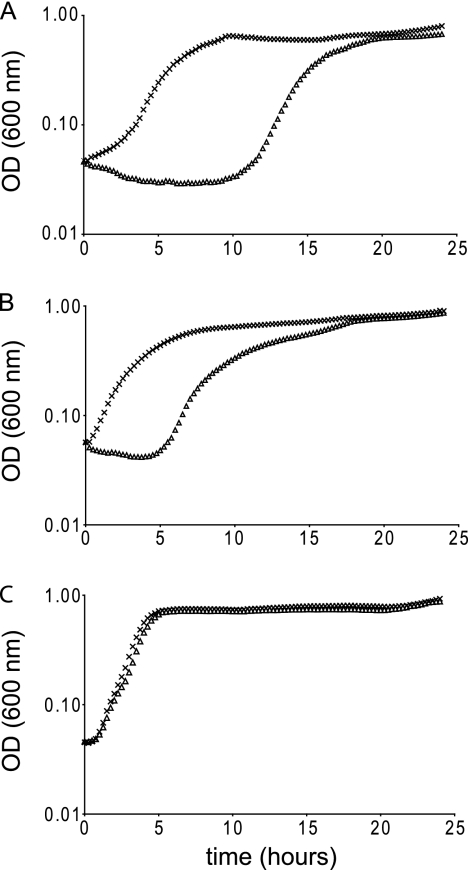
The impact of pH on the antibacterial properties of synthetic cereulide (6) was assessed using a drop dilution plate assay. In congruence with the results with valinomycin, Gram-positive bacteria appeared to be most susceptible at high pH (data not shown), and pH 8.5 was selected for subsequent experiments. Selected strains were incubated in BHI (pH 8.5, 30°C) in the absence and presence of 9 μM cereulide, and growth (OD600) was followed for 24 h in a 96-well spectrophotometer. All tested Gram-positive strains proved sensitive to the presence of cereulide as reflected in increased lag times, with the exception of the two emetic B. cereus strains used in this study (data not shown). Figure 3 illustrates the growth inhibitory effect of 9 μM cereulide on B. subtilis 168 and B. cereus ATCC 10987, with the emetic B. cereus strains (Fig. 3 and data not shown) showing resistance to cereulide. Interestingly, B. cereus ATCC 14579 revealed to be less susceptible to both valinomycin and cereulide but did not exhibit full resistance to cereulide, in contrast to the tested emetic B. cereus strains. Similar to data obtained in aerobic cultures, no growth effects were noticed for Gram-negative E. coli and S. Typhimurium exposed to cereulide (data not shown).
Fig. 3.
Impact of cereulide on growth of selected Bacillus strains at pH 8.5. Bacillus subtilis 168 (A), Bacillus cereus ATCC 10987 (B), and emetic Bacillus cereus F4810/72 (C) were grown for 24 h in BHI at 30°C in the presence (triangles) and absence (crosses) of 9 μM cereulide.
Bactericidal and bacteriostatic activity.
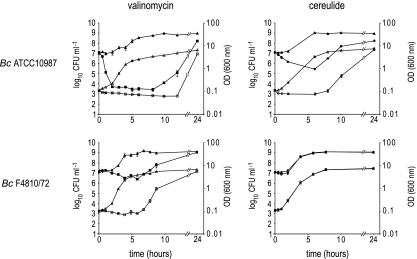
Exposure to valinomycin or cereulide induced bacteriostatic conditions in selected Gram-positive bacteria. Close examination of the different growth curves revealed small drops in OD600 during the lag phase. To investigate if this phenomenon is related to changes in cell viability, 1 × 107 CFU ml−1 of selected strains were exposed to 9 μM valinomycin or 9 μM cereulide or 1% DMSO as a control. Exposures took place in BHI at pH 8.5, and samples were collected at regular time intervals. Next to measuring the OD at 600 nm, samples were plated on BHI to monitor viable counts. B. cereus ATCC 10987 (Fig. 4) and B. subtilis (data not shown) appeared to be highly sensitive to valinomycin as reflected in the 3-log reduction of the viable count in the first hours of exposure. B. cereus ATCC 14579 (data not shown) and the emetic strain B. cereus F4810/72 (Fig. 4) were revealed to be less susceptive to the bactericidal properties of valinomycin, showing a 2-log reduction and less than 1-log reduction in viable counts, respectively. Noteworthy, increasing the pH to 9.5 enhances the bactericidal effects of valinomycin, resulting in a 4.5-log reduction for B. cereus ATCC 10987, 4-log reduction for B. cereus ATCC 14579, and 3.5-log reduction for B. cereus F4810/72. Exposure of B. cereus ATCC 10987 cells to cereulide at pH 8.5 resulted in a 1.5-log reduction, confirming its bactericidal properties (Fig. 4). Interestingly, the emetic strain B. cereus F4810/72 proved to be, although to a lesser extent compared to other nonemetic Bacillus strains, sensitive to valinomycin (Fig. 4). Yet, growth and survival seemed unaffected when exposed to cereulide (Fig. 3 and 4), indicating innate immunity of the producer cells to cereulide.
Fig. 4.
Bactericidal or bacteriostatic properties of valinomycin and cereulide at pH 8.5. OD600 values (open symbols) and viable counts (closed symbols) of Bacillus cereus ATCC 10987 and Bacillus cereus F4810/72 cultures exposed to 9 μM valinomycin (squares) or 9 μM cereulide (circles) in BHI. The nonexposed control cultures are depicted with triangles.
The addition of valinomycin and cereulide results in loss of membrane potential.
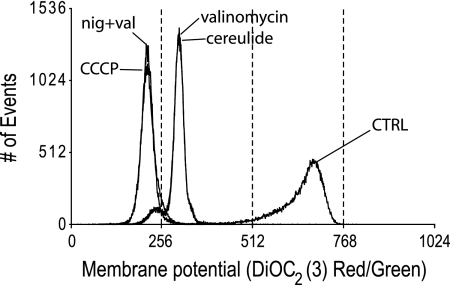
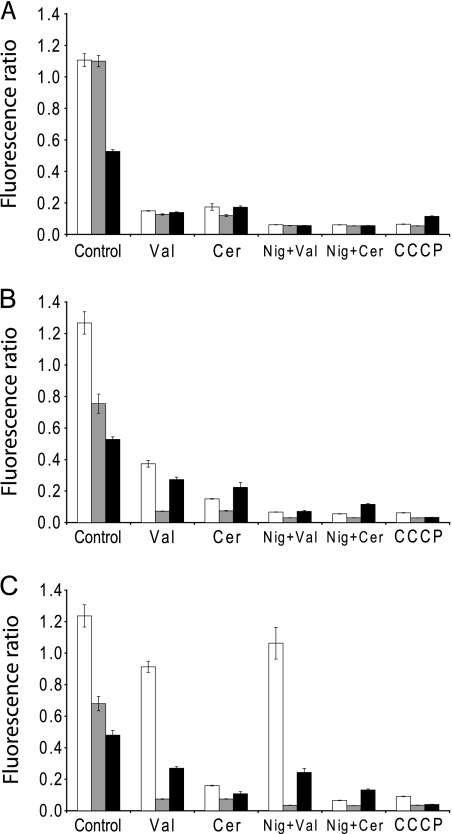
The K+ ionophore valinomycin is widely used to study membrane energetics and causes dissipation of the membrane potential (ΔΨ). The impact of cereulide on bacterial energetics has not been reported up to now, although activity was tested against eukaryotic cells and isolated mitochondria. The bacteriostatic and bactericidal activity of cereulide and valinomycin at high pH may be attributed to the fact that, e.g., ΔΨ is the main or sole component of the PMF under these conditions (7). The effect of valinomycin and cereulide on membrane (de)polarization of Gram-positive bacteria was investigated by exposing the cells to these compounds in the presence of 50 mM KCl and 100 mM NaCl at pH 5.5, 7, or 8.5 and subsequent staining with the ΔΨ probe DiOC2(3) and quantification with flow cytometry. Figure 5 shows a fluorescence histogram revealing the detrimental effect on ΔΨ in B. subtilis cells exposed to either valinomycin or cereulide, as indicated by the downshift in red emission of the fluorescent dye, signifying extensive depolarization of the cells. Using the proton ionophore CCCP, complete dissipation of ΔΨ was achieved, whereas exposure to 1% DMSO as a control had no effect. In the presence of the K+/H+ exchanger nigericin that dissipates the ΔpH (7), the addition of valinomycin or cereulide resulted in complete dissipation of the ΔΨ at all pH values tested. Similar results were obtained with cells from B. cereus ATCC 10987, L. innocua, and L. monocytogenes (data not shown). In Fig. 6, the responses of two nonemetic and one emetic B. cereus strain at different pH values are shown. Although growth effects are predominately linked to higher pH values, cells were readily (partially) depolarized by valinomycin and cereulide at all pH values, although the extent varied per strain. B. cereus ATCC 10987 demonstrated a dramatic loss of ΔΨ, whereas B. cereus ATCC 14579 and F4810/72 displayed residual polarization at higher pH values, signifying maintenance of a ΔΨ. The latter data are in line with the observed resistance to valinomycin and cereulide in the growth experiments.
Fig. 5.
FACS DiOC2(3) red/green fluorescence histogram overlays showing the effect of selected compounds on the membrane potential of B. subtilis. Cells were exposed for 5 min to DMSO (CTRL), valinomycin, cereulide, CCCP, and the combination of valinomycin with nigericin. Treated cells are stained for 30 min with 30 μM DiOC2(3) probe.
Fig. 6.
Impact of pH and exposure to ionophores on bacterial membrane potential. B. cereus ATCC 14579 (white bars), B. cereus ATCC 10987 (gray bars), and B. cereus F4810/72 (black bars) were exposed to DMSO (control), protonophore CCCP, valinomycin, and cereulide without and with nigericin. Exposures were performed at pH 5.5 (A), pH 7 (B), and pH 8.5 (C).
DISCUSSION
This study showed that the B. cereus emetic toxin cereulide, like its structural homologue valinomycin, produced by soil-dwelling Streptomyces spp., dissipates the membrane potential in Gram-positive bacteria at a broad pH range. Notably, significant inhibition of growth and cellular inactivation are observed only at alkaline pH values, a condition where the membrane potential is the main component of the proton motive force, whereas at neutral pH values, and even more pronounced at acid pH values, the pH gradient (pH inside − pH outside) is the dominant factor (7, 46, 47). In this way, bacteria maintain a similar magnitude of proton motive force at a broad pH range with various contributions of the membrane potential and pH gradient, allowing generation of an increasing pH gradient at acidic conditions, thereby maintaining the intracellular pH at neutral to alkaline pH values (7). Under aerobic and anaerobic conditions, the PMF is generated by H+-pumping electron transfer chain activity and H+ ATPase, respectively (25). In aerobic respiratory conditions, ATP is generated mainly via H+ influx via H+-ATPase, whereas in anaerobic conditions, ATP generated via substrate-level phosphorylation is used to extrude protons via this H+-ATPase. Despite basic differences in energy generation in anaerobic and aerobic conditions, growth of sensitive facultative anaerobic bacteria is affected in both conditions.
All tested Gram-positive bacteria, including Bacillus spp., Listeria spp., and Staphylococcus aureus, show inhibition of growth, although to different extents, whereas none of the tested Gram-negative bacteria are affected. This is in line with previous data obtained with valinomycin and other ionophores, which showed that Gram-negative bacteria are protected by the presence of an outer membrane that prevents access of these compounds to the inner membrane, acting as a selective permeability barrier between the cytoplasm and the outside environment (25, 61). Notably, enterotoxic B. cereus strain ATCC 14579 showed increased resistance to both valinomycin and cereulide. One could speculate that resistance to these compounds may involve activation of, e.g., antibiotic and/or multidrug resistance mechanisms in this strain. Obviously, more detailed experiments are required to elucidate the underlying mechanisms. Notably, emetic B. cereus displayed reduced sensitivity to valinomycin and appeared to be resistant to cereulide. This is in line with expectations where antimicrobial peptide and ionophore-producing microorganisms are protected by so-called innate immunity (43, 59). An obvious candidate for such a role is the putative cereulide secretion machinery encoded by genes located on the cer plasmid (15, 58). Further experiments including targeted deletion mutants will allow for the identification and characterization of genes conferring (innate) immunity of producer cells.
Production of cereulide could provide producing strains with a competitive advantage toward other Gram-positive bacteria in alkaline environments. An interesting question thus arises if and where environments exist with alkaline pH and sufficient potassium levels and whether emetic B. cereus strains have been isolated from these. Recent papers and reviews highlighted the unique features of emetic B. cereus and discussed their presence in a wide range of environments, including soil and plant rhizosphere and specifically paddy rice fields (3, 5, 28, 30, 50, 70). Potassium levels in soil are generally low and in the range of 0.1 to 4 mM (35, 40, 60, 63, 77). However, potassium levels in (organic) manure, plants, and the guts of insects can reach values up to 200 mM (27, 34, 76, 77). The potassium concentration and pH in the soil could transiently increase in decaying plant material which involves microbial metabolism of proteinaceous compounds, resulting in production of ammonia and a concomitant increase of pH. Environments known to reach high pH values are the mid- and hindgut of insects (14, 19, 30). Here, pH values >8 have been determined. However, up to now emetic B. cereus strains are not regarded as regular inhabitants of insects. Whether they can occupy this niche in certain conditions remains to be clarified. The fact that cereulide can inhibit Gram-positive bacteria that may occupy similar niches as emetic B. cereus may offer an explanation for maintenance of the plasmids carrying the genetic information for production of this dodecadepsipeptide. The so-called pXO1-like virulence megaplasmids have been sequenced and characterized, revealing the presence of a 24-kb ces gene cluster coding for the enzyme machinery mediating synthesis, modification, and secretion of cereulide (15, 58). The valinomycin-encoding gene cluster vlm from Streptomyces spp. (51) was shown to be structurally highly similar to the ces gene cluster (32, 44), but comparative sequence analysis revealed them to be highly divergent from each other at the DNA level. It was suggested that the vlm and ces gene clusters may share a relatively distant common ancestor, but these two gene clusters have since evolved independently (44). The capacity to produce valinomycin may provide competitive advantage to Streptomyces spp. in a range of environments, and a similar situation may occur in cereulide-producing emetic B. cereus. This could offer an explanation for maintenance of B. cereus cer genes in a range of environments outside the human host and would fit with the optimum temperature found for cereulide production, i.e., at a range from 5 to 20°C (20, 23, 65, 72, 73). The fact that cereulide production is activated at high cell densities, i.e., in the transition phase (38), may point to an additional role in intercellular signaling and/or cannibalism when nonproducing cells are targeted cells and killed, finally leading to a release of amino acids that subsequently serve as nutrients for the surviving population as described for stationary-phase cultures of B. subtilis (37). Alternatively, cereulide could play a role in intercellular signaling, thereby affecting cellular differentiation in the targeted subpopulation, resulting in phenotypic differentiation within emetic B. cereus populations. Lopez et al. (36) recently showed that exposure of B. subtilis cells to nystatin and surfactin resulted in loss of intracellular K+ with concomitant activation of KinC, a membrane protein kinase. This kinase controls activation of a set of genes involved in the production of extracellular polysaccharides that constitute the matrix of the biofilm, thus stimulating biofilm formation in B. subtilis. Since cereulide, like valinomycin, acts as a K+ ionophore, efflux of K+ from the cells down a concentration gradient will result in lowering of intracellular K+ levels, which could act as a signal for sensor kinases present in B. cereus group members, conceivably affecting cell physiology, including activation of pathways associated with cellular differentiation, such as production of matrix components and/or sporulation. Recently, evidence has been provided that transition state regulator AbrB, which is linked to biofilm formation in B. subtilis (24), controls production of cereulide in B. cereus F4810/72 (38). The role of cereulide production in biofilm formation of emetic B. cereus remains to be elucidated and is therefore currently studied in our laboratory.
In conclusion, our data show that cereulide, the toxin produced by emetic B. cereus, dissipates the membrane potential in sensitive Gram-positive bacteria, thereby providing a possible biological function of this K+ ionophore in the environment, i.e., outside the human host. Whether cereulide, besides its putative role in microbial warfare, has a role in intercellular signaling, cellular differentiation, and biofilm formation in emetic B. cereus remains to be elucidated.
Footnotes
Published ahead of print on 25 February 2011.
REFERENCES
- 1. Agata N., et al. 1994. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol. Lett. 121:31–34 [DOI] [PubMed] [Google Scholar]
- 2. Agata N., Ohta M., Mori M., Isobe M. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17–19 [DOI] [PubMed] [Google Scholar]
- 3. Altayar M., Sutherland A. D. 2006. Bacillus cereus is common in the environment but emetic toxin producing isolates are rare. J. Appl. Microbiol. 100:7–14 [DOI] [PubMed] [Google Scholar]
- 4. Bartoszewicz M., Hansen B. M., Swiecicka I. 2008. The members of the Bacillus cereus group are commonly present contaminants of fresh and heat-treated milk. Food Microbiol. 25:588–596 [DOI] [PubMed] [Google Scholar]
- 5. Berg G., Eberl L., Hartmann A. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7:1673–1685 [DOI] [PubMed] [Google Scholar]
- 6. Biesta-Peters E. G., et al. 2010. Quantification of the emetic toxin cereulide in food products by liquid chromatography-mass spectrometry using synthetic cereulide as a standard. Appl. Environ. Microbiol. 76:7466–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Booth I. R. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49:359–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brockmann H., Geeren H. 1957. Valinomycin II. Antibiotika aus Actinomyceten XXXVII. Die konstitution des valinomycins. Justus Liebig Annalen Der Chemie 603:216–232 [Google Scholar]
- 9. Brown R., Brennan J., Kelley C. 1962. An antifungal agent identical with valinomycin. Antibiot. Chemother. 12:482–487 [PubMed] [Google Scholar]
- 10. Butaye P., Devriese L. A., Haesebrouck F. 2003. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on Gram-positive bacteria. Clin. Microbiol. Rev. 16:175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y. Q. 2006. Deciphering the biosynthetic codes for the potent anti-SARS-CoV cyclodepsipeptide valinomycin in Streptomyces tsusimaensis ATCC 15141. Chembiochem 7:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dierick K., et al. 2005. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 43:4277–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dommel M. K., et al. 2010. Identification of the main promoter directing cereulide biosynthesis in emetic Bacillus cereus and its application for real-time monitoring of ces gene expression in foods. Appl. Environ. Microbiol. 76:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dow J. A. 1992. pH gradients in Lepidopteran midgut. J. Exp. Biol. 172:355–375 [DOI] [PubMed] [Google Scholar]
- 15. Ehling-Schulz M., et al. 2006. Cereulide synthetase gene cluster from emetic Bacillus cereus: structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ehling-Schulz M., Fricker M., Scherer S. 2004. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol. Nutr. Food Res. 48:479–487 [DOI] [PubMed] [Google Scholar]
- 17. Ehling-Schulz M., et al. 2006. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol. Lett. 260:232–240 [DOI] [PubMed] [Google Scholar]
- 18. Eisenman G. 1968. Ion permeation of cell membranes and its models. Fed. Proc. 27:1249–1251 [PubMed] [Google Scholar]
- 19. Fazito do Vale V., Pereira M. H., Gontijo N. F. 2007. Midgut pH profile and protein digestion in the larvae of Lutzomyia longipalpis (Diptera: Psychodidae). J. Insect Physiol. 53:1151–1159 [DOI] [PubMed] [Google Scholar]
- 20. Finlay W. J., Logan N. A., Sutherland A. D. 2000. Bacillus cereus produces most emetic toxin at lower temperatures. Lett. Appl. Microbiol. 31:385–389 [DOI] [PubMed] [Google Scholar]
- 21. Firakova S., Proksa B., Sturdikova M. 2007. Biosynthesis and biological activity of enniatins. Pharmazie 62:563–568 [PubMed] [Google Scholar]
- 22. Granum P. E., Lund T. 1997. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 157:223–228 [DOI] [PubMed] [Google Scholar]
- 23. Haggblom M. M., Apetroaie C., Andersson M. A., Salkinoja-Salonen M. S. 2002. Quantitative analysis of cereulide, the emetic toxin of Bacillus cereus, produced under various conditions. Appl. Environ. Microbiol. 68:2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamon M. A., Stanley N. R., Britton R. A., Grossman A. D., Lazazzera B. A. 2004. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol. Microbiol. 52:847–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harold F. M. 1972. Conservation and transformation of energy by bacterial membranes. Bacteriol. Rev. 36:172–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harold F. M., Baarda J. R. 1967. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J. Bacteriol. 94:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harvey W. R., Nedergaard S. 1964. Sodium-independent active transport of potassium in isolated midgut of Cecropia silkworm. Proc. Natl. Acad. Sci. U. S. A. 51:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoton F. M., et al. 2009. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 1:177–183 [DOI] [PubMed] [Google Scholar]
- 29. Jaaskelainen E. L., Haggblom M. M., Andersson M. A., Salkinoja-Salonen M. S. 2004. Atmospheric oxygen and other conditions affecting the production of cereulide by Bacillus cereus in food. Int. J. Food Microbiol. 96:75–83 [DOI] [PubMed] [Google Scholar]
- 30. Jensen G. B., Hansen B. M., Eilenberg J., Mahillon J. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5:631–640 [DOI] [PubMed] [Google Scholar]
- 31. Kotiranta A., Lounatmaa K., Haapasalo M. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189–198 [DOI] [PubMed] [Google Scholar]
- 32. Kroten M. A., Bartoszewicz M., Swiecicka I. 2010. Cereulide and valinomycin, two important natural dodecadepsipeptides with ionophoretic activities. Pol. J. Microbiol. 59:3–10 [PubMed] [Google Scholar]
- 33. Lehninger A. L., Nelson D. L., Cox M. M. 1993. Principles of biochemistry: with an extended discussion of oxygen-binding proteins, 2nd ed. Worth Publishers, New York, NY [Google Scholar]
- 34. Leigh R. A., Jones R. G. W. 1984. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 97:1–13 [Google Scholar]
- 35. Liebeke M., Brozel V. S., Hecker M., Lalk M. 2009. Chemical characterization of soil extract as growth media for the ecophysiological study of bacteria. Appl. Microbiol. Biotechnol. 83:161–173 [DOI] [PubMed] [Google Scholar]
- 36. Lopez D., Fischbach M. A., Chu F., Losick R., Kolter R. 2009. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 106:280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopez D., Kolter R. 2010. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol. Rev. 34:134–149 [DOI] [PubMed] [Google Scholar]
- 38. Lucking G., Dommel M. K., Scherer S., Fouet A., Ehling-Schulz M. 2009. Cereulide synthesis in emetic Bacillus cereus is controlled by the transition state regulator AbrB, but not by the virulence regulator PlcR. Microbiology 155:922–931 [DOI] [PubMed] [Google Scholar]
- 39. Luxananil P., Atomi H., Panyim S., Imanaka T. 2001. Isolation of bacterial strains colonizable in mosquito larval guts as novel host cells for mosquito control. J. Biosci. Bioeng. 92:342–345 [DOI] [PubMed] [Google Scholar]
- 40. Maathuis F. J. 2009. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12:250–258 [DOI] [PubMed] [Google Scholar]
- 41. Magarvey N. A., Ehling-Schulz M., Walsh C. T. 2006. Characterization of the cereulide NRPS alpha-hydroxy acid specifying modules: activation of alpha-keto acids and chiral reduction on the assembly line. J. Am. Chem. Soc. 128:10698–10699 [DOI] [PubMed] [Google Scholar]
- 42. Mahler H., et al. 1997. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 336:1142–1148 [DOI] [PubMed] [Google Scholar]
- 43. Martin J. F., Demain A. L. 1980. Control of antibiotic biosynthesis. Microbiol. Rev. 44:230–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matter A. M., Hoot S. B., Anderson P. D., Neves S. S., Cheng Y. Q. 2009. Valinomycin biosynthetic gene cluster in Streptomyces: conservation, ecology and evolution. PLoS One 4:e7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mikkola R., Saris N. E., Grigoriev P. A., Andersson M. A., Salkinoja-Salonen M. S. 1999. Ionophoretic properties and mitochondrial effects of cereulide: the emetic toxin of Bacillus cereus. Eur. J. Biochem. 263:112–117 [DOI] [PubMed] [Google Scholar]
- 46. Mitchell P. 1991. Foundations of vectorial metabolism and osmochemistry. Biosci. Rep. 11:297–346 [DOI] [PubMed] [Google Scholar]
- 47. Mitchell P. 1977. Vectorial chemiosmotic processes. Annu. Rev. Biochem. 46:996–1005 [DOI] [PubMed] [Google Scholar]
- 48. Mitchell P. D. 2004. Foundations of vectorial metabolism and osmochemistry. Biosci. Rep. 24:386–435 [DOI] [PubMed] [Google Scholar]
- 49. Novo D., Perlmutter N. G., Hunt R. H., Shapiro H. M. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35:55–63 [DOI] [PubMed] [Google Scholar]
- 50. Pandey A., Palni L. M., Bisht D. 2001. Dominant fungi in the rhizosphere of established tea bushes and their interaction with the dominant bacteria under in situ conditions. Microbiol. Res. 156:377–382 [DOI] [PubMed] [Google Scholar]
- 51. Perkins J. B., Guterman S. K., Howitt C. L., Williams V. E., II, Pero J. 1990. Streptomyces genes involved in biosynthesis of the peptide antibiotic valinomycin. J. Bacteriol. 172:3108–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perlman D., Bodanszk M. 1971. Biosynthesis of peptide antibiotics. Annu. Rev. Biochem. 40:449–464 [DOI] [PubMed] [Google Scholar]
- 53. Pressman B. C. 1976. Biological applications of ionophores. Annu. Rev. Biochem. 45:501–530 [DOI] [PubMed] [Google Scholar]
- 54. Pressman B. C. 1965. Induced active transport of ions in mitochondria. Proc. Natl. Acad. Sci. U. S. A. 53:1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pressman B. C. 1968. Ionophorous antibiotics as models for biological transport. Fed. Proc. 27:1283–1288 [PubMed] [Google Scholar]
- 56. Pressman B. C. 1973. Properties of ionophores with broad range cation selectivity. Fed. Proc. 32:1698–1703 [PubMed] [Google Scholar]
- 57. Rajkovic A., et al. 2008. Heat resistance of Bacillus cereus emetic toxin, cereulide. Lett. Appl. Microbiol. 46:536–541 [DOI] [PubMed] [Google Scholar]
- 58. Rasko D. A., et al. 2007. Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the Bacillus cereus-group plasmids, including Bacillus anthracis pXO1. J. Bacteriol. 189:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Riley M. A., Wertz J. E. 2002. Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56:117–137 [DOI] [PubMed] [Google Scholar]
- 60. Russell E. J., Wild A. 1988. Russell's soil conditions and plant growth, 11th ed. Longman Scientific & Technical, Harlow, Essex, England [Google Scholar]
- 61. Ryabova I. D., Gorneva G. A., Ovchinnikov Y. A. 1975. Effect of valinomycin on ion transport in bacterial cells and on bacterial growth. Biochim. Biophys. Acta 401:109–118 [DOI] [PubMed] [Google Scholar]
- 62. Ryoo I. J., et al. 2006. Selective cytotoxic activity of valinomycin against HT-29 human colon carcinoma cells via down-regulation of GRP78. Biol. Pharm. Bull. 29:817–820 [DOI] [PubMed] [Google Scholar]
- 63. Schroeder J. I., Ward J. M., Gassmann W. 1994. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu. Rev. Biophys. Biomol. Struct. 23:441–471 [DOI] [PubMed] [Google Scholar]
- 64. Seshachalam D., Frahm D. H., Ferraro F. M. 1973. Cation reversal of inhibition of growth by valinomycin in Streptococcus pyogenes and Clostridium sporogenes. Antimicrob. Agents Chemother. 3:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shaheen R., et al. 2006. Potential of selected infant food formulas for production of Bacillus cereus emetic toxin, cereulide. Int. J. Food Microbiol. 107:287–294 [DOI] [PubMed] [Google Scholar]
- 66. Shemyakin M. M., et al. 1965. The structure-antimicrobial relation for valinomycin depsipeptides. Experientia 21:548–552 [DOI] [PubMed] [Google Scholar]
- 67. Shinagawa K., Konuma H., Sekita H., Sugii S. 1995. Emesis of rhesus monkeys induced by intragastric administration with the HEp-2 vacuolation factor (cereulide) produced by Bacillus cereus. FEMS Microbiol. Lett. 130:87–90 [DOI] [PubMed] [Google Scholar]
- 68. Shinagawa K., Ueno Y., Hu D., Ueda S., Sugii S. 1996. Mouse lethal activity of a HEp-2 vacuolation factor, cereulide, produced by Bacillus cereus isolated from vomiting-type food poisoning. J. Vet. Med. Sci. 58:1027–1029 [DOI] [PubMed] [Google Scholar]
- 69. Shiota M., et al. 2010. Rapid detoxification of cereulide in Bacillus cereus food poisoning. Pediatrics 125:e951–e955 [DOI] [PubMed] [Google Scholar]
- 70. Stenfors Arnesen L. P., Fagerlund A., Granum P. E. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 71. Thorsen L., Azokpota P., Hansen B. M., Hounhouigan D. J., Jakobsen M. 2010. Identification, genetic diversity and cereulide producing ability of Bacillus cereus group strains isolated from Beninese traditional fermented food condiments. Int. J. Food Microbiol. 142:247–250 [DOI] [PubMed] [Google Scholar]
- 72. Thorsen L., Budde B. B., Henrichsen L., Martinussen T., Jakobsen M. 2009. Cereulide formation by Bacillus weihenstephanensis and mesophilic emetic Bacillus cereus at temperature abuse depends on preincubation conditions. Int. J. Food Microbiol. 134:133–139 [DOI] [PubMed] [Google Scholar]
- 73. Thorsen L., et al. 2006. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 72:5118–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Turnbull P. C., Kramer J. M., Jorgensen K., Gilbert R. J., Melling J. 1979. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am. J. Clin. Nutr. 32:219–228 [DOI] [PubMed] [Google Scholar]
- 75. Vilain S., Luo Y., Hildreth M. B., Brozel V. S. 2006. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl. Environ. Microbiol. 72:4970–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Walker D. J., Leigh R. A., Miller A. J. 1996. Potassium homeostasis in vacuolate plant cells. Proc. Natl. Acad. Sci. U. S. A. 93:10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Y., Wu W. H. 2010. Plant sensing and signaling in response to K+-deficiency. Mol. Plant. 3:280–287 [DOI] [PubMed] [Google Scholar]
- 78. Yabutani M., Agata N., Ohta M. 2009. A new rapid and sensitive detection method for cereulide-producing Bacillus cereus using a cycleave real-time PCR. Lett. Appl. Microbiol. 48:698–704 [DOI] [PubMed] [Google Scholar]
- 79. Zwietering M. H., Jongenburger I., Rombouts F. M., van 't Riet K. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56:1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]