Abstract
We have investigated the impacts of 63 different low-molecular-weight compounds, most of them plant derived, on the in vitro expression of two antifungal biosynthetic genes by the plant-protecting rhizobacterium Pseudomonas fluorescens CHA0. The majority of the compounds tested affected the expression of one or both antifungal genes. This suggests that biocontrol activity in plant-beneficial pseudomonads is modulated by plant-bacterium signaling.
Certain strains of root-colonizing fluorescent pseudomonads are able to provide efficient protection of crop plants against a variety of soilborne phytopathogenic fungi, notably by the secretion of extracellular antimicrobial secondary metabolites into the rhizosphere (11, 17). Pseudomonas fluorescens strain CHA0 produces the two well-characterized antifungal compounds, 2,4-diacetylphloroglucinol (DAPG) (15) and pyoluteorin (PLT) (18), which are major determinants of biocontrol activity in this strain and in many other pseudomonads with disease-suppressive capacity (12). There is evidence that strain CHA0 maintains these antifungal compounds at a fine-tuned balance that can be regulated in the rhizosphere by plant-derived factors (2, 8, 21).
Plant roots release into the rhizosphere a wide variety of low-molecular-weight compounds (3) with prominent functions in plant defense signaling and in some symbiotic and pathogenic plant-microbe interactions. Root exudates are composed mainly of sugars and amino acids but also contain phenolic compounds. Because of their abundance and importance in plant-soil-microbe systems (1, 22), it has been hypothesized that plant-derived compounds may also play a relevant role in interaction with beneficial pseudomonads by modulating the expression of antifungal compounds.
The aim of this study was to screen a large number of plant-derived compounds for their potential to regulate the expression of DAPG and PLT biosynthetic genes in the well-characterized root-associated biocontrol strain P. fluorescens CHA0. For this purpose, green fluorescent protein (GFP)-based reporters were used to monitor the expression of phlA and pltA, two genes involved in DAPG and PLT biosynthesis, respectively (2). The same reporters were used in previous work by Baehler et al. (2), who have shown that their expression in growing cultures strongly correlates with DAPG and PLT production. Emphasis was placed on compounds related to plant defense or plant development or compounds known to be involved in plant-microbe interactions. Most of the selected compounds occur in the rhizosphere. Since biocontrol activity can also occur in the upper parts of the plant (16), some compounds found in aerial parts of the plant were also considered in this study. The obtained results demonstrate that numerous plant-derived compounds may play a considerable role in the regulation of biocontrol gene expression by P. fluorescens CHA0.
P. fluorescens strain CHA0 (23) was cultivated according to the method of de Werra et al. (9). For monitoring of antifungal gene expression, derivatives of P. fluorescens strain CHA0 carrying rhizosphere-stable plasmids with transcriptional fusions of a stable variant of the gfp gene to the DAPG biosynthetic gene phlA (pME7100) (2) or the PLT biosynthetic gene pltA (pME7109) (2) were used. Compounds were purchased from Sigma-Aldrich Chemie GmbH (Schnelldorf, Germany), Fluka Chemie GmbH (Buchs, Switzerland), or Toronto Research Chemical Inc. (North York, Canada). All stock solutions of compounds were prepared in methanol.
Gene expression studies with P. fluorescens CHA0 or its derivatives carrying the different gfp-based transcriptional fusions were carried out according to the method of de Werra et al. (9). Briefly, 10 ml of OS minimal medium with 47 mM glycerol as a carbon source (2) was inoculated with 20 μl of exponential-growth-phase LB cultures of the bacterial strains diluted to an optical density at 600 nm (OD600) of 0.05 and supplemented with the corresponding plant-derived compound at a final concentration of 100 μM. Compound stock solutions in methanol were added at 1% of the final volume. Control cultures received the same amount of pure methanol. For each treatment, six replicates of 200 μl each were prepared in a microtiter plate. OD600 as a parameter of growth and green fluorescence (excitation at 480 nm and emission at 520 nm) as a parameter of antifungal gene expression were measured with a Spectrafluor Plus microplate reader (Tecan Group Ltd., Männedorf, Switzerland) throughout the exponential and stationary growth phases. For each individual measurement, the green fluorescence value was divided by the corresponding OD600 value, giving the specific fluorescence per cell expressed in relative fluorescence units (RFU) (2). Based on their ability to induce or repress the expression of the phlA or pltA gene at the early stationary growth phase, percentages of expression change of the compounds were determined; “toxic” means bacterial growth was inhibited by the compound.
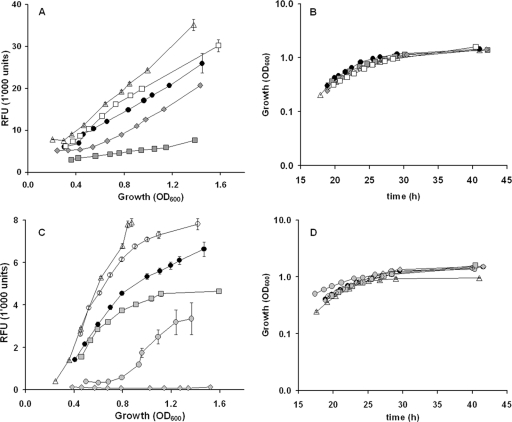
In vitro effects of 63 compounds, mostly produced by plants or plant-associated microorganisms, on the expression of DAPG and PLT biosynthetic genes in P. fluorescens CHA0 were evaluated. All three repetitions of the experiment presented similar expression curves, summarized in Table 1. Detailed expression and growth curves in response to selected compounds are shown in Fig. 1. Among the 63 compounds tested, 25 had an effect on DAPG biosynthetic gene expression (5 inducing and 20 repressing), 27 had an effect on the PLT biosynthetic gene expression (17 inducing and 10 repressing), and 23 compounds did not have any effect on expression of either of the two antifungal genes (Table 1). Umbelliferone was the compound which exhibited the strongest inhibition of phlA. Umbelliferone is also known to inhibit nod genes in Rhizobium (5). Interestingly, in contrast to the strong inhibitory effect of umbelliferone, the similar molecule coumarin, which differs from umbelliferone only in the lack of the hydroxyl group had no effect on phlA expression (Table 1). It seems that only small differences in chemical structure between compounds may result in completely different responses of the antifungal gene phlA. No specific chemical structures were identified which generally induced or repressed phlA gene expression. The four compounds 2,4-diacetylphloroglucinol, monoacetylphloroglucinol, phloroglucinol, and resorcinol were found to have a complete repressive effect on the expression of the pltA gene (Table 1). Interestingly, all these compounds are based on a resorcinol structure. However, molecules also having a resorcinol basis but with more complex structures, such as genistein or phloridzin, did not have any repressive effect on pltA expression (Table 1). Except for hydroquinone (isomer of resorcinol) and pectin, which were found to completely inhibit and slightly reduce bacterial growth, respectively, all other compounds did not have a significant effect on the proliferation of bacterial cells (Fig. 1; also data not shown). Pectin was the compound with the strongest inducing effect on pltA expression. Since pectin is generally present in plant cell walls, it is likely that this compound is present in the vicinity of roots either deriving from root particles or from decaying plants.
Table 1.
Compounds used in this study and their effects on expression of the phlA and pltA genes of P. fluorescens CHA0
Antifungal gene expression was studied in OS glycerol medium using CHA0 derivatives carrying phlA-gfp and pltA-gfp fusions on plasmids pME7100 and pME7109, respectively.
Compounds are indicated with their common name and/or chemical formula when necessary.
Induction or repression of reporter gene expression at the early stationary growth phase. Expression measurements are relative to cultures treated with methanol only. The following compounds did not have any effects on the expression of either of the two antifungal genes: 2-hydroxy-acetophenone, vanillin, syringaldehyde, p-hydroxy-benzoic acid, vanillic acid, isovanillic acid, syringic acid, p-coumaric acid, ferulic acid, coniferyl alcohol, biochanin A, naringenin, epicatechin, quercetin dehydrate, 4-quinolinol, digalacturonic acid, and esculin hydrate.
Fig. 1.
Kinetics of the expression of 2,4-diacetylphloroglucinol (A) or pyoluteorin (C) biosynthetic genes in growing cultures of Pseudomonas fluorescens CHA0. Gene expression as relative fluorescence units (RFU) per bacterial density (A and C) or growth (B and D) was measured in CHA0 carrying a phlA-gfp reporter fusion on pME7100 (A and B) or a pltA-gfp fusion on pME7109 (C and D). Bacterial cultures were amended with 100 μM indole-3-acetic acid (open triangles), 100 μM acetovanillone (open squares), 100 μM acetyl salicylate (gray diamonds), or 100 μM umbelliferone (gray squares) (A) or with 10 ppm of pectin (open triangles), 100 μM m-coumaric acid (open circles), 100 μM protocatechuic acid (gray squares), 100 μM 2,4-dihydroxy-acetophenone (gray circles), or 100 μM resorcinol (gray diamonds) (C). Black circles indicate control cultures with methanol only (A, B, C, and D). The strains were grown in OS glycerol medium at 30°C. Data represent means (± standard errors) of data for six replicate cultures. The experiment was repeated twice with similar results.
Collectively, these data indicate that many different plant-derived compounds are able to modify the expression of genes involved in the production of secondary metabolites which play an important role in the biocontrol activity of P. fluorescens CHA0. In some cases, an antagonistic expression of DAPG and PLT genes has been observed. This phenomenon was already described by Baehler et al. (2) for mutants overproducing one antifungal, which led to the repression of the other.
Certainly, bacterial and plant signals, mainly phenolic compounds, play a major role in interactions between plants and soil microorganisms (4, 13). Plant phenolics have traditionally been considered inducers of virulence genes in plant-pathogenic bacteria, such as Agrobacterium (5), as signal molecules in root-nodule bacteria (6, 24), or mostly as defense molecules in plant-pathogen interactions (10, 20). Some phenolics act as phytoanticipins or phytoalexins and are involved in preformed plant defense or are accumulated in plant tissue upon pathogen attack or other plant stress (7, 19).
To date, few studies have validated the role of plant-derived compounds, mainly phenolics, in the interactions between plants, fungal plant pathogens, and beneficial bacteria suppressing fungal diseases and their effect on antifungal compound production by the bacteria. Our results show that the majority of compounds tested can impact on the disease-suppressive bacterium P. fluorescens CHA0 by modulating the expression of the DAPG, the PLT, or both biosynthetic genes. Interestingly, well-known plant signals like salicylate, jasmonate, and methyl jasmonate, which are key components of plant defense, all slightly reduced phlA gene expression, whereas the plant hormone indole-3-acetic acid induced the expression of this antifungal gene. In an earlier study (8), we suggested that plant phenolics involved in stress response and defense against pathogens might be responsible for the alteration of antifungal gene expression in P. fluorescens CHA0 in the rhizosphere of pathogen-attacked and mechanically injured plants. Similarly, a study performed by Jousset et al. (14) showed that upon pathogen infection, the quantity of certain plant phenolics in root exudates was increased and phlA expression in CHA0 on plant roots was upregulated.
The results obtained in this study provide a further indication that plant-derived compounds released into the rhizosphere are part of a complex regulatory network and may act as signals modulating antifungal compound production and biocontrol activity in plant-beneficial rhizobacteria.
Acknowledgments
We gratefully acknowledge financial support from the Swiss National Science Foundation (project no. 3100A0-105881).
Footnotes
Published ahead of print on 25 February 2011.
REFERENCES
- 1. Badri D. V., Weir T. L., van der Lelie D., Vivanco J. M. 2009. Rhizosphere chemical dialogues: plant-microbe interactions. Curr. Opin. Biotechnol. 20:642–650 [DOI] [PubMed] [Google Scholar]
- 2. Baehler E., Bottiglieri M., Péchy-Tarr M., Maurhofer M., Keel C. 2005. Use of green fluorescent protein-based reporters to monitor balanced production of antifungal compounds in the biocontrol agent Pseudomonas fluorescens CHA0. J. Appl. Microbiol. 99:24–38 [DOI] [PubMed] [Google Scholar]
- 3. Bais H. P., Weir T. L., Perry L. G., Gilroy S., Vivanco J. M. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57:233–266 [DOI] [PubMed] [Google Scholar]
- 4. Bauer W. D., Mathesius U. 2004. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 7:429–433 [DOI] [PubMed] [Google Scholar]
- 5. Bolton G. W., Nester E. W., Gordon M. P. 1986. Plant phenolic compounds induce expression of the Agrobacterium tumefaciens loci needed for virulence. Science 232:983–985 [DOI] [PubMed] [Google Scholar]
- 6. Dakora F. D. 2003. Defining new roles for plant and rhizobial molecules in sole and mixed plant cultures involving symbiotic legumes. New Phytol. 158:39–49 [Google Scholar]
- 7. Dakora F. D., Phillips D. A. 1996. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 49:1–20 [Google Scholar]
- 8. de Werra P., Baehler E., Huser A., Keel C., Maurhofer M. 2008. Detection of plant-modulated alterations in antifungal gene expression in Pseudomonas fluorescens CHA0 on roots by flow cytometry. Appl. Environ. Microbiol. 74:1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Werra P., Péchy-Tarr M., Keel C., Maurhofer M. 2009. Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 75:4162–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dixon R. A. 2001. Natural products and plant disease resistance. Nature 411:843–847 [DOI] [PubMed] [Google Scholar]
- 11. Haas D., Défago G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307–319 [DOI] [PubMed] [Google Scholar]
- 12. Haas D., Keel C. 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41:117–153 [DOI] [PubMed] [Google Scholar]
- 13. Hirsch A. M., et al. 2003. Molecular signals and receptors: controlling rhizosphere interactions between plants and other organisms. Ecology 84:858–868 [Google Scholar]
- 14. Jousset A., et al. 2011. Plants respond to pathogen infection by enhancing the antifungal gene expression of root associated bacteria. Mol. Plant Microbe Interact. 24:352–358 [DOI] [PubMed] [Google Scholar]
- 15. Keel C., et al. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol. Plant Microbe Interact. 5:4–13 [Google Scholar]
- 16. Kloepper J. W., et al. 1999. Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australas. Plant Pathol. 28:21–26 [Google Scholar]
- 17. Lugtenberg B., Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63:541–556 [DOI] [PubMed] [Google Scholar]
- 18. Maurhofer M., Keel C., Haas D., Défago G. 1994. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. Eur. J. Plant Pathol. 100:221–232 [Google Scholar]
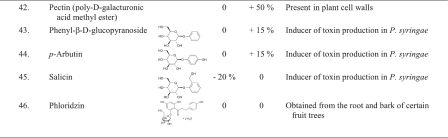
- 19. Ndakidemi P. A., Dakora F. D. 2003. Legume seed flavonoids and nitrogenous metabolites as signals and protectants in early seedling development. Funct. Plant Biol. 30:729–745 [DOI] [PubMed] [Google Scholar]
- 20. Nicholson R. L., Hammerschmidt R. 1992. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 30:369–389 [Google Scholar]
- 21. Notz R., et al. 2001. Biotic factors affecting expression of the 2,4-diacetylphloroglucinol biosynthesis gene phlA in Pseudomonas fluorescens biocontrol strain CHA0 in the rhizosphere. Phytopathology 91:873–881 [DOI] [PubMed] [Google Scholar]
- 22. Siqueira J. O., Nair M. G., Hammerschmidt R., Safir G. R. 1991. Significance of phenolic compounds in plant-soil-microbial systems. Crit. Rev. Plant Sci. 10:63–121 [Google Scholar]
- 23. Stutz E. W., Défago G., Kern H. 1986. Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology 76:181–185 [Google Scholar]
- 24. van Rhijn P., Vanderleyden J. 1995. The Rhizobium-plant symbiosis. Microbiol. Rev. 59:124–142 [DOI] [PMC free article] [PubMed] [Google Scholar]