Abstract
The persistence of Listeria monocytogenes in food-associated environments represents a key factor in transmission of this pathogen. To identify persistent and transient strains associated with production of fermented meat sausages in northern Portugal, 1,723 L. monocytogenes isolates from raw material and finished products from 11 processors were initially characterized by random amplification of polymorphic DNA (RAPD), PCR-based molecular serotyping, and epidemic clone characterization, as well as cadmium, arsenic, and tetracycline resistance typing. Pulsed-field gel electrophoresis (PFGE) typing of 240 representative isolates provided evidence for persistence of L. monocytogenes for periods of time ranging from 10 to 32 months for all seven processors for which isolates from different production dates were available. Among 50 L. monocytogenes isolates that included one representative for each PFGE pattern obtained from a given sample, 12 isolates showed reduced invasion efficiency in Caco-2 cells, including 8 isolates with premature stop codons in inlA. Among 41 isolates representing sporadic and persistent PFGE types, 22 isolates represented lysogens. Neither strains with reduced invasion nor lysogens were overrepresented among persistent isolates. While the susceptibility of isolates to lysogenic phages also did not correlate with persistence, it appeared to be associated with molecular serotype. Our data show the following. (i) RAPD may not be suitable for analysis of large sets of L. monocytogenes isolates. (ii) While a large diversity of L. monocytogenes subtypes is found in Portuguese fermented meat sausages, persistence of L. monocytogenes in this food chain is common. (iii) Persistent L. monocytogenes strains are diverse and do not appear to be characterized by unique genetic or phenotypic characteristics.
INTRODUCTION
Listeria monocytogenes is a facultative intracellular food-borne pathogen capable of causing a disease known as listeriosis in humans and a wide range of animals (10, 60, 62). L. monocytogenes is not only present in a variety of different environments, but it also has the ability to grow at refrigeration temperatures (70), in a wide pH range (approximately 4 to 9.6) (53, 54), and at high salt concentrations (up to 10%) (41). Despite the implementation of intensive control measures, eradication and control of L. monocytogenes in food processing environments remain considerable challenges. Several studies indicate that the most important source of food contamination with L. monocytogenes is via cross-contamination from the equipment and general environment of the processing plants, where some L. monocytogenes strains can persist over extended periods, while others are recovered only sporadically (30, 33, 37, 38, 44, 47). While several authors attempted to identify specific characteristics, e.g., biofilm formation or sanitizer resistance capabilities, that may be associated with L. monocytogenes persistence in processing plants (1, 6, 39, 49), little attention has been given to the potential contributions of prophages to the ability of L. monocytogenes to establish persistence. In many pathogens, these bacterial viruses are recognized as important contributors to virulence, in the form of bacterial lysogens, or as vectors in horizontal gene transfer; in addition, the presence of prophages may also confer resistance to certain phages (7, 8).
Meat preserved in the form of fermented sausages is typical in the rural areas of Portugal and can have a positive economic impact in these areas. Previous studies of different traditional fermented meat sausages produced in northern Portugal (i.e., alheira, salpicão de Vinhais, and chouriça de Vinhais) showed that these products are often contaminated with L. monocytogenes. Frequent contamination and high counts of L. monocytogenes have been reported in the finished products for alheira (17, 19), and in the production stages of salpicão de Vinhais and chouriça de Vinhais, but not in the finished products. These differences are probably due to differences in processing, including the fact that the production of salpicão de Vinhais and chouriça de Vinhais involves a longer smoking process (3 to 4 weeks) and entails less handling and manual labor than the production of alheira does (18). Briefly, for production of alheira, various meats are boiled in water with salt and spices, and thinly sliced bread, soaked in the broth formed during boiling the meat, is added to the mixture of small meat pieces, spices, and olive oil and/or fat drippings. The resulting paste is stuffed into the intestinal casings of cattle and submitted to a smoking process, usually for no longer than 8 days. Alheira must be cooked before consumption. The essential ingredients of chouriça de Vinhais and salpição de Vinhais include raw pork meat from Bísaro, an autochthonous pig breed, salt, spices, and regional wine. The process for making these sausages includes an initial seasoning stage (48 h at 4°C), stuffing the mixture into natural pork casings, and a final stage of curing by traditional smoking at 20 to 30°C for approximately 3 weeks for chouriça and 4 weeks for salpicão. In the production of salpicão, only pork loin meat is used and stuffed into large intestines, while in chouriça, meat and pork fat are used and stuffed into small intestines. These products are typically consumed without further cooking. The aim of this study was to characterize a collection of 1,723 L. monocytogenes isolates recovered from traditional northern Portuguese fermented meat sausages in order to (i) assess genetic diversity of L. monocytogenes found in these types of products, (ii) evaluate L. monocytogenes contamination patterns in the production of these products, and (iii) investigate selected genetic and phenotypic characteristics of L. monocytogenes strains identified as persistent.
MATERIALS AND METHODS
Isolate information.
Overall, a total of 1,723 L. monocytogenes isolates obtained from either (i) traditional fermented meat sausages (final products) from northern Portugal or (ii) raw products and products at different stages of processing were included in this study. Samples of traditional fermented meat sausages (finished products), representing products from 11 processors (processors A to K; Tables 1 and 2), were collected either at retail establishments or at processing plants. For 7 processors, only isolates from finished product samples were tested (Table 1), including four processors where isolates were obtained from samples collected on two different dates (between 14 and 32 months apart). Some of these sampling data have previously been reported in a study by Ferreira et al. (19), which did not include any isolate characterization (Table 1).
Table 1.
Subtype patterns for L. monocytogenes isolates from processors where only final products were tested
| Processor and producta | Sampling date (mo-yr)b | No. of isolates with a specific subtype | Molecular serotype group | EC marker(s)c | Resistance patternd | RAPD type(s) (no. of isolates) | No. of isolates for further PFGE characterization | PFGE profile(s) of isolates further characterized |
|---|---|---|---|---|---|---|---|---|
| Processor A | ||||||||
| Alheira (P; n = 130)** | May-04 | 127 | D | ECI−, ECII− | Cds Asr Tets | a (79), b (14), c (12), d (7), e (3), f (3), g (3), h (3), i (1), j (1), k (1) | 16 | 358, 383 |
| 2 | B | NT | Cdr Ass Tets | b (2) | 1 | 346 | ||
| 1 | D | ECI−, ECII− | Cdr Arr Tets | m (1) | 1 | 362 | ||
| Alheira (P; n = 40)** | Jul-05 | 21 | D | ECI−, ECII− | Cds Ass Tets | a (9), b (7), g (4), n (1) | 4 | 358 |
| 15 | B | NT | Cdr Ass Tets | l (11), o (4) | 2 | 346 | ||
| 3 | D | ECI−, ECII− | Cdr Asr Tets | l (2), o (1) | 3 | 358 | ||
| 1 | B | Cds Ass Tets | p (1) | 1 | 346 | |||
| Processor D | ||||||||
| Alheira (P; n = 38)** | May-04 | 28 | A | ECIII− | Cdr Ass Tets | a (13), b (1), c (14) | 5 | 356 |
| 8 | B | NT | Cdr Ass Tets | d (7), e (1) | 3 | 372, 346 | ||
| 2 | D | ECI−, ECII− | Cds Asr Tets | f (2) | 2 | 358 | ||
| Alheira (P; n = 13)** | Jul-05 | 12 | A | ECIII− | Cdr Ass Tets | a (12) | 2 | 356 |
| 1 | D | ECI−, ECII− | Cds Asr Tets | g (1) | 1 | 384 | ||
| Processor E | ||||||||
| Alheira (R; n = 8)* | Sep-03 | 7 | B | NT | Cdr Ass Tets | a (7) | 2 | 344, 380 |
| 1 | D | ECI−, ECII− | Cds Ass Tets | b (1) | 1 | 379 | ||
| Alheira (R; n = 12)** | Mar-05 | 12 | B | NT | Cdr Ass Tets | c (12) | 2 | 344 |
| Processor F | ||||||||
| Alheira (R; n = 61)** | May-04 | 53 | D | ECI+ | Cdr Ass Tets | a (53) | 2 | 360 |
| 4 | A | ECIII− | Cdr Ass Tets | e (2), f (1), g (1) | 4 | 342 | ||
| 3 | D | ECI+ | Cds Ass Tets | a (1), b (1), c (1) | 3 | 367, 360 | ||
| 1 | D | ECI+ | Cdr Asr Tets | d (1) | 1 | 360 | ||
| Alheira (P; n = 7)** | Jan-07 | 6 | B | NT | Cdr Ass Tets | h (4), i (2) | 3 | 373 |
| 1 | D | ECI+ | Cdr Ass Tets | a (1) | 1 | 360 | ||
| Processor H | ||||||||
| Alheira (P; n = 19)* | Sep-03 | 16 | B | NT | Cdr Ass Tetr | a (15), b (1) | 5 | 352, 371, 378 |
| 2 | D | ECI−, ECII− | Cdr Ass Tetr | b (1), c (1) | 2 | 377 | ||
| 1 | A | ECIII− | Cdr Ass Tets | d (1) | 1 | 376 | ||
| Processor J | ||||||||
| Alheira (P; n = 136)** | May-04 | 129 | C | NT | Cdr Ass Tets | a (127), b (2) | 6 | 361 |
| 7 | C | NT | Cds Ass Tets | a (4), c (1), d (1), e (1) | 6 | 361 | ||
| Processor K | ||||||||
| Alheira (P; n = 3)* | May-04 | 3 | D | ECI−, ECII− | Cdr Ass Tetr | a (2), b (1) | 3 | 362 |
L. monocytogenes prevalence data for some samples (processor A, 2005; processor D, 2005; processor E, 2005; processor K) have been reported previously by Ferreira et al. (19); in addition, characterization of a subset of isolates from some samplings (processor A, 2004; processor D, 2004; processor E, 2003; and processor H and processor J) have been reported previously by Felício et al. (17). Samples were collected either at processing plants (P) or retail stores (R) as indicated at the beginning of the parentheses (the number of samples is shown by n). For each processor and each time point, two composite samples were tested for L. monocytogenes, and isolates from all positive samples were further characterized; the number of the two composite samples that were positive for L. monocytogenes is indicated by the number of asterisks.
The sampling dates are shown in an abbreviated month-year form (e.g., May-04 stands for May 2004).
Isolates with molecular serotypes B and C were not tested (NT) for epidemic clone (EC) markers, as these groups do not contain EC strains. ECI−, epidemic clone I negative; ECI+, epidemic clone I positive.
The resistance pattern to cadmium (Cd), arsenic (As), and tetracycline (Tet) is shown as follows: superscript r for resistance and superscript s for sensitive.
Table 2.
Subtype patterns for L. monocytogenes isolates from processors where final products and samples from different processing stages were tested
| Processor and producta | Sampling date (mo-yr) | No. of isolates with a specific subtype | Molecular serotype group | EC markerb | Resistance pattern | RAPD type(s) (no. of isolates) | No. of isolates for further PFGE characterization | PFGE profile(s) of isolates further characterized |
|---|---|---|---|---|---|---|---|---|
| Processor B | ||||||||
| Alheira (R; n = 96)** | Nov-03 | 92 | B | NT | Cdr Ass Tets | a (80), b (8), c (2), d (2) | 6 | 349 |
| 4 | B | NT | Cds Ass Tets | c (2), d (1), e (1) | 3 | 369, 370, 374 | ||
| Alheira production (P; n = 151) | Mar-05 | 66 | B | NT | Cdr Ass Tets | a (46), b (11), c (3), d (2), e (2), f (1), g (1) | 11 | 349, 370 |
| 63 | A | ECIII− | Cdr Ass Tets | i (45), j (10), k (3), l (2), m (2), n (1) | 9 | 365 | ||
| 11 | D | ECI+ | Cdr Asr Tets | b (3), q (3), g (2), r (1), s (1), t (1) | 6 | 368 | ||
| 6 | D | ECII+ | Cds Ass Tets | a (4), o (1), p (1) | 3 | 385 | ||
| 2 | A | ECIII− | Cdr Asr Tets | i (2) | 2 | 365 | ||
| 1 | B | NT | Cds Ass Tets | h (1) | 1 | 349 | ||
| 1 | B | NT | Cdr Asr Tets | h (1) | 1 | 349 | ||
| 1 | D | ECI+ | Cdr Ass Tets | b (1) | 1 | 368 | ||
| Processor C | ||||||||
| Alheira (P; n = 151)** | May-04 | 47 | D | ECII+ | Cds Ass Tets | a (20), b (11), c (6), d (3), e (2), f (3), g (2) | 10 | 130 |
| 14 | B | NT | Cdr Ass Tets | a (3), h (5), i (4), j (2) | 6 | 359, 382 | ||
| Alheira production (P; n = 62) | Apr-05 | 42 | D | ECII+ | Cds Ass Tets | m (34), n (5), o (2), p (1) | 7 | 130 |
| 20 | D | ECI+ | Cdr Ass Tets | q (20) | 2 | 381 | ||
| Alheira production (P; n = 522) | Jan-07 | 489 | D | ECII+ | Cds Ass Tets | 33 unique patterns | 53 | 130 |
| 31 | B | NT | Cdr Ass Tets | 2 unique patterns | 3 | 344 | ||
| 2 | B | NT | Cds Ass Tets | 1 unique pattern | 2 | 373 | ||
| Processor G | ||||||||
| Alheira (R; n = 47)** | Dec-04 | 24 | D | ECI+ | Cds Ass Tets | a (22), b (1), c (1) | 3 | 360 |
| 23 | D | ECI+ | Cdr Ass Tets | a (21), e(1), d (1) | 3 | 360 | ||
| Alheira production (P; n = 107) | Dec-06 | 84 | D | ECI+ | Cdr Ass Tets | f (83), g (1) | 5 | 360 |
| 13 | D | ECI+ | Cds Ass Tets | f (13) | 3 | 360 | ||
| 10 | B | NT | Cdr Ass Tets | f (10) | 2 | 373 | ||
| Processor I | ||||||||
| Alheira production (P; n = 149) | Jul-05 | 149 | A | ECIII− | Cdr Ass Tets | a (146), b (3) | 7 | 356 |
| Chouriça de Vinhais production (P; n = 30) | Jul-05 | 25 | C | NT | Cdr Ass Tets | c (25) | ||
| 5 | C | NT | Cds Ass Tets | c (5) | 2 | 375 | ||
| Salpicão de Vinhais production (P; n = 31) | Jul-05 | 13 | C | NT | Cdr Asr Tets | c (13) | 2 | 375 |
| 12 | C | NT | Cdr Ass Tets | c (11), e (1) | 2 | 375 | ||
| 4 | A | ECIII− | Cdr Ass Tets | d (4) | 1 | 356 | ||
| 2 | C | NT | Cds Ass Tets | c (2) | 1 | 375 |
Samples represented either finished products (labeled “alheira”), collected at processing plants (P) or retail stores (R), or samples from various stages of processing, collected at processing plants (P); these samples are indicated by the word “production” after the product name. For finished products, two composite samples were tested for L. monocytogenes for each processor and each time point, and isolates from all positive samples were further characterized. The number of the two composite samples that were positive for L. monocytogenes is indicated by the number of asterisks. For samples from various stages of processing, detailed descriptions are provided in Table 4. L. monocytogenes prevalence data for some samples (processor G, 2004; processor I, samples of salpicão de Vinhais and chouriça de Vinhais, 2005) have been reported previously by Ferreira et al. (18); in addition, characterization of isolates from some samplings (processor B, 2003; processor C, 2004) have been reported previously by Felício et al. (17).
Isolates with molecular serotypes B and C were not tested (NT) for EC markers, as these groups do not contain EC strains.
For four processors (processors B, C, G, and I; Table 2), L. monocytogenes isolates recovered at different processing stages of alheira production (3 processors) and at different processing stages of alheira, salpicão de Vinhais, and chouriça de Vinhais production (one processor) were used. For two of these processors, isolates from samples collected on three sampling dates were available, while for two processors, isolates from samples collected on two sampling dates were available (Table 2); some of these sampling data have previously been reported in a study by Ferreira et al. (18, 19).
While sample collection and L. monocytogenes isolation for some samplings that provided isolates for this study have previously been reported (Tables 1 and 2), isolates from additional sample collections conducted as part of the study reported here were also used (Tables 1 and 2). L. monocytogenes isolation for these samples was performed essentially as described previously (17) using an approach that included selective enrichment in Fraser broth as well as enumeration of L. monocytogenes by 5-tube most-probable-number (MPN) and direct plating (see reference 17 for details on the methodology). For all products (e.g., finished product and product at different stages of processing), two samples were tested (except for some samples from processor I [see Table 4]); for finished products, each sample tested represented aliquots from 6 different sausages. For each testing procedure (i.e., MPN, direct plating, and enrichment) that yielded presumptive Listeria colonies on PALCAM medium (17), five presumptive Listeria colonies were used to determine whether they actually were L. monocytogenes; for MPN, up to 5 colonies from each tube that yielded presumptive Listeria colonies were tested. All confirmed L. monocytogenes isolates obtained by this approach (i.e., 1,723 isolates) were used here for initial subtype characterization by random amplification of polymorphic DNA (RAPD), PCR-based molecular serotyping, epidemic clone characterization, and cadmium, arsenic, and tetracycline resistance typing (as detailed below). For some samplings (indicated in Tables 1 and 2), a small number of isolates (i.e., approximately 10% of isolates obtained from a given sample) have previously been characterized by selected molecular subtyping methods (see reference 17); these isolates were not included among the isolates characterized here.
Table 4.
L. monocytogenes PFGE profiles for isolates obtained from different production stages of fermented sausages for four processors
| Processor and producta | Sampling date (mo-yr) | Sampleb | PFGE profile(s) (no. of isolates) |
|---|---|---|---|
| Processor B | |||
| Alheira | Mar-05 | Casing* | 365 (1) |
| Cooked meat mixture* | 349 (1) | ||
| Paste** | 349 (5), 365 (4), 368 (3), 370 (1), 385 (3) | ||
| Alheira (final product)** | 365(6), 368 (4), 370 (6) | ||
| Processor C | |||
| Alheira | Apr-05 | Alheira before smoking* | 381 (2) |
| Alheira after 3 days of smoking** | 130 (7) | ||
| Jan-07 | Casing** | 130 (5) | |
| Cooked beef meat** | 130 (5) | ||
| Cooked pork meat** | 130 (1) | ||
| Cooked meat mixture* | 130 (2) | ||
| Paste** | 130 (10), 344 (2) | ||
| Alheira before smoking** | 130 (7) | ||
| Alheira after 1 day of smoking** | 130 (11), 373 (1) | ||
| Alheira (final product)** | 130 (12), 344 (1), 373 (1) | ||
| Processor G | |||
| Alheira | Dec-06 | Casing** | 360 (2), 374 (1) |
| Cooked meat mixture** | 360 (4), 374 (1) | ||
| Paste* | 360 (1) | ||
| Alheira before smoking* | 360 (1) | ||
| Processor I | |||
| Alheira | Dec-06 | Paste | 356 (3) |
| Alheira before smoking | 356 (2) | ||
| Alheira after 2 days of smoking | 356 (1) | ||
| Allheira (final product)** | 356 (1) | ||
| Salpicão de Vinhais | Dec-06 | Seasoned raw pork | 375 (1) |
| Seasoned raw pork (2nd day) | 375 (4) | ||
| Salpicão (before smoking) | 356 (1) | ||
| Chouriça de Vinhais | Dec-06 | Seasoned raw pork | 375 (1) |
| Seasoned raw pork (2nd day) | 375 (1) |
For three processors, only samples from alheira production were tested; for processor I, samples for three product types (alheira, salpicão de Vinhais, and chouriça de Vinhais) from the production chain were tested.
For processors B, C, and G, two samples were tested for L. monocytogenes at each sampling date, and isolates from all positive samples were further characterized. The number of asterisks indicates whether one or two of the two samples were positive for L. monocytogenes; for processor I, only one sample for each processing stage was tested (except for finished product).
RAPD-PCR.
Bacterial lysates for random amplification of polymorphic DNA were prepared by growing bacteria for 12 to 16 h in brain heart infusion (BHI) broth at 37°C, followed by centrifugation of a 1-ml aliquot of the resulting culture at 16,250 × g for 5 min. The resulting cell pellet was washed two times in 0.9% (wt/vol) saline solution and resuspended in 500 μl of sterile distilled water. After the suspensions were boiled at 100°C for 15 min, they were diluted with water to an A600 of 0.75. RAPD-PCR was performed with 1 μl of the cell lysate in a 25-μl reaction mixture containing 1× Taq buffer (MBI Fermentas, Mundolsheim, France), 2.5 mM MgCl2 (MBI Fermentas), 0.2 mM (each) deoxynucleotides (ABGene, Surrey, United Kingdom), and 1 U of Taq polymerase (MBI Fermentas). For each isolate, separate RAPD-PCRs were performed with either primer UBC 155 (5′-CTG GCG GCT G-3′) (16) or primer OPM-01 (5′-GTT GGT GGC T-3′) (34); these primers were used at final concentrations of 1.4 μM and 1.96 μM, respectively. Amplification reactions were performed in a Thermocycler (Bio-Rad MyCycler and thermocycler firmware; both from Bio-Rad, Richmond, CA) using the following steps: (i) one cycle of 94°C for 2 min; (ii) 39 cycles, with 1 cycle consisting of 94°C for 1 min, 35.5°C for 2 min, and 72°C for 2 min; and (iii) a final cycle of 72°C for 10 min. The amplified products were resolved by electrophoresis on 1.5% agarose gels; a 100-bp PCR molecular ruler (Bio-Rad Laboratories) was used as a molecular size standard. The gels were photographed under UV transillumination. Initial clustering of RAPD patterns was performed with GelCompar II version 4.0 (Applied Maths, Sint-Martens-Latem, Belgium) using the unweighted-pair group matching algorithm and the Dice correlation coefficient. Using visual refinement, unique RAPD patterns for each primer were designated with a number; UBC 155 and OPM-01 patterns were also combined to yield a single RAPD profile, which was designated with a letter. The reproducibility of RAPD assays was assessed by preparing two independent cell lysates and performing RAPD-PCR (performed on different days) for approximately 10% of L. monocytogenes isolates, which were selected conveniently to represent isolates from all processors; for both primers, RAPD profiles were found to consistently yield patterns with comparable main bands. There was variation in the minor bands, including differences in relative intensity and appearance or disappearance of bands; therefore, these low-intensity bands were not taken into consideration when the RAPD patterns were compared. RAPD typing of the L. monocytogenes positive-control strain NCTC 11994, which was included in every RAPD-PCR run, was consistently reproducible for both primers.
Molecular serotyping.
Detection of serotype-specific marker genes for L. monocytogenes strains was performed using a multiplex PCR assay previously reported by Doumith et al. (13). This assay differentiates isolates into five major subtypes, each of which represents more than one serotype, including (i) subtype A (which includes serotypes 1/2a and 3a); (ii) subtype B (serotypes 1/2b, 3b, and 7); (iii) subtype C (serotypes 1/2c and 3c); (iv) subtype D (serotypes 4b, 4d, and 4e); and (v) subtype E (serotypes 4a and 4c).
Resistance to cadmium, arsenic, and tetracycline.
Isolates were screened for resistance to cadmium (Cd), arsenic (As), and tetracycline (Tet) at concentrations of 75 μg ml−1, 500 μg ml−1, and 8 μg ml−1, as previously described (42, 69). Isolates were classified as resistant (r superscript) or sensitive (s superscript) to each compound, and results were combined to generate a unique resistance profile (e.g., if one isolate was resistant to cadmium and sensitive to arsenic and tetracycline, the results were expressed as Cdr Ass Tets).
PCR screen for EC-associated genetic markers.
A previously described multiplex PCR assay (9) was used to test all isolates for genetic markers for the three previously reported L. monocytogenes major epidemic clones (ECs). Only molecular serotype D isolates were screened for epidemic clone I (ECI) and ECII genetic markers (as these two ECs represent serotype 4b strains), while only molecular serotype A isolates were screened for the ECIII genetic marker (as this EC represents a serotype 1/2a strain).
PFGE.
A subset of 240 L. monocytogenes isolates, representing at least one representative of each combined subtype pattern for each sample (Tables 1 and 2), was selected for further characterization by pulsed-field gel electrophoresis (PFGE); “combined subtype patterns” were based on RAPD, resistance typing, molecular serotyping, and EC determination. PFGE was performed according to the standard CDC PulseNet protocol (22) using restriction enzymes ApaI (Roche Molecular Biochemicals, Indianapolis, IN) and AscI (New England BioLabs, Ipswich, MA) and a CHEF Mapper XA (Bio-Rad Laboratories, Hercules, CA). PFGE images for individual isolates were processed to enhance contrast and reduce background. Similarity clustering was performed with the Applied Maths BioNumerics version 3.5 software package (Applied Maths, Sint-Martens-Latem, Belgium), using the unweighted-pair group matching algorithm and the Dice correlation coefficient with a tolerance of 1.5% and an optimization of 1.5%. Classification of isolates into different ApaI and AscI patterns was visually validated. ApaI and AscI PFGE pattern data were used to assign a combined PFGE profile to each isolate; PFGE profiles were designated by numbers (e.g., 130). Isolate information and subtyping data are freely available through the Pathogen Tracker 2.0 database (http://www.pathogentracker.net).
Caco-2 cell invasion assays.
Caco-2 cell invasion assays were performed as previously described (46) with minor adjustments. A standard laboratory control strain of L. monocytogenes (10403S) and an isogenic ΔinlA strain were included as controls in each invasion assay (Table 3). At least two independent invasion assays were performed for each isolate. Results were reported as percent invasion efficiency (i.e., number of bacteria recovered/number of bacteria inoculated), normalized to the values obtained for the control strain 10403S (set at 100%).
Table 3.
L. monocytogenes control and host strains
| L. monocytogenes strain | Serotype | Lineage | Purpose(s) | Reference |
|---|---|---|---|---|
| 10403S | 1/2a | II | Control included in invasion, swarming, and inlA qRT-PCR assays | 4 |
| FSL W3-084 | 1/2a | II | 10403S ΔinlA mutant, control for invasion assay | 46 |
| FSL H6-199 | 1/2a | II | 10403S ΔflaA mutant, control for swarming assay | 50 |
| FSL J1-123 | 4b | I | Control for inlA qRT-PCR assays | 58 |
| FSL R2-503 | 1/2b | I | Control for inlA qRT-PCR assays | 58 |
| Mack | 1/2a | II | Host strain for phage isolation | 26 |
| FSL F2-695 | 4a | IIIA | Host strain for phage isolation | 57 |
| F2365 | 4b | I | Host strain for phage isolation | 45 |
inlA sequencing.
L. monocytogenes isolates that showed Caco-2 cell invasion efficiencies of <20% (relative to the control strain 10403S) were screened for the presence of premature stop codons (PMSCs) in the inlA gene, which encodes a protein critical for invasion of Caco-2 cells. Primer sequences, PCR conditions, and sequencing strategies have previously been detailed (46). Initially, an approximately 800-bp fragment in the 3′ end of inlA fragment was sequenced; if no PMSCs were detected in this fragment, DNA sequencing of the remaining inlA open reading frame was performed.
Motility assays.
Swarming on semisoft agar was evaluated for four isolates showing reduced invasiveness in Caco-2 cells but no PMSC in inlA. One individual colony of each L. monocytogenes isolate to be tested was used to needle inoculate tryptic soy broth plates containing 0.4% agar, followed by incubation for 48 h at either 24, 30, or 37°C. L. monocytogenes 10403S and 10403S ΔflaA strains were included as controls (Table 3). The plates were scanned with an Epson Perfection 1650 photo scanner (Epson America, Inc., Long Beach, CA), and the area of the swarming halo around the point of inoculation was measured using the image analysis program SigmaScan Pro 5.0 (Systat Software Inc., San Jose, CA). Results are reported as the mean of the swarming area observed in four independent experiments.
Quantitative reverse transcription-PCR (qRT-PCR) analysis of inlA transcript levels.
inlA transcript levels were evaluated for the four isolates that showed reduced invasiveness in Caco-2 cells but did not have a PMSC in inlA (i.e., isolates FSL F8-110, FSL F8-131, FSL F8-132, and FSL F8-151) and three control strains representing two different lineages and three different serotypes (1/2a, 1/2b, and 4b; Table 3). For RNA extraction, the bacteria were grown as described above for the Caco-2 cell invasion assays. RNA Protect bacterial reagent (Qiagen, Valencia, CA) was added to the cultures according to the manufacturer's instructions to stabilize the mRNA. After centrifugation at 4°C for 5 min at 4,200 × g, the supernatant was removed and cells were resuspended in 1 ml of TRI reagent (Applied Biosystems). The cell suspension was mixed with 0.1-mm acid-washed zirconium beads, and cells were disrupted in a minibeadbeater (Biospec Products, Bartlesville, OK) for 4 min. After centrifugation for 5 min at 9.3 × g at 4°C, the supernatant was added to 100 μl of 1-bromo-3-cyclopropane (Sigma). The aqueous phase was subsequently collected into a new centrifuge tube, and nucleic acids were precipitated with isopropanol and then washed with ethanol. The pellet was resuspended in 100 μl of nuclease-free water (Qiagen). After treatment with RQ1 DNase (Promega) in the presence of RNasin (Promega, Madison, WI), total RNA was purified using the RNeasy minikit (Qiagen) according to the manufacturer's protocol. RNA was eluted from the column using RNase-free water, and UV spectrophotometry (Nanodrop, Wilmington, DE) was used to quantify RNA and assess the purity of the RNA.
qRT-PCR was used to quantify transcript levels for the target gene (inlA) and the housekeeping gene (rpoB), using previously reported primers and probes (49). qRT-PCR was performed on an ABI Prism 7000 sequence detection system (Applied Biosystems) essentially as previously described (63). Two RNA extractions were performed, and transcript levels for inlA were expressed as mean of log cDNA copy numbers, which were normalized by subtracting the log cDNA copy numbers observed for the rpoB housekeeping gene.
Prophage induction and phage propagation.
A subset of 41 L. monocytogenes isolates representing both sporadic (n = 22) and persistent (n = 19) L. monocytogenes strains associated with 7 processors (processors A to G; Fig. 1 shows isolates and their PFGE patterns) was used to test for lysogen induction after UV treatment. Briefly, single colonies of isolates were inoculated in 5 ml of Luria-Bertani broth with 50 mM morpholinepropanesulfonic acid (MOPS), 1% (wt/vol) glucose, 10 mM CaCl2, and 10 mM MgCl2 (LB-MOPS-Glu-salts). After overnight incubation at 30°C with shaking (220 rpm), followed by exposure of 1 ml of culture to UV light for 2 min and subsequent incubation at room temperature in the dark for 3 h, 100-μl aliquots of the culture were mixed with 100 μl of one of three propagating strains in separate tubes (i.e., strains F2365, Mack, and FSL F2-695 [Table 3] grown in LB-MOPS-Glu-salts at 30°C as described above) and subsequently incubated with 2 ml of LB-MOPS-Glu-salts. After overnight incubation at 30°C with shaking (220 rpm), the cultures were filtered using a 0.22-μm syringe membrane filter (Nalgene Labware, Rochester, NY), and 5 μl of the filtrate was spotted onto soft-agar layer lawns prepared with a 1:10 dilution of the propagating strain. The plates were incubated overnight at room temperature and evaluated for growth inhibition zones.
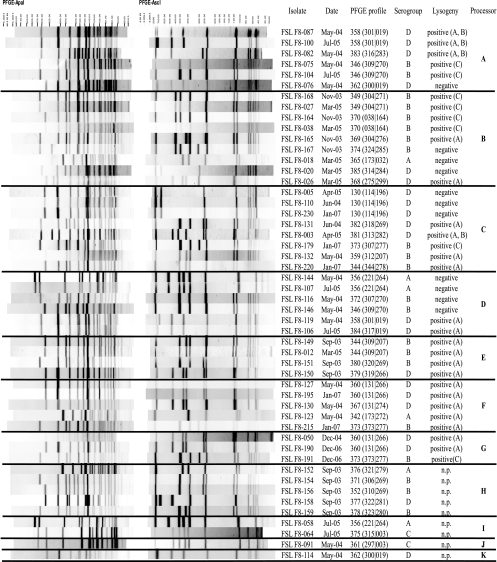
Fig. 1.
ApaI and AscI PFGE restriction patterns for selected L. monocytogenes isolates obtained from processors A to K. Isolates were selected to include one isolate for each PFGE profile obtained from a given sample at a given processor (Tables 2 and 3). ApaI and AscI restriction patterns are shown on the left side of the figure, followed by (i) the isolate identification (ID) number, (ii) sampling date, (iii) PFGE profile designation, (iv) molecular serogroup, (v) results for prophage induction, (vi) processor. The sampling dates are shown in the month-year form and abbreviated (e.g., May-04 stands for May 2004). For PFGE profiles, the ApaI and AscI PFGE profile designations are shown in the parentheses, with the ApaI and AscI PFGE profile designations shown before and after the vertical line, respectively. Molecular serogroup A includes serotypes 1/2a and 3a, molecular serogroup B includes serotypes 1/2b, 3b, and 7, molecular serogroup C includes serotypes 1/2c and 3c, and molecular serogroup D includes serotypes 4b, 4d, and 4e. For prophage induction results, first the result (positive or negative) is shown. For isolates that contained lysogenic phages, the propagating strain that yielded a prophage is shown as follows: A, strain F2365; B, strain FSL F2-695; and C, Mack strain. For some isolates, prophage induction was not performed (n.p.). The processor from which isolates were obtained is indicated on the right-hand side in boldface letters (A to K).
For each filtrate that yielded zones of inhibition, phage isolation was attempted on the same propagating strain that yielded the zone of inhibition. Briefly, a sterile glass pipette was used to remove an agar plug from the zone of inhibition (which would contain a phage if inhibition was caused by phage-mediated lysis). After the plug was dissolved in phosphate-buffered saline (PBS) and serial dilutions were prepared, different dilutions were plated for phage isolation using the soft-agar layer method. For this procedure, 0.1 ml of the PBS with the dissolved agar plug was mixed with 0.3 ml of its propagating strain (i.e., an overnight culture that was diluted in 1:10) and 4 ml of soft agar (0.75%) containing LB-MOPS-Glu-salts, which was laid over a base of 1.5% agar containing LB-MOPS (in 100-mm-diameter petri dishes). The plates were incubated overnight at room temperature, and phages were further purified, from single plaques, by two consecutive passages.
For each purified phage, lysates were prepared from plates that were inoculated with phage, using the soft-agar layer method. Four plates that showed confluent lysis after overnight incubation at room temperature were used for phage harvest with 10 ml of sterile PBS (per plate). With the help of a cell scraper, the top agar layer was removed into PBS and transferred to a centrifuge tube, followed by the addition of chloroform (to a final concentration of 2% [vol/vol]) and centrifugation at 4°C with 4,200 × g for 15 min. The supernatant was then filtered with a 0.22-μl syringe membrane filter (Nalgene Labware), and stock preparations were maintained at 4°C.
Phage host range and L. monocytogenes phage susceptibility determinations.
The 41 L. monocytogenes isolates used for phage isolation (as well as the three propagating strains) were used along with the 26 lysogenic phages isolated to determine (i) the host ranges for the 26 phages and (ii) phage susceptibility patterns for the 44 L. monocytogenes isolates. For these experiments, L. monocytogenes isolates were grown at 30°C with shaking in LB-MOPS, and mixtures of 300 μl of 1:10 dilutions of cultures and 4 ml of melted soft agar (0.75%) containing LB-MOPS were then laid on 1.5% agar containing LB-MOPS. After this overlay was solidified, 3 μl of each prophage lysate was spotted onto the plate, and the plates were incubated at room temperature for 12 to 16 h. Lysis patterns for each phage were classified on a scale ranging from 0 to 3 as follows: (i) 0 indicates that no plaques or zone of clearance was observed; (ii) 1 indicates a turbid zone of clearance; (iii) 2 indicates semiconfluent lysis; and (iv) 3 indicates confluent lysis. Two independent experiments were performed, and the scores were averaged and represented as a heat map (see Fig. 5) using the R software environment for statistical computing and graphics (version 2.9.1; R Development Core Team, Foundation for Statistical Computing, Vienna, Austria [http://www.R-project.org]).
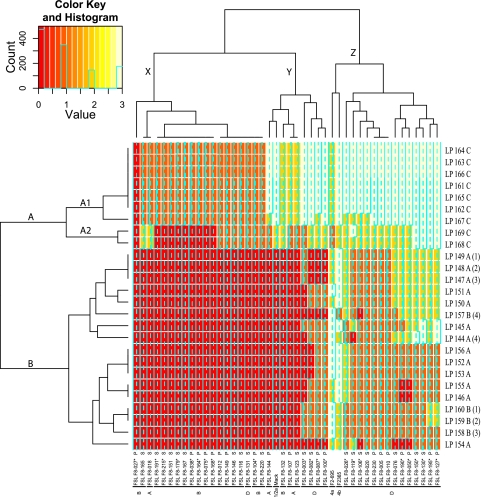
Fig. 5.
Heat map and hierarchical clustering of the lytic activities of the 26 prophages isolated here against the 41 L. monocytogenes isolates and the three propagating strains. The lysis intensity is represented by a color: red indicates no lytic activity (scored as 0); orange indicates weak lysis (scored as 1); yellow indicates semiconfluent lysis (scored as 2); light yellow or white indicates confluent lysis (scored as 3). The branch lengths and pattern on the vertical axis on the left of the figure demonstrate the relatedness between prophages. Prophage designations start with the letters LP, while the final three letters, A, B, or C, indicate the propagating strain used, i.e., F2365, FSL F2-695, or Mack, respectively. As four isolates yielded prophages on two host strains, these prophages are indicated by a number in parentheses; 1, 2, 3, and 4 in parentheses indicate prophages obtained from isolates FSL F8-082, FSL F8-087, FSL F8-100, and FSL F8-003, respectively. On the horizontal axis at the top of the figure, the similarity between L. monocytogenes isolates is presented according to the lytic pattern observed. The molecular serotype for each isolate is indicated first as follows: A, serotypes 1/2a and 3a; B, serotypes 1/2b, 3b, and 7; and D, serotypes 4b, 4d, and 4e. The isolate designation comes next, followed by the letters P and S, which indicate whether an isolate represents a persistent (P) or sporadic (S) strain. Isolates that yielded phages after UV induction are indicated by an asterisk after the isolate designation.
Statistical analysis.
To compare differences in normalized inlA transcript levels and swarming ability among L. monocytogenes isolates, a one-way analysis of variance (ANOVA), including a comparison of least-squares means and Tukey's Studentized residuals to correct for multiple comparisons was performed. ANOVA was also used to determine if there was a statistically significant association between isolate invasion efficiency and (i) persistence, (ii) molecular serotype, or (iii) lineage. Chi-square tests were run to determine whether there was a relationship between L. monocytogenes persistence and (i) presence of an inducible prophage, (ii) molecular serogroup, and (iii) lineage. ANOVA and chi-square tests were performed using Statistical Analysis System JMP software (SAS Institute, Cary, NC). The nonparametric Wilcoxon test (R software environment for statistical computing and graphics) was used to determine whether persistent and nonpersistent strains differed in phage susceptibility. The statistical significance was set at P < 0.05.
RESULTS
Characterization of 1,723 L. monocytogenes isolates by PCR-based molecular serotyping and epidemic clone (EC) marker identification, resistance profiling, and RAPD-PCR.
Among a total of 1,723 L. monocytogenes isolates from 11 processors (processors A to K; Tables 1 and 2), molecular serotyping identified group D, which is typically serotype 4b (979 isolates representing 9 of the 11 processors), group B, which is typically serotype 1/2b (288 isolates representing 8 processors), group A, which is typically serotype 1/2a (263 isolates representing 5 processors), and group C, which is typically serotype 1/2c (193 isolates representing 2 processors). While all group A isolates were negative for the epidemic clone III (ECIII) genetic marker, the ECI and ECII markers were found in 234 and 584 group D isolates, respectively (isolates from four processors and two processors, respectively); for two processors, both isolates with ECI and isolates with ECII markers were identified (processors B and C; Table 2). Interestingly, ECI marker-positive isolates with the same PFGE type were obtained from processors F and G.
Cadmium, arsenic, and tetracycline resistance profiling identified Cdr Ass Tets (875 isolates from 10 processors) and Cds Ass Tets (645 isolates from eight processors) as the most frequent profiles; additional profiles included Cdr Asr Tets (32 isolates from four processors), Cds Asr Tets (154 isolates from three processors), and Cdr Ass Tetr (18 isolates from one processor). The combination of resistance profiles and molecular serotyping allowed for differentiation of isolates in 14 groups (Tables 1 and 2).
As the need for extensive visual analyses did not permit for reliable analysis of all 1,723 RAPD patterns in a single dendrogram, analysis of RAPD patterns was performed using individual dendrograms for isolates from each processor. Between two (processor K; Table 2) and 53 (processor C; Table 2) combined RAPD profiles (based on patterns for both primers UBC 155 and OPM-01) were obtained for isolates from a given processor. Results obtained with the four typing techniques were combined to generate a single typing profile for each isolate from a given processor. One to five isolates from each typing profile were then conveniently selected for further characterization by PFGE, for a total of 240 isolates.
Characterization of 240 representative isolates by PFGE.
ApaI and AscI PFGE analyses yielded 27 and 22 patterns (Fig. 1), respectively, yielding a total of 32 PFGE profiles (based on combined analysis of ApaI and AscI patterns). Two to seven different PFGE profiles were obtained among isolates from a given processor, with the exception of processors J and K, where only one PFGE profile was found (Tables 1 and 2). Overall, characterization of isolates by PFGE showed lower diversity compared to RAPD, and in many cases, isolates with different RAPD profiles exhibited the same PFGE profile. For example, for processor C, 70 isolates representing 46 different RAPD profiles were all classified into PFGE profile 130 (Table 1).
For all seven processors (processors A to G) where isolates were obtained on multiple sample collection dates, at least some L. monocytogenes isolates collected on different sampling dates showed the same PFGE profile, suggesting persistence of a given strain. Times between the sampling dates that yielded isolates with the same PFGE profile ranged from 14 months (processors A and D) to 32 months (processors C and F). The patterns of persistence included the following: (i) three processors that showed persistence of a single strain (PFGE profiles 130, 356, and 344 in isolates from processors C, D, and E, respectively), which was not found to persist in another processor; (ii) two processors that each showed evidence for persistence of two strains (PFGE profiles 358 and 346 for processor A and profiles 349 and 370 for processor B); and (iii) two processors where the same strain persisted (PFGE profile 360 was isolated over multiple sampling dates from products of processors F and G with a time between sampling dates of 32 and 24 months, respectively). While we thus found that strains with 8 different PFGE profiles showed evidence for persistence in association with different processors, only one PFGE profile was associated with strains that persisted in more than one processor. In addition, we found that four PFGE profiles (profiles 344, 346, 358, and 356) that were associated with persistent strains were also isolated from samples associated with another processor (where they did not persist) on a single date. For example, PFGE profile 344 was isolated from processor C samples collected on a single date, while a strain with this PFGE type appears to also have persisted in processor E.
Interestingly, for 6 processors (processors A though F; Fig. 1) some L. monocytogenes isolates recovered in different years were found to have closely related PFGE types, which differed by less than 3 bands in their patterns with a given enzyme. For example, PFGE profile 383, which was isolated from processor A samples collected in May 2004, and profile 358, which was isolated from processor A samples collected in May 2004 and July 2005, differed by 3 bands each in the AscI pattern and ApaI pattern. Also, PFGE profile 132, representing isolates from processor C samples collected in May 2004, and profile 220, representing isolates from processor C samples in January 2007, showed the same AscI pattern and differed by 1 band in the ApaI pattern. PFGE profiles 367 and 360, both representing isolates from processor F (Table 1), differed by 1 band in their AscI patterns and had the same ApaI patterns.
Use of PFGE data to track L. monocytogenes in different production stages.
For four processors, isolates were obtained from products in different processing stages of alheira, chouriça de Vinhais, and salpicão de Vinhais (Table 4). Isolates from alheira production in plant B showed five different PFGE profiles, including the following: (i) profile 349, represented by isolates from cooked meat mixture and paste; (ii) profile 365, represented by isolates from casings, paste, and final product; and (iii) profiles 368 and 370, represented by isolates from the paste and final product. As isolates with PFGE profiles 349 and 370 were recovered over multiple samplings (Table 4), our data indicate that contamination from environmental sites of persistence may occur early in production and be carried through the processing steps.
Sampling different stages of alheira production in processor C showed that isolates with PFGE profile 130 were recovered from all stages of production, from casing samples and cooked meats to final products, in January 2007. As isolates with this PFGE profile were also recovered from alheira samples in May 2004 and April 2005 (Table 2), these data may also indicate that, in January of 2007, contamination from environmental sites of persistence occurred early in production and was carried through the processing steps. Sampling at different stages of alheira production in processor G plant showed that isolates with PFGE profile 360 were recovered from all stages of production in December 2004, again suggesting that contamination occurred early in production and persisted.
Sampling at different stages of production of alheira, salpicão de Vinhais, and chouriça de Vinhais in the processor I plant showed that isolates with a single PFGE profile (profile 356) were recovered throughout alheira production (Table 4). An isolate with the same PFGE profile was also recovered from salpicão de Vinhais (before smoking), which is produced in the same area and with the same equipment as alheira. Isolates with a second PFGE profile (profile 375) were obtained from the seasoned raw pork for chouriça de Vinhais and salpicão de Vinhais (both right after seasoning and 2 days after seasoning), suggesting a common source of contamination (likely the raw meats used for these products).
Identification and characterization of isolates with reduced invasion efficiency in Caco-2 cells.
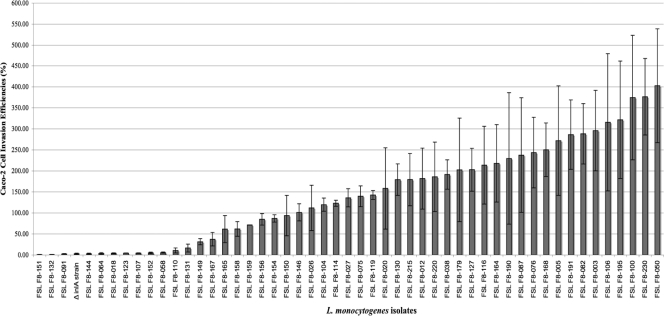
Fifty L. monocytogenes isolates were characterized for invasion efficiency in Caco-2 cells; these isolates were selected to include one isolate for each PFGE profile obtained from a given sample (the isolates and their PFGE patterns are shown in Fig. 1). The average invasion efficiencies (relative to the laboratory control strain 10403S) for these 50 isolates ranged from 0.49 to 402.6% (Fig. 2). Twelve isolates representing 10 different PFGE types showed reduced invasion phenotype, defined as a relative invasion efficiency of <20% (Table 5); this cutoff was used, because previous data (17) suggested that only isolates with <20% invasion efficiency show premature stop codons (PMSCs) in the inlA gene, which encodes a key protein responsible for invasion of intestinal epithelial cells.
Fig. 2.
Caco-2 cell invasion efficiencies for 50 selected L. monocytogenes isolates (representing one representative for each PFGE pattern obtained from a given sample). Values represent average invasion efficiencies (normalized to that of the control strain 10403S) for 2 or 3 independent replicates; the error bars indicate standard deviations or ranges. A ΔinlA strain (Table 3) was included as an invasion-attenuated control.
Table 5.
Characteristics of L. monocytogenes isolates that showed reduced invasion efficiencies in Caco-2 cells
| Isolate | Invasion efficiency (%)a | PFGE profile | Processor | Molecular serotype | Description of mutation found in inlAb | PMSC mutation typec | Length of predicted protein (aa) | References for PMSCs |
|---|---|---|---|---|---|---|---|---|
| FSL F8-151 | 0.49 | 380 | E | B | No mutation foundd | NAe | 800 | NA |
| FSL F8-132 | 0.57 | 359 | C | B | No mutation foundf | NA | 800 | NA |
| FSL F8-091 | 2.05 | 361 | J | C | Deletion of A (nt 1637) | 12 | 576 | 17, 29, 56, 67, 68 |
| FSL F8-144 | 2.49 | 356 | D | A | Deletion of A (nt 12) | 4 | 9 | 17, 52, 67, 68 |
| FSL F8-064 | 3.32 | 375 | I | C | Deletion of A (nt 1637) | 12 | 576 | 17, 29, 56, 67, 68 |
| FSL F8-018 | 3.64 | 365 | B | A | C→T substitution (nt 1474) | 6 | 492 | 17, 48, 56, 67, 68 |
| FSL F8-123 | 4.07 | 342 | F | A | C→T substitution (nt 1474) | 6 | 492 | 17, 48, 56, 67, 68 |
| FSL F8-107 | 4.09 | 356 | D | A | Deletion of A (nt 12) | 4 | 9 | 17, 52, 67, 68 |
| FSL F8-152 | 4.62 | 376 | H | A | C→T substitution (nt 1474) | 6 | 492 | 17, 48, 56, 67, 68 |
| FSL F8-058 | 5.33 | 356 | I | A | Deletion of A (nt 12) | 4 | 9 | 17, 52, 67, 68 |
| FSL F8-110 | 10.23 | 130 | C | D | Deletion of 3 aa (nt 2212 to 2220)g | NA | 797 | NA |
| FSL F8-131 | 16.17 | 382 | C | B | No mutation foundh | NA | 800 | NA |
Values represent average invasion efficiencies (normalized to that of the control strain 10403S) from 2 or 3 independent replicates.
nt, nucleotide; aa, amino acids.
Premature stop codon (PMSC) mutations were classified by the method of Van Stelten et al. (67).
Strain FSL F8-151 showed reduced swarming compared to strain 10403S at 37°C; swarming at this temperature was similar to that for a ΔflaA control strain (Fig. 3).
NA, not applicable.
Strain FSL F8-132 showed reduced swarming compared to strain 10403S at 24, 30, and 37°C; swarming at these temperatures was similar to that for a ΔflaA control strain (Fig. 3). This strain also showed reduced inlA transcript levels (Fig. 4).
Strain FSL F8-110 showed reduced inlA transcript levels (Fig. 4).
Strain FSL F8-131 showed reduced swarming compared to strain 10403S at 37°C; swarming at this temperature was similar to that for a ΔflaA control strain.
Premature stop codon mutations in inlA were identified in 8 of the 12 isolates that showed invasion efficiencies of <20% (Table 5). Three isolates with PFGE type 356 (two from processor D and one from processor I) carried inlA PMSC type 4, which has been previously observed in isolates from the United States (52, 68) and Portugal, namely, in one isolate with the same PFGE type collected from processor D on another sampling date (17). Three isolates representing 3 different PFGE profiles (profiles 365, 342, and 376) carried inlA PMSC mutation type 6 (Table 4); Felício et al. (17) reported this mutation in 2 L. monocytogenes isolates with PFGE type 342 recovered from two different processors (not included in this study) and in one isolate with PFGE type 365 obtained from processor G in October 2003. Two isolates (with PFGE profiles 375 and 361) carried inlA PMSC mutation type 12 (Table 5); this mutation was also previously identified by Felício et al. (17) in two isolates with PFGE profile 361 obtained from the same processor J samples that yielded one of the two isolates with this PMSC here.
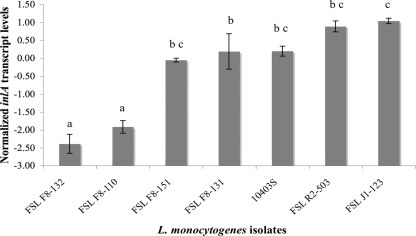
The four isolates that showed no inlA PMSC mutations, despite their reduced invasion efficiency, included isolate FSL F8-110, which showed an in-frame deletion of InlA amino acids (aa) 738, 739, and 749, located in the membrane anchor domain. This 3-aa deletion is unlikely to be responsible for the reduced invasion efficiency, as Lecuit et al. (36) showed that a mutant strain with a deletion in the preanchor region (between aa 714 and aa 766) presented an invasiveness level comparable to that of a strain with full-length internalin. Importantly though, both this isolate (FSL F8-110) and isolate FSL F8-132 showed reduced inlA transcript levels, while isolates FSL F8-131 and FSL F8-151 demonstrated inlA transcript levels comparable to those observed for control strains 10403S, FSL R2-503, and FSL J1-123 (Fig. 3).
Fig. 3.
Normalized inlA transcript levels for four L. monocytogenes isolates with reduced invasion phenotype in Caco-2 cells and three control strains. The control strains included L. monocytogenes 10403S, FSL J1-123, and FSL R2-503, representing two different lineages and three different serotypes (serotypes 1/2a, 1/2b, and 4b; Table 3). The transcript levels are expressed as log inlA cDNA copy number normalized to the log cDNA copy number of the rpoB housekeeping gene (log10 copy number inlA − log10 copy number rpoB). Values represent average transcript levels based on two independent RNA extract preparations for each isolate; error bars represent the data range. Means with the same letter are not statistically different from each other (overall α = 0.05, Tukey's correction).
Isolate FSL F8-132 showed reduced motility at 24, 30 and 37°C, with values comparable to that obtained for the ΔflaA control strain. Two other invasion-attenuated isolates (FSL F8-131 and FSL F8-151) showed significantly higher motility at 24 and 30°C compared to the ΔflaA control strain but lower motility compared to strain 10403S at 24 and 37°C. At 37°C, the invasion efficiency values for these strains were similar to the value for the ΔflaA control strain (Fig. 4). The invasion-deficient isolate FSL F8-110, which showed reduced inlA transcript levels, showed motility levels comparable to that obtained for control strain 10403S, at the three temperatures tested (Fig. 4).
Fig. 4.
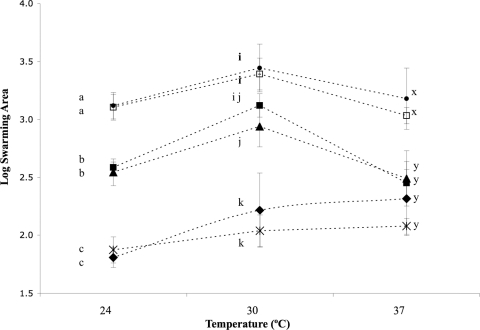
Swarming abilities of four L. monocytogenes strains that showed reduced invasiveness in Caco-2 cells but did not have a premature stop codon (PMSC) in inlA. L. monocytogenes strain 10403S and an isogenic ΔflaA strain were included as controls (Table 3). Points represent the logarithmic mean swarming area from four independent replicates for each isolate; error bars represent standard deviations. Means within each temperature with the same letter are not statistically different from each other (overall α = 0.05, Tukey's correction). The strains are indicated by symbols as follows: •, FSL F8-110; □, 10403S; ▪, FSL F8-131; ▴, FSL F8-151; ♦, FSL F8-132; ×, ΔflaA mutant.
Association between invasion efficiency and genetic lineage and persistence.
While ANOVA showed no significant association between invasion efficiency and persistence (i.e., whether an isolate presented a persistent or nonpersistent isolate), ANOVA analyses also showed significant effects on invasion efficiency of either molecular serogroup or lineage (with molecular serotypes 1/2b and 4b classified into lineage I and molecular serotypes 1/2a and 1/2c classified into lineage II). Specifically, lineage I isolates were significantly more invasive than lineage II isolates (P < 0.0001); serogroup A strains were significant less invasive than either serogroup B or D strains (this analysis did not include serogroup C, as there were no serogroup C isolates among plants with multiple samplings). Chi-square tests also showed that there was no significant association (P > 0.05) between persistence and either molecular serogroup or lineage.
Isolation and characterization of lysogenic phages among persistent and nonpersistent strains.
A total of 41 isolates, including 19 isolates representing strains that persisted in a given processor and 22 isolates representing strains that did not show evidence for persistence, were tested for lysogen induction. Filtrates of UV-treated cultures from 30 isolates had the ability to inhibit the growth of a host strain. Inhibition caused by filtrates of eight isolates (FSL F8-012, FSL F8-123, FSL F8-131, FSL F8-132, FSL F8-149, FSL F8-151, FSL F8-165, and FSL F8-220) appears to be caused by listeriocidal substances (e.g., a monococin, which represents a defective phage particle [74]), rather than lysogenic phages, as the filtrates from these eight isolates (i) did not form plaques (they showed only a zone of inhibition) and (ii) did not allow for propagation of an inhibitory agent (while the other 22 isolates allowed for phage propagation). The 22 isolates that yielded lysogenic phages by the induction approach employed here included (i) nine isolates (representing six PFGE types) for which phages were obtained only on the host strain F2365, (ii) nine isolates (representing four PFGE types) for which phages were obtained only on the host strain Mack, and (iii) four isolates (representing three PFGE types) for which phages were obtained on two host strains (i.e., F2365 and FSL F2-695), thus yielding 8 phage isolates. These 22 L. monocytogenes isolates thus yielded 26 phage isolates for further characterization. For the four L. monocytogenes isolates that yielded phage isolates on two hosts, one phage plaque from each host was propagated for further characterization, recognizing that it is possible that these bacteria contained a lysogenic phage with the ability to infect both host strains. Overall, 12 of 19 isolates representing persistent strains and 10 of 22 isolates representing nonpersistent strains yielded lysogens; there was no significant association between lysogeny and persistence (P = 0.254 by the chi-square test), recognizing that some lysogens may not have been detected with the approach used.
The 26 phages isolated as described above were also characterized for their ability to lyse a panel of 44 L. monocytogenes isolates, including (i) strains F2365, Mack, and FSL F2-695, which were also used as hosts for phage isolation, and (ii) the 41 L. monocytogenes isolates (representing persistent and sporadic strains) that were used for lysogeny screening. Overall, the phages represented 15 distinct lysis patterns, which cluster into two main lysis groups (Fig. 5). All phages in lysis group A were isolated on host strain Mack; the 7 phages in group A1 (Fig. 5) showed strong lysis against the vast majority of L. monocytogenes classified into molecular serogroup D (typically serotype 4b), while showing weak lysis of most serogroup A and B strains (representing serotypes 1/2a and 1/2b, respectively). The phages in group A2 showed semiconfluent lysis of only some serogroup B strains and showed reduced lysis (compared to group A1) for a number of serogroup D host strains. All phages in lysis group B were isolated on hosts F2365 or FSL F2-695; these phages showed no lysis with the vast majority of serogroup A and B strains (representing serotypes 1/2a and 1/2b, respectively) and typically showed semiconfluent lysis with serogroup D host strains.
All phages showed no lytic infection (score of 0) or a very weak infection (score of 1) against the strain they were induced from. Phage host range data also allowed us to compare the phages that were isolated, from the same L. monocytogenes isolate, on two different host strains. Three phage pairs obtained from the same L. monocytogenes each represented two phages with distinct host ranges (Fig. 5), while one phage pair represented two phages with very similar host ranges, suggesting isolation of two phages representing the same prophage (possibly with some mutations) on the two host strains.
Susceptibility of persistent and nonpersistent isolates against lysogenic phages.
The lysogenic phages isolated here were also used to probe the phage susceptibility profiles of 41 persistent and nonpersistent L. monocytogenes. These 41 isolates represented 26 different phage resistance patterns, which grouped into 3 distinct clades (Fig. 5). Clade X represents predominantly isolates with serogroup B (typically serotype 1/2b), which are resistant to lysis with all clade B phages and show weak lysis with most clade A phages. L. monocytogenes clade Y includes 4 isolates each in molecular serogroups A and D and one isolate in molecular serogroup B; isolates in this clade are resistant or show weak lysis with clade B phages and show semiconfluent or confluent lysis with clade A phages. L. monocytogenes clade Z exclusively represents serogroup D (typically serotype 4b), which largely show semiconfluent or weak lysis with clade B phages, while predominantly showing confluent or semiconfluent lysis with clade A phages. Overall, serogroup D isolates were found to be sensitive to lysis by a larger number of phages compared to serogroup A and B isolates, which all showed no lysis with 17/26 phages tested (Fig. 5).
Among the 19 persistent isolates characterized here for phage resistance, 8 were resistant or showed only weak lysis with all phages, while 11 showed confluent lysis with at least some phages (typically clade A phages). Statistical analysis with Wilcoxon test showed that there was no significant relationship (P = 0.8825) between isolate persistence (i.e., whether a isolate represented a persistent or nonpersistent strain) and phage susceptibility (expressed as proportion of phages an isolate was resistant to).
DISCUSSION
Characterization of 1,723 L. monocytogenes isolates from 11 processors of traditional fermented sausages in northern Portugal allowed us to identify L. monocytogenes strains with evidence of persistence for >1 year in all 7 processors where isolates were collected over at least two samplings. Further characterization of selected isolates representing persistent and nonpersistent strains showed that a number of isolates were characterized by (i) a reduced ability to invade human intestinal epithelial cells, commonly caused by a premature stop codon in inlA and (ii) the presence of mobilizable prophages. Overall, our data show the following. (i) RAPD may not be suitable for analysis of large sets of L. monocytogenes isolates. (ii) While a large diversity of L. monocytogenes subtypes is found in Portuguese fermented meat sausages, the persistence of L. monocytogenes in this food chain is common. (iii) L. monocytogenes bacteria persisting for >1 year in a given facility often appear to show genetic diversification that can yield closely related but distinct PFGE patterns. (iv) Persistent L. monocytogenes strains do not appear to be characterized by unique genetic or phenotypic characteristics (e.g., molecular serotype, reduced virulence, or phage resistance).
RAPD may not be suitable for analysis of large sets of L. monocytogenes isolates.
While molecular subtyping of L. monocytogenes can provide valuable information not only for human disease surveillance but also for improved control of this pathogen along the food chain (22, 23), more widespread application of these methods is still hampered by a variety of factors, including, but not limited to, (i) the considerable cost of subtyping, (ii) the time required for subtype analysis, and (iii) the technical expertise required for many subtyping methods. While a variety of molecular subtyping methods are available for L. monocytogenes, many of them, including PFGE, which is often considered the gold standard for subtyping of L. monocytogenes (20, 22), show the challenges and disadvantages detailed above. RAPD-PCR (72) continues to be a potentially attractive alternative for subtyping, as it allows for relatively easy, rapid, and inexpensive high-throughput subtyping of bacterial isolates. RAPD was used here as an initial high-throughput subtype screen, which allowed characterization of a large number of L. monocytogenes isolates from each sample, as it has been shown in a number of studies that a single sample may contain multiple L. monocytogenes subtypes (12). Combination of initial RAPD and subsequent PFGE characterization of L. monocytogenes isolates representing different RAPD types from each sample indeed showed considerable L. monocytogenes subtype diversity in a given sample, with up to five different PFGE profiles detected among isolates from a single sample.
While the use of RAPD as an initial high-throughput subtyping screen thus allowed for characterization of a large number of isolates, not only were RAPD patterns extremely difficult to interpret but they also showed considerable variability among isolates from samples associated with a single processor (e.g., identification of 53 RAPD profiles among isolates associated with processor C) as well as among isolates from a single sample. It is striking that in many of the cases where a large number of different RAPD types were associated with a single processor or sample, PFGE, which is a highly discriminatory subtyping method for L. monocytogenes (3), classified isolates with a number of different RAPD patterns into the same PFGE profile. While these findings raise questions about the reproducibility of RAPD, consistent with several studies that previously reported problems with RAPD reproducibility (5, 43, 64, 66, 71), it is also possible that, at least in some cases, isolates that showed identical PFGE profiles but different RAPD patterns may truly represent distinct strains. This is supported by the observation that, sometimes, isolates with identical PFGE profiles but different RAPD patterns also showed different Cd As Tet resistance patterns, suggesting that these isolates may differ in the presence or absence of mobile genetic elements (e.g., plasmids carrying Cd resistance genes [35] or transposons carrying tet resistance genes [55]). While our data thus strongly suggest, consistent with other studies (5, 64), that RAPD, due to often poor reproducibility, may be of limited value for subtype studies that require characterization of large sets of isolates, our data also further support that, in many cases, a combination of subtyping methods may be needed. For example, a combination of a low-cost high-throughput method with a highly standardized secondary subtyping method may be needed in some cases to allow for testing of a large number of isolates from a given sample, followed by confirmation on a smaller set of isolates with a highly standardized subtyping method.
While a large diversity of L. monocytogenes subtypes is found in Portuguese fermented meat sausages, persistence of L. monocytogenes in this food chain is common.
Use of multiple subtyping methods revealed considerable subtype diversity, including presence of epidemic clones I and II, among L. monocytogenes isolates from Portuguese fermented meat sausages, processing plant environments, and raw materials. L. monocytogenes isolates also showed considerable variation in the ability to invade human Caco-2 intestinal epithelial cells; isolates from eight processors representing 10 different PFGE types showed attenuated Caco-2 invasion phenotypes. These reduced invasion phenotypes appear to be due to different mechanisms, including the following: (i) different premature stop codon (PMSC) mutations in the inlA gene, which encodes a protein (internalin A) that promotes L. monocytogenes internalization into human epithelial cells (36); (ii) reduced transcription of inlA; and (iii) reduced swarming. While PMSC mutations in inlA have been described extensively in both clinical and food L. monocytogenes isolates collected worldwide, with at least 18 different naturally occurring inlA mutations identified so far (17, 25, 27, 29, 46, 48, 52, 56, 59, 67, 68), only limited previous evidence (58) exists for attenuated invasion associated with reduced inlA transcript levels or reduced swarming. While swarming motility has previously been shown to contribute to the ability of L. monocytogenes to adhere to and invade Caco-2 cells, as well as its ability to cause disease in a mouse infection model (2, 11, 50), further characterization of isogenic mutants will be necessary to confirm that specific mechanisms (e.g., reduced swarming) are responsible for reduced invasion efficiency.
Among a total of 32 L. monocytogenes PFGE types identified, seven PFGE types were found in isolates from more than one processing plant, which could be explained by the geographic proximity of the plants studied (which were all located in two cities approximately 40 km apart) or by possible common sources of raw materials. In addition, some of these PFGE types could simply represent common and widely distributed strains as reported by Fugett et al. (20), who identified some common and widely distributed L. monocytogenes PFGE types among 495 isolates from human clinical cases, foods, ruminant farms, and urban and natural environments in New York State in the United States. Importantly, PFGE data also indicated persistence of specific L. monocytogenes strains in 7 different processing facilities, with time of persistence ranging from at least 14 months to at least 32 months. Persistence may indicate deficiencies in sanitation procedures or potential niches in the processing environment where L. monocytogenes is protected from sanitation. While persistence of L. monocytogenes in meat processing plants (15, 37, 38, 61) and in other plants (44, 47, 73) has previously been reported, identification of different PFGE types (as well as different molecular serotypes and lineages) associated with persistence in different plants (except for one PFGE type associated with persistence in two plants) clearly suggests that many different L. monocytogenes strains have the ability to establish persistent populations in processing plants.
L. monocytogenes bacteria persisting for >1 year in a given processing plant often appear to show genetic diversification that can yield closely related but distinct PFGE patterns.
L. monocytogenes with closely related PFGE types (i.e., PFGE types that differed by less than three bands) were isolated on different sampling dates from six processors. This suggests sufficient genomic diversification of L. monocytogenes during persistence to yield distinct but closely related PFGE patterns, consistent with the “3-band rule” proposed by Tenover et al. (65). These findings are consistent with observations from a number of investigations of listeriosis outbreaks, where multiple isolates with closely related PFGE types were isolated from processing plants, foods, and human clinical cases epidemiologically linked to an outbreak (21, 23); diversification during these outbreaks may occur both during human infection and during bacterial growth in the processing plant where food contamination occurred. Diversification of L. monocytogenes during persistence has also been reported by Orsi et al. (51), who found that short-term evolution of a L. monocytogenes strain that persisted in a food processing plant over 12 years involved prophage diversification and plasmid loss or gain. Similar to the findings by Orsi et al. (51), we also identified isolates sharing the same PFGE type but presenting prophages with different host ranges, further suggesting that phages contribute to rapid bacterial diversification.
Persistent L. monocytogenes strains do not appear to be characterized by unique genetic or phenotypic characteristics.
While L. monocytogenes persistence in food processing plants and other food-associated environments has been well documented (30, 33, 37, 38, 44, 47), it still remains largely unclear whether persistent strains share unique genetic or phenotypic characteristics or whether establishment of persistence is largely a random process with most L. monocytogenes strains being able to establish persistence if they are introduced into an appropriate location at an opportune time. While some authors have reported that isolates representing persistent subtypes appear to possess specific characteristics that facilitate persistence, such as resistance to disinfectants (1, 39), improved ability to adhere to food contact surfaces and to form biofilms (6, 40), others have found no evidence that persistent strains adhere better to surfaces (28) or are more resistant to disinfectants (14, 31). Initial analysis of genotypic characteristics of persistent and sporadic isolates obtained here not only showed that persistent strains represented considerable phenotypic and genetic diversity but also found no associations between either cadmium, arsenic, or tetracycline resistance, molecular serotype classification, L. monocytogenes invasion efficiency in Caco-2 cells, or inlA PMSC and persistent strains.
While a number of studies (6, 28, 39) have evaluated sanitizer resistance and biofilm formation abilities of persistent L. monocytogenes isolates, limited information is available on possible associations between phage resistance and prophage carriage and the ability of L. monocytogenes bacteria of distinct genotypes to establish persistence. Lysogenic conversion by temperate phages plays an important role in horizontal gene transfer and has played a crucial role in the evolution of several food-borne pathogens by facilitating horizontal transfer of virulence, antibiotic resistance, or toxin genes (7). Some studies (reviewed in reference 8) have also suggested that prophages can enhance the survival and fitness of the lysogen in the environment. Possible mechanisms for this enhanced fitness include conferral, via lysogenic conversion, of immunity against lytic infection by other phages. While we identified a considerable number of L. monocytogenes isolates with mobilizable prophages, consistent with previous studies (24), we found no association between lysogeny and persistence. We did confirm though that lysogens showed immunity against lysis with the homologous prophage, suggesting the possibility of at least some selective advantage for lysogens.
Evaluation of susceptibility profiles of persistent and nonpersistent L. monocytogenes isolates against the isolated prophages also found no association between phage resistance and persistence, even though we identified an association between molecular serotype groups and phage resistance. Specifically, isolates with molecular serogroup D (typically serotype 4b), including lysogenic and nonlysogenic isolates, were susceptible to more phages compared to serogroup A and B (typically serotypes 1/2a and 1/2b, respectively) isolates. These findings are consistent with a study by Kim et al. (32), which demonstrated that strains of the serotype 4b complex recovered from the environment of a turkey processing plant were typically susceptible to wide-host-range phages isolated from the same plant, while strains of other serotypes (serotype 1/2a or 3a and 1/2b or 3b) were resistant to infection by these phages. Overall, these findings suggest differences in phage susceptibility between L. monocytogenes serotypes, with 1/2a and 1/2b strains typically resistant to a larger number of phage isolates. Future experiments characterizing phage resistance of persistent and nonpersistent L. monocytogenes isolates with large sets of diverse lytic and nontransducing phages will be necessary to further characterize phage resistance attributes of persistent strains and to determine the potential for identification of lytic phages that can help control L. monocytogenes persistence.
ACKNOWLEDGMENTS
This project was partially supported by a grant from the National Integrated Food Safety Initiative (Special Emphasis grant 2005-51110-03278), USDA Special Research grants (2006-34459-16952 and 2008-34459-19043), and Hatch Funds (project NYC-143445) of the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture. This work was partially supported by funding from the Fundação para a Ciência e a Tecnologia (FCT)/FEDER, project POCTI/AGG/39587/2001, FCT project PTDC/AGR-ALI/64662/2006 (Listeria monocytogenes in foods: contributing data for risk assessment), and a Ph.D. grant to V. Ferreira (SFRH/BD/24240/2005).
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 4 March 2011.
REFERENCES
- 1. Aase B., Sundheim G., Langsrud S., Rørvik L. M. 2000. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 62:57–63 [DOI] [PubMed] [Google Scholar]
- 2. Bigot A., et al. 2005. Role of FliF and FliI of Listeria monocytogenes in flagellar assembly and pathogenicity. Infect. Immun. 73:5530–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bille J., Rocourt J. 1996. WHO International multicenter Listeria monocytogenes subtyping study - rationale and set-up of the study. Int. J. Food Microbiol. 32:251–262 [DOI] [PubMed] [Google Scholar]
- 4. Bishop D. K., Hinrichs D. J. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005–2009 [PubMed] [Google Scholar]
- 5. Boerlin P., Bannerman E., Ischer F., Rocourt J., Bille J. 1995. Typing Listeria monocytogenes: a comparison of random amplification of polymorphic DNA with 5 other methods. Res. Microbiol. 146:35–49 [DOI] [PubMed] [Google Scholar]
- 6. Borucki M. K., Peppin J. D., White D., Loge F., Call D. R. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brabban A. D., Hite E., Callaway T. R. 2005. Evolution of foodborne pathogens via temperate bacteriophage-mediated gene transfer. Foodborne Pathog. Dis. 2:287–303 [DOI] [PubMed] [Google Scholar]
- 8. Brüssow H., Canchaya C., Hardt W. D. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y., Knabel S. J. 2007. Multiplex PCR for simultaneous detection of bacteria of the genus Listeria, Listeria monocytogenes, and major serotypes and epidemic clones of L. monocytogenes. Appl. Environ. Microbiol. 73:6299–6304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalton C. B., et al. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 336:100–105 [DOI] [PubMed] [Google Scholar]
- 11. Dons L., et al. 2004. Role of flagellin and the two-component CheA/CheY system of Listeria monocytogenes in host cell invasion and virulence. Infect. Immun. 72:3237–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Döpfer D., et al. 2008. Assessing genetic heterogeneity within bacterial species isolated from gastrointestinal and environmental samples: how many isolates does it take? Appl. Environ. Microbiol. 74:3490–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doumith M., Buchrieser C., Glaser P., Jacquet C., Martin P. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 42:3819–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Earnshaw A. M., Lawrence L. M. 1998. Sensitivity to commercial disinfectants, and the occurrence of plasmids within various Listeria monocytogenes genotypes isolated from poultry products and the poultry processing environment. J. Appl. Microbiol. 84:642–648 [DOI] [PubMed] [Google Scholar]
- 15. Eifert J. D., et al. 2005. Molecular characterization of Listeria monocytogenes of the serotype 4b complex (4b, 4d, 4e) from two turkey processing plants. Foodborne Pathog. Dis. 2:192–200 [DOI] [PubMed] [Google Scholar]
- 16. Farber J. M., Addison C. J. 1994. RAPD typing for distinguishing species and strains in the genus Listeria. J. Appl. Microbiol. 77:242–250 [DOI] [PubMed] [Google Scholar]
- 17. Felício M. T., Hogg T., Gibbs P., Teixeira P., Wiedmann M. 2007. Recurrent and sporadic Listeria monocytogenes contamination in alheiras represents considerable diversity, including virulence-attenuated isolates. Appl. Environ. Microbiol. 73:3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferreira V., et al. 2009. Microbiological profile of Salpicão de Vinhais and Chouriça de Vinhais from raw materials to final products: traditional dry sausages produced in the North of Portugal. Inno. Food Sci. Emerg. Technol. 10:279–283 [Google Scholar]
- 19. Ferreira V., et al. 2007. Characterisation of alheiras, traditional sausages produced in the North of Portugal, with respect to their microbiological safety. Food Control 18:436–440 [Google Scholar]
- 20. Fugett E. B., Schoonmaker-Bopp D., Dumas N. B., Corby J., Wiedmann M. 2007. Pulsed-field gel electrophoresis (PFGE) analysis of temporally matched Listeria monocytogenes isolates from human clinical cases, foods, ruminant farms, and urban and natural environments reveals source-associated as well as widely distributed PFGE types. J. Clin. Microbiol. 45:865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilmour M. W., et al. 2010. High-throughput genome sequencing of two Listeria monocytogenes clinical isolates during a large foodborne outbreak. BMC Genomics 11:120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graves L. M., Swaminathan B. 2001. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 65:55–62 [DOI] [PubMed] [Google Scholar]
- 23. Graves L. M., et al. 2005. Microbiological aspects of the investigation that traced the 1998 outbreak of listeriosis in the United States to contaminated hot dogs and establishment of molecular subtyping-based surveillance for Listeria monocytogenes in the PulseNet network. J. Clin. Microbiol. 43:2350–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hagens S., Loessner M. J. 2007. Bacteriophages of Listeria, p. 265–279 In Goldfine H., Shen H. (ed.), Listeria monocytogenes pathogenesis and host response. Springer, New York, NY [Google Scholar]
- 25. Handa-Miya S., et al. 2007. Nonsense-mutated inlA and prfA not widely distributed in Listeria monocytogenes isolates from ready-to-eat seafood products in Japan. Int. J. Food Microbiol. 117:312–318 [DOI] [PubMed] [Google Scholar]
- 26. Hodgson D. A. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312–323 [DOI] [PubMed] [Google Scholar]
- 27. Jacquet C., et al. 2004. A molecular marker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. J. Infect. Dis. 189:2094–2100 [DOI] [PubMed] [Google Scholar]
- 28. Jensen A., et al. 2008. Processing plant persistent strains of Listeria monocytogenes appear to have a lower virulence potential than clinical strains in selected virulence models. Int. J. Food Microbiol. 123:254–261 [DOI] [PubMed] [Google Scholar]
- 29. Jonquières R., Bierne H., Mengaud J., Cossart P. 1998. The inlA gene of Listeria monocytogenes LO28 harbors a nonsense mutation resulting in release of internalin. Infect. Immun. 66:3420–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kabuki D. Y., Kuaye A. Y., Wiedmann M., Boor K. J. 2004. Molecular subtyping and tracking of Listeria monocytogenes in Latin-style fresh-cheese processing plants. J. Dairy Sci. 87:2803–2812 [DOI] [PubMed] [Google Scholar]
- 31. Kastbjerg V., Gram L. 2009. Model systems allowing quantification of sensitivity to disinfectants and comparison of disinfectant susceptibility of persistent and presumed nonpersistent Listeria monocytogenes. J. Appl. Microbiol. 106:1667–1681 [DOI] [PubMed] [Google Scholar]
- 32. Kim J. W., Siletzky R. M., Kathariou S. 2008. Host ranges of Listeria-specific bacteriophages from the turkey processing plant environment in the United States. Appl. Environ. Microbiol. 74:6623–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lappi V. R., et al. 2004. Longitudinal studies on Listeria in smoked fish plants: impact of intervention strategies on contamination patterns. J. Food Prot. 67:2500–2514 [DOI] [PubMed] [Google Scholar]
- 34. Lawrence L. M., Harvey J., Gilmour A. 1993. Development of a random amplification of polymorphic DNA typing method for Listeria monocytogenes. Appl. Environ. Microbiol. 59:3117–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lebrun M., Loulergue J., Chaslus-Dancla E., Audurier A. 1992. Plasmids in Listeria monocytogenes in relation to cadmium resistance. Appl. Environ. Microbiol. 58:3183–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lecuit M., Ohayon H., Braun L., Mengaud J., Cossart P. 1997. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun. 65:5309–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. López V., et al. 2008. Different contamination patterns of lineage I and II strains of Listeria monocytogenes in a Spanish broiler abattoir. Poult. Sci. 87:1874–1882 [DOI] [PubMed] [Google Scholar]
- 38. Lundén J. M., Autio T. J., Sjoberg A. M., Korkeala H. J. 2003. Persistent and nonpersistent Listeria monocytogenes contamination in meat and poultry processing plants. J. Food Prot. 66:2062–2069 [DOI] [PubMed] [Google Scholar]
- 39. Lundén J., Autio T., Markkula A., Hellström S., Korkeala H. 2003. Adaptive and cross-adaptive responses of persistent and non-persistent Listeria monocytogenes strains to disinfectants. Int. J. Food Microbiol. 82:265–272 [DOI] [PubMed] [Google Scholar]
- 40. Lundén J. M., Miettinen M. K., Autio T. J., Korkeala H. J. 2000. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Prot. 63:1204–1207 [DOI] [PubMed] [Google Scholar]
- 41. Mcclure P. J., Roberts T. A., Oguru P. O. 1989. Comparison of the effects of sodium chloride, pH and temperature on the growth of Listeria monocytogenes on gradient plates and in liquid medium. Lett. Appl. Microbiol. 3:95–99 [Google Scholar]
- 42. McLauchlin J., et al. 1997. Subtyping of Listeria monocytogenes on the basis of plasmid profiles and arsenic and cadmium susceptibility. J. Appl. Microbiol. 83:381–388 [DOI] [PubMed] [Google Scholar]
- 43. Meunier J. R., Grimont P. A. 1993. Factors affecting reproducibility of random amplified polymorphic DNA fingerprinting. Res. Microbiol. 144:373–379 [DOI] [PubMed] [Google Scholar]
- 44. Miettinen M. K., Bjorkroth K. J., Korkeala H. J. 1999. Characterization of Listeria monocytogenes from an ice cream plant by serotyping and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 46:187–192 [DOI] [PubMed] [Google Scholar]
- 45. Nelson K. E., et al. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32:2386–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nightingale K. K., Windham K., Martin K. E., Yeung M., Wiedmann M. 2005. Select Listeria monocytogenes subtypes commonly found in foods carry distinct nonsense mutations in inlA, leading to expression of truncated and secreted internalin A, and are associated with a reduced invasion phenotype for human intestinal epithelial cells. Appl. Environ. Microbiol. 71:8764–8772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norton D. M., et al. 2001. Molecular studies on the ecology of Listeria monocytogenes in the smoked fish processing industry. Appl. Environ. Microbiol. 67:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olier M., et al. 2002. Assessment of the pathogenic potential of two Listeria monocytogenes human fecal carriage isolates. Microbiology 148:1855–1862 [DOI] [PubMed] [Google Scholar]
- 49. Oliver H., Orsi R., Wiedmann M., Boor K. 2010. Listeria monocytogenes σB has a small core regulon and a conserved role in virulence, but differential contributions to stress tolerance, across a diverse collection of strains. Appl. Environ. Microbiol. 76:4216–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O'Neil H. S., Marquis H. 2006. Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion. Infect. Immun. 74:6675–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orsi R. H., et al. 2008. Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genomics 9:539–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orsi R. H., Ripoll D. R., Yeung M., Nightingale K. K., Wiedmann M. 2007. Recombination and positive selection contribute to evolution of Listeria monocytogenes inlA. Microbiology 153:2666–2678 [DOI] [PubMed] [Google Scholar]
- 53. Petran R. L., Zottola E. A. 1989. A study of factors affecting growth and recovery of Listeria monocytogenes Scott A. J. Food Sci. 54:458–460 [Google Scholar]
- 54. Phan-Thanh L. 1998. Physiological and biochemical aspects of the acid survival of Listeria monocytogenes. J. Gen. Appl. Microbiol. 44:183–191 [DOI] [PubMed] [Google Scholar]
- 55. Poyart-Salmeron C., et al. 1992. Genetic basis of tetracycline resistance in clinical isolates of Listeria monocytogenes. Antimicrob. Agents Chemother. 36:463–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ragon M., et al. 2008. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 4:e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roberts A., et al. 2006. Genetic and phenotypic characterization of Listeria monocytogenes lineage III. Microbiology 152:685–693 [DOI] [PubMed] [Google Scholar]
- 58. Roberts A. J., Williams S. K., Wiedmann M., Nightingale K. K. 2009. Some Listeria monocytogenes outbreak strains demonstrate significantly reduced invasion, inlA transcript levels, and swarming motility in vitro. Appl. Environ. Microbiol. 75:5647–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rousseaux S., Olier M., Lemaitre J. P., Piveteau P., Guzzo J. 2004. Use of PCR-restriction fragment length polymorphism of inlA for rapid screening of Listeria monocytogenes strains deficient in the ability to invade Caco-2 cells. Appl. Environ. Microbiol. 70:2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salamina G., et al. 1996. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol. Infect. 117:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Senczek D., Stephan R., Untermann F. 2000. Pulsed-field gel electrophoresis (PFGE) typing of Listeria strains isolated from a meat processing plant over a 2-year period. Int. J. Food Microbiol. 62:155–159 [DOI] [PubMed] [Google Scholar]
- 62. Slutsker L., Schuchat A. 1999. Listeriosis in humans, p. 75–95 In Ryser E. T., Marth E. H. (ed.), Listeria, listeriosis and food safety, 2nd ed. Marcel Dekker, Inc., New York, NY [Google Scholar]
- 63. Sue D., Fink D., Wiedmann M., Boor K. J. 2004. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 150:3843–3855 [DOI] [PubMed] [Google Scholar]
- 64. Swaminathan B., Barrett T. J. 1995. Amplification methods for epidemiologic investigations of infectious diseases. J. Microbiol. Methods 23:129–139 [Google Scholar]
- 65. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tyler K. D., Wang G., Tyler S. D., Johnson W. M. 1997. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J. Clin. Microbiol. 35:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Stelten A., Simpson J. M., Ward T. J., Nightingale K. K. 2010. Revelation by single-nucleotide polymorphism genotyping that mutations leading to a premature stop codon in inlA are common among Listeria monocytogenes isolates from ready-to-eat foods but not human listeriosis cases. Appl. Environ. Microbiol. 76:2783–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Stelten A., Nightingale K. K. 2008. Development and implementation of a multiplex single-nucleotide polymorphism genotyping assay for detection of virulence-attenuating mutations in the Listeria monocytogenes virulence-associated gene inlA. Appl. Environ. Microbiol. 74:7365–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vaz-Velho M., Duarte G., McLauchlin J., Gibbs P. 2001. Characterization of Listeria monocytogenes isolated from production lines of fresh and cold-smoked fish. J. Appl. Microbiol. 91:556–562 [DOI] [PubMed] [Google Scholar]
- 70. Walker S. J., Archer P., Banks J. G. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157–162 [DOI] [PubMed] [Google Scholar]
- 71. Wernars K., et al. 1996. The WHO multicenter study on Listeria monocytogenes subtyping: random amplification of polymorphic DNA (RAPD). Int. J. Food Microbiol. 32:325–341 [DOI] [PubMed] [Google Scholar]
- 72. Williams J. G. K., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18:6531–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wulff G., Gram L., Ahrens P., Vogel B. F. 2006. One group of genetically similar Listeria monocytogenes strains frequently dominates and persists in several fish slaughter- and smokehouses. Appl. Environ. Microbiol. 72:4313–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zink R., Loessner M. J., Scherer S. 1995. Characterization of cryptic prophages (monocins) in Listeria and sequence analysis of a holin/endolysin gene. Microbiology 141:2577–2584 [DOI] [PubMed] [Google Scholar]