Abstract
Clostridium difficile spores can survive extended heating at 71°C (160°F), a minimum temperature commonly recommended for adequate cooking of meats. To determine the extent to which higher temperatures would be more effective at killing C. difficile, we quantified (D values) the effect of moist heat at 85°C (145°F, for 0 to 30 min) on C. difficile spores and compared it to the effects at 71 and 63°C. Fresh (1-week-old) and aged (≥20-week-old) C. difficile spores from food and food animals were tested in multiple experiments. Heating at 85°C markedly reduced spore recovery in all experiments (5 to 6 log10 within 15 min of heating; P < 0.001), regardless of spore age. In ground beef, the inhibitory effect of 85°C was also reproducible (P < 0.001), but heating at 96°C reduced 6 log10 within 1 to 2 min. Mechanistically, optical density and enumeration experiments indicated that 85°C inhibits cell division but not germination, but the inhibitory effect was reversible in some spores. Heating at 63°C reduced counts for fresh spores (1 log10, 30 min; P < 0.04) but increased counts of 20-week-old spores by 30% (15 min; P < 0.02), indicating that sublethal heat treatment reactivates superdormant spores. Superdormancy is an increasingly recognized characteristic in Bacillus spp., and it is likely to occur in C. difficile as spores age. The potential for reactivation of (super)dormant spores with sublethal temperatures may be a food safety concern, but it also has potential diagnostic value. Ensuring that food is heated to >85°C would be a simple and important intervention to reduce the risk of inadvertent ingestion of C. difficile spores.
INTRODUCTION
Clostridium difficile is a spore-forming enteric pathogen associated with increasing outbreak frequency and disease severity in humans worldwide (23, 27, 41). Since 2006, molecular studies have shown that subtypes of C. difficile isolates recovered from food animals and retail foods are genetically indistinguishable from human-derived strains (namely, PCR ribotypes 027, 077, and 078), indicating that foods could be a source of inadvertent infection for humans (3, 30, 32–34, 38). Although ingestion of spores may not necessarily result in disease induction, the presence of C. difficile spores in a large proportion of raw and ready-to-eat meat products in North America (6 to 42%) highlights the need to consider this potential risk factor in disease epidemiology (30, 38). Identification of effective measures to reduce C. difficile exposure, especially among higher-risk individuals (e.g., the elderly or individuals consuming antimicrobials or antacids), would further reduce the likelihood of this organism becoming an important food-borne pathogen.
Although interventions to control food contamination with C. difficile may be applied both during production and at harvest, little is known about this pathogen in food animals and their environment. Thus, until additional information about the epizootiology and ecology of this pathogen is better understood, postharvest strategies, such as proper cooking and handling, may be among the most effective measures to control food-borne exposure to C. difficile in the short term. Minimal internal meat cooking temperatures ranging from 63 to 85°C (145 to 185°F) have been promoted by government and industry organizations to control other food-borne pathogens (e.g., Salmonella spp., Escherichia coli O157) (5, 12, 40). For ground meats, a frequent target is to reach 71°C and maintain that temperature for a few seconds to reduce between 6 and 7 log10 units of most recognized non-spore-forming pathogens (12, 39). However, C. difficile produces spores which are more thermotolerant than vegetative cells. Recent thermoresistance studies on C. difficile where spores were heated at 71°C for 2 h (31), as well as studies with other clostridia (35), indicate that C. difficile spores survive cooking temperatures recommended for ground meats, and if heated spores survive, they could possibly proliferate during the postcooking chilling phase (35). Previous thermal studies with C. difficile have been single-time-point studies and conducted largely with human-derived strains (24, 46). However, effective comparisons often required multiple-time-point experiments and the determination of D values (19). The main objective of the present study was to quantify and compare the inhibitory effects of moist heat at 63, 71, 85, and 96°C on C. difficile spores, from food animals and retail foods, in liquid media, and in ground beef. In addition, we tested similar thermal treatments on spores aged under different conditions and preliminarily determined whether the mechanism of thermal inhibition was by impairing spore germination or by impairing cell division after germination.
MATERIALS AND METHODS
Clostridium difficile strains.
Progressively, three experiment types—(i) thermal inhibition in liquid culture media, (ii) mechanism of thermal inhibition, and (iii) thermal inhibition on ground beef and gravy—were conducted with multiple C. difficile strains (n = 22; 13 genotypes). Genotypes tested included toxigenic PCR ribotypes 014, 027, 033, 077, and 078 and toxigenic strain ATCC 9569 (see Fig. S1 in the supplemental material). All strains, except the ATCC strain, were isolated from food animals and foods and represent genotypes of public health relevance in North America (32, 33).
Spore production and aging.
Prior to testing, spores were produced by using the nutrient exhaustion method (47). To enhance heterogeneity and thus external validity (29), spores were produced in two nutritious broths. In brief, frozen C. difficile isolates were revived and purified on tryptic soya agar (Acumedia, Lansing, MI) supplemented with 5% defibrinated sheep blood (Hemostat Laboratories, Dixon, CA) (blood agar). Single, 72-hour-old colonies were used to inoculate broth supplemented with sodium taurocholate and antibiotics for C. difficile as in previous studies (2, 22, 32, 45). Alternatively, for some experiments, spores were produced in antibiotic-free brain heart infusion broth supplemented with 5% yeast extract (Acumedia, Lansing, MI) (BHI broth). Inoculated broths were incubated anaerobically at 37°C for 10 days to allow C. difficile to grow and produce spores following nutrient exhaustion of the media. In all cases, spores were harvested (7,000 × g for 10 min) after 10 days of incubation and resuspended in plastic transparent tubes using phosphate-buffered saline (PBS) and left on a bench top to age at room temperature (23 ± 3°C). If the spores were heat tested within 2 days of production, these spores were considered fresh spores. However, to simulate aging conditions likely to occur in food, feces, or the environment, some spores were left at this temperature an additional 20 or 52 weeks: these are referred to as aged spores.
Thermal inhibition in liquid media.
Thermal testing was conducted in PBS containing the spore suspensions using conventional PCR microtubes (210 ± 10 μl) which were submerged in automatic water baths synchronized to the desired temperatures using the average of two external thermometers (EU620-0918; WVN International). Temperatures tested were 63 and 85°C, which encompass the thermal spectrum of widely publicized food safety guidelines for household minimal internal cooking of meat meals (5, 12, 40). Based on pilot trials, timing started 10 to 15 s after the PCR tubes were immersed in the water bath, when the temperature inside tested tubes reached target temperatures. Then, at specific time intervals (0, 15, and 30 min for 63°C and 0, 3, 6, 9, 12, 15, and 30 min for 85°C), the heated aliquots were removed, cooled in icy water, and enumerated using the spread-plating method on blood agar. Colonies were plated in duplicate and counted after 72 h of anaerobic incubation at 37°C. To confirm that survivor colonies corresponded to the strains tested, PCR ribotyping (4, 32, 33) was used to verify that the genotypes of the inoculum and heated recovered organisms were identical. The D values (decimal reduction times; i.e., time needed to inhibit 90% of spores) were determined using at least two linear equation fits of the inhibition curves excluding the graph shoulders, i.e., the initial and final time points (19). Because precedent enumeration studies of C. difficile in feces indicate that spore recoverability during storage decreases over time (43), we also determined if refrigeration (4 ± 2°C) would reduce the recoverability of heated and nonheated spores over time. Thus, replicate aliquots of all nonheated strains and replicate aliquots of heated PCR ribotype 078 strain (85°C) at multiple time points (3, 6, 9, 12 and 15 min) were enumerated after 14 and 18 days of refrigeration. Paired analysis and D values derived from PCR ribotype 078 aliquots were used to assess variability.
Mechanism of thermal inhibition.
To determine if heat inactivation of C. difficile spores was due to impaired germination or to inhibition of cell division following germination, as has been reported for Bacillus species (13, 15), we first used germination studies to determine if heated spores could germinate in a nutritious broth and then followed those studies with outgrowth studies to determine if these (germinated) spores would then be able to divide to produce visible colonies on blood agar. Normally, during early stages of germination, often within 30 min of incubation, most spores release dipicolinic acid and calcium from their core, changing its refractancy and reducing the optical density of the liquid medium that contains them (25, 26). If germination pathways are affected with heat, no germination-related optical density (at 600 nm [OD600]) changes would occur in the incubation broth. If germination occurred normally and the cell had normal division, the formation of colonies in agar surfaces would also be as expected. However, if heat impaired cell division mechanisms, a reduced number of colonies would then be visible or not at all apparent on the agar.
In brief, the complementary germination and outgrowth experiments were conducted as follows. First, aged spores from C. difficile PCR ribotypes 027, 078, and 077 and the toxigenic strain ATCC 9569 were resuspended and heat shocked individually in BHI broth at 63, 71, and 85°C for 15 min. To prevent inadvertent spore germination, spores suspensions were maintained in icy water in a cold room set at 4°C. Spore suspensions were seeded in replicates (210 μl; OD600, 0.78 ± 0.03) in microtiter plate (Corning Incorporated, Corning, NY) wells to monitor the OD600 during subsequent anaerobic incubation in the BHI at 37°C. Although germination of C. difficile spores is not impaired with room air (17), the wells were layered with 40 μl of 0.2-μm (pore size)-filtered prereduced heavy mineral oil (28) to minimize potential confounding effects due to air diffusion into the BHI during the OD600 readings outside the anaerobic chamber (<4 min). An enzyme-linked immunosorbent assay (ELISA) reader (Spectra max 340PC; Molecular Devices, Sunnyvale, CA) was used to assess the OD600 changes of the spore suspensions after heat shock (time zero) and thereafter at 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 7 h of anaerobic incubation. Nonheated spores (controls) were concurrently tested to document normal-germination OD600 changes. The mean OD600 time point values were derived from results from at least four replicates. To quantify the ability of heated spores to divide and produce countable colonies (outgrowth study), spores were plated and enumerated on blood agar before and after incubation in BHI. However, because the production of daughter vegetative cells from spores could have been possible during the 7 h of incubation, the BHI broths were treated with 96% ethanol (1:1, vol/vol, 30 min) (7), washed, and resuspended in PBS prior to enumeration to eliminate potential vegetative cells and infer the number of dormant spores capable of normal division.
Thermal inhibition in ground beef and gravy.
Thermal resistance data from PBS or culture broth studies should not be directly extrapolated to food matrices due to the physicochemical complexity of foods (18). Therefore, we also quantified the extent of spore inhibition using retail ground beef (2-kg packages purchased at a local retailer) and a beef gravy model (5% beef extract, 1.7% soluble starch, 1.5% proteose peptone, and 0.5% yeast extract) (20). In these experiments, the strains studied in the above-mentioned germination outgrowth study were tested following a protocol for E. coli O157 reported by Juneja et al. (20) with minor modifications.
In brief, Juneja et al. (20) used a mixture of four strains of E. coli (a “cocktail”), experimentally inoculated single random aliquots of ground beef (3 g), and individually heated those aliquots using Whirlpack bags and a water bath. Here, we used the same Whirlpack bag technique, but we assessed the effect of heating for a 1:1:1:1 (107 spores · ml−1) mixture of four C. difficile genotypes (PCR ribotype 027, 078, and 077 and an ATCC toxigenic strain). We determined the D values in a complete block design using three food matrices (i.e., a nutritious fat-free gravy model, retail “lean” 7% fat ground beef, and retail 30% fat ground beef) (18) and four heating temperatures (63, 71, 85, and the purportedly more effective 96°C). The 96°C was chosen as a subboiling water temperature for the city of Wooster, OH, where the altitude-adjusted boiling temperature is about 98.8°C. These experiments were conducted in duplicate with spore enumeration conducted in triplicate; the spores used here had been aged for 52 weeks at the time of testing. We used Juneja's gravy model to control and quantify the effect of spore entrapment within the beef, which shrinks with heating. Following experimental inoculation of the 3-gram beef aliquots, these were heated for 0, 2, 5, 7.5, 10, 15, 20, and 30 min and then mixed with 3 ml of PBS (1:1, vol/wt), stomached for 1 min, and enumerated as mentioned above for D value determination. To control for the natural level of C. difficile contamination that could be present within the retail meats, we also quantified the spore concentration of noninoculated nonheated aliquots of both ground beef types purchased.
Statistics.
Univariate and multivariable analyses of repeated measures of transformed (log10) and normalized enumeration data were conducted as indicated by Davis (9), using STATA (College Station, TX, v.10) and Minitab 15 (Upper Saddle River, NJ). Comparisons among D values were analyzed with generalized linear regression; relevant descriptive statistics and 95% confidence intervals (95% CI) with pooled errors are reported. Percentages of effect were computed as 10 exponentiated to the log10 unit of interest.
RESULTS
Thermal inhibition in liquid media.
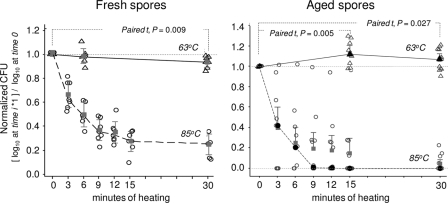
Controlling for strain variability, heating C. difficile spores in PBS at 63°C for 30 min was not as effective as heating at 85°C in reducing the spore counts (Fig. 1). However, the effect of heating at 63°C depended on whether the spores were fresh or aged. While heating fresh spores at 63°C resulted in a steady significant inhibitory effect over time (median reduction of 0.37 log10, i.e., 57.3% spore reduction after 30 min, 95% CI = 0.13, 0.67 log10; generalized linear model [GLM]; P = 0.002), heat treatment of aged spores resulted in a 30% increased recovery compared to baseline counts within 15 min of heating (GLM, P = 0.049). Heating at 85°C resulted in much faster inhibition for all strains (99.9998% of spores after 15 min) with the estimated D value at 85°C ranging from 6.0 to 8.5 min, irrespective of spore aging (median reduction of 5.6 log10, 95% CI = 4.48, 5.94 log10; GLM; P < 0.0001; Fig. 1).
Fig. 1.
Effect of moist heat in phosphate-buffered saline at 63 and 85°C on viability of fresh (1-week-old) and aged (20-week-old) spores of C. difficile. Open symbols represent isolate means; gray squares with interval bars represent 95% CI of the means.
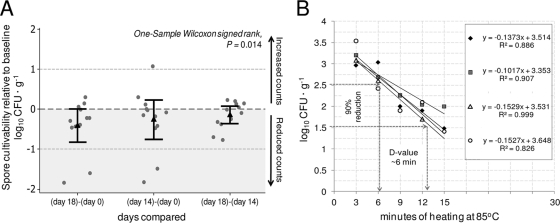
When the effect of refrigeration on the recovery of nonheated aged spores was assessed, storage significantly reduced the spore counts by day 18 (median reduction of 0.28 log10, i.e., 42.5% of spores, 95% CI = 0.045, 0.915 log10; Wilcoxon signed; P = 0.014; Fig. 2A). At the strain level, two isolates had a 2-log10 reduction by days 14 and 18. Complementarily, repeated quantification of heated aliquots of PCR ribotype 078 at 85°C showed that refrigeration did not affect spore enumeration and the D values therein derived (Fig. 2B).
Fig. 2.
Effect of storage on cultivability of aged C. difficile spores during 18 days of refrigeration at 4°C. (A) Results for nonheated spores illustrate reduced cultivability over time (12 strains; triangles and interval bars represent means and 95% CI). (B) Results for spores heated at 85°C indicate reproducible estimation and cultivability (PCR ribotype 078 strain; regression fits shown for 0, 3, 14, and 18 days).
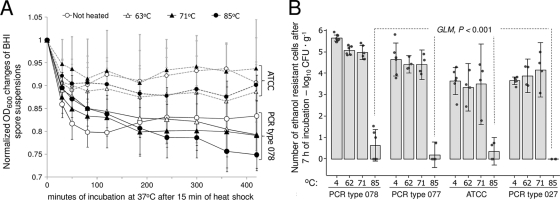
Heat treatment at 85°C affects cell division but not spore germination.
Assessment of heat-shocked spores (15 min) in BHI during 7 h of incubation demonstrated that the OD600 germination curves of all heated strains were normal, as they were similar to the germination curves of nonheated controls (GLM; P > 0.1; Fig. 3A). However, when we assessed the effect of heat shock on cell division, determined as the number of countable colonies on agar after normal germination (curves), multivariable analysis indicated that 15 min of heat shock at 85°C was the only temperature that significantly reduced the number of countable colonies. On average, 85°C induced a 4.2 log10 reduction (99.994% of spores) compared to results for nonheated controls (95% CI = 3.9, 4.5; GLM; P < 0.0001; Fig. 3B).
Fig. 3.
Germination and outgrowth studies of C. difficile to determine if 15 min of heat shock impairs spore germination or the subsequent cell division. (A) Germination study. Optical density (OD600) reductions of heavily inoculated BHI spore suspensions, incubated at 37°C for 7 h, are similar for heated and nonheated spores, indicating normal germination of heated spores. Only PCR ribotype 078 and the ATCC strain are shown; results are means ± standard deviations. (B) Outgrowth study. Enumeration of ethanol-resistant cells from aliquots in Fig. 3A after 7 h of incubation. Countable colonies on blood agar indicate adequate cell division. Reduced counts after heat shock with 85°C (and normal germination shown in Fig. 3A) indicate impaired cell division. Dots represent replica averages; results are means (solid bars) ± 95% CI.
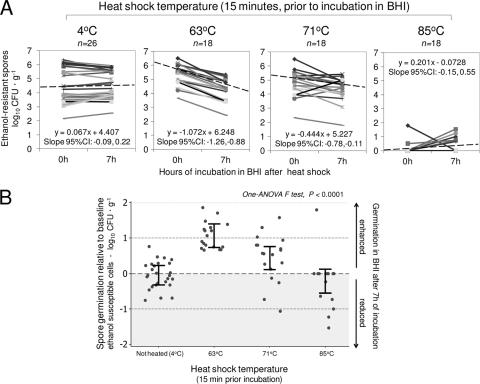
Thermal inhibition at 85°C can revert during incubation in broth.
Paired analysis (before versus after 7 h of incubation in BHI) of ethanol-resistant spores showed that 85°C resulted in complete inhibition of most isolates immediately after 15 min of heat shock (Fig. 4A, panel 85°C, 0 h). However, when those heat shocked spores (85°C) were left incubating in BHI, the number of countable colonies after 7 h of incubation increased significantly (Fig. 4A, panel 85°C, 7 h), indicating that the thermal inhibitory effect observed immediately after heat shock can be reversed during subsequent incubation. In contrast, heat shock with 63 and 71°C increased the rate of spore recovery compared to that of nonheated controls immediately after heat shock. These two temperatures also enhanced the rate of spore germination (assessed as the number of ethanol-susceptible cells) during the subsequent 7 h of incubation in BHI. The highest rate of germination was observed with 63°C (0.4 log10 increase, 95% CI = 0.1, 0.9; GLM; P < 0.01; Fig. 4B).
Fig. 4.
Effect of heat shock on immediate cultivability and subsequent germination of C. difficile spores determined by enumeration of ethanol-resistant cells before and after 7 h of incubation in BHI. (A) Line plots represent paired enumeration of heat-shocked and nonheated individual spore replicas. Linear equations and extended dashed lines represent average effects (slopes). The highest and steepest dashed line (63°C) indicates immediate enhanced cultivability and subsequently increased spore germination. The panel for 85°C shows immediate inhibition and subsequent increased cultivability. (B) Spore germination relative to baseline. Normalized paired differences (before and after BHI incubation). Heat shock with 63 and 71°C enhanced germination in spores that otherwise would have remained dormant after an additional 7 h of incubation in BHI and 72 h in blood agar at 37°C (here referred to as superdormant spores). Dots represent average difference per replica; means ± 95% CI (see also Table S1 in the supplemental material).
Thermal inhibition of C. difficile in ground beef and gravy.
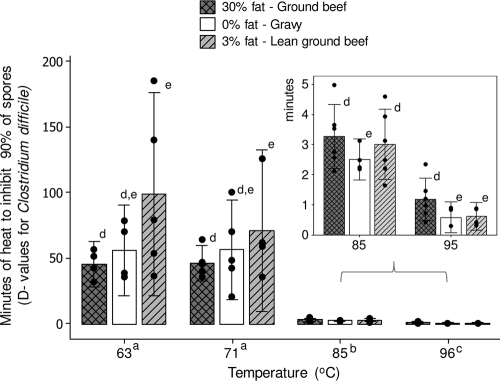
Quantification of C. difficile contamination in the ground beef packages indicated that natural contamination was present in both products (different processing plants), varied within the packages, and ranged from 0 to between 0.5 and 3.3 log10 · g−1. Mathematically, the highest level of natural contamination corresponded to less than 0.02% of the spore concentration achieved with experimental contamination and was deemed minimal to bias the estimation and interpretation of the D values. Multivariable analysis of D value data indicated that 85 and 96°C have the highest inhibitory effects against C. difficile experimentally inoculated in beef matrices (GLM; P < 0.001; Fig. 5). In contrast, 63 and 71°C had comparable and minimal effects, respectively, in inhibiting the C. difficile mixture (see D values in Table S2 in the supplemental material). Although significant differences were observed among the matrices tested (which varied in up to 30% in fat content), the direction of such effects varied with temperature, indicating matrix-temperature interactions (Fig. 5).
Fig. 5.
Minutes of heat needed to inhibit a mixture of aged C. difficile spores heated in three food matrices. Dots indicate individual D value estimates; results are means ± 95% CI. Identical superscripts connect variables with nonsignificant differences (i.e., GLM; P > 0.05), whereas different superscripts indicate significance. See also Table S2 in the supplemental material for coefficient estimates.
DISCUSSION
This study quantified the inhibitory effects of moist heat at 63, 71, 85, and 96°C on C. difficile spores in liquid media and in ground beef. Our quantitative report also evaluated for the first time fresh and aged spores, the latter of which are likely to occur naturally in the environment and within contaminated foods. To contribute to food safety validation protocols, temperatures tested included widely publicized food safety guidelines of minimal internal cooking temperatures for meat meals (5, 12, 40). Briefly, publicized temperatures range from 63°C (for 30-min batch milk pasteurization, for preparation of fish, and for preparation of steaks/roasts of beef, veal, and lamb as “medium-rare”) to 85°C (for whole poultry and turkey meals). However, cooking at 71°C is often cited in the “safe handling instructions” of most retail meat labels and is indicated for “well-done” cooking of beef, veal and lamb steaks, for ground beef, and for pork products (5, 6, 10, 37, 40). Of concern, recent data indicate that 71°C results in only a 2 log10 reduction in C. difficile spores after heating for 2 h (31).
Under the conditions of our experiments, moist-heat treatment at 85°C, irrespective of spore aging and test media, markedly inhibited (up to 6 log10) spores from most C. difficile strains within 15 min of heat treatment. Considering that the concentration of C. difficile spores in naturally contaminated retail raw meat products may be less than 3.3 log10 · g−1 of product, which is in agreement with Weese et al. (42), cooking at least 85°C could markedly reduce human exposure to C. difficile. Here we found that heating aliquots containing less than 4 log10 at 85°C in liquid media always yielded no cultivable spores after 15 min of heating. For other more susceptible food-borne pathogens, such as Salmonella spp., it is known that there is a greater risk of pathogen persistence in the final ready-to-eat meat products after heat treatment if the initial pathogen load in the raw products is high (39). However, it is unknown whether this applies to C. difficile because it is unknown whether C. difficile proliferates in retail meals and if bacterial proliferation reaches more than 4 log10 · g−1. Likewise, it is uncertain to what extent spore aging within food matrices parallels the aging process induced in culture broths in our study and the subsequent magnitude of thermal tolerance.
To date, most studies assessing C. difficile germination and sporulation have used freshly prepared spores, leaving aged spores largely uncharacterized. As aging of C. difficile spores could occur in the environment or in foods, studying the effect of heat is critical for understanding spore survivability patterns. Here, we showed that 85°C was markedly inhibitory for aged and fresh spores; however, of food safety relevance was the finding that temperatures of 63 and 71°C enhanced germination in aged spores. The food safety concerns are that heat treatment is a well-known germination factor that triggers the synchronic activation of most spore-forming organisms associated with food and that spore germination during the subsequent chilling period is possible in other clostridia (35). Furthermore, sublethal heat shock of spores in other clostridia is known to significantly enhance the thermal tolerance of surviving spores (21).
Storage under refrigeration resulted in lower spore counts over time. Although there are no comparable studies assessing aging of C. difficile in pure suspensions under refrigeration, our finding is comparable to that of a study assessing the C. difficile recoverability in inoculated feces (43). In that study, the rate of isolation of C. difficile decreased over time (43). Although another similar study stated that there was little effect (11), our findings indicate that factors associated with decreased recovery (e.g., superdormancy) might be enhanced during storage. Superdormancy is a recently described characteristic in Bacillus species (13) and a rising concern for the food industry, as superdormant Bacillus spores have higher moist-heat resistance, have lower water core content than regular spores, and can recover from heat stress injuries during refrigeration (14, 15, 44). Understanding the properties of superdormancy in Bacillus spp. could also indicate ways to reduce the potential impact of C. difficile on food safety.
To date, some epidemiological studies assessing C. difficile contamination of foods have used thermal treatment (80°C for 10 min) to reduce contaminants and facilitate spore recovery (38). However, the observed inhibition with 85°C in our study indicates that culture-based studies based on thermal methods for spore selection that use temperatures between 71 and 85°C might introduce selection bias, favoring more thermoresistant spores. Hence, it is advisable to use temperatures below 71°C, with 63°C being a pasteurization temperature potentially beneficial for spore selection in diagnostic settings.
Thermal inhibition at 85°C is a promising preventive measure, since cooking guidelines at this temperature are readily available for preparation of whole-turkey meals and could be easily adopted. However, this study indicates that more than a few seconds are required to lower the number of C. difficile spores to the desired 5 to 6 log10 reduction. It is expected that extended cooking at 85°C could maintain the nutritional quality of the cooked foods, but it is uncertain how extended heating would impact the nutritional and palatability characteristics of nonturkey meals. A balance between food safety risks, nutritional value, and food sensory quality needs to be taken into consideration. The marked inhibitory effect of 96°C documented here further highlights the potential benefits of revising cooking time and temperature recommendations, at least for individuals at risk. This study generated the first D values for C. difficile in a food matrix. These data can be used for policy and management decision making relevant to food safety and infection control strategies to disinfect hospital reusable materials (1).
Comparing our estimated D values at 85°C (<8 min) to those reported for other clostridia, it becomes evident that C. difficile appears to be more susceptible to heat. For instance, the D values at 100°C for C. perfringens, the most common spore-forming food-borne pathogen in the United States (36), in beef gravy vary from 16 to 21 min (21), and in distilled water the D value at 85°C was 55 min (16). Thus, cooking guidelines for industrial purposes against C. perfringens (8) may also be a good basis for the refinement of household guidelines against C. difficile.
In summary, we quantified the inhibitory effect of heat on C. difficile spores, estimated D values (63 to 96°C) with confidence intervals for ground beef and gravy, and mechanistically determined that the inhibitory effect of heat shock at 85°C, the highest recommended internal minimal temperature for cooking, is due to inhibition of cell division but not germination and that it can be reversible. Spore germination and recoverability were enhanced with 63°C, especially in aged spores. However, with concern, but as expected, we documented the sublethal effect of 71°C and its moderate effect to enhance germination. Thermoresistance experimentation also indicated that spore superdormancy, an increasingly recognized characteristic in Bacillus species, is likely to occur in C. difficile as spores age. Ensuring that food is heated to more than 85°C would be a simple and important intervention to reduce the risk of inadvertent ingestion of C. difficile spores.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly supported by state and U.S. federal funds allocated to the Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH. A.R.-P. is a research fellow supported by the Public Health Preparedness for Infectious Diseases (Targeted Investments in Excellence) Program, The Ohio State University.
Special thanks to Pamela Schlegel and Jennifer Brock for technical assistance and Michele Williams for comments during the final revision of the manuscript.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 11 March 2011.
REFERENCES
- 1. Alfa M. J., Olson N., Buelow-Smith L. 2008. Simulated-use testing of bedpan and urinal washer disinfectors: evaluation of Clostridium difficile spore survival and cleaning efficacy. Am. J. Infect. Control 36:5–11 [DOI] [PubMed] [Google Scholar]
- 2. Aspinall S. T., Hutchinson D. N. 1992. New selective medium for isolating Clostridium difficile from faeces. J. Clin. Pathol. 45:812–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakri M. M., Brown D. J., Butcher J. P., Sutherland A. D. 2009. Clostridium difficile in ready-to-eat salads, Scotland. Emerg. Infect. Dis. 15:817–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bidet P., Barbut F., Lalande V., Burghoffer B., Petit J. C. 1999. Development of a new PCR-ribotyping method for Clostridium difficile based on ribosomal RNA gene sequencing. FEMS Microbiol. Lett. 175:261–266 [DOI] [PubMed] [Google Scholar]
- 5. CFIA 14 July 2010, posting date Canadian Food Inspection Agency. Food thermometer food safety tips: preventing foodborne illness. http://www.inspection.gc.ca/english/fssa/concen/tipcon/thermoe.shtml
- 6. CIPHI 19 December 2003, posting date Canadian Institute of Public Health Inspectors. Food safety factors for cooking Christmas turkey. http://www.ciphi.ca/pdf/turkey.pdf
- 7. Clabots C. R., Gerding S. J., Olson M. M., Peterson L. R., Gerding D. N. 1989. Detection of asymptomatic Clostridium difficile carriage by an alcohol shock procedure. J. Clin. Microbiol. 27:2386–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crouch E., Golden N. 1 September 2005, posting date Risk assessment for Clostridium perfringens in ready-to-eat and partly cooked meat and poultry products. USDA—Food Safety Inspection Service. http://www.fsis.usda.gov/PDF/CPerfringens_Risk_Assess_Sep2005.pdf [DOI] [PubMed]
- 9. Davis C. S. 2002. Statistical methods for the analysis of repeated measurements, p. 415 Springer, New York, NY [Google Scholar]
- 10. FDA 26 April 2010, posting date United States Food and Drug Administration. Grade “A” Pasteurized Milk Ordinance—Public Health Service/Food and Drug Administration (2009 revision). http://www.fda.gov/downloads/Food/FoodSafety/Product-SpecificInformation/MilkSafety/NationalConferenceonInterstateMilkShipmentsNCIMSModelDocuments/UCM209789.pdf
- 11. Freeman J., Wilcox M. H. 2003. The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces. J. Clin. Pathol. 56:126–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FSA 31 July 2007, posting date Food Standards Agency, United Kingdom. Advisory Committee on the Microbiological Safety of Food: report on the safe cooking of burgers. http://www.food.gov.uk/news/newsarchive/2007/jul/burgers and http://www.food.gov.uk/multimedia/pdfs/acmsfburgers0807.pdf
- 13. Ghosh S., Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghosh S., Setlow P. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J. Appl. Microbiol. 108:582–590 [DOI] [PubMed] [Google Scholar]
- 15. Ghosh S., Zhang P., Li Y. Q., Setlow P. 2009. Superdormant spores of Bacillus species have elevated wet-heat resistance and temperature requirements for heat activation. J. Bacteriol. 191:5584–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heredia N. L., Garcia G. A., Luevanos R., Labbe R. G., Garcia-Alvarado J. S. 1997. Elevation of the heat resistance of vegetative cells and spores of Clostridium perfringens type A by sublethal heat shock. J. Food Prot. 60:998–1000 [DOI] [PubMed] [Google Scholar]
- 17. Jump R. L. P., Pultz M. J., Donskey C. J. 2007. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob. Agents Chemother. 51:2883–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juneja V. K., Eblen B. S., Marks H. M. 2001. Modeling non-linear survival curves to calculate thermal inactivation of salmonella in poultry of different fat levels. Int. J. Food Microbiol. 70:37–51 [DOI] [PubMed] [Google Scholar]
- 19. Juneja V. K., Eblen B. S., Ransom G. M. 2001. Thermal inactivation of Salmonella spp. in chicken broth, beef, pork, turkey, and chicken: determination of D- and Z-values. J. Food Sci. 66:146–152 [Google Scholar]
- 20. Juneja V. K., Klein P. G., Marmer B. S. 1998. Heat shock and thermotolerance of Escherichia coli O157:H7 in a model beef gravy system and ground beef. J. Appl. Microbiol. 84:677–684 [DOI] [PubMed] [Google Scholar]
- 21. Juneja V. K., Novak J. S., Huang L., Eblen B. S. 2003. Increased thermotolerance of Clostridium perfringens spores following sublethal heat shock. Food Control 14:163–168 [Google Scholar]
- 22. Levett P. N., Margaritis-Bassoulis G. 1985. Enrichment media for isolation of Clostridium difficile from faeces. Eur. J. Clin. Microbiol. 4:135–136 [DOI] [PubMed] [Google Scholar]
- 23. McFarland L. V. 2008. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 5:40–48 [DOI] [PubMed] [Google Scholar]
- 24. Meisel-Mikolajczyk F., Kaliszuk-Kaminska E., Martirosian G. 1995. Study of the thermoresistance of Clostridium difficile spores. Med. Dosw. Mikrobiol. 47:177–181 (In Polish.) [PubMed] [Google Scholar]
- 25. Paredes-Sabja D., Bond C., Carman R. J., Setlow P., Sarker M. R. 2008. Germination of spores of Clostridium difficile strains, including isolates from a hospital outbreak of Clostridium difficile-associated disease (CDAD). Microbiology 154:2241–2250 [DOI] [PubMed] [Google Scholar]
- 26. Paredes-Sabja D., Torres J. A., Setlow P., Sarker M. R. 2008. Clostridium perfringens spore germination: characterization of germinants and their receptors. J. Bacteriol. 190:1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pepin J., et al. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591–1597 [DOI] [PubMed] [Google Scholar]
- 28. Rafii F., Park M. 2005. Effects of gyrase mutation on the growth kinetics of ciprofloxacin-resistant strains of Clostridium perfringens. Anaerobe 11:201–205 [DOI] [PubMed] [Google Scholar]
- 29. Richter S. H., Garner J. P., Wurbel H. 2009. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat. Methods 6:257–261 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Palacios A., et al. 2009. Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg. Infect. Dis. 15:802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez-Palacios A., Reid-Smith R. J., Staempfli H. R., Weese J. S. 2010. Clostridium difficile survives minimal temperature recommended for cooking ground meats. Anaerobe 16:540–542 [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez-Palacios A., Staempfli H. R., Duffield T., Weese J. S. 2007. Clostridium difficile in retail ground meat, Canada. Emerg. Infect. Dis. 13:485–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez-Palacios A., et al. 2006. Clostridium difficile PCR ribotypes in calves, Canada. Emerg. Infect. Dis. 12:1730–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rupnik M. 2007. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin. Microbiol. Infect. 13:457–459 [DOI] [PubMed] [Google Scholar]
- 35. Sanchez-Plata M. X., et al. 2005. Predictive model for Clostridium perfringens growth in roast beef during cooling and inhibition of spore germination and outgrowth by organic acid salts. J. Food. Prot. 68:2594–2605 [DOI] [PubMed] [Google Scholar]
- 36. Scallan E., et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silvestre D., Ruiz P., Martinez-Costa C., Plaza A., Lopez M. C. 2008. Effect of pasteurization on the bactericidal capacity of human milk. J. Hum. Lact. 24:371–376 [DOI] [PubMed] [Google Scholar]
- 38. Songer J. G., et al. 2009. Clostridium difficile in retail meat products, U. S. A., 2007. Emerg. Infect. Dis. 15:819–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. USDA 7 February 1999, posting date Food Safety and Inspection Agency—United States Department of Agriculture. Time-temperature tables for cooking ready-to-eat poultry products. http://www.fsis.usda.gov/oppde/rdad/fsisnotices/rte_poultry_tables.pdf
- 40. USDA 12 January 2011, posting date United States Department of Agriculture—Food Safety and Inspection Service. Food safety education—is it done yet? http://www.fsis.usda.gov/is_it_done_yet/brochure_text/index.asp#SMIT
- 41. Warny M., et al. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084 [DOI] [PubMed] [Google Scholar]
- 42. Weese J. S., Avery B. P., Rousseau J., Reid-Smith R. J. 2009. Detection and enumeration of Clostridium difficile in retail beef and pork. Appl. Environ. Microbiol. 75:5009–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weese J. S., Staempfli H. R., Prescott J. F. 2000. Survival of Clostridium difficile and its toxins in equine feces: implications for diagnostic test selection and interpretation. J. Vet. Diagn. Invest. 12:332–336 [DOI] [PubMed] [Google Scholar]
- 44. Wei J., et al. 2010. Superdormant spores of Bacillus species germinate normally with high pressure, peptidoglycan fragments, and bryostatin. J. Bacteriol. 192:1455–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson K. H., Kennedy M. J., Fekety F. R. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15:443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wultanska D., Pituch H., Obuch-Woszczatynski P., Meisel-Mikolajczyk F., Luczak M. 2004. Comparative study of thermoresistance spores of Clostridium difficile strains belonging to different toxigenicity groups. Med. Dosw. Mikrobiol. 56:155–159 (In Polish.) [PubMed] [Google Scholar]
- 47. Young M., Mandelstam J. 1979. Early events during bacterial endospore formation. Adv. Microb. Physiol. 20:103–162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.