Abstract
The selection of highly productive cell lines remains a key step for manufacturing therapeutic proteins. Microengraving was used to screen chemically mutagenized populations of Pichia pastoris for increased production of an Fc fragment. Clones retrieved following three rounds of mutagenesis yielded titers 2.65-fold greater than those of the parental strain.
INTRODUCTION
Therapeutic proteins account for more than $99 billion of drug revenues annually worldwide (14) and account for a quarter of all new drugs for the treatment of diseases ranging from autoimmunity to cancer (1, 15). The pharmaceutical industry uses both microbial and cultured mammalian cells to produce these biologic drugs in large quantities. Fermentation titer remains a dominant determinant of the cost of recombinant proteins (3), and thus, identifying clonal master cell lines that maximize protein expression and secretion is critical for bioprocess development (1, 11).
Advances in robotics, automated liquid handling, and high-throughput imaging of microtiter plates have enabled new technologies for microbial colony picking. For example, the Genetix QPix and Aviso CellCelector can pick thousands of microbial colonies per day. Both, however, require highly sophisticated, costly equipment, and neither system allows the screening of colonies directly for secreted proteins. Furthermore, cells must be incubated in semisolid medium and cannot be screened immediately after cultivation by fermentation. Flow cytometry (FC) has also been used to select both mammalian and microbial strains based on intracellular, surface-bound, or locally encapsulated reporting molecules that link protein production with the expression of fluorescent markers (8, 12). These approaches are rapid and relatively inexpensive, but the analysis required to identify and translate beneficial genetic factors into production strains can be costly and require significant effort.
Here we report a method that uses microengraving for clonal selection to screen production-ready hosts rapidly for the secretion of a desired recombinant protein. Microengraving is a bioanalytical process that isolates and quantitatively measures the rates of protein secretion for thousands of single cells simultaneously (6). Cells are deposited into an array of subnanoliter wells at a density of ∼1 cell per well, and the array is then sealed to a glass slide uniformly coated with a reagent to capture specific secreted products of interest (e.g., antibodies). Following a short incubation of the sealed array (1 to 2 h), the glass is removed to yield a protein microarray comprising the secreted proteins captured from each individual well. Using this method, cells with desired phenotypes are identified and recovered in less than 24 h using off-the-shelf components typically available in university core facilities for microscopy or microarray processing. The miniaturized format of each microengraving assay also helps conserve reagents and thus limits costs—a typical screen costs less than a single enzyme-linked immunosorbent assay (ELISA) using a 96-well microtiter plate.
Recently, we adapted microengraving to study Pichia pastoris, an alternative host organism for the production of therapeutic proteins and antibodies (2, 5). We determined that secretion of heterologous proteins in this yeast fluctuates dynamically (7). Here, a parental strain of wild-type P. pastoris cells secreting a human Fc fragment under the control of a constitutive promoter (glyceraldehyde 3-phosphate dehydrogenase) was grown in 10 ml yeast extract-peptone-dextrose (YPD), subjected to random mutagenesis for diversification using 3% aqueous ethyl methanesulfonate (EMS) (13), and then allowed to recover for 24 h under standard growth conditions (5) (Fig. 1A). The mutagenized cells were analyzed by microengraving to screen for single cells secreting the Fc fragment at the highest rates (7), with ∼10,000 single cells screened per assay. The 10 most productive cells were recovered, propagated, and frozen to create new clonal stocks. These 10 frozen lines were then used to inoculate 10 new cultures, which were grown to stationary phase and pooled prior to a subsequent round of chemical mutagenesis.
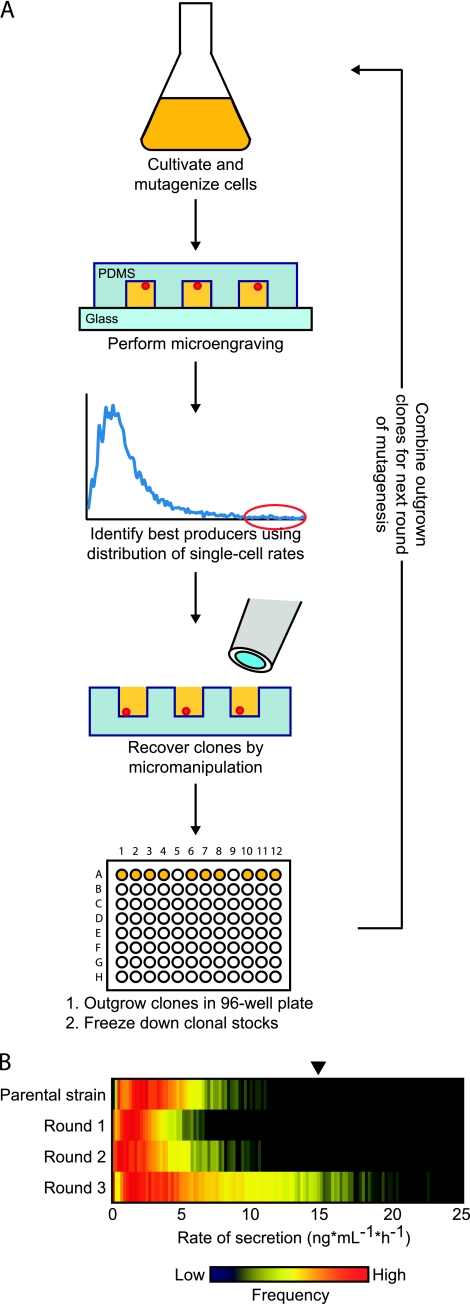
Fig. 1.
(A) Iterative process of mutagenesis and microengraving enables generation and identification of single cells with improved rates of heterologous protein secretion. Wild-type P. pastoris cells constitutively secreting a human Fc fragment were subjected to chemical mutagenesis and then analyzed by microengraving to characterize the rates of protein secretion from single cells quantitatively. Cells with the highest rates of secretion were recovered by micromanipulation, grown, and frozen. These clonal stocks were regrown and pooled before each subsequent round of mutagenesis and screening. PDMS, polydimethylsiloxane. (B) Distribution of single-cell rates of secretion obtained by microengraving using polyclonal populations comprising 10 clones recovered in each round of chemical mutagenesis. Frozen stocks of recovered clones were used to inoculate separate 10-ml cultures of YPD and grown to mid-log phase before pooling and analysis by microengraving. The black arrow indicates a rate five times the median rate of secretion of the parental strain.
Initially, mutagenesis with EMS produced a less productive population of cells (Fig. 1B). This outcome was not unexpected, since it is unlikely that cell survival under stringent mutagenesis conditions (∼70% kill rate) should correlate with enhanced productivity prior to screening. The distributions of the rates of secretion by single cells following the second and third rounds of mutagenesis and screening show that secretion was improved iteratively during our screening process. Following the third round of mutagenesis, we observed significant improvement in Fc secretion of the polyclonal population over the wild-type parental strain (Table 1). This mutagenized population surpassed the parental strain in its median (1.5 times) and mean (1.68 times) rates of secretion. The percentage of highly productive cells—those with rates of secretion greater than five times the median of the parental strain—also increased 6% compared to that in the nonmutagenized population.
Table 1.
Frequency and average rate of Fc-fragment secretion by single cells in wild-type P. pastoris and mutagenized polyclonal daughter populations
| Strain or round of mutagenesis | Frequency of secreting cells (%) | Rate of secretion (ng · ml−1 · h−1) |
|
|---|---|---|---|
| Median | Mean | ||
| Nonmutagenized parent strain | 69 | 3.0a | 3.8 |
| 1 | 80 | 1.8 | 2.4 |
| 2 | 73 | 2.2 | 3.1 |
| 3 | 84 | 4.5a | 6.3 |
These data were subjected to a Mann-Whitney test (P < 0.0001).
After three rounds of mutagenesis, the selected clones were further characterized by ELISA to determine which clonal lines produced more Fc fragment than the parental strain. Frozen clonal stocks were plated on solid medium, and a single colony from each plate was used to inoculate a 10-ml culture of YPD and grown overnight at 25°C. Aliquots of these cultures (optical density at 600 nm of 0.1) were used to inoculate a 250-ml culture for each clone. Supernatants were collected from each culture following 60 h of growth and assayed by ELISA to determine Fc titers. The parental strain was grown under the same conditions for reference.
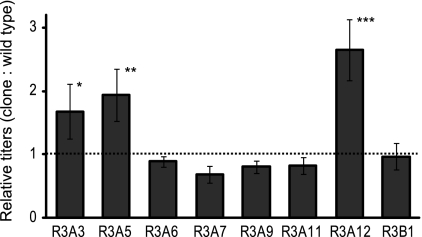
The secreted Fc titer of each mutant clone was compared with that of the parental strain to assess each strain's relative productivity (Fig. 2). Three of the eight clones selected in the third round of mutagenesis demonstrated improved production of the Fc fragment; clones A3 (168%), A5 (193%), and A12 (265%) all exhibited titers of the Fc fragment consistently higher than that of the parental strain. Sequencing of the inserted gene confirmed that none of the selected clones contained mutations within the Fc fragment itself (data not shown), and thus, improved production is not due to alterations or truncation of the product. Further characterization of these clones by gene expression analysis or whole-genome sequencing may reveal genes responsible for the increased productivity seen. Since protein expression and secretion exhibit epigenetic variations (7, 10), the clones that did not outperform the parent strain were likely selected during screening at a point inconsistent with their true steady-state rate of secretion.
Fig. 2.
Comparison of relative productivities of clonal strains recovered after three rounds of chemical mutagenesis. Fc titers in culture supernatants were assessed by ELISA following the growth of each clone and compared to that of the parental strain. Each bar represents the mean ratio of a given clone's titer relative to that of the parental strain from seven independent outgrowth experiments. Error bars indicate standard errors. The dashed line shows the ratio above which a clone's secretion output was considered improved. The significance of the increased productivity was determined by Student's t test (*, P < 0.1; **, P < 0.05; ***, P < 0.01).
In summary, screening of single cells using microengraving to quantify protein secretion is an effective and expedient method for identifying and isolating highly productive clonal lines. Cells, either microbial or mammalian, can be grown in any medium composition prior to their analysis by microengraving—even bioreactors (K. R. Love, V. Panagiotou, B. Jiang, T. A. Stadheim, and J. C. Love, unpublished work)—thus enabling early identification of cell lines using cultivation conditions more similar to those employed in manufacturing. Increasing the data obtained in each assay for each cell is straightforward: As many as four parameters can be scored for each secreted protein simultaneously (4), including posttranslational modifications like glycosylation (9). The multiplexing capabilities of microengraving should allow the integration of process analytical technologies with initial clonal selection and greatly accelerate the time line for upstream process development.
Acknowledgments
We thank Timothy Politano for experimental assistance. J.C.L. is a Latham Family Career Development Professor.
This work was supported by Merck & Co., Inc.
GlycoFi, Inc., is a wholly owned subsidiary of Merck & Co., Inc.
Footnotes
Published ahead of print on 4 March 2011.
REFERENCES
- 1. Browne S. M., Al-Rubeai M. 2007. Selection methods for high-producing mammalian cell lines. Trends Biotechnol. 25:425–432 [DOI] [PubMed] [Google Scholar]
- 2. Cereghino J. L., Cregg J. M. 2000. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 24:45–66 [DOI] [PubMed] [Google Scholar]
- 3. Farid S. S. 2007. Process economics of industrial monoclonal antibody manufacture. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 848:8–18 [DOI] [PubMed] [Google Scholar]
- 4. Han Q., Bradshaw E. M., Nilsson B., Hafler D. A., Love J. C. 2010. Multidimensional analysis of the frequencies and rates of cytokine secretion from single cells by quantitative microengraving. Lab. Chip 10:1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li P. Z., et al. 2007. Expression of recombinant proteins in Pichia pastoris. Appl. Biochem. Biotechnol. 142:105–124 [DOI] [PubMed] [Google Scholar]
- 6. Love J. C., Ronan J. L., Grotenbreg G. M., van der Veen A. G., Ploegh H. L. 2006. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat. Biotechnol. 24:703–707 [DOI] [PubMed] [Google Scholar]
- 7. Love K. R., Panagiotou V., Jiang B., Stadheim T. A., Love J. C. 2010. Integrated single-cell analysis shows Pichia pastoris secretes protein stochastically. Biotechnol. Bioeng. 106:319–325 [DOI] [PubMed] [Google Scholar]
- 8. Mattanovich D., Borth N. 2006. Applications of cell sorting in biotechnology. Microb. Cell Fact. 5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park S., et al. 2010. Array-based analysis of secreted glycoproteins for rapid selection of a single cell producing a glycoprotein with desired glycosylation. Anal. Chem. 82:5830–5837 [DOI] [PubMed] [Google Scholar]
- 10. Pilbrough W., Munro T. P., Gray P. 2009. Intraclonal protein expression heterogeneity in recombinant CHO cells. PLoS One 4:e8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seth G., Charaniya S., Wiaschin K. F., Hu W. S. 2007. In pursuit of a super producer—alternative paths to high producing recombinant mammalian cells. Curr. Opin. Biotechnol. 18:557–564 [DOI] [PubMed] [Google Scholar]
- 12. Sleiman R. J., Gray P. P., McCall M. N., Codamo J., Sunstrom N. A. S. 2008. Accelerated cell line development using two-color fluorescence activated cell sorting to select highly expressing antibody-producing clones. Biotechnol. Bioeng. 99:578–587 [DOI] [PubMed] [Google Scholar]
- 13. Spencer J. F., Spencer D. M. 1996. Mutagenesis in yeast. Methods Mol. Biol. 53:17–38 [DOI] [PubMed] [Google Scholar]
- 14. Walsh G. 2010. Biopharmaceutical benchmarks 2010. Nat. Biotechnol. 28:917–924 [DOI] [PubMed] [Google Scholar]
- 15. Woodnutt G., Violand B., North M. 2008. Advances in protein therapeutics. Curr. Opin. Drug Discov. Devel. 11:754–761 [PubMed] [Google Scholar]