Abstract
Mycobacteria are among the microorganisms least susceptible to biocides but cause devastating diseases, such as tuberculosis, and increasingly opportunistic infections. The exceptional resistance of mycobacteria to toxic solutes is due to an unusual outer membrane, which acts as an efficient permeability barrier, in synergy with other resistance mechanisms. Porins are channel-forming proteins in the outer membrane of mycobacteria. In this study we used the alamarBlue assay to show that the deletion of Msp porins in isogenic mutants increased the resistance of Mycobacterium smegmatis to isothiazolinones (methylchloroisothiazolinone [MCI]/methylisothiazolinone [MI] and octylisothiazolinone [2-n-octyl-4-isothiazolin-3-one; OIT]), formaldehyde-releasing biocides {hexahydrotriazine [1,3,5-tris (2-hydroxyethyl)-hexahydrotriazine; HHT] and methylenbisoxazolidine [N,N′-methylene-bis-5-(methyloxazolidine); MBO]}, and the lipophilic biocides polyhexamethylene biguanide and octenidine dihydrochloride 2- to 16-fold. Furthermore, the susceptibility of the porin triple mutant against a complex disinfectant was decreased 8-fold compared to wild-type (wt) M. smegmatis. Efficacy testing in the quantitative suspension test EN 14348 revealed 100-fold improved survival of the porin mutant in the presence of this biocide. These findings underline the importance of porins for the susceptibility of M. smegmatis to biocides.
INTRODUCTION
Biocides are chemical agents that inactivate microorganisms and include skin and mucosa antiseptics, disinfectants of surfaces and medical devices, and preservatives of food, feed, pharmaceuticals, and other industrial products (11, 20). They comprise a wide variety of chemical classes, such as phenols, aldehydes, biguanides, surface-active agents, halogens, and others (20). It is generally assumed that the majority of biocidal molecules and their formulations target multiple sites of the bacterial cell (19). This general mode of action depends on the physicochemical nature of the given molecule. Some biocides act as membrane destabilizers, and others are alkylating or oxidizing agents or intercalate with nucleic acids. Exceptions include triclosan and isothiazolinones, which inhibit specific enzymes (3, 20, 34).
Whereas the vast majority of Gram-positive and Gram-negative bacteria pose no potential challenge for most biocidal substances, mycobacteria are among the microorganisms least susceptible to biocides (17, 33). However, mycobacteria are important human pathogens, with Mycobacterium tuberculosis remaining a major cause of global mortality and with nontuberculosis mycobacteria increasingly causing opportunistic infections in hospitals and in other environments (9, 30). Therefore, mycobacteria are important organisms to study in infection control, and a better understanding of the mode of action of biocides against mycobacteria will help to fight mycobacterial infections more effectively. The exceptional resistance of mycobacteria to toxic solutes is due to an outer membrane of unusual lipid composition and architecture (23), which acts as an efficient permeability barrier (2), in synergy with other resistance mechanisms, such as efflux or enzymatic inactivation of the target molecule (25). Porins are water-filled channel proteins in the outer membrane of mycobacteria (21). MspA was discovered as the major porin (24) and was later found to be the most abundant protein of Mycobacterium smegmatis (29). Deletion of mspA reduced outer membrane permeability toward glucose (38), phosphate (44), and amino acids (40), indicating that MspA represents the major general diffusion pathway in M. smegmatis. The loss of MspA and MspC, a porin very similar to MspA, reduced the growth rate of M. smegmatis (40, 44), indicating that the influx of hydrophilic nutrients through Msp porins is required for normal growth. Importantly, it was shown that the Msp porins also provide an entry pathway for some antibiotics and drugs (6, 41). These results gave rise to the hypothesis that porins might also be involved in the susceptibility of mycobacteria to biocides. This assumption has been confirmed recently for glutardialdehyde (42). However, it is unknown how mycobacteria take up other biocides (34) and whether these biocidal agents enter mycobacteria by diffusing directly through the lipid bilayers of the cell envelope or by using porins for this purpose. In this study we examined the role of porins in the susceptibility of M. smegmatis to biocides of different chemical classes using the alamarBlue assay and a quantitative suspension test (EN 14348).
MATERIALS AND METHODS
Bacterial strains and standard growth conditions.
To assess the porin-dependent efficacy of biocides against M. smegmatis, a set of well-characterized isogenic porin mutants was chosen. Construction of the porin mutants M. smegmatis ML02 (the ΔmspA mutant), ML10 (the ΔmspA ΔmspC mutant), and ML16 (the ΔmspA ΔmspC ΔmspD mutant) was described previously (38, 40). Unless stated otherwise, all strains were cultured on Middlebrook 7H10 agar plates supplemented with 0.2% glycerol or under agitation in Middlebrook 7H9 liquid medium supplemented with 0.2% glycerol and 0.05% Tween 80 at 37°C. All media, chemicals, and the OADC enrichment were purchased from BD Biosciences, Difco Laboratories, and Merck.
Biocides for susceptibility testing.
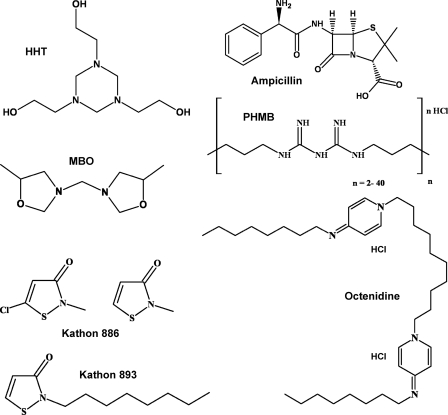
Formaldehyde-releasing biocides hexahydrotriazine [1,3,5-tris (2-hydroxyethyl)-hexahydrotriazine; HHT] (Grotan BK) and methylenbisoxazolidine (N,N-methylene-bis-5-methyl-oxazolidine; MBO) (Grotan OX) and a mycobactericidal disinfectant formulation consisting of the active ingredients phenoxy-propanol (35%, wt/wt), cocospropylene-diamine-guanidine diacetate (14%, wt/wt), and benzalkonium chloride (2.5%, wt/wt) (Gigasept Instru AF) were provided by Schülke & Mayr (Germany). The isothiazolinones Kathon 886 and Kathon 893 were purchased from Rohm & Haas (Germany) and Thor (Germany). Polyhexamethylene biguanide hydrochloride (PHMB) solution (also termed Vantocil IB) was from Avecia (Germany), and octenidine dihydrochloride (octenidine) was from Schülke & Mayr GmbH (Germany). See Fig. 1 for the chemical structures.
Fig. 1.
Chemical structures of antimicrobial compounds used in this study. Ampicillin served as standard for solutes whose uptake is porin mediated. Formaldehyde-releasing biocides (MBO and HHT) are commonly used for technical preservations. Kathon 893 (octylisothiazolinone) and Kathon 886 (mixture of methylchloroisothiazolinone and methylisothiazolinone) are used for preservation of cosmetics and metalworking fluids. The lipophilic and cationic biocides PHMB (polyhexamethylene biguanide) and octenidine dihydrochloride are commonly used in hygiene and antiseptic products. The physicochemical properties of these biocides are listed in Table 1.
Susceptibility determination by MABA.
A microplate-based alamarBlue assay (MABA) was performed to determine the MIC as described in reference 5, with slight modifications (6). All solutions of antimicrobial compounds were made freshly and were sterilized by filtration (0.2-μm pore size) prior to each assay. Stock solutions of solid substances were prepared with ultrapure water. Biocide and antibiotic stock solutions were diluted with 7H9-T medium (without Tween 80) to the required test concentrations. In brief, well-dispersed cell inocula (optical density of 600 nm [OD600] of 0.01 to 0.02) were exposed to serially diluted biocidal agents. The microplates were incubated overnight at 37°C under shaking (300 rpm). After addition of alamarBlue (Biosource, Belgium), incubation was continued for 3 to 4 h until the control wells containing no biocides turned from blue to pink due to the metabolic reduction of the redox-active dyes. Fluorescence was measured with a Synergy HT reader (Bio-Tek) at 590 nm after excitation at 530 nm in the top-reading mode. Biocide concentrations that reduced the fluorescence by more than 90% were considered MICs as defined previously (5).
Quantitative suspension test EN 14348.
Quantitative suspension tests were carried out according to the EN 14348 testing method for the evaluation of mycobactericidal activity of chemical disinfectants in the medical area, including instrument disinfectants (phase 2/step 1) (8). Cell suspensions were prepared from three agar plates according to EN specifications. Removal of cell aggregates was achieved by grinding the suspension with a cooled, sterile agitate mortar at 4°C. The suspension was left to settle for 30 min, and the supernatant was adjusted to 2 × 109 to 5 × 109 CFU/ml with sterile, purified water. Complete homogenization and integrity of the cells were subsequently monitored by fluorescence staining (LIVE/DEAD BacLight bacterial viability kit; Molecular Probes, OR) according to the instructions of the manufacturer. For fluorescence microscopy, an Olympus BX61 microscope equipped with a U-WIBA filter cube (excitation filter, 460- to 490-nm band pass; barrier filter, 510- to 550-nm narrow band pass) was used.
The disinfectant formulation (Gigasept Instru AF) was diluted with standardized hard water (CEN-HW; 300 ppm CaCO3) to 1.25-fold of the desired test concentrations to achieve final concentrations of 0.0625% and 0.125% after addition of the cell inoculum. Experiments were carried out in the presence of 0.3% bovine serum albumin (BSA) (“clean conditions”) with contact times of 5, 15, and 30 min. The antimicrobial efficacy of the disinfectant formulation was quenched in a neutralizer solution consisting of 3.0% (wt/vol) saponin, 1.0% (wt/vol) Tween 80, 0.5% (wt/vol) sodium thiosulfate, and 0.1% (wt/vol) histidine in 0.08 M phosphate buffer, pH 7.0.
Briefly, 1 ml of the cell inoculum preincubated with 1 ml organic load was added to 8 ml Gigasept Instru AF. After specified contact times, 1-ml aliquots were removed and quenched in 9 ml neutralizer solution. Further decimal dilutions were prepared in neutralizer and spread doubly on 7H10 plates to perform conventional plate counting. The level of mycobactericidal activity was calculated according to the guidelines (logarithmical cell count difference of RF = log N0 − log Na, where RF is the reduction factor and N0 and Na are the numbers of CFU per milliliter before and after exposure to disinfectant, respectively). Additionally, standardized internal controls were carried out to verify the nontoxicity of hard water, BSA, and neutralizer exposure to each strain.
RESULTS AND DISCUSSION
Susceptibility of M. smegmatis and isogenic porin mutants to biocides by alamarBlue assay.
We showed previously that antibiotics cross the mycobacterial cell envelope either by porin-facilitated diffusion or by direct diffusion through the lipid membranes, with the entry route being dependent on the size and the hydrophobicity of the solute (6, 41). The aim of this study was to examine which pathways biocides use to penetrate the cell walls of mycobacteria. To examine the role of porins in the susceptibility of mycobacteria to biocides, a well-characterized set of isogenic porin mutants of M. smegmatis was employed. These mutants lack the major porin gene mspA (ML02), mspA and mspC (ML10), and mspA, mspC, and mspD (ML16) (40). The efficacies of biocides with various physicochemical properties and modes of action (Table 1 and Fig. 1) and of a more complex mycobactericidal disinfectant formulation against M. smegmatis and the isogenic porin mutants were determined by the microplate-based alamarBlue assay (MABA). MICs were defined as the concentration of the antimicrobial agent which caused a reduction of cell viability by ≥90% (Table 1). Ampicillin is a small and hydrophilic antibiotic which diffuses through porins in M. smegmatis (6, 41). In our study, ampicillin served as a standard for solutes whose uptake was largely porin mediated. Deletion of the major porin MspA (ΔmspA; strain ML02) reduced the susceptibility of M. smegmatis against ampicillin 2-fold (Table 1). The lack of additional porins in the mutant strains ML10 (the ΔmspA ΔmspC mutant) and ML16 (the ΔmspA ΔmspC ΔmspD mutant) enhanced the ß-lactam resistance of M. smegmatis 16-fold (Table 1). This increased resistance to ampicillin has been attributed to the drastic reduction in the number of porins from 1,000 to less than 60 porins/μm2 cell wall (6, 40).
Table 1.
Physicochemical properties of biocides and their MICs for M. smegmatis SMR5 and isogenic porin mutants
| Antimicrobial substance | Molecular mass (g/mol) | Partition coefficient (log Pow)a,f | MIC for M. smegmatis (μg/ml)b |
Resistance factorc | |||
|---|---|---|---|---|---|---|---|
| Wild type | ML02 | ML10 | ML16 | ||||
| Ampicillin | 349 | 0.43 | 125 | 250 | 2,000 | 2,000 | 16 |
| OIT (Kathon 893) | 213 | 3.89 | 0.94 | 1.88 | 15 | 15 | 16 |
| MCI/MId (Kathon 886) | 150/115 | 0.72/0.48 | 0.18 | 0.35 | 0.7 | 0.7 | 4 |
| MBO (Grotan OX) | 186 | 0.45 | 0.01 | 0.02 | 0.02 | 0.02 | 2 |
| HHT (Grotan BK) | 219 | −2.16 | 0.01 | 0.02 | 0.02 | 0.02 | 2 |
| PHMBe | 400–8,000 | ND | 5 | 5 | 10 | 10 | 2 |
| Octenidine dihydrochloride | 624 | 11.16 | 1 | 2 | 2 | 2 | 2 |
| Gigasept Instru AF | Formulation | ND | 6.25 | 12.5 | 50 | 50 | 8 |
Partition coefficients were calculated using ALOGPS 2.1 (available from the Virtual Computational Chemistry Laboratory at http://www.vcclab.org).
MICs were determined by MABA (see Materials and Methods) and represent biocide concentrations that reduced viability of the cells by ≥90%.
The resistance factor R is the quotient of the MIC of the triple porin knockout strain M. smegmatis ML16 and the MIC of the M. smegmatis wild type.
Kathon 886 contains a mixture of the active ingredients methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI) in a ratio of 3:1.
PHMB is a mixture of hexamethylene biguanide hydrochloride polymers (n = 2 to 40) with an average chain length of 12 units. The partition coefficient cannot be calculated due to the polycationic nature of the molecules.
ND, the partition coefficient cannot be determined for mixtures of compounds.
Role of porins in the efficacy of isothiazolinones against M. smegmatis.
Isothiazolinones are broad-spectrum biocidal agents used to control the growth of bacteria, fungi, and algae in cooling water systems, storage tanks, emulsions, or paints. Methylchloroisothiazolinone (MCI) is used as a preservative in many water-based cosmetics because of its effectiveness against Gram-positive and Gram-negative bacteria, yeast, and fungi (32). Kathon 886 is a mixture (3:1) of MCI and methylisothiazolinone (MI) and killed M. smegmatis efficiently with a MIC of 0.2 μg/ml (Table 1), in line with previously reported MICs ranging from 0.2 to 15 μg/ml against different bacteria (3, 7). The porin mutants ML02, ML10, and ML16 were 2- to 4-fold less susceptible to MCI/MI (Table 1). It is likely that methylchloroisothiazolinone and methylisothiazolinone diffuse through the pores of Msp porins of M. smegmatis due to their small sizes and their hydrophilicity (Fig. 1). This hypothesis is supported by the observation that Pseudomonas aeruginosa isolates not expressing the outer membrane porin OprD lacked susceptibility to the more effective methylchloroisothiazolinone (4).
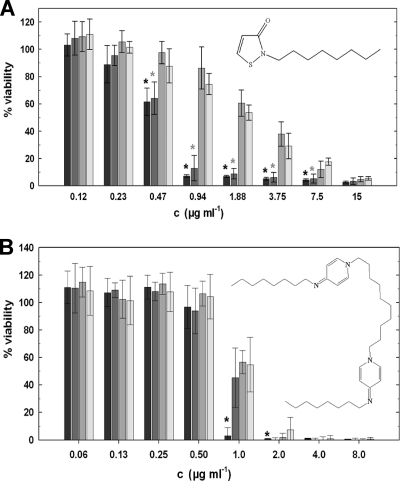
Octylisothiazolinone (2-n-octyl-4-isothiazolin-3-one [OIT]) is a biocide that is used in technical applications, such as paints or metalworking fluids for suppressing growth of bacteria and fungi (16). We tested the role of porins in the susceptibility of M. smegmatis to OIT (Kathon 893). Deletion of mspA reduced the susceptibility of M. smegmatis to OIT 2-fold (Fig. 2A). Deletion of the two porins MspA and MspC, which are the only Msp porins expressed under laboratory growth conditions (40), enhanced the resistance of M. smegmatis to OIT 16-fold (Table 1 and Fig. 2A). However, octylisothiazolinone is more hydrophobic and larger than methylisothiazolinone and methylchloroisothiazolinone (Fig. 1). Increased hydrophobicity and larger solute size are molecular properties known to slow down diffusion through water-filled channel proteins in general (27, 28) and in particular through Msp pores, in which 16 aspartate residues form the constriction zone (10). Hence, the reduced susceptibility of porin mutants to octylisothiazolinone might be increased by an indirect effect. It was shown that the loss of porins decreases the permeability of the outer membrane of M. smegmatis to the hydrophobic chenodeoxycholate, accounting for the increased resistance of porin mutants to hydrophobic antibiotics, such as rifampin and erythromycin, which are both too large to fit through the Msp pores (41). However, it cannot be excluded that octylisothiazolinone may not be sufficiently hydrophobic to diffuse through lipid membranes at appreciable rates and still depends on porins to enter M. smegmatis cells. Nonfunctional MspA pores would provide a tool to distinguish between these uptake mechanisms. These results not only demonstrate that porins play an important role in the susceptibility of M. smegmatis to isothiazolinones but also indicate that their targets are intracellular, i.e., not on the cell surface.
Fig. 2.
Porin-dependent susceptibility of M. smegmatis to Kathon 893 and octenidine dihydrochloride. After exposure to various concentrations (“c”) of octylisothiazolinone (Kathon 893) (A) and the cationic surfactant octenidine (B), the viability of M. smegmatis wild type (black bars) and the isogenic porin mutants ML02 (the ΔmspA mutant, dark gray bars), ML10 (the ΔmspA ΔmspC mutant, medium gray bars), and ML16 (the ΔmspA ΔmspC ΔmspD mutant, light gray bars) was determined by the alamarBlue assay. Significant differences in the efficacy of the biocides against the wild type in comparison to at least one of the porin deletion strains are denoted with black asterisks. Gray asterisks indicate significantly different susceptibilities between the single porin mutant ML02 and the double or triple porin mutants. Significance was calculated using the paired Student t test (P ≤ 0.05). The experiments were done in triplicate. Error bars represent standard deviations.
Role of porins in the efficacy of formaldehyde-releasing biocides against M. smegmatis.
MBO (Grotan OX) and HHT (Grotan BK) are formaldehyde-releasing biocides used, for example, in metalworking fluids (18, 31, 39). Contamination of metalworking fluids with environmental mycobacteria represents an increasing health problem in the metalworking industry (13). M. smegmatis was very susceptible to these biocides, with MICs ranging from 0.01 to 0.02 μg/ml (Table 1). Deletion of the porin genes mspA, mspC, and mspD decreased susceptibility of M. smegmatis 2-fold. This finding is in apparent contrast to previous results which showed a drastic loss of susceptibility of M. smegmatis porin mutants to glutardialdehyde (42). The molecule sizes or hydrophilicity levels of these biocides are quite similar and cannot account for this discrepancy. However, it is likely that MBO and HHT act primarily by releasing formaldehyde, which can easily permeate through membranes (37) in contrast to glutardialdehyde. Direct permeation through the outer membrane might be influenced by the lack of proteins in the porin mutants as discussed previously (6, 41). In particular the absence of MspA might considerably change the interactions of lipids in the outer membrane of M. smegmatis, considering that it is the most abundant protein in M. smegmatis (29). Such an effect might also account for the moderate loss of susceptibility against formaldehyde in porin mutants of Escherichia coli and Serratia marcescens (14).
Role of porins in the efficacy of lipophilic biocides against M. smegmatis.
Polyhexamethylene biguanide (PHMB) and octenidine are lipophilic biocides. PHMB is a fast-acting, broad-spectrum biocide effective at low concentrations against bacteria and viruses and is used as a preservative in cosmetics, personal care products, fabric softeners, contact lens solutions, and hand washes and as a sanitizer in swimming pools and hot tubs due to its stability in sunlight and its resistance against heat and pH fluctuations (1). Octenidine dihydrochloride is a cationic lipophilic biocide of high molecular weight (Fig. 1; Table 1) and is an efficient antiseptic for skin, mucous membranes, and wounds (15). The efficacy of the bispyridine derivative octenidine was reduced 2-fold by deletion of the main M. smegmatis porin MspA, but not by further deletions of mspC and mspD (Table 1; Fig. 2B). Likewise, loss of porins caused only a 2-fold increase in MIC values from 5 μg/ml to 10 μg/ml by exposure to PHMB (Table 1). These lipophilic biocides might cross the mycobacterial outer membrane directly, because it is known that the rate of permeation of lipophilic substances through lipid membranes increases with an increasing n-octanol–water partition coefficient (log Pow) (26). This may explain the minor role of porins in the efficacy of these biocides. However, it was proposed that relative hydrophobic polymeric biguanides, which constitute PHMB, may enter Gram-negative bacteria by destabilizing the outer membrane in a synergistic way, termed self-promoted uptake (12). However, it is unknown whether a similar process exists in mycobacteria (22).
Role of porins in the efficacy of a complex disinfectant formulation against M. smegmatis.
In addition to pure biocide solutions, we examined the influence of Msp porins on the mycobacterial susceptibility against a complex mycobactericidal disinfectant formulation consisting of the biocides phenoxypropanol, cocospropylene-diamine-guanidine diacetate, and benzalkonium chloride (Gigasept Instru AF). We observed a 2-fold decreased susceptibility of the ΔmspA porin mutant ML02 and an 8-fold decreased susceptibility of the double and triple porin mutant strains to Gigasept Instru AF (Table 1). Benzalkonium chloride, which belongs to the group of surface-active quaternary ammonium compounds, is relatively hydrophobic and acts primarily on lipids of bacterial membranes (12). Cocospropylene-diamine-guanidine diacetate is a relatively hydrophilic, surface-active polymeric guanidinium salt of high molecular weight. Hence, both of these compounds are unlikely to cross the outer membrane of M. smegmatis via porins. Thus, we assume that uptake of the hydrophilic and small bactericidal biocide phenoxypropanol (152 g/mol; log Pow = 1.66) is impaired by the lack of porins, resulting in the observed decrease in susceptibility of M. smegmatis porin mutants to the disinfectant formulation.
Susceptibility of M. smegmatis and isogenic porin mutants to biocides in the quantitative suspension test EN 14348.
To examine whether the reduced expression of porins would also affect the susceptibility of M. smegmatis to biocides under more complex conditions, quantitative suspension tests according to the EN 14348 testing method (8), used for the evaluation of the mycobactericidal efficacy of disinfectants, were carried out (8) with a low organic load (0.3% BSA, clean conditions). The aggregation of mycobacterial cells is a known problem in disinfectant testing (43). The M. smegmatis cultures were monitored prior to each experiment by fluorescence microscopy to ensure that they contained dispersed, viable cells with infrequent cell clumps smaller than 10 cells (not shown). The initial cell count of the cultures ranged from 2 × 109 to 5 × 109 CFU/ml for both M. smegmatis SMR5 and ML16. Control experiments verified the nontoxicity and efficacy of the neutralizer, standard hard water, and BSA (data not shown).
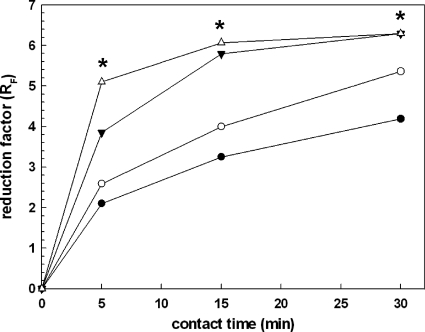
Exposure to 0.0625% of the disinfectant Gigasept Instru AF reduced the viability of wild-type M. smegmatis 10,000-fold within a contact time of 5 min, whereas viability of the porin mutant ML16 was reduced only 100-fold (Fig. 3). In these quantitative suspension experiments, wild-type M. smegmatis was on average 100- to 1,000-fold more sensitive to disinfectant exposure than the porin triple mutant. Treatment with 0.125% of the disinfectant formulation led to an almost complete inactivation of the M. smegmatis SMR5 cell inoculum (Fig. 3). Only 0.0001% M. smegmatis SMR5 cells survived. As observed for the lower concentration, the ML16 strain was less susceptible to the disinfectant formulation at all contact times. Taken together, these findings make evident a strong correlation between the susceptibility of M. smegmatis to the disinfectant formulation and porin density. Thus, consistent with the data obtained in alamarBlue assays, outer membrane porins are important for the susceptibility of M. smegmatis also under more complex conditions, as used in the quantitative suspension test EN 14348.
Fig. 3.
Porin-dependent susceptibility of M. smegmatis to a disinfectant formulation in quantitative suspension tests (EN 14348). Homogenized cell suspensions of M. smegmatis wild type (triangles) and the ΔmspA ΔmspC ΔmspD mutant (ML16, circles) were exposed to 0.0625% (closed symbols) or 0.125% (open symbols) of the disinfectant formulation. After 5, 15, and 30 min, the antimicrobial activity was quenched with a neutralizer, and surviving cells were recovered on agar plates. The antimicrobial activity was expressed as logarithmical cell count reduction (reduction factor [RF]) of the initial cell inoculum. Black asterisks denote RF values, which are in fact higher but cannot be displayed due to the experimental setup demanded by the EN standard.
Efficacy of the disinfectant Gigasept Instru AF against the standard test organisms Mycobacterium terrae and Mycobacterium avium was demonstrated in the quantitative suspension test EN 14348 (phase 2/step 1) and in the quantitative carrier test EN 14563 (phase 2/step 2) at a concentration of 1.5% of the disinfectant formulation for 60 min of contact time or at a concentration of 3% with 15 min of contact time (35, 36). Thus, a 24-fold higher concentration than that of M. smegmatis is necessary to kill M. avium and M. terrae, the latter being the surrogate organism for M. tuberculosis in European disinfectant testing procedures. According to the results of this study, the lower susceptibility of M. avium and M. terrae might be caused by a lower number of porins in the outer membrane of slow-growing mycobacteria, as pointed out earlier (22, 23).
Conclusions.
In this study we showed that a lack of Msp porins, channel-forming proteins in the outer membrane, reduces the susceptibility of M. smegmatis against different classes of widely used biocides. This is of particular interest since Msp porins are widespread (21). For example, MspA-like porins have been identified in Mycobacterium chelonae (42), Mycobacterium ulcerans, and M. avium (21). Infections with these mycobacteria are increasingly common but difficult to treat (9, 30). The findings of this study underline the importance of porins for the susceptibility of M. smegmatis to chemically very different biocides. Better knowledge of the mode of how biocides enter mycobacterial cells and of their mode of action might help to prevent and treat mycobacterial infections more efficiently.
ACKNOWLEDGMENTS
We thank Mirjana Jevtić for excellent technical assistance.
This work was supported by Schülke & Mayr GmbH, Norderstedt, Germany, and by grant AI063432 from the National Institutes of Health to M.N.
Footnotes
Published ahead of print on 11 March 2011.
REFERENCES
- 1. Allen M. J., White G. F., Morby A. P. 2006. The response of Escherichia coli to exposure to the biocide polyhexamethylene biguanide. Microbiology 152:989–1000 [DOI] [PubMed] [Google Scholar]
- 2. Brennan P. J., Nikaido H. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29–63 [DOI] [PubMed] [Google Scholar]
- 3. Chapman J. S., Diehl M. A. 1995. Methylchloroisothiazolone-induced growth inhibition and lethality in Escherichia coli. J. Appl. Bacteriol. 78:134–141 [DOI] [PubMed] [Google Scholar]
- 4. Chapman J. S., Diehl M. A., Fearnside K. B. 1998. Preservative tolerance and resistance. Int. J. Cosmet. Sci. 20:31–39 [DOI] [PubMed] [Google Scholar]
- 5. Collins L., Franzblau S. G. 1997. Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danilchanka O., Pavlenok M., Niederweis M. 2008. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob. Agents Chemother. 52:3127–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diehl M. A., Chapman J. S. 1999. Association of the biocide 5-chloro-2-methyl-isothiazol-3-one with Pseudomonas aeruginosa and Pseudomonas fluorescens. Int. Biodeterior. Biodegradation 44:191–199 [Google Scholar]
- 8. European Committee for Standardisation 2009. European standard EN 14563: chemical disinfectants: quantitative carrier test for evaluation of mycobactericidal activity of chemical disinfectants for instruments used in medical area. Test method and requirements (phase2/step2). European Committee for Standardisation, Brussels, Belgium [Google Scholar]
- 9. Falkinham J. O., III 2009. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 107:356–367 [DOI] [PubMed] [Google Scholar]
- 10. Faller M., Niederweis M., Schulz G. E. 2004. The structure of a mycobacterial outer-membrane channel. Science 303:1189–1192 [DOI] [PubMed] [Google Scholar]
- 11. Fraise A. P. 2002. Biocide abuse and antimicrobial resistance—a cause for concern? J. Antimicrob. Chemother. 49:11–12 [DOI] [PubMed] [Google Scholar]
- 12. Gilbert P., Moore L. E. 2005. Cationic antiseptics: diversity of action under a common epithet. J. Appl. Microbiol. 99:703–715 [DOI] [PubMed] [Google Scholar]
- 13. Gordon T., Nadziejko C., Galdanes K., Lewis D., Donnelly K. 2006. Mycobacterium immunogenum causes hypersensitivity pneumonitis-like pathology in mice. Inhal. Toxicol. 18:449–456 [DOI] [PubMed] [Google Scholar]
- 14. Heinzel M. 1998. Phenomena of biocide resistance in microorganisms. Int. Biodeterior. Biodegradation 41:225–234 [Google Scholar]
- 15. Hübner N. O., Siebert J., Kramer A. 2010. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol. Physiol. 23:244–258 [DOI] [PubMed] [Google Scholar]
- 16. Kramer A., Reichwagen S., Widulle H., Heldt P. 2008. Isothiazoline, p. 819–820 In Assadian O., Kramer A. (ed.), Wallhäußers praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung, 1st ed. Georg Thieme Verlag, Stuttgart, Germany [Google Scholar]
- 17. Lambert P. A. 2002. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. Appl. Microbiol. 92(Suppl.):46S–54S [PubMed] [Google Scholar]
- 18. Madan V., Beck M. H. 2006. Occupational allergic contact dermatitis from N,N-methylene-bis-5-methyl-oxazolidine in coolant oils. Contact Dermatitis 55:39–41 [DOI] [PubMed] [Google Scholar]
- 19. Maillard J. Y. 2002. Bacterial target sites for biocide action. J. Appl. Microbiol. 92:16S–27S [PubMed] [Google Scholar]
- 20. McDonnell G., Russell A. D. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niederweis M. 2008. Mycobacterial porins, p. 153–165 In Daffé M., Reyrat J.-M. (ed.), The mycobacterial cell envelope. ASM Press, Washington, DC [Google Scholar]
- 22. Niederweis M. 2003. Mycobacterial porins—new channel proteins in unique outer membranes. Mol. Microbiol. 49:1167–1177 [DOI] [PubMed] [Google Scholar]
- 23. Niederweis M., Danilchanka O., Huff J., Hoffmann C., Engelhardt H. 2010. Mycobacterial outer membranes: in search of proteins. Trends Microbiol. 18:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niederweis M., et al. 1999. Cloning of the mspA gene encoding a porin from Mycobacterium smegmatis. Mol. Microbiol. 33:933–945 [DOI] [PubMed] [Google Scholar]
- 25. Nikaido H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 12:215–223 [DOI] [PubMed] [Google Scholar]
- 26. Nikaido H., Jarlier V. 1991. Permeability of the mycobacterial cell wall. Res. Microbiol. 142:437–443 [DOI] [PubMed] [Google Scholar]
- 27. Nikaido H., Rosenberg E. Y. 1981. Effect of solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J. Gen. Physiol. 77:121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nikaido H., Rosenberg E. Y. 1983. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J. Bacteriol. 153:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ojha A., Hatfull G. F. 2007. The role of iron in Mycobacterium smegmatis biofilm formation: the exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol. Microbiol. 66:468–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Primm T. P., Lucero C. A., Falkinham J. O., III 2004. Health impacts of environmental mycobacteria. Clin. Microbiol. Rev. 17:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rasschaert V., Goossens A. 2002. Conjunctivitis and bronchial asthma: symptoms of contact allergy to 1,3,5-tris (2-hydroxyethyl)-hexahydrotriazine (Grotan BK). Contact Dermatitis 47:116. [DOI] [PubMed] [Google Scholar]
- 32. Reinhard E., et al. 2001. Preservation of products with MCI/MI in Switzerland. Contact Dermatitis. 45:257–264 [DOI] [PubMed] [Google Scholar]
- 33. Russell A. D. 1996. Activity of biocides against mycobacteria. Soc. Appl. Bacteriol. Symp. Ser. 25:87S–101S [PubMed] [Google Scholar]
- 34. Russell A. D. 2003. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 52:750–763 [DOI] [PubMed] [Google Scholar]
- 35. Schlösser A., Matin A. 2005. Prüfbericht Gigasept Instru AF: praxisnaher quantitativer keimträgertest zur bestimmung der mykobakteriziden wirkung chemischer desinfektionsmittel für instrumente im humanmedizinischen Bereich gemäß prEN 14563. L+S AG, Bad Bocklet, Bavaria, Germany [Google Scholar]
- 36. Schlösser A., Matin A. 2005. Prüfbericht Gigasept Instru AF: quantitativer suspensionsversuch zur bestimmung der mykobakteriziden wirkung chemischer desinfektionsmittel für instrumente im humanmedizinischen Bereich gemäß prEN 14348. L+S AG, Bad Bocklet, Bavaria, Germany [Google Scholar]
- 37. Scott D. C., Newton S. M., Klebba P. E. 2002. Surface loop motion in FepA. J. Bacteriol. 184:4906–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stahl C., et al. 2001. MspA provides the main hydrophilic pathway through the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 40:451–464 (Author's correction, 457:1509.) [DOI] [PubMed] [Google Scholar]
- 39. Steinhauer K., Goroncy-Bermes P. 2008. Treatment of water-based metalworking fluids to prevent hypersensitivity pneumonitis associated with Mycobacterium spp. J. Appl. Microbiol. 104:454–464 [DOI] [PubMed] [Google Scholar]
- 40. Stephan J., et al. 2005. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Mol. Microbiol. 58:714–730 [DOI] [PubMed] [Google Scholar]
- 41. Stephan J., Mailaender C., Etienne G., Daffe M., Niederweis M. 2004. Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48:4163–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svetlíková Z., et al. 2009. Role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob. Agents Chemother. 53:4015–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woelk E., Goroncy-Bermes P., Sand W. 2003. Influence of storage on monodispersed cells of Mycobacterium terrae used for quantitative carrier test prEN 14563. Appl. Environ. Microbiol. 69:6932–6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolschendorf F., Mahfoud M., Niederweis M. 2007. Porins are required for uptake of phosphates by Mycobacterium smegmatis. J. Bacteriol. 189:2435–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]