Abstract
The Krox-20 gene encodes a zinc finger transcription factor, which has been shown previously, by targeted inactivation in the mouse, to be required for the development of rhombomeres (r) 3 and 5 in the segmented embryonic hindbrain. In the present work, Krox-20 was expressed ectopically in the developing chick hindbrain by use of electroporation. We demonstrate that Krox-20 expression is sufficient to confer odd-numbered rhombomere characteristics to r2, r4, and r6 cells, presumably in a cell-autonomous manner. Therefore, Krox-20, appears as the major determinant of odd-numbered identity within the hindbrain. In addition, we provide evidence for the existence of a non cell-autonomous autoactivation mechanism allowing recruitment of Krox-20-positive cells from even-numbered territories by neighboring Krox-20-expressing cells. On the basis of these observations, we propose that Krox-20 regulates multiple, intertwined steps in segmental patterning: Initial activation of Krox-20 in a few cells leads to the segregation, homogenization, and possibly expansion of territories to which Krox-20 in addition confers an odd-numbered identity.
Keywords: Hindbrain, rhombomere, segmentation, Krox-20, Hox, Eph, kreisler
The morphogenesis of the vertebrate hindbrain involves a transient segmentation process along the anterior–posterior (AP) axis, which leads to the generation of 7–8 metameres called rhombomeres (r; Vaage 1969; Lumsden 1990). This subdivision presages the differentiation of neurons in segment-specific patterns and underlies the repeated organization of branchiomotor cranial nerves (Lumsden and Keynes 1989). It also participates in the specification of neural crest cells and in the establishment of their pathways of migration, therefore playing a crucial role in craniofacial organization (Bronner-Fraser 1995; Kontges and Lumsden 1996). Segmentation involves a restriction of cell intermingling at rhombomere boundaries due to alternating cell-surface properties that cause r3/r5 cells to be immiscible with r2/r4/r6 cells (Fraser et al. 1990; Guthrie and Lumsden 1991; Guthrie et al. 1993). This restriction of cell lineages is thought to be required for each segment to maintain a specific pattern of gene expression and thus a distinct AP identity.
Numerous genes present spatially restricted patterns of expression along the AP axis in the hindbrain, with limits corresponding to prospective or established boundaries between adjacent rhombomeres (Lumsden and Krumlauf 1996). Loss-of-function studies have shown that several of these genes play essential roles in the control of hindbrain segmentation (for review, see Schneider-Maunoury et al. 1998). Among them, Krox-20, which encodes a zinc finger transcription factor, is activated in two transverse stripes, which prefigure and subsequently coincide with r3 and r5 (Wilkinson et al. 1989). Targeted inactivation of Krox-20 leads to a progressive elimination of r3 and r5, indicating that this gene is essential to the maintenance of these hindbrain territories (Swiatek and Gridley 1993; Schneider-Maunoury et al. 1993, 1997). In addition, Krox-20 has been shown to directly activate the transcription of several Hox genes in r3 and/or r5 (Hoxb2, Hoxa2, and Hoxb3; Sham et al. 1993; Nonchev et al. 1996; Manzanares et al., unpubl.). Because the combinatorial expression of Hox genes is thought to determine AP positional identity (Lumsden and Krumlauf 1996), this suggests that Krox-20 also plays an important role in the specification of rhombomere identity. Finally, Krox-20 has been shown to directly activate the transcription of at least one of the Eph tyrosine kinase receptor genes expressed in r3 and r5, EphA4 (Gilardi-Hebenstreit et al. 1992; Theil et al. 1998). Bidirectional interactions between the Eph receptors and their ephrin transmembrane ligands, which are present in even-numbered rhombomeres, have been implicated in the segregation of cells between odd- and even-numbered rhombomeres (Mellitzer et al. 2000). Therefore, Krox-20 is also involved in the control of lineage restrictions in the hindbrain.
To gain further insight into the role of Krox-20 in the specification of rhombomere identity, we performed gain-of-function experiments in the chick embryo hindbrain using in ovo electroporation. We show that ectopic expression of Krox-20 can convert even-numbered rhombomere cells into odd-numbered identity (r3 or r5). Unexpectedly, this analysis also revealed that Krox-20 can propagate its own expression by a non cell-autonomous mechanism. This latter phenomenon is likely to play an important role in the establishment of r3 and r5 territories during hindbrain segmentation.
Results
Krox-20 ectopic expression in the hindbrain neuroepithelium
To ectopically express Krox-20 in the hindbrain during the period of segmentation, we used the procedure of electroporation in the chick embryo neural tube (Itasaki et al. 1999). This method allows comparison between the electroporated side and the non-electroporated side, which can be used as a control. Because, in the chick, Krox-20 transcripts were first detected at stage HH8 (Hamburger and Hamilton 1951) in pre-r3 and HH9 in pre-r5 (Nieto et al. 1991; Irving et al. 1996) electroporation was performed at stages HH8–HH10. To set up the conditions, we first electroporated a construct in which the Escherichia coli LacZ gene is driven by a regulatory element composed of the Rous sarcoma virus long terminal repeat promoter enhanced by a human type 5 adenovirus inverted terminal repeat (pAdRSVβ-gal). In these conditions, the presence of β-galactosidase was detected in isolated cells in the neuroepithelium as early as 6 h after electroporation and up to at least 48 h. β-Galactosidase-positive cells were observed only in the electroporated side, in the neuroepithelium, neural crest streams, to a lower extent in non-neural ectoderm, and very rarely in mesodermal tissues (data not shown). They did not show any obvious bias in distribution along the AP axis, often covering the entire hindbrain and part of the midbrain and of the spinal cord, whereas their frequency was usually much higher in the dorsal part of the neural tube (Fig. 1A).
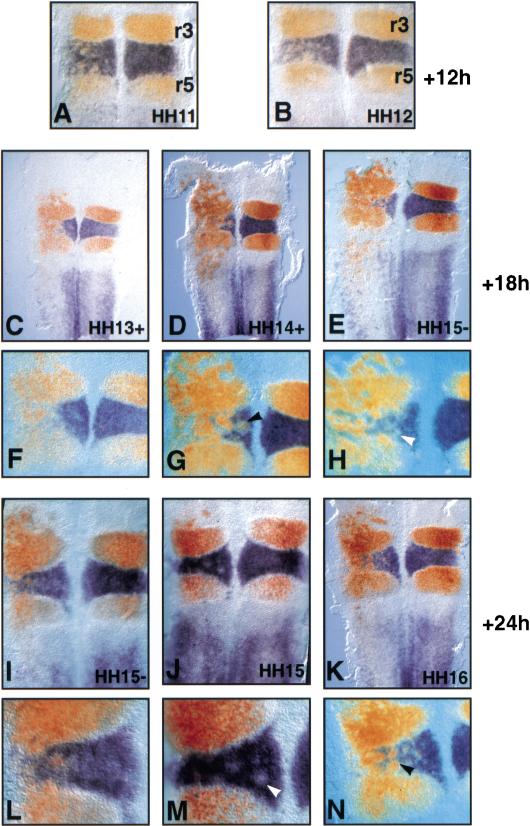
Figure 1.
Ectopic Krox-20 expression leads to EphA4 induction and follistatin and Hoxb1 repression. Flat-mounted hindbrains (A–E,H–J) or whole mounts (F,G) from chick embryos electroporated with LacZ (A), wild-type (B–G), or R409W mutant (H–J) mouse Krox-20 expressing plasmids between stages HH8 and HH10 (A–E,H–J) or between stages HH10 and HH11 (F,G). The embryos were collected 24 h after electroporation (18 h for D and 16 h for F and G) and the expression of the indicated markers was analyzed by X-Gal staining (A), immunochemistry (B,C,F–H) or in situ hybridization (D,E,I,J). (A) Analysis of β-galactosidase distribution after electroporation with a LacZ expression plasmid. (B) Analysis of Krox-20 expression with an antibody that recognizes both mouse and chicken proteins after electroporation with a Krox-20 expression plasmid. Note that ectopic Krox-20 is present in isolated cells with a distribution similar to that of β-galactosidase (A). (C) EphA4 ectopic expression is detected in large patches of cells in r2 and r4. (D) follistatin expression is severely down-regulated upon Krox-20 ectopic expression in the hindbrain, including r5, where endogenous Krox-20 is also present. The patchy appearance of the follistatin-positive domain on the control side is normal at this stage. (E) Hoxb1 is repressed following ectopic Krox-20 expression. Large patches of Hoxb1-negative cells are observed within the entire domain of normal expression, including r4 and r7. (F,G) EphA4 activation in rhombomere 2 (arrowheads) is not due to cell migration from r3 because, in (G), the embryo was cut immediately after electroporation at the level of prospective rhombomere 2 and the two parts were kept separated. (H,I,J) Ectopic expression of the Krox-20 mutant allele (R409W) does not induce EphA4 (H), nor does it repress follistatin (I) or Hoxb1 (J). In I, the red staining corresponds to in situ hybridization with a mouse-specific Krox-20 probe, revealing the transfected cells. Electroporated side is on the left.
The mouse Krox-20 gene was placed under the control of the same regulatory elements. Unless otherwise indicated, two constructs, encoding either the wild-type protein or a carboxy-terminal Myc-tagged version, have been used equivalently during the course of this study. No differences were observed in terms of phenotypic consequences between these two constructs. Electroporation of the Krox-20-expressing constructs and detection of the protein with an antibody recognizing both the mouse and chick proteins revealed efficient ectopic expression, with patterns largely similar to the β-galactosidase patterns described above (Fig. 1A,B).
Krox-20 activates EphA4 in a restricted AP domain within the hindbrain
To investigate the consequences of Krox-20 ectopic expression on hindbrain segmentation and specification, we first analyzed the expression of EphA4, known to constitute a direct target of Krox-20 (Theil et al. 1998). On the electroporated side, EphA4 mRNA (Fig. 2) and protein (Fig. 1C,F,G) were found outside of the normal expression domain, which is restricted to r3 and r5 (Irving et al. 1996; Hirano et al. 1998). This ectopic pattern presented highly reproducible features: (1) EphA4 was always expressed at a level similar to that observed in r3 and r5. Consistently, no overexpression was observed in r3 and r5. (2) Cells expressing EphA4 ectopically were almost never isolated, but rather grouped in large patches. (3) EphA4 ectopic activation was strictly restricted rostrally to a limit likely corresponding to the r1/r2 boundary according to morphological criteria. (4) The efficiency of EphA4 activation, in terms of both frequency and size of the patches, generally followed a decreasing rostral to caudal gradient from r2 to r7/r8, with expression in this caudal region observed only in some of the embryos older than HH14+ (Fig. 2D,E,K).
Figure 2.
Time-course of the effects of Krox-20 ectopic expression on Hoxb1 and EphA4 expression. Flat-mounted hindbrains from embryos electroporated with Krox-20, incubated for the indicated period of time and hybridized in situ with Hoxb1 (purple) and EphA4 (red) probes. The developmental stages of the harvested embryos are indicated (bottomright of A–E, and I–K). (F–H,L–N) Higher magnifications of the embryos shown in C–E and I–K, respectively. At early stages, EphA4 expression fills all Hoxb1-negative patches in r4, but not in the caudal domain of Hoxb1 expression. Later, EphA4 is down-regulated in basal Hoxb1-negative patches in r4 (white arrowheads in H and M). Note the formation of a thin unstained boundary at the interface between adjacent rhombomeres as well as between EphA4-positive and Hoxb1-positive domains within r4 (black arrowheads in G and N). The apparent overlap in EphA4 and Hoxb1 labeling in some areas in M is due to cytoplasmic overlap of different cells. Electroporated side is on the left.
Krox-20 ectopic expression affects the molecular identity of even-numbered rhombomeres
Next, we examined the expression of genes expressed in even-numbered rhombomeres. Around stage HH14, follistatin is normally expressed in r2 and r6, and at a lower level in r4 and r5 (Graham and Lumsden 1996). Krox-20 loss-of-function mutation has been shown previously to lead to ectopic activation of follistatin in r3 in the mouse (Seitanidou et al. 1997). Consistently, Krox-20 electroporation led to a general and drastic downregulation of follistatin in even-numbered rhombomeres (Fig. 1D). We also observed a slight reduction of the low level of follistatin in r5.
Hoxb1, a major determinant of r4 identity (Studer et al. 1996), is expressed at high levels in r4 and at lower levels in r7/r8 and the spinal cord (Sundin and Eichele 1990) at the stages of our analyses. Following Krox-20 electroporation, Hoxb1 expression was dramatically altered, with the appearance of patches of negative cells within r4 and the caudal expression domain (Fig. 1E). Therefore, like in the case of follistatin, ectopic expression of Krox-20 leads to repression of Hoxb1 transcription.
Because, in even-numbered rhombomeres, Krox-20 up-regulates an odd-numbered rhombomere marker (EphA4) and down-regulates even-numbered rhombomere markers (follistatin and Hoxb1), it was essential to determine whether these effects involved the same or different cells. Therefore, we recorded the expression of EphA4 and Hoxb1 simultaneously by double in situ hybridization and performed a time course analysis. Typical results are shown in Figure 2 and can be summarized as follows: (1) at all stages examined, Hoxb1 and EphA4 expression domains remained exclusively in the entire hindbrain. (2) In r4, EphA4 and Hoxb1 expression domains were strictly complementary in all samples until stage HH14 (Fig. 2A,B). At later stages, the most ventral r4 Hoxb1-negative patches down-regulated EphA4, a phenomenon reminiscent of the normal EphA4 down-regulation in ventral r5 (Fig. 2H,M, white arrowheads). In addition, a thin zone negative for both markers appeared at the interface between the Hoxb1-positive r4 domains and r3, r5 or the EphA4-positive ectopic patches (Fig. 2G,N, black arrowheads). During normal development, such double negative zones were also observed after HH14–HH15 at rhombomere boundaries between r3, r4 and r5 (Fig. 2I–K, control side). (3) In contrast to r4, down-regulation of Hoxb1 in its caudal expression domain was rarely accompanied by activation of EphA4. More precisely, the caudal EphA4 up-regulation occurred only after stage HH14–HH15 and never completely filled the Hoxb1-negative domains, being restricted to a few cells in r7/r8 (Fig. 2E,K).
In conclusion, Krox-20 ectopic expression leads to the appearance of patches of EphA4-positive cells within even-numbered rhombomeres. These patches are Hoxb1-negative in r4 and, therefore, may present molecular characteristics of odd-numbered rhombomeres.
Krox-20 transforms even- into odd-numbered rhombomere identity
To analyze the molecular identity of the EphA4-positive patches more precisely, we studied the expression of additional regional markers. mafB/kr is normally expressed in r5 and r6 (Eichmann et al. 1997). We performed double in situ hybridizations to detect mafB/kr mRNA together with EphA4 mRNA. The mafB/kr pattern was not affected by Krox-20 ectopic expression (Fig. 3A). Therefore, the EphA4-positive patches in r2 and r4 do not express mafB/kr, whereas those in r6 still do. Consistently, double in situ hybridization with the mafB/kr and Hoxb1 probes showed that the Hoxb1-negative patches in r4 and r7 were also negative for mafB/kr (Fig. 3B).
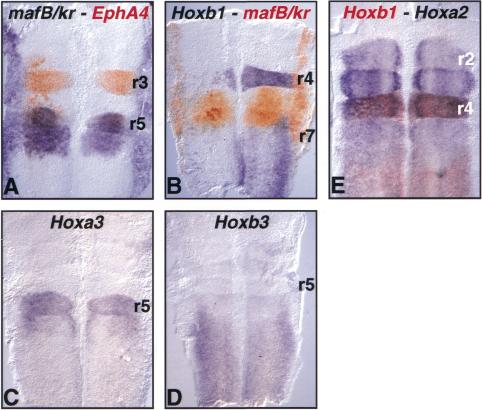
Figure 3.
Krox-20 misexpression does not affect mafB/kr, Hoxa2, Hoxa3, and Hoxb3 expression. Flat mounts of hindbrains of embryos electroporated with Krox-20, incubated for 24 h (18 h in A and B) and hybridized with the indicated probes. (A) EphA4-positive patches (red) in r2 and r4 do not express mafB/kr (purple) whereas those in r6 maintain this expression. (B) MafB/kr expression (red) is not affected by Krox-20 expression. In particular, the Hoxb1-negative domains in r4 and r7 do not activate mafB/kr. (C,D) Krox-20 ectopic expression does not lead to significant modifications in the patterns of Hoxa3 or Hoxb3 expression. (E) Hoxa2 expression (purple) is also maintained in r4 in the patches negative for Hoxb1 (red). Electroporated side is on the left.
In control embryos, Hoxa3 is expressed in r5/r6 and at a lower level in r7/r8, whereas Hoxb3 is expressed in r7/r8 and more caudal and at a much lower level in r5 and r6 (Grapin-Botton et al. 1995). These expression patterns were not significantly modified after electroporation with the Krox-20 expression construct (n=15 and 6 respectively, Fig. 3,C,D). Double in situ hybridization for Hoxb1 and Hoxa2, which is normally expressed from r2 to r7/r8 in the hindbrain, revealed that no modification in Hoxa2 expression was observed in electroporated embryos and, in particular, that the Hoxb1-negative patches were still positive for Hoxa2 (Fig. 3E).
In summary, our combined data indicate that, in r2 and r4, the EphA4-positive patches are positive for Hoxa2 and negative for Hoxb1, Hoxa3, Hoxb3, mafB/k, and possibly also follistatin. In r6, the EphA4-positive patches are positive for Hoxa2, Hoxa3, and mafB/kr and negative for Hoxb1. These expression patterns are consistent with odd-numbered rhombomere molecular identity.
The presence of patches of cells with r3- or r5-like identities in the vicinity of r3 or r5 may have resulted from two different processes: a change of identity of cells within even-numbered rhombomeres, or an inappropriate migration of odd-numbered rhombomere cells into adjacent even-numbered rhombomeres. To resolve this issue, electroporations were performed at stage HH10, after establishment of lineage restrictions between rhombomeres (Fraser et al. 1990; Birgbauer and Fraser 1994). Immediately afterward, the embryos were sectioned transversally within presumptive r2, and anterior and posterior parts were not allowed to reassociate to prevent any cell migration from r3 into the rostral hindbrain. EphA4 immunodetection was performed after 16 h of incubation, and most embryos that appeared to have been effectively sectioned within r2 showed ectopic expression in the most caudal part of the anterior half of the neural tube, while the entire r3 was clearly present in the posterior half as shown by the EphA4 pattern (Fig. 1G). These data indicate that, in r2, the formation of patches with r3-like identity involves a change of identity induced by Krox-20 ectopic expression.
Changes of identity require Krox-20 DNA-binding activity
To investigate whether the different consequences of Krox-20 ectopic expression were actually dependent on the capacity to bind DNA of the protein, we introduced a point mutation resulting in an arginine to tryptophan substitution in the third zinc finger of the DNA-binding domain (R409W). This mutation was identified in a human peripheral myelinopathy (Warner et al. 1998) and leads to a Schwann cell phenotype similar to that observed in Krox-20 null mice (Topilko et al. 1994). Subsequently, this mutation was shown to completely abolish Krox-20 DNA binding in vitro (Warner et al. 1999). Chick embryos were electroporated with an R409W mutant Krox-20 construct and analyzed for the expression of EphA4 (Fig. 1H), follistatin (Fig. 1I), and Hoxb1 (Fig. 1J). In contrast to the wild-type construct, no alterations in the expression patterns of these genes were observed with the mutant construct (n ≥ 5 for each of them), although the levels of ectopic Krox-20 mRNAs and proteins were similar (cf. Fig. 1I with Fig. 5, below, and data not shown). This demonstrates that the DNA-binding activity of Krox-20 is required for each of the observed phenotypic consequences of its ectopic expression.
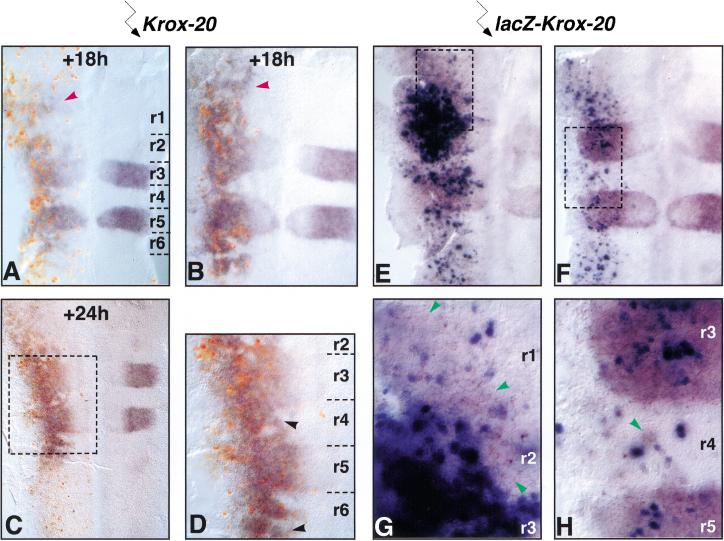
Figure 5.
Expression of exogenous Krox-20 leads to non-autonomous activation of endogenous Krox-20. Flat-mounted hindbrains from embryos electroporated with mouse Krox-20 (A–D) or a construct (pAdRSVβgalKrox20) directing co-transcription of LacZ and mouse Krox-20 (E–H). They were analyzed by in situ hybridization with chicken-specific (purple) and mouse-specific (red, AD) Krox-20 probes, and Bluo-Gal staining (dark blue, E–H). (A,B) HH13 embryos harvested 18 h after the electroporation. (Red arrowheads) Patches of endogenous Krox-20 expression in r1. (C) HH15 embryo harvested 24 h after the electroporation. (D) Higher magnification view of the embryo shown in C. While mouse Krox-20 is expressed in a punctuate manner along the neural tube, chicken Krox-20 is ectopically activated in cell patches restricted to the r1–r7 region. The extent of chicken Krox-20 expression appears to broaden at later stages, and positive patches are observed in absence of close mouse Krox-20-expressing cells (black arrowheads in D). (E,F) HH13+ embryos harvested 18 h after electroporation. (G,H) Higher magnification views of the embryos shown in E and F. Chicken Krox-20 is expressed in Bluo-Gal-negative cells (examples are indicated by green arrowheads). Electroporated side is on the left.
Non cell-autonomous consequences of Krox-20 exogenous expression
A striking and common characteristic of the alterations in gene expression (activation or repression) following ectopic expression of Krox-20 was their occurrence not in isolated cells but in patches. Because electroporation is expected to hit isolated cells (Fig. 1A), the existence of such patches may be explained by at least three nonexclusive mechanisms: (1) aggregation of transfected cells that may have acquired adhesion properties different from their neighbors; (2) proliferation of transfected cells in the absence of cell intermingling; (3) non cell-autonomous modifications of gene expression around the transfected cells. The presence of cell patches early after electroporation (Fig. 2A,B) argues rather in favor of the last possibility, at least during this early period.
To investigate the issue directly, we performed double labeling experiments to detect both the target genes and the exogenous Krox-20 mRNA, using an in situ hybridization probe derived from the 3′-UTR of the mouse Krox-20 mRNA, which did not cross-hybridize with chicken mRNA (Fig. 4A and data not shown). Double in situ hybridization with this probe and the EphA4 probe on Krox-20-electroporated embryos indicated that the mouse Krox-20 gene is expressed in isolated cells often within or bordering the EphA4-positive patches (Fig. 4A). Strikingly, the large majority of the cells expressing EphA4 ectopically were negative for mouse Krox-20 mRNA. We then performed double labeling of Hoxb1 by in situ hybridization and of the exogenous Krox-20 protein by immunodetection of the Myc tag epitope. In accordance with the previous result, exogenous Krox-20 was detected in isolated cells within or bordering the Hoxb1-negative domains (Fig. 4E). Again, most of the cells repressing Hoxb1 in r4 or r7 appeared negative for mouse Krox-20. Therefore, exogenous Krox-20 expression appears to enforce modifications in gene expression by a non cell-autonomous mechanism.
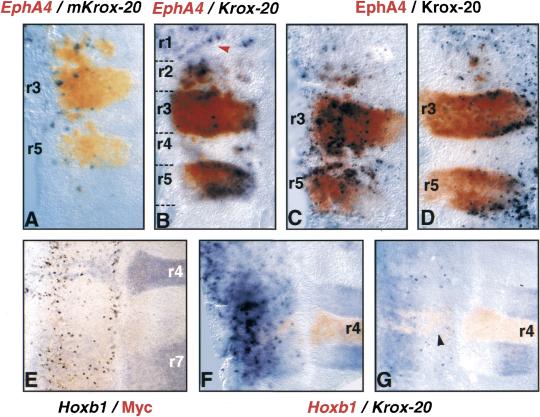
Figure 4.
Non cell-autonomous effects on EphA4 and Hoxb1 expression. Flat-mounted hindbrains from embryos electroporated with mouse Krox-20 and revealed by in situ hybridization (A,B,E–G) or immunochemistry (C–E) with the indicated probes or antibodies. (A) Double in situ hybridization performed with an EphA4 probe (red) and a mouse-specific Krox-20 probe (purple) which labels only electroporated cells. Note that the mouse Krox-20 probe labels isolated cells within the EphA4-positive patches. (B) Double in situ hybridization performed on an embryo harvested 24 h after electroporation with an EphA4 probe (red) and a Krox-20 probe (purple), which hybridizes with both the endogenous chicken and the exogenous mouse mRNAs. Note the complete overlap between both labelings (brown color, compare with red/orange staining in A), except in r1, where only Krox-20 is detected (red arrowhead). (C,D) Double immunochemistry performed on embryos harvested 14 h (C) and 20 h (D) following electroporation with antibodies directed against EphA4 (red) and Krox-20 (purple). The latter antibody recognizes both the chick and mouse Krox-20. Note again that all EphA4-positive territories express Krox-20. (E) Hoxb1 in situ hybridization (purple) combined with anti-Myc immunochemistry (brown) on an embryo electroporated with the Myc-tagged Krox-20. (F,G) Double in situ hybridization performed on embryos harvested at stages HH14− and HH14+ respectively with Hoxb1 (red) and Krox-20 (purple) probes. The Krox-20 probe recognizes both chick and mouse mRNAs. Note the presence of white patches (Hoxb1– and Krox-20-negative) within basal r4 (black arrowhead in G). Electroporated side is on the left.
These experiments raised the question of the mediator of the modifications in gene expression in cells that do not express the exogenous Krox-20 gene. A possibility was that these cells actually expressed the endogenous Krox-20 gene. To address this question, we first performed another series of double-labeling experiments in which we used an in situ hybridization probe or an antibody recognizing both chicken and mouse Krox-20 mRNA or protein. Detection of Krox-20 and EphA4 transcripts 24 h after Krox-20 electroporation indicated that the cells in the EphA4-positive patches in r2, r4, and r6 expressed Krox-20, the uniform brown staining in r3, r5, and the ectopic patches resulting from the superposition of orange/red EphA4 and purple Krox-20 stainings (Fig. 4, cf. B with A). Furthermore, this uniform color established that both EphA4 and Krox-20 are expressed at similar levels in r3/r5 and in the ectopic patches, apart from isolated cells expressing very high levels of Krox-20. These latter cells presumably correspond to those expressing the exogenous mouse Krox-20. As expected from previous experiments, in r1, cells were observed expressing Krox-20 ectopically but not EphA4 (Fig. 4B, red arrowhead), confirming that Krox-20 is not able to activate EphA4 rostrally to r2. Similar results were obtained when the embryos were incubated for only 14 h (Fig. 4C) or 20 h (Fig. 4D) after Krox-20 electroporation. In conclusion, these experiments indicate that the cells expressing EphA4 ectopically also express Krox-20, even at early stages of activation, and, for most of them, this expression is likely to result from activation of the endogenous chicken gene.
Consistent with the above data, double in situ hybridization for Krox-20 and Hoxb1 demonstrated that Krox-20 transcripts are present presumably in all cells within Hoxb1-negative patches in r4 when the embryos are collected before stage HH14− (Fig. 4F). Similar to what was observed in the case of EphA4 ectopic expression (Fig. 2H,M), after stage HH14 the Hoxb1-negative patches in ventral r4 also appeared mostly negative for Krox-20 expression (Fig. 4G, black arrowhead), paralleling the down-regulation of endogenous Krox-20 in basal r3 and r5 (Fig. 4G).
In conclusion, these data suggest that EphA4 up-regulation in r2, r4, and r6 and Hoxb1 repression in r4 require the presence of Krox-20 in a cell-autonomous manner, as the expression of the latter is always observed, at least transiently, in the patches. In contrast, they indicate that ectopic expression of exogenous Krox-20 results in non cell-autonomous activation of the endogenous Krox-20 gene.
Spatially and temporally restricted, non cell-autonomous Krox-20 autoactivation
To investigate the autoregulation of Krox-20, a chicken-specific probe was required. For this purpose, we screened a chicken genomic BAC library with a Krox-20 DNA-binding domain cDNA probe (Nieto et al. 1991) and isolated the entire gene. The nucleotide sequence was established (GeneBank accession no. AF291747), and the inferred protein amino acid sequence was shown to present a 65% similarity to the mouse Krox-20 sequence (data not shown). The chicken Krox-20 gene was used to derive a 3′-UTR probe presenting no sequence similarity to the corresponding region of the mouse gene, thus specifically recognizing the chicken mRNA (Fig. 5 and data not shown).
To establish the existence of non cell-autonomous activation of Krox-20, we performed double in situ hybridization experiments with mouse- and chicken-specific Krox-20 probes on Krox-20-electroporated embryos. As expected from previous experiments, mouse Krox-20 labeling was restricted to isolated cells evenly spread along the neural tube, whereas chicken Krox-20 was expressed at a uniform level in r3/r5 and in large cell patches outside of these rhombomeres (Fig. 5A–D). These patches were often bordered by or contained mouse Krox-20-expressing cells. These data strongly suggest that expression of exogenous Krox-20 in isolated cells leads to activation of the endogenous gene in surrounding, nonelectroporated cells.
To exclude the possibility that these surrounding cells may have also received the plasmid, but subsequently lost it or failed to express the gene at a detectable level, we performed an additional experiment. A bicistronic expression vector containing the LacZ gene (with a nuclear localization signal) downstream of the RSV promoter, followed by an internal ribosome entry site (IRES) and the Krox-20 gene, was electroporated into the chick hindbrain. β-Galactosidase activity was revealed by Bluo-Gal staining and endogenous chicken Krox-20 mRNA by in situ hybridization. In these conditions, we observed, within even-numbered rhombomeres, Bluo-Gal-positive cells surrounded by Bluo-Gal-negative, Krox-20-positive patches (Fig. 5E–H, green arrowheads). In the hindbrain neuroepithelium, the β-galactosidase protein shows a half-life exceeding 12 h (M. Frain and P. Charnay, unpubl.), and the detection of its activity is very sensitive. In addition, our construct ensures that the first cistron (LacZ) is expressed at a higher level than the second one (Krox-20; Mizuguchi et al. 2000). Therefore, essentially all cells having expressed exogenous Krox-20, even at low levels, should be detected by Bluo-Gal staining. We conclude that ectopic activation of endogenous Krox-20 expression can occur in cells that never expressed the exogenous gene, establishing the non cell-autonomous character of Krox-20 ectopic activation.
Interestingly, the distribution of the cell patches expressing endogenous Krox-20 was different from that observed for the EphA4-positive patches (cf Fig. 5A,B with Figs. 1, 3, and 4). While ectopic expression of EphA4 was restricted to the region caudal to the r1/r2 boundary, induction of endogenous Krox-20 was observed up to the mesencephalic/metencephalic boundary at least, spreading largely within r1 (Fig. 5A,B, red arrowheads). Endogenous Krox-20 expression was restricted caudally, in a way similar to ectopic EphA4, with a low level expression observed caudally to r6 only in embryos older than HH14–HH15, whereas mouse Krox-20-positive cells were observed much more posteriorly (Fig. 5C). These data indicate that the capacity of autoactivation of Krox-20 through the non cell-autonomous mechanism is approximately restricted to the hindbrain territory.
Comparison of endogenous Krox-20 patterns between embryos allowed to develop for 18, 24, or 32 h after electroporation suggested an extension of the domains of expression with time, while the number of exogenous Krox-20-expressing cells decreased (Fig. 5A–D and data not shown). After 24 or 32 h (Fig. 5C,D, arrowheads, and data not shown), ectopic patches of endogenous expression were often detected in the absence of mouse Krox-20-positive cells in their vicinity. These data suggest that some of the electroporated cells die, while endogenous expression is maintained within the patches and possibly extends to previously negative territories. Cell aggregation might also contribute to the increase in size of the Krox-20-positive patches.
Krox-20 misexpression affects early neurogenesis
Finally, we asked whether the molecular alterations of rhombomeric identity due to Krox-20 ectopic expression were followed by long-term cellular manifestations. In the hindbrain, neurogenesis is regulated in a segment-specific manner. In particular, between HH12 and HH15, r3 and r5 display a marked delay, compared with even-numbered r2, r4, and r6, in the timing of neuronal differentiation and axonal growth. Therefore, we performed an analysis of neurofilament expression, a marker of differentiated neurons, on embryos harvested at around stages HH13–HH15, 24 h after Krox-20 electroporation. At these stages, the most differentiated neurons are located in the basal plate of even-numbered rhombomeres and extend their axons in the descending medial longitudinal fasciculus (mlf). Other populations, such as postmitotic dorsal interneurons or basal columnar motor and sensory efferent neurons fated to project toward the dorsolateral cranial nerve exit points, are still largely restricted to even-numbered rhombomeres. In electroporated embryos, neurogenesis was found to be severely delayed and impaired on the experimental side, although not completely prevented. Most noticeable was a significant reduction in the number of neurons in even-numbered, and to a lower extent in odd-numbered rhombomeres (Fig. 6A,B). Co-labeling with an anti-EphA4 antibody demonstrated that the severity of this phenotype correlated with the extent of EphA4 induction and showed a reduced density of neuronal cell bodies in EphA4-expressing territories (Fig. 6C,D). Axons growing from cell bodies located outside of the EphA4-positive patches avoided those, to stay within the EphA4-negative remnants of even-numbered rhombomeres (Fig. 6D, arrowheads). Finally, the few neurons located in the EphA4-positive patches appeared impaired in their ability to develop processes (Fig. 6D, cf. EphA4-positive patches with r3/r5 on the control side). These data indicate that the modifications of molecular identity are correlated with defects in neurogenesis timing and axonal progression. They also suggest that the delayed neurogenesis in odd-numbered rhombomeres is a consequence of Krox-20 expression.
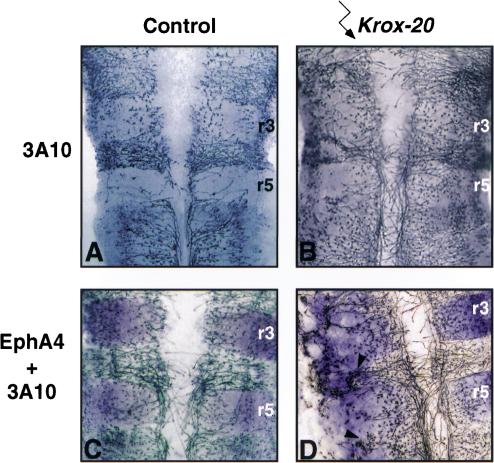
Figure 6.
Early neurogenesis is affected by ectopic Krox-20 expression. Flat-mounted hindbrains from control (A,C) and Krox-20 electroporated (B,D) embryos. (A,B) Immunochemistry with an antibody directed against neurofilaments (3A10), which reveals cell bodies and growing axons of differentiated neurons. The control embryo in A was electroporated with a mutant Krox-20 allele (R409W). In B, neurogenesis is impaired and delayed on the electroporated side. (C,D) Double labeling with antibodies directed against neurofilaments and EphA4. The embryo in C was not electroporated. In the Krox-20 electroporated case (D), the density of neurofilament staining is lower in EphA4-positive patches in r4 and r6 and in contrast reinforced in their vicinity (arrowheads). Electroporated side is on the left.
Discussion
In this study, we have used gain-of-function experiments to establish the role of Krox-20 in the specification of odd-numbered rhombomere identity in the hindbrain. In addition, we have discovered that Krox-20 is able to promote its own expression non cell-autonomously. We propose a model for hindbrain development in which this mechanism is involved in the homogenization and possibly expansion of odd-numbered rhombomeres.
Krox-20 is sufficient to enforce odd-numbered rhombomere identity in the r2-r6 region in a cell-autonomous manner
Krox-20 misexpression results in marked modifications of patterns of gene expression in the hindbrain. We argue that these patterns primarily reflect changes in cell identity rather than inappropriate cell migrations. Indeed we have shown that the presence of EphA4-positive patches within r2 cannot be due solely to migration of cells from r3. Our data, however, do not exclude a contribution of cell migration to the formation of the ectopic patches.
Thus, ectopic expression of Krox-20 in the r2–r6 region is sufficient in even-numbered rhombomeres to convert the gene expression pattern into that of an odd-numbered rhombomere. More precisely, in r2 and r4, the affected cells express Krox-20, EphA4, and Hoxa2 and are negative for Hoxb1, Hoxa3, Hoxb3, mafB/kr, and follistatin, consistent with an r3-like identity. In r6, the converted cells express Krox-20, EphA4, mafB/kr, Hoxa2, and Hoxa3, and are negative for Hoxb1, consistent with an r5-like identity. Moreover, analysis of neurogenesis in the EphA4-positive patches in r2, r4 and r6 indicates that it is delayed, a characteristics of odd-numbered rhombomeres. Together, these data indicate that Krox-20 can enforce odd-numbered identity in the r2–r6 region. The occurrence of r5 characteristics in r6 patches is likely to result from the presence of the product of the mafB/kr gene which, together with Krox-20, may specify r5 identity (Manzanares et al. 1999; M. Manzanares, unpubl.). Finally, in r1, ectopic expression of Krox-20 also leads to the formation of Krox-20-positive cell patches, whereas none of the tested Krox-20 targets is activated.
Krox-20 has been previously implicated in the direct transcriptional activation of several rhombomere-specific genes (Hoxb2, Hoxa2, Hoxb3, and EphA4; Sham et al. 1993; Nonchev et al. 1996; Theil et al. 1998; Manzanares et al. unpubl.). The present study is consistent with these data, and, together, they suggest that the specification of odd-numbered rhombomere identity by Krox-20 is a cell-autonomous phenomenon. Our analysis also revealed that Krox-20 can repress Hoxb1. This observation was not expected as the inactivation of Krox-20 does not lead to an extension of r4 (Schneider-Maunoury et al. 1993). In addition, it has been proposed that Hoxb1 and Hoxa1 repress Krox-20 (Barrow et al. 2000). Together, these facts suggest that expressions of Krox-20 and Hoxb1 are mutually exclusive, presumably preventing the appearance of cells with an aberrant molecular identity.
Non cell-autonomous Krox-20 autoregulation
This work has also provided evidence for non cell-autonomous autoactivation of Krox-20 expression. This conclusion is based on the observation that, following electroporation of the mouse gene, mouse Krox-20 mRNA is restricted to isolated cells, whereas the endogenous gene appears expressed in large cell patches that can be observed from r1 to r7/r8. Two other mechanisms could have been invoked to explain this observation: cell migration from odd-numbered rhombomeres and loss of exogenous Krox-20 expression in transfected cells or their progeny. We have already provided arguments against the involvement of cell migration in the early formation of the EphA4-positive patches, which are also Krox-20-positive. The observation of abundant endogenous Krox-20 expression in r1 within 14 h after electroporation is also inconsistent with this possibility. Loss of exogenous Krox-20 expression is also not supported by our data. Indeed, double labeling experiments after co-transcription of lacZ and Krox-20 in electroporated cells indicate that the cells expressing only the endogenous Krox-20 gene have not previously expressed the exogenous gene.
As indicated, the formation of the Krox-20-positive patches is restricted to the hindbrain, whereas ectopic expression of the mouse gene can be observed beyond these AP limits. This observation can be explained by the existence of positively acting factors restricted to the r1–r7/r8 region or negatively acting factors present outside of the hindbrain and regulating signaling by Krox-20-positive cells or responsiveness to the signal by Krox-20-negative cells. Caudally, the limits of action of exogenous Krox-20 appear to correlate in terms of induction of both EphA4 and endogenous Krox-20. In contrast, rostrally, the domain of induction of EphA4 is more restricted than that of endogenous Krox-20, as EphA4 is never expressed in r1. The same is true for Hoxa2, another direct target of Krox-20 (Fig. 3E). These data suggest that EphA4 or Hoxa2 activation by Krox-20 requires additional factor(s) absent in the part of the CNS rostral to r2 or is subject to repression by factor(s) specifically present in this region.
A model for odd-numbered rhombomere formation
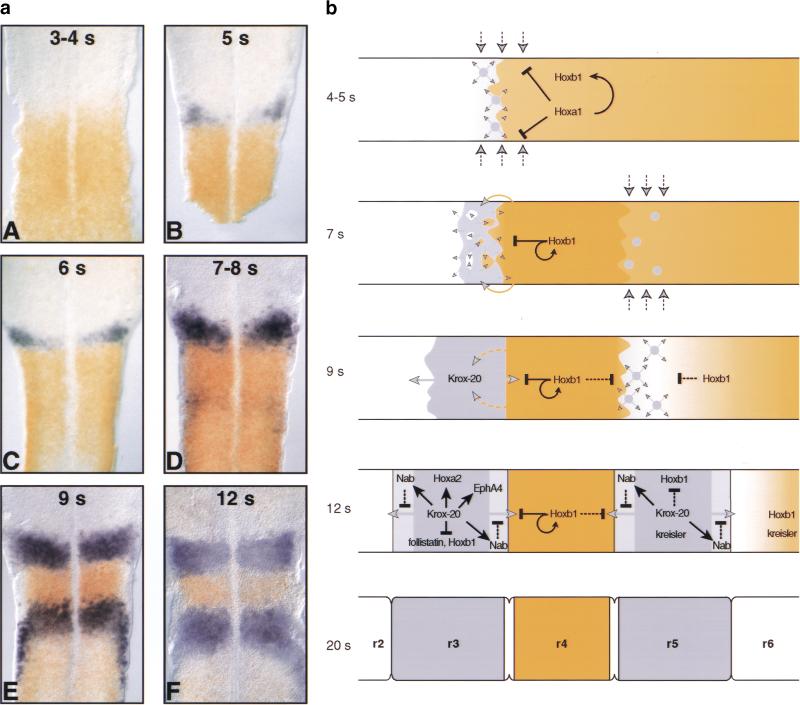
The discovery of the non cell-autonomous autoinduction of Krox-20 raises the issue of the functional significance of this phenomenon during hindbrain development. We propose that it plays an essential role in the establishment and homogenization of r3 and r5 and present a model for hindbrain patterning in the chick, integrating expression and genetic data derived from the mouse (Fig. 7b). This model accounts for the evolution of the Krox-20 and Hoxb1 expression patterns (Fig. 7a) and is depicted in the following steps.
Figure 7.
A model for chick hindbrain patterning. (a) Time-course of Krox-20 and Hoxb1 expression in the developing chick hindbrain. (A–F) Flat mounts of embryos at the indicated somite stages of development, subjected to double in situ hybridization with chicken Krox-20 (purple) and Hoxb1 (red) probes. (b) Schematic representation of the development of Krox-20 and Hoxb1 expression and of putative genetic interactions. For a detailed description, see Discussion. Hoxa1/Hoxb1- and Krox-20-expressing territories are represented in orange and purple-grey, respectively. (Light purple-grey) High level expression of Nab1 and Nab2 within the Krox-20-positive territories. (Black arrows and bars) Established (solid stem) or putative (dashed stem) cell-autonomous induction and repression/inhibition, respectively. (Purple-grey arrowheads) Different putative signals involved in Krox-20 activation and originating from unidentified tissues (dashed stem), Krox-20-expressing cells (purple-grey stem) and prospective r4 (orange stem). Somite stages are indicated on the left.
(1) Early after the onset of somitogenesis, Hoxa1 and Hoxb1 expression extends within the unsegmented neural plate up to an anterior limit corresponding to the prospective r3/r4 boundary (Fig. 7A; Murphy and Hill 1991; Barrow et al. 2000). At around the 4- to 5-somite stages, Krox-20 is induced in a narrow stripe of cells immediately rostral to the Hoxa1 domain (Fig. 7B,b; Schneider-Maunoury et al. 1993; Irving et al. 1996; Barrow et al. 2000). The signals responsible for this activation have not been identified, although FGFs may be involved (Marin and Charnay 2000). Hoxa1 and Hoxb1 have been proposed to repress Krox-20 expression in a cell-autonomous manner (Barrow et al. 2000) and therefore, may antagonize appearance of Krox-20-positive cells within their expression domain.
(2) At around the 7-somite stage, a second, more caudal band of sparse cells activates Krox-20 expression (Fig. 7C,D,b. Schneider-Maunoury et al. 1993). Meanwhile, in prospective r3, Krox-20-expressing cells activate the expression of other r3-specific genes and repress Hoxb1 and follistatin expression presumably in a cell-autonomous manner. In addition, Krox-20-expressing cells induce neighboring cells to activate Krox-20 expression in a non cell-autonomous manner. This recruitment leads to homogenization and possibly extension of r3. This step is in accordance with the analysis of LacZ expression in Krox-20LacZ/LacZ knock-in mouse mutant embryos, where β-galactosidase-positive cells appear scattered as compared with heterozygous embryos (Schneider-Maunoury et al. 1993). In addition, we have demonstrated previously that in homozygous Hoxa1 mutant embryos, cell patches with r2 identity are observed within r3, and this phenotype is markedly enhanced when the embryos are also heterozygous for the Krox-20LacZ mutation (Helmbacher et al. 1998). We now propose that this genetic interaction involves the promotion, by a signal derived from prospective r4, of the induction of Krox-20 expression in neighboring cells by Krox-20-positive cells. Therefore, signaling from Krox-20- and Hoxa1/Hoxb1-expressing cells synergizes to enforce recruitment of additional r3 cells. This mechanism may ensure, on the one hand, that the territory immediately rostral to r4 expresses Krox-20 and, on the other hand, that the expansion of r3 is restricted anteriorly because of limited diffusion of the r4-derived signal.
(3) At around the 9-somite stage, r3 has largely increased in size and is becoming a homogeneous, well-defined, Krox-20-positive territory, presumably due to the combination of recruitment of adjacent cells with segregation from even-numbered cells governed by Eph receptors–ephrins interactions, themselves controlled by Krox-20 (Fig. 7a, E and 7b; Mellitzer et al. 1999; Xu et al. 1999). In prospective r5, levels of Hoxa1 and Hoxb1 have decreased, which may allow efficient recruitment of additional Krox-20-positive cells by Krox-20-expressing cells and progressive homogenization of the rhombomere.
(4) By the 12-somite stage, Krox-20 has activated the expression of Nab1 and Nab2 (Mechta-Grigoriou et al. 2000). These genes encode specific antagonists of Krox-20 activity and their expression is reinforced at the vicinity of rhombomere interfaces (MechtaGrigoriou et al. 2000). We propose that this reinforcement may also participate in preventing further expansion of r3 and r5.
(5) After the 20-somite stage, the antagonistic action of the Nab proteins on Krox-20 may also be involved in enforcing cells located at the level of the r3/r4 and r4/r5 interfaces to adopt specific fates, leading to the formation of Krox-20- and Hoxb1-negative boundaries.
In conclusion, our work suggests that Krox-20 is involved in multiples steps in hindbrain patterning, both in proper segmentation and in acquisition of AP identity. The ground-state fate of the prospective r2–r6 region may be to acquire even-numbered rhombomere identity. Activation of Krox-20 in a few cells leads to the formation, homogenization, extension, and later stabilization of territories, which segregate, from even-numbered domains. In parallel, these prospective odd-numbered rhombomeres also acquire their proper identity under the control of Krox-20. Therefore, segmentation and identity specification are largely intertwined in the developing vertebrate hindbrain.
Materials and methods
Plasmid constructs
The Krox-20 expression plasmid, pAdRSVKrox20, was constructed by cloning of a 5-kb AvrII–SpeI genomic fragment (Chavrier et al. 1989) containing the gene plus 1.2 kb of 3′ flanking sequences, into the pAdRSVβgal plasmid (Le Gal La Salle et al. 1993), after removal of the β-galactosidase coding sequence. pAdRSVKrox20Myc differs from pAdRSVKrox20 by the insertion of a Myc epitope coding sequence (5′-GAACAGAAACT TATCTCAGAGGAAGACCTT-3′) just before the Krox-20 STOP codon. The two constructs are expressed at similar levels and equivalently activate transcription as assayed in transiently transfected cells according to Vesque and Charnay (1992; data not shown). pAdRSVKrox20R409W was constructed by directed mutagenesis with the oligonucleotide 5′-GGCCGCAAGTTT GCCTGGAGTGACGAAAGG-3′ using the Transformer Site-Directed Mutagenesis Kit (Clontech). pAdRSVβgalKrox20 was constructed by cloning of the Krox-20 gene downstream of the encephalomyocarditis virus IRES (Ghattas et al. 1991) followed by insertion of the resulting fragment downstream of the LacZ gene in pAdRSVβgal. For all constructs, cloning junctions were checked by sequencing, and the molecular weight of the encoded proteins was verified by immunoblots performed on extracts of transiently transfected 293 cells.
In ovo electroporation
Commercial fertilized hens eggs were incubated typically for 30 h, up to stages HH8–HH10, before injection. DNA was resuspended at a concentration of 1 μg/μL in 10 mM Tris (pH 8) solution, and 0.025% Fast-Green (Sigma) was added. The DNA solution was injected into the embryo neural tube by use of a stretched glass capillary, anteriorward from the level of approximately the third somite. A drop of L15 medium (Life Technologies) was poured onto the egg membrane and electroporation was performed with a BTX820 electroporator (Quantum) and CUY611 platinum-coated electrodes (Tr Tech) as described (Itasaki et al. 1999), using the following parameters: four pulses of 25 V and 50 ms at a frequency of 1 Hz. Embryos were harvested in phosphate buffered saline (PBS) and fixed in paraformaldehyde (PFA, 4% in PBS) for 2–3 h or 8–24 h for whole-mount immunochemistry or in situ hybridization respectively, then dehydrated in methanol. For detection of β-galactosidase activity, embryos were fixed in PFA for 45 min and stained with X-Gal as described (Schneider-Maunoury et al. 1993), or with Bluo-Gal (Sigma) following manufacturer conditions. When subsequent in situ hybridization was required, staining was performed in 0.2% PFA, and the embryos were post-fixed overnight in 4% PFA. Unless otherwise indicated, the embryos were electroporated between stages HH8 and HH10 and collected 24 h later. In the type of experiment presented in Figure 1G, embryos were electroporated at stage HH10 and immediately cut transversally at the presumed level of r2 (according to morphological landmarks), using a tungsten needle. The resulting anterior part was then slightly rotated to avoid subsequent healing of the two parts of the neural tube.
Whole-mount immunohistochemistry
Immunochemical detection of proteins was performed on dissected neural tubes with the following primary antibodies and dilutions: rabbit polyclonal antibodies directed against Krox-20 (BAbCO, 1:500) or EphA4 (Becker et al. 1995; 1:20,000), and mouse monoclonal antibodies directed against EphA4 (Hirano et al. 1998; 1:20), chick neurofilaments, (DSHB 3A10, 1:50) or the Myc epitope (9E10, 1:5). The following secondary antibodies were used at a 1:200 dilution: peroxidase-coupled goat antibody directed against mouse IgG (Sigma), alkaline phosphatase-coupled goat antibody directed against mouse IgG (Vector), biotinylated donkey antibody directed against rabbit IgG (Amersham), or biotinylated hamster antibody directed against mouse IgG (Vector). The biotinylated antibodies were detected using streptavidin-horse radish peroxidase (Amersham, 1:500). Peroxidase activity was revealed with diaminobenzidine (Sigma; brown staining). Occasionally, the staining was enhanced with nickel ammonium (dark blue staining) according to Adams (1981). Alkaline phosphatase activity was detected using 4-nitroblue tetrazolium chloride/5-bromo 4-chloro 3-indolyl phosphate (NBT/BCIP, Roche; purple staining). In some cases, 2-(4-) 5-(4-nitrophenyl) 3-phenyltetrazolium chloride (INT/BCIP, Roche; orange/red staining) was used instead.
Whole-mount in situ hybridization
In situ hybridization was performed essentially as described (Wilkinson and Nieto 1993), using digoxigenin-labeled riboprobes. For double hybridization, one of the probe was labeled with fluorescein-UTP. Digoxigenin and fluorescein were then detected sequentially using alkaline phosphatase-coupled antibodies (Roche, 1:2000). NBT/BCIP (purple) staining was always carried out first, and the antibody was stripped in 0.1 M glycine-HCl (pH 2.2). The embryo was then incubated with the second antibody and stained with INT/BCIP (orange/red). The riboprobes were as follows: cHoxb1 (Guthrie et al. 1992), mKrox-20 (Wilkinson et al. 1989), Krox-20 zinc finger coding region (Nieto et al. 1991), cEphA4 (Sajjadi and Pasquale 1993), cfollistatin (Graham and Lumsden 1996), cHoxa3, cHoxb3 (Grapin-Botton et al. 1995), cHoxa2 (Prince and Lumsden 1994). mKrox-20–3′ is a 500-bp NheI–NsiI fragment derived from the 3′- UTR of mouse Krox-20 (Chavrier et al. 1988) and presenting no similarity to the chicken gene. cKrox-20–3′ is a 700-bp StuI–PstI fragment derived from the 3′-UTR of chicken Krox-20 and presenting no similarity to the mouse gene (this work).
Isolation of the chicken Krox-20 gene
A chicken genomic BAC library (Crooijmans et al. 2000) provided by the UK HGMP Resource Centre as high-density gridded filters was screened with a 150-bp cDNA probe, presumably of chick origin, encoding part of the Krox-20 DNA-binding domain (Nieto et al. 1991). Three strongly hybridizing clones were identified, and a common 10-kb HindIII fragment containing the entire Krox-20 gene was subcloned into BlueScript (Stratagene) and used for restriction mapping and nucleotide sequencing. This analysis revealed that the original cDNA probe used for BAC screening was only 89% identical to the corresponding sequence of the chicken gene, while it showed 98% identity to the Xenopus gene. Therefore, this sequence is more likely to be of Xenopus origin.
Acknowledgments
We thank J. Ghislain, F. Mechta, S. Schneider-Maunoury, C. Vesque, and M. Wassef for critical reading of the manuscript. We are grateful to M. Perricaudet for providing us with the pAdRSVβgal expression construct, A. Grappin-Botton, R. Krumlauf, A. Lumsden, V. Prince, M. Sieweke, and D. Wilkinson for the kind gift of probes, H. Tanaka and the Developmental Studies Hybridoma Bank (University of Iowa) for antibodies. F.G. and E.T. were supported by fellowships from MENRT and LNCC respectively. This work was supported by grants from INSERM, MENRT, EC, ARC and AFM.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL charnay@wotan.ens.fr; FAX 33 1 44 32 39 88.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.189801 .
References
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Stadler HS, Capecchi MR. Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development. 2000;127:933–944. doi: 10.1242/dev.127.5.933. [DOI] [PubMed] [Google Scholar]
- Becker N, Gilardi-Hebenstreit P, Seitanidou T, Wilkinson D, Charnay P. Characterisation of the Sek-1 receptor tyrosine kinase. FEBS Lett. 1995;368:353–357. doi: 10.1016/0014-5793(95)00652-p. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Fraser SE. Violation of cell lineage restriction compartments in the chick hindbrain. Development. 1994;120:1347–1356. doi: 10.1242/dev.120.6.1347. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Patterning of the vertebrate neural crest. Perspect Dev Neurobiol. 1995;3:53–62. [PubMed] [Google Scholar]
- Chavrier P, Zerial M, Lemaire P, Almendral J, Bravo R, Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988;7:29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Janssen-Timmen U, Mattei MG, Zerial M, Bravo R, Charnay P. Structure, chromosome location, and expression of the mouse zinc finger gene Krox-20: Multiple gene products and coregulation with the proto-oncogene c-fos. Mol Cell Biol . 1989;9:787–797. doi: 10.1128/mcb.9.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooijmans RP, Vrebalov J, Dijkhof RJ, van der Poel JJ, Groenen MA. Two-dimensional screening of the Wageningen chicken BAC library. Mamm Genome. 2000;11:360–363. doi: 10.1007/s003350010068. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Grapin-Botton A, Kelly L, Graf T, Le Douarin N M, Sieweke M. The expression pattern of the mafB/kr gene in birds and mice reveals that the kreisler phenotype does not represent a null mutant. Mech Dev. 1997;65:111–122. doi: 10.1016/s0925-4773(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Ghattas IR, Sanes JR, Majors JE. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991;11:5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi-Hebenstreit P, Nieto MA, Frain M, Mattei MG, Chestier A, Wilkinson DG, Charnay P. An Eph-related receptor protein tyrosine kinase gene segmentally expressed in the developing mouse hindbrain. Oncogene. 1992;7:2499–2506. [PubMed] [Google Scholar]
- Graham A, Lumsden A. Interactions between rhombomeres modulate Krox-20 and follistatin expression in the chick embryo hindbrain. Development. 1996;122:473–480. doi: 10.1242/dev.122.2.473. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Bonnin MA, McNaughton LA, Krumlauf R, Le Douarin NM. Plasticity of transposed rhombomeres: Hox gene induction is correlated with phenotypic modifications. Development. 1995;121:2707–2721. doi: 10.1242/dev.121.9.2707. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Lumsden A. Formation and regeneration of rhombomere boundaries in the developing chick hindbrain. Development. 1991;112:221–229. doi: 10.1242/dev.112.1.221. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Muchamore I, Kuroiwa A, Marshall H, Krumlauf R, Lumsden A. Neuroectodermal autonomy of Hox-2.9 expression revealed by rhombomere transpositions. Nature. 1992;356:157–159. doi: 10.1038/356157a0. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Prince V, Lumsden A. Selective dispersal of avian rhombomere cells in orthotopic and heterotopic grafts. Development. 1993;118:527–538. doi: 10.1242/dev.118.2.527. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Helmbacher F, Pujades C, Desmarquet C, Frain M, Rijli FM, Chambon P, Charnay P. Hoxa1 and krox-20 synergize to control the development of rhombomere 3. Development. 1998;125:4739–4748. doi: 10.1242/dev.125.23.4739. [DOI] [PubMed] [Google Scholar]
- Hirano S, Tanaka H, Ohta K, Norita M, Hoshino K, Meguro R, Kase M. Normal ontogenic observations on the expression of Eph receptor tyrosine kinase, Cek8, in chick embryos. Anat Embryol. 1998;197:187–197. doi: 10.1007/s004290050130. [DOI] [PubMed] [Google Scholar]
- Irving C, Nieto MA, DasGupta R, Charnay P, Wilkinson DG. Progressive spatial restriction of Sek-1 and Krox-20 gene expression during hindbrain segmentation. Dev Biol. 1996;173:26–38. doi: 10.1006/dbio.1996.0004. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, Krumlauf R. ‘Shocking’ developments in chick embryology: Electroporation and in ovo gene expression. Nat Cell Biol. 1999;1:E203–E207. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Robert JJ, Berrard S, Ridoux V, Stratford-Perricaudet LD, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990;13:329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Manzanares M, Trainor PA, Nonchev S, Ariza-McNaughton L, Brodie J, Gould A, Marshall H, Morrison A, Kwan CT, Sham MH, Wilkinson DG, Krumlauf R. The role of kreisler in segmentation during hindbrain development. Dev Biol. 1999;211:220–237. doi: 10.1006/dbio.1999.9318. [DOI] [PubMed] [Google Scholar]
- Marin F, Charnay P. Hindbrain patterning: FGFs regulate krox20 and mafB/kr expression in the otic/preotic region. Development. 2000;127:4925–4935. doi: 10.1242/dev.127.22.4925. [DOI] [PubMed] [Google Scholar]
- Mechta-Grigoriou F, Garel S, Charnay P. Nab proteins mediate a negative feedback loop controlling Krox-20 activity in the developing hindbrain. Development. 2000;127:119–128. doi: 10.1242/dev.127.1.119. [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- ————— Control of cell behaviour by signalling through Eph receptors and ephrins. Curr Opin Neurobiol. 2000;10:400–408. doi: 10.1016/s0959-4388(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther. 2000;1:376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- Murphy P, Hill RE. Expression of the mouse labial-like homeobox-containing genes, Hox 2.9 and Hox 1.6, during segmentation of the hindbrain. Development. 1991;111:61–74. doi: 10.1242/dev.111.1.61. [DOI] [PubMed] [Google Scholar]
- Nieto, M.A., Bradley, L.C., and Wilkinson, D.G. 1991. Conserved segmental expression of Krox-20 in the vertebrate hindbrain and its relationship to lineage restriction. Development (Suppl.) 59–62. [PubMed]
- Nonchev S, Vesque C, Maconochie M, Seitanidou T, Ariza-McNaughton L, Frain M, Marshall H, Sham MH, Krumlauf R, Charnay P. Segmental expression of Hoxa-2 in the hindbrain is directly regulated by Krox-20. Development. 1996;122:543–554. doi: 10.1242/dev.122.2.543. [DOI] [PubMed] [Google Scholar]
- Prince V, Lumsden A. Hoxa-2 expression in normal and transposed rhombomeres: Independent regulation in the neural tube and neural crest. Development. 1994;120:911–923. doi: 10.1242/dev.120.4.911. [DOI] [PubMed] [Google Scholar]
- Sajjadi FG, Pasquale EB. Five novel avian Eph-related tyrosine kinases are differentially expressed. Oncogene. 1993;8:1807–1813. [PubMed] [Google Scholar]
- Schneider-Maunoury S, Topilko P, Seitandou T, Levi G, Cohen-Tannoudji M, Pournin S, Babinet C, Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993;75:1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S, Gilardi-Hebenstreit P, Charnay P. How to build a vertebrate hindbrain. Lessons from genetics. CR Acad Sci Iii. 1998;321:819–834. doi: 10.1016/s0764-4469(99)80022-5. [DOI] [PubMed] [Google Scholar]
- Seitanidou T, Schneider-Maunoury S, Desmarquet C, Wilkinson DG, Charnay P. Krox-20 is a key regulator of rhombomere-specific gene expression in the developing hindbrain. Mech Dev. 1997;65:31–42. doi: 10.1016/s0925-4773(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Sham MH, Vesque C, Nonchev S, Marshall H, Frain M, Gupta RD, Whiting J, Wilkinson D, Charnay P, Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993;72:183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Sundin OH, Eichele G. A homeodomain protein reveals the metameric nature of the developing chick hindbrain. Genes Dev. 1990;4:1267–1276. doi: 10.1101/gad.4.8.1267. [DOI] [PubMed] [Google Scholar]
- Theil T, Frain M, Gilardi-Hebenstreit P, Flenniken A, Charnay P, Wilkinson DG. Segmental expression of the EphA4 (Sek-1) receptor tyrosine kinase in the hindbrain is under direct transcriptional control of Krox-20. Development. 1998;125:443–452. doi: 10.1242/dev.125.3.443. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Vaage S. The segmentation of the primitive neural tube in chick embryos (Gallus domesticus). A morphological, histochemical and autoradiographical investigation. Ergeb Anat Entwicklungsgesch. 1969;41:3–87. [PubMed] [Google Scholar]
- Vesque C, Charnay P. Mapping functional regions of the segment-specific transcription factor Krox-20. Nucleic Acids Res. 1992;20:2485–2492. doi: 10.1093/nar/20.10.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen L, Koob KG, Lupski JR. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet. 1998;18:382–384. doi: 10.1038/ng0498-382. [DOI] [PubMed] [Google Scholar]
- Warner LE, Svaren J, Milbrandt J, Lupski J R. Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum Mol Genet. 1999;8:1245–1251. doi: 10.1093/hmg/8.7.1245. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Chavrier P, Bravo R, Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989;337:461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]