Abstract
Bacteria in their natural environments frequently exist as mixed surface-associated communities, protected by extracellular material, termed biofilms. Biofilms formed by the human pathogen Campylobacter jejuni may arise in the gastrointestinal tract of animals but also in water pipes and other industrial situations, leading to their possible transmission into the human food chain either directly or via farm animals. Bacteriophages are natural predators of bacteria that usually kill their prey by cell lysis and have potential application for the biocontrol and dispersal of target bacteria in biofilms. The effects of virulent Campylobacter specific-bacteriophages CP8 and CP30 on C. jejuni biofilms formed on glass by strains NCTC 11168 and PT14 at 37°C under microaerobic conditions were investigated. Independent bacteriophage treatments (n ≥ 3) led to 1 to 3 log10 CFU/cm2 reductions in the viable count 24 h postinfection compared with control levels. In contrast, bacteriophages applied under these conditions effected a reduction of less than 1 log10 CFU/ml in planktonic cells. Resistance to bacteriophage in bacteria surviving bacteriophage treatment of C. jejuni NCTC 11168 biofilms was 84% and 90% for CP8 and CP30, respectively, whereas bacteriophage resistance was not found in similarly recovered C. jejuni PT14 cells. Dispersal of the biofilm matrix by bacteriophage was demonstrated by crystal violet staining and transmission electron microscopy. Bacteriophage may play an important role in the control of attachment and biofilm formation by Campylobacter in situations where biofilms occur in nature, and they have the potential for application in industrial situations leading to improvements in food safety.
INTRODUCTION
Campylobacter jejuni is a Gram-negative thermotolerant microaerobic pathogen that causes human gastroenteritis worldwide. In the United States, it is estimated there are 2 to 3 million people infected each year (http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter/technical.html#incidence), whereas over 190,000 cases were reported in 2008 in the European Union (www.efsa.europa.eu/de/scdocs/doc/1533.pdf). Infection usually causes self-limiting diarrhea but can occasionally lead to serious illnesses, such as Guillain-Barré syndrome and reactive arthritis (45). The majority of human disease is attributed to C. jejuni, while infection by other members of the Campylobacter genus is relatively infrequent. Consumption of undercooked or contaminated meat or poultry is generally believed to be the cause of a significant proportion of the total number of infections (49).
Bacteria in their natural environments frequently form biofilms that are comprised of single or multiple bacterial species embedded in a surface extracellular polymeric matrix, which can include polysaccharides, protein, nucleic acid, phospholipid, and teichoic acid (9, 11, 33, 46). Campylobacters may be considered part of the commensal communities found in the intestines of poultry (20) and many other animals, where they form densely packed parcels of cells within the luminal crypts, attached to the mucus but not directly to the epithelium (4). In the laboratory, Campylobacter has been shown to be capable of forming three distinct types of biofilm (24): the surface-attached type, a pellicle type in liquid culture, and an unattached aggregate or floc type, probably similar to that observed in the luminal crypts in vivo. Biofilm formation may help the bacteria to overcome environmental stresses, such as aerobic conditions, desiccation, heating, disinfectants, and acidic conditions (32, 37), and thereby increase their potential to cause disease. Campylobacter strains have been demonstrated to attach or associate with biofilms containing multiple organisms, such as Gram-positive bacteria. For example, campylobacters comprise part of the mixed microflora of biofilms present in water lines and on many types of surface, including materials commonly used in industrial settings, such as polyvinyl chloride (PVC) and stainless steel (7, 39, 40, 47, 50). Campylobacters have also been demonstrated to form monoculture biofilms on various surfaces under microaerobic (14, 19, 24, 25, 31, 37) and aerobic (38) conditions. Under conditions of biofilm formation, Campylobacter strains have been reported to be enhanced in their ability to resist antimicrobial agents (24, 25). The formation of biofilms has therefore been suggested as a mechanism by which campylobacters are able to survive harsh environments and is probably a contributing factor in their persistence and colonization of the digestive tracts of poultry and other animal species.
Bacteriophages represent an alternative to conventional antimicrobial agents to control food-borne microorganisms. The abilities of bacteriophages to reduce food-borne bacteria as a means of bio-sanitization and phage therapy have been investigated. Campylobacter bacteriophages have been shown to reduce viable Campylobacter cells on chicken skin (2, 18) and on raw and cooked beef (5). Bacteriophage therapy has been shown to reduce Campylobacter in the intestines of broiler chickens over a period sufficient for birds to be prepared for retail (15, 16, 28, 41, 48). The acquisition of bacteriophage resistance is commonly cited as a disadvantage of bacteriophage therapy; however, implementation of the treatment of broiler chickens at the end of the rearing cycle should ensure maximal effect of the treatment and the removal of resistant survivors upon depopulation of the barn (16).
The application of bacteriophage to reduce biofilms of several different bacterial species has been demonstrated (21, 22, 44). Moreover, engineered bacteriophage enzymes have been employed to disperse biofilms by breaking down components of the extracellular polymeric matrix (30). However, when stainless-steel-adherent Escherichia coli O157:H7 cells were treated with bacteriophages, there was a reduction in the viability of the exposed adhered cells but not in the cells within the biofilm itself (43).
The effect of bacteriophage treatment of Campylobacter biofilms had not previously been studied, and therefore this study was undertaken to assess the ability of Campylobacter-specific bacteriophage to prey on bacteria in situ and disperse Campylobacter biofilms. Bacteriophage treatment of bacteria is known to involve nonlinear kinetic phenomena (35), and it is likely these will be influenced by the presence of biofilms formed in vitro or in vivo.
MATERIALS AND METHODS
Bacterial growth conditions and strains.
Campylobacter jejuni NCTC 11168 and PT14 (17) were routinely grown on blood agar base number 2 (BAB; Oxoid, Basingstoke, United Kingdom) supplemented with 5% defibrinated horse blood (TCS, Buckingham, United Kingdom) at 42°C under microaerobic conditions for 18 h. Cultures were resuspended in Mueller-Hinton (MH) broth (Oxoid) at an A600 of 0.3 to 0.4 using a sterile swab and then incubated (42°C under microaerobic conditions for 18 h) to use as inocula for biofilms. The microaerobic atmosphere was achieved using CampyGen (Oxoid) gas packs placed in sealed incubation boxes or using anaerobic jars employing gas replacement (85% N2, 5% O2, and 10% H2).
Bacteriophage propagation.
The bacteriophages CP8 and CP30, originally isolated from United Kingdom poultry excreta, were selected from laboratory stocks, on the basis that they were well characterized and had been shown to have different lytic profiles (28, 41). They were propagated on the bacterial hosts to which they were to be applied by the soft agar overlay method using NZCYM agar as previously described (2, 8).
Attachment and biofilm formation.
Overnight Campylobacter cultures were diluted to approximately 105 CFU/ml based on optical density (A600, ∼0.01) with fresh MH broth. A 10-ml aliquot of the culture was dispensed into a petri dish containing six glass coverslips (18 by 18 mm; Scientific Laboratory Supplies Ltd., Nottingham, United Kingdom) on which to form the biofilms, which were then incubated (at 37°C under microaerobic conditions for 120 h).
Bacteriophage treatment of biofilms.
The glass coverslips containing Campylobacter biofilms were moved to a clean petri dish for phage treatment, and the residual unattached planktonic cells (∼90%) were collected in a tube for independent treatment with phage. Bacteriophage stock solutions were diluted in fresh MH broth to a final concentration of approximately 106 or 109 PFU/ml, from which 100 μl of bacteriophage suspension was placed onto each glass surface and corresponding planktonic phase in a microcentrifuge tube and then incubated (at 37°C under microaerobic conditions for 0, 2, 4, 7, and 24 h). Control samples were mock treated with fresh sterile MH broth instead of phage suspension. All experiments were performed using triplicate slides with a minimum of three independent biological replicates. The data are presented as mean counts (± standard deviation), and their significance was determined using t tests (P < 0.05).
Enumeration of viable bacterial cells.
Bacterial cells attached to the glass surfaces were resuspended in maximal recovery diluent (MRD; Oxoid) using a pipette to detach bound cells. Serial dilutions (10-fold) were performed; the samples were dispensed in triplicate onto campylobacter blood-free selective agar (CCDA; Oxoid) containing additional agar to 2% (wt/vol) agar to prevent swarming and incubated (under microaerobic conditions at 42°C for 42 h), and the colonies were counted. Planktonic cells were serially diluted and enumerated in the same way.
Enumeration of bacteriophage.
Bacterial lawns of C. jejuni NCTC 11168 or PT14 were prepared and bacteriophage were enumerated using serial dilutions as previously described (2, 28) from suspensions as described above. The number of PFU per cm2 of surface area of glass coverslip was calculated.
Crystal violet staining of biofilms.
Crystal violet is a vital dye which binds to carbohydrate, protein, and nucleic acid and is therefore useful for staining biofilm material (27). The Campylobacter biofilms attached to glass coverslips were transferred to a clean petri dish, where they were washed carefully two times with 2 ml of MRD, at ambient temperature, using a wide-tip pipette, prior to staining. One hundred microliters of 0.1% (wt/vol) crystal violet (Sigma Aldrich) was added onto each glass coverslip and allowed to stain for 1 h. The biofilms were then washed three times with sterile reverse osmosis (RO) water. The bound crystal violet was extracted from the biofilms by dissolving in 2 ml of 95% ethanol. The absorbance of the extract was measured at 570 nm.
Determination of the frequency of phage resistance.
Colonies (n = 100) produced by viable cells extracted from the biofilms posttreatment with bacteriophages were subcultured on blood agar plates. Bacterial lawns were prepared from these subcultures for bacteriophage propagation (28). Phage stocks at the routine test dilution (105 PFU/ml) (17) were applied as 10-μl aliquots to these lawns. An isolate was considered to be resistant to the applied bacteriophage if fewer than 20 plaques were produced.
Motility determination.
The motility of the viable Campylobacter isolates above was assessed by measuring the degree of swarming on 0.4% (wt/vol) Mueller-Hinton agar plates (incubated at 42°C under microaerobic conditions for 48 h) (42).
Transmission electron microscopy.
Biofilms attached to glass coverslips were fixed and capsular material was stained with 1% (wt/vol) Alcian blue 8GX (Sigma-Aldrich) and 3% glutaraldehyde prepared in 0.075 M sodium cacodylate (Sigma-Aldrich) buffer, pH 7.4, and postfixed in 1% osmium tetroxide in the same buffer (26). Following this, a thin layer of 1% molten agar (wt/vol; Oxoid) that had been cooled to 45°C was applied to cover the fixed biofilms and allowed to solidify. The agar-encased biofilms could then be removed from the glass coverslips and cut into 2- to 3-mm pieces to allow processing using standard procedures, by dehydration in ethanol, infiltration, and embedding in resin (TAAB Laboratories, Aldermaston, United Kingdom). Ultrathin sections were stained with uranyl acetate and lead citrate before observation using a Tecnai Biotwin transmission electron microscope (FEI) operated at an accelerating voltage of 100 kV.
RESULTS
Viability of Campylobacter cells within biofilms following bacteriophage treatment.
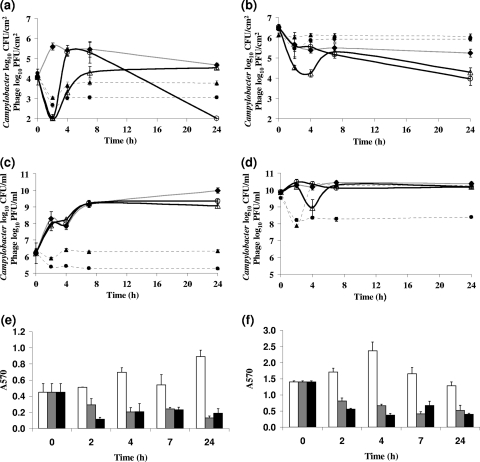
Biofilms that were formed on glass surfaces for 5 days (time of maximum observed attachment; unpublished observation) were treated with either bacteriophages CP8 or CP30, at a multiplicity of infection (MOI) of approximately 1 PFU per viable Campylobacter cell. Planktonic cultures from these biofilms were recovered and treated simultaneously at an equivalent MOI. Viable cell counts from the C. jejuni NCTC 11168 biofilm were significantly reduced by 3 log10 CFU/cm2 at 2 h postinfection by either of the phages compared to those of non-bacteriophage-treated biofilms for each of three independent experiments (P < 0.05; a representative data set is presented in Fig. 1 a). Following the initial fall in the viable count, an increase to approximately log10 5.5 occurred at 2 h and 4 h for the mock- and CP8-treated cultures, respectively. The rapid recovery in the number of viable cells could not occur by normal cell division alone and therefore may be associated with the reattachment of Campylobacter-containing biofilm that had been detached by the mock or bacteriophage treatment. The CP8-treated culture experienced a further decline in the viable cell numbers over the next 24 h to barely detectable levels for each of the three replicate experiments. Bacteriophage treatments of C. jejuni PT14 biofilms with CP8 resulted in a 1 log10 CFU/cm2 reduction in viable count over the 24-h period compared to the level in the mock-treated control (Fig. 1b). Treatment of C. jejuni PT14 biofilms with bacteriophage CP30, by comparison, produced a 2.5 log10 CFU/cm2 reduction in the viable count 4 h postinfection, before experiencing a temporary recovery at 8 h. No significant reductions beyond that recorded for the control culture were observed over the 4 h postinfection for C. jejuni PT14 biofilms treated with CP8 bacteriophage (Fig. 1b) for each of the three independent replicate experiments. Planktonic cells removed from the surfaces of the C. jejuni NCTC 11168 and PT14 biofilms were treated in parallel with bacteriophages CP8 and CP30. Of these, only C. jejuni PT14 treated with CP30 showed a significant reduction of 1 log10 CFU/ml (P < 0.05) in the viable count at 4 h for each of the three replicate experiments, before recovery to levels recorded for mock-treated controls (representative data sets are presented in Fig. 1c and d).
Fig. 1.
Viability of C. jejuni NCTC 11168 (left) and PT14 (right) following bacteriophage treatment of biofilms (a and b) compared to that following treatment of planktonic cultures (c and d). ○, bacteriophage CP8-treated cells; ▵, bacteriophage CP30-treated cells; ⧫, mock-treated cells. Bacteriophage titers are shown as broken lines: •, CP8; ▴, CP30. The bar charts (e and f) compare the effects of bacteriophage treatment of biofilms of C. jejuni NCTC 11168 (left) and PT14 (right), quantified using crystal violet staining: white bars, mock-treated biofilm; gray bars, CP8-treated biofilm; black bars, CP30-treated biofilm. The data are mean counts ± standard deviation (n = 3).
Bacteriophage titers following application to the biofilms declined probably as a result of binding host cells but thereafter remained static over the experimental period (broken lines in Fig. 1a and b). However, it is notable that bacteriophage CP8 and CP30 bind the C. jejuni NCTC 11168 host in the biofilm or planktonic phase to a greater degree than C. jejuni PT14 (Fig. 1a to d).
Evidence for reduction of biofilms following bacteriophage treatment.
Reductions in the biofilm matrix density following bacteriophage treatment were assessed by measuring the optical density of ethanol-solubilized material after staining with crystal violet and comparing these with mock-treated controls. The results showed that bacteriophage treatment of either C. jejuni PT14 or 11168 significantly reduced the attached biofilms material at the 2- to 24-h time points after treatment (P < 0.05; Fig. 1e and f).
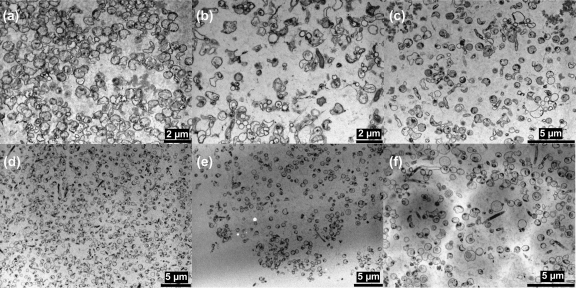
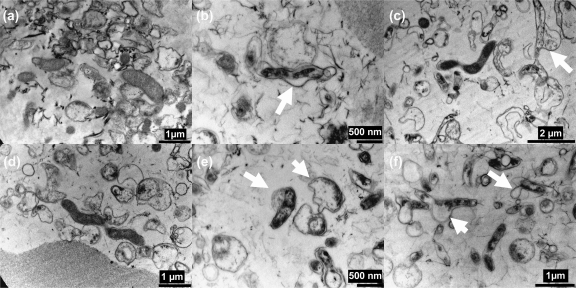
Bacteriophage-treated and untreated Campylobacter biofilms were examined by transmission electron microscopy (TEM). Alcian blue staining allowed visualization of the capsular polysaccharide surrounding vegetative cells within the biofilm matrix (26). Examination of the TEM images revealed that the bacteriophage treatment disrupted the biofilms formed by both Campylobacter strains. The TEM images also allowed detailed examination of the structure of the Campylobacter biofilms which revealed them to consist of coccoid bacteria with few vegetative rod-shaped cells. Evidence of disruption of the biofilms occurred after 2 h, following bacteriophage treatment, with the biofilms showing a reduction in the densities of coccoid and rod-shaped cells (Fig. 2). Interestingly, in the bacteriophage-treated biofilms it was possible to identify Campylobacter cells where the membranes were distorted and swelling outwards as if on the point of lysis (indicated by arrows in Fig. 3 b, c, e, and f) that were not observable in the non-bacteriophage-treated biofilms (Fig. 3a and d).
Fig. 2.
Transmission electron microscopy to show the effect of treatment with bacteriophage CP8 and CP30 on biofilms of Campylobacter jejuni NCTC 11168 (a, b, and c) and PT14 (d, e, and f): control without phage treatment (a and d), bacteriophage CP8 treatment after 2 h (b and e), and bacteriophage CP30 treatment after 2 h (c and f).
Fig. 3.
Transmission electron microscopy to show the effect of treatment with bacteriophage CP8 (b and e) and CP30 (c and f) on Campylobacter jejuni NCTC 11168 (a, b, and c) and PT14 (d, e, and f) biofilms after 24 h compared to effects in controls without phage treatment (a and d). Arrows indicate regions of the Campylobacter cells showing membrane distortion.
Frequency of phage resistance in bacteriophage-treated biofilms.
Viable cells recovered from the biofilms following bacteriophage treatment were tested for acquired resistance. There was very clear disparity between the sensitivity to bacteriophage of the two different strains of Campylobacter tested following bacteriophage treatment. All of the isolated viable cells from the C. jejuni PT14 biofilms remained susceptible to both the bacteriophages applied. In contrast, only 16% and 10% of the viable cells recovered from C. jejuni 11168 bacteriophage-treated biofilms remained susceptible to bacteriophages CP8 and CP30, respectively. This difference is not reflected in the planktonic cells recovered in these experiments, in which the C. jejuni PT14 biofilms retained 8% and 9% susceptible bacteria for CP8 and CP30 bacteriophage, respectively, with 9% and 11% being the respective figures for C. jejuni 11168. The motility of the posttreatment isolates was not affected on the basis of microscopic observation or the degree of swarming. Three of each of the bacteriophage-resistant types recovered after CP8 or CP30 treatment of C. jejuni 11168 were selected and tested for their ability to make biofilms on glass coverslips. The C. jejuni 11168 isolates resistant to CP8 were impaired in the ability to form biofilm based on the observation of significant reductions in the biofilm matrix density (estimated by staining with crystal violet and optical density measurements of the ethanol-solubilized material) compared to that of the progenitor strain (P < 0.05). The C. jejuni 11168 isolates resistant to CP30 appeared to form biofilm, but these were only recoverable as unattached aggregates or flocs rather than remaining adherent to the glass slide.
DISCUSSION
Members of the genus Campylobacter represent a significant burden to public health, so novel treatments to reduce the spread of infection are of fundamental importance. Most bactericidal treatments are tested on the basis of their ability to kill planktonic cells, but their effects may be reduced where the target bacterium is protected within a biofilm. Bacteriophages have the potential to negate the protection afforded by biofilms either by direct penetration and infection of the cells at the biofilm surface or via infiltration of bacteriophage-infected bacteria into the interior of the biofilm and their subsequent lysis to release bacteriophage in situ.
The selection of a particular bacteriophage to use as a treatment is an important issue for phage therapy and bio-sanitization applications, since bacteriophages and their effects are diverse. Not least the bacteriophage should be virulent and not temperate if it is to have a significant effect. Virulent Campylobacter bacteriophage can be isolated from chicken excreta and from poultry meat (3, 10, 15, 29). Bacteriophages CP8 and CP30 are virulent members of Myoviridae with proven stability and efficacy in reducing Campylobacter numbers by 2 log10 CFU/g in experimental Campylobacter-colonized chickens (28, 41). These bacteriophages were therefore selected for this study in order to assess if bacteriophage application could reduce or eliminate either Campylobacter biofilms or the bacteria within them.
Treatment of Campylobacter biofilms attached to glass surfaces with bacteriophages CP8 or CP30 resulted in substantial reductions of viable cells compared to levels in mock-treated controls. Interestingly, the response to bacteriophage treatment was dependent on the host strain forming the biofilm, with differences in both the speed and degree of reduction of the viable count. It is evident that the Campylobacter strains have differing capacities for biofilm formation, which could influence the effect of the bacteriophages. For example, C. jejuni PT14 produced a far greater quantity of biofilm on glass than did C. jejuni NCTC 11168, as measured by crystal violet staining. The bacteriophages were most effective at 2 h postinfection of C. jejuni NCTC 11168 biofilms in their ability to reduce the viable count, with reductions of 2.5 log10 CFU/cm2 or greater at the limit of detection (Fig. 1a). Bacteriophage CP30 was similarly effective at this time point against C. jejuni PT14 despite the increase in the biofilm matrix associated with this strain. However, treatment of C. jejuni PT14 with bacteriophage CP8 did not significantly reduce the viable Campylobacter count compared to that in the mock-treated control. At 24 h postinfection, CP8 treatment of C. jejuni NCTC 11168 biofilm reduced the viable count below the detection limit but CP30 treatment results were not significantly different from the control. Bacteriophages CP8 and CP30 significantly reduced the viable count compared to that in the mock-treated control at 24 h postinfection of the C. jejuni PT14 biofilm (P < 0.01). The initial fall in viable count at 2 h was followed by a fast recovery at 4 to 8 h postinfection. An increase of almost 4 log10 CFU/cm2 in 2 h was recorded for CP8-treated C. jejuni NCTC 11168. It is not likely that such a rapid recovery in the number of viable cells could occur by normal cell division alone, and therefore it may be associated with the reattachment of Campylobacter-containing biofilm that had been detached by the bacteriophage treatment. To account for the recovery in viable count, it may be postulated that not all of the campylobacters were compromised but remained viable and associated with sloughed biofilm material. If left undisturbed for longer periods, this material could become reattached, although in the long term, viability was reduced due to the actions of the bacteriophage irrespective of the initial detachment. TEM images provide support for the contention that the biofilms were dispersed in the presence of bacteriophage after 2 h (Fig. 2). Higher-resolution TEM images at 24 h post-bacteriophage treatment also clearly show that campylobacters are subject to cell lysis within the biofilm matrix (Fig. 3). Regardless of whether the viability of the campylobacters persists in the short term (4 h), the immediate effect of bacteriophage-mediated biofilm dispersal would be to create a window of opportunity for the removal of Campylobacter biofilms. Bacteriophage could be used to initiate biofilm dispersion in industrial settings that could be followed by thorough pressure flushing to purge water conduits.
The numbers of viable bacteriophage found in the biofilms and planktonic cultures showed an initial binding phase characterized by a decrease in numbers, then a recovery, followed by a period where numbers appeared to remain static. At first sight it might seem strange that the reduction in viable cells in the biofilms from 4 h to 24 h under treatment by phage CP8 should not be accompanied by a rise in phage numbers. This could be an example of what is called passive (or inundation) therapy (34) in which the weight of numbers of phage is sufficient to reduce cell counts without a need for significant levels of phage replication. Even if this is not the case, phages that infect Camplylobacter generally have a small burst size of around 2 to 3 (8), and so it would require only a small extra loss of phage to prevent significant amplification of phage numbers. It should be noted that phage amplification depends not only on cell lysis but also on the proportion of free phage able to successfully reach and infect another susceptible bacterium, which in turn depends on the density of bacteria and the movement within the medium. Mean burst size could be attenuated by the fact that the majority of Campylobacter cells in the mature biofilms are likely to be in stationary phase and growing relatively slowly. Moreover, movement through the biofilm may be impaired by proximity to cell debris from the lysis event (36), so that debris may be encountered and bound to at a higher rate than in planktonic systems. Experiments where phage was administered to Campylobacter-colonized chickens also appear to confirm this stasis in phage titers, as there appears to be a threshold above which phage numbers do not increase despite being continually added to the system through replication (28). It is perhaps not surprising that the phage-bacteria kinetics show somewhat different properties in biofilms compared to those observed for planktonic systems or in vivo. Clearly a formal mathematical model, based on more-refined data, will need to be developed to properly understand the kinetic properties of the system.
Bacteriophages have been shown to diffuse through the biofilms of other bacteria (6), and it has also been reported that biofilm formation does not provide additional protection against bacteriophage attack (43). Our data support these observations, since campylobacters within the biofilm are subject to bacteriophage-mediated lysis. However, it is also reported that the effects of bacteriophage on attached and planktonic cells are similar (43). In contrast, our work indicates that bacteriophage were actually more effective at reducing numbers of viable cells when campylobacters were associated in a biofilm than when they were associated with planktonic cells. However, caution is required in drawing mechanistic interpretations from these contrasting results: phage-bacteria kinetics are determined by density thresholds (34), whereas in most empirical studies the standard practice is to control experiments using MOI (which is a ratio, not a density). Further experiments, with control of the initial densities, will be needed to untangle the differing balances of processes in biofilm and planktonic populations. This will not be trivial, as we anticipate that localized high densities of host and bacteriophage may arise within the biofilm, and these could give rise to physiological differences between the bacterial populations. Further, the planktonic cells in the overlaying static culture are likely to be in stationary phase, which may not support phage replication, and notably exhibit a high frequency of phage resistance in these experiments (82 to 92%).
The mechanism by which the bacteriophages were able to break down the biofilm is unknown. It seems possible that bacteriophage CP8 and CP30 can reduce the extracellular matrix produced by C. jejuni in biofilms through enzymatic means. Bacteriophages have been shown to produce enzymes that can degrade exopolysaccharide, which forms the major component of the biofilm matrix (22, 23). An alternative to phage-encoded enzymatic degradation is that the Campylobacter bacteriophages may break down the biofilm indirectly, through bacterial cell lysis with the subsequent release of bacterial enzymes that degrade the biofilm matrix. It is clear from the TEM images that the bacteriophage not only lysed their targets but also caused a breakdown of the biofilm matrix.
In the laboratory, bacteria that survive bacteriophage infection often develop resistance to subsequent attack through mutation. Moreover, phage resistance of bacteria within biofilms has been reported to be due to the loss of phage receptor sites that become incorporated into the cell envelope (13). This is contrary to what has been observed for campylobacters surviving phage therapy in chickens, where the environment of the gut counterselects resistant types that are physiologically compromised (28, 42). It was therefore of interest to examine if the bacteria recovered from biofilms were resistant to the test bacteriophage. There were clear differences in the frequency of acquired bacteriophage resistance dependent on the host strain used. All of the viable C. jejuni PT14 isolates recovered from the biofilm retained sensitivity to both bacteriophages. In contrast, only a minority (10 to 16%) of the viable C. jejuni NCTC 11168 isolates from the treated biofilm retained sensitivity to the bacteriophages. Treatment of planktonic bacterial cells with bacteriophages in vitro results in the selection of resistant mutants (1). Such spontaneous resistant mutants generated by Campylobacter bacteriophage following in vitro treatment of broth-cultured cells are often deficient in motility (12) and as a consequence would be unlikely to be able to survive outside the laboratory environment. The incidence of resistant phenotypes following bacteriophage treatment in vivo was found to be relatively low (4%) (28, 42), where the isolates were noted to be motile, probably due to the requirement of a competent motility phenotype for colonization (20). The frequency of resistance to bacteriophage in Campylobacter isolated from the biofilms of the two strains did not therefore match the observations made for phage infection either in vitro or in vivo. The incidence of resistance to bacteriophage was high for C. jejuni 11168 biofilms, but unlike the survivors from in vitro planktonic infections, the isolates were fully motile. However, the bacteriophage-resistant types recovered from C. jejuni 11168 biofilms were impaired in their ability to either form or maintain biofilms compared to the parental type strain. No resistant isolates were recovered from bacteriophage-treated biofilms of C. jejuni PT14, a pattern more reminiscent of that observed in vivo, during phage therapy of chickens (10, 28, 42).
In conclusion, we have shown the potential for use of lytic bacteriophages against campylobacters as a treatment to control biofilm formation. The viruses within the biofilm could not only effectively target and lyse campylobacters but were also able to disperse the extracellular matrix forming the biofilm. Further studies to identify the components involved in this action will allow further understanding of the interaction between bacteriophage and host within biofilms. More refined experiments, together with kinetic modeling, will be needed to better understand how the phage-bacteria interactions differ in biofilms and planktonic populations and the kinetic consequences of those differences. Our preliminary results show promise, not only because the viruses within the biofilm effectively target and lyse campylobacters but also because they are able to disperse the extracellular matrix forming the biofilm.
ACKNOWLEDGMENTS
This study was supported by a grant from the Royal Thai Government.
We thank Marie Smith, Advanced Microscopy Unit, Queens Medical Centre, University of Nottingham, for technical assistance with transmission electron microscopy.
Footnotes
Published ahead of print on 25 March 2011.
REFERENCES
- 1. Adams M. H. (ed.). 1959. Bacteriophages. Interscience Publishers Inc., New York, NY [Google Scholar]
- 2. Atterbury R. J., Connerton P. L., Dodd C. E., Rees C. E., Connerton I. F. 2003. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6302–6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atterbury R. J., Connerton P. L., Dodd C. E., Rees C. E., Connerton I. F. 2003. Isolation and characterization of Campylobacter bacteriophages from retail poultry. Appl. Environ. Microbiol. 69:4511–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beery J. T., Hugdahl M. B., Doyle M. P. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bigwood T., Hudson J. A., Billington C., Carey-Smith G. V., Heinemann J. A. 2008. Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiol. 25:400–406 [DOI] [PubMed] [Google Scholar]
- 6. Briandet R., et al. 2008. Fluorescence correlation spectroscopy to study diffusion and reaction of bacteriophages inside biofilms. Appl. Environ. Microbiol. 74:2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buswell C. M., et al. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cairns B. J., Timms A. R., Jansen V. A., Connerton I. F., Payne R. J. 2009. Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog. 5:e1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chmielewski R. A. N., Frank J. F. 2003. Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. Food Safety 2:22–32 [DOI] [PubMed] [Google Scholar]
- 10. Connerton P. L., et al. 2004. A longitudinal study of Campylobacter jejuni bacteriophage and their hosts from broiler chickens. Appl. Environ. Microbiol. 70:3877–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 12. Coward C., et al. 2006. Phase-variable surface structures are required for infection of Campylobacter jejuni by bacteriophages. Appl. Environ. Microbiol. 72:4638–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donlan R. M. 2009. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 17:66–72 [DOI] [PubMed] [Google Scholar]
- 14. Dykes G. A., Sampathkumar B., Korber D. R. 2003. Planktonic or biofilm growth affects survival, hydrophobicity and protein expression patterns of a pathogenic Campylobacter jejuni strain. Int. J. Food Microbiol. 89:1–10 [DOI] [PubMed] [Google Scholar]
- 15. El-Shibiny A., Connerton P. L., Connerton I. F. 2005. Enumeration and diversity of campylobacters and bacteriophages isolated during the rearing cycles of free-range and organic chickens. Appl. Environ. Microbiol. 71:1259–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Shibiny A., et al. 2009. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 72:733–740 [DOI] [PubMed] [Google Scholar]
- 17. Frost J. A., Kramer J. M., Gillanders S. A. 1999. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goode D., Allen V. M., Barrow P. A. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69:5032–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunther N. W., IV, Chen C. Y. 2009. The biofilm forming potential of bacterial species in genus Campylobacter. Food Microbiol. 26:44–51 [DOI] [PubMed] [Google Scholar]
- 20. Hendrixson D. R., DiRita V. J. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471–484 [DOI] [PubMed] [Google Scholar]
- 21. Hibma A. M., Jassim S. A. A., Griffiths M. W. 1997. Infection and removal of L-forms of Listeria monocytogenes with bred bacteriophage. Int. J. Food Microbiol. 34:197–207 [DOI] [PubMed] [Google Scholar]
- 22. Hughes K. A., Sutherland I. W., Clark J., Jones M. V. 1998. Bacteriophage and associated polysaccharide depolymerases—novel tools for study of bacterial biofilms. J. Appl. Microbiol. 85:583–590 [DOI] [PubMed] [Google Scholar]
- 23. Hughes K. A., Sutherland I. W., Jones M. V. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144:3039–3047 [DOI] [PubMed] [Google Scholar]
- 24. Joshua G. W., Gutherie-Irons C., Karlyshev A. V., Wren B. W. 2006. Biofilm formation in Campylobacter jejuni. Microbiology 152:387–396 [DOI] [PubMed] [Google Scholar]
- 25. Kalmokoff M., et al. 2006. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J. Bacteriol. 188:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karlyshev A. V., McCrossan M. V., Wren B. W. 2001. Demonstration of polysaccharide capsule in Campylobacter jejuni using electron microscopy. Infect. Immun. 69:5921–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim S.-H., Wei C.-I. 2007. Biofilm formation by multidrug-resistant Salmonella enterica serotype Typhimurium phage type DT104 and other pathogens. J. Food. Prot. 70:22–29 [DOI] [PubMed] [Google Scholar]
- 28. Loc Carrillo C., et al. 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71:6554–6563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loc Carrillo C. M., Connerton P. L., Pearson T., Connerton I. F. 2007. Free-range layer chickens as a source of Campylobacter bacteriophage. Antonie Van Leeuwenhoek 92:275–284 [DOI] [PubMed] [Google Scholar]
- 30. Lu T. K., Collins J. J. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 104:11197–11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moe K. K., et al. 2010. The mode of biofilm formation on smooth surfaces by Campylobacter jejuni. J. Vet. Med. Sci. 72:411–416 [DOI] [PubMed] [Google Scholar]
- 32. Murphy C., Carroll C., Jordan K. N. 2006. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 100:623–632 [DOI] [PubMed] [Google Scholar]
- 33. O'Toole G., Kaplan H., Kolter R. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79 [DOI] [PubMed] [Google Scholar]
- 34. Payne J. H., Jansen V. A. 2000. Phage therapy: the peculiar kinetics of self-replicating pharmaceuticals. Clin. Pharmacol. Ther. 68:225–230 [DOI] [PubMed] [Google Scholar]
- 35. Payne J. H., Jansen V. A. 2001. Understanding bacteriophage therapy as a density-dependent kinetic process. J. Theor. Biol. 208:37–48 [DOI] [PubMed] [Google Scholar]
- 36. Rabinovitch A., Aviram I., Zaritsky A. 2003. Bacterial debris—an ecological mechanism for coexistence of bacteria and their viruses. J. Theor. Biol. 224:377–383 [DOI] [PubMed] [Google Scholar]
- 37. Reeser R. J., Medler R. T., Billington S. J., Jost B. H., Joens L. A. 2007. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 73:1908–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reuter M., Mallett A., Pearson B. M., van Vliet A. H. M. 2010. Biofilm formation in Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 76:2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanders S. Q., Boothe D. H., Frank J. F., Arnold J. W. 2007. Culture and detection of Campylobacter jejuni within mixed microbial populations of biofilms on stainless steel. J. Food Prot. 70:1379–1385 [DOI] [PubMed] [Google Scholar]
- 40. Sanders S. Q., Frank J. F., Arnold J. W. 2008. Temperature and nutrient effects on Campylobacter jejuni attachment on multispecies biofilms on stainless steel. J. Food Prot. 71:271–278 [DOI] [PubMed] [Google Scholar]
- 41. Scott A. E., Timms A. R., Connerton P. L., El-Shibiny A., Connerton I. F. 2007. Bacteriophage influence Campylobacter jejuni types populating broiler chickens. Environ. Microbiol. 9:2341–2353 [DOI] [PubMed] [Google Scholar]
- 42. Scott A. E., et al. 2007. Genome dynamics of Campylobacter jejuni in response to bacteriophage predation. PLoS Pathog. 3:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharma M., Ryu J.-H., Beuchat L. R. 2005. Inactivation of Escherichia coli O157:H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J. Appl. Microbiol. 99:449–459 [DOI] [PubMed] [Google Scholar]
- 44. Sillankorva S., Oliveira R., Vieira M. J., Sutherland I. W., Azeredo J. 2004. Bacteriophage Φ S1 infection of Pseudomonas fluorescens planktonic cells versus biofilms. Biofouling 20:133–138 [DOI] [PubMed] [Google Scholar]
- 45. Stern N. J. 2001. Campylobacter, p. 61–68 In Labbé R. G., García S. (ed.), Guide to foodborne pathogens. John Wiley & Sons, New York, NY [Google Scholar]
- 46. Sutherland I. W. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9 [DOI] [PubMed] [Google Scholar]
- 47. Trachoo N., Frank J. F., Stern N. J. 2002. Survival of Campylobacter jejuni in biofilms isolated from chicken houses. J. Food Prot. 65:1110–1116 [DOI] [PubMed] [Google Scholar]
- 48. Wagenaar J. A., Van Bergen M. A., Mueller M. A., Wassenaar T. M., Carlton R. M. 2005. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 109:275–283 [DOI] [PubMed] [Google Scholar]
- 49. Wilson D. J., et al. 2008. Tracing the source of campylobacteriosis. PLoS Genet. 4:e1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zimmer M., Barnhart H., Idris U., Lee M. D. 2003. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. 47:101–107 [DOI] [PubMed] [Google Scholar]