Abstract
Enzymatic processes are useful for industrially important sugar production, and in vitro two-step isomerization has proven to be an efficient process in utilizing readily available sugar sources. A hypothetical uncharacterized protein encoded by ydaE of Bacillus licheniformis was found to have broad substrate specificities and has shown high catalytic efficiency on d-lyxose, suggesting that the enzyme is d-lyxose isomerase. Escherichia coli BL21 expressing the recombinant protein, of 19.5 kDa, showed higher activity at 40 to 45°C and pH 7.5 to 8.0 in the presence of 1.0 mM Mn2+. The apparent Km values for d-lyxose and d-mannose were 30.4 ± 0.7 mM and 26 ± 0.8 mM, respectively. The catalytic efficiency (kcat/Km) for lyxose (3.2 ± 0.1 mM−1 s−1) was higher than that for d-mannose (1.6 mM−1 s−1). The purified protein was applied to the bioproduction of d-lyxose and d-glucose from d-xylose and d-mannose, respectively, along with the thermostable xylose isomerase of Thermus thermophilus HB08. From an initial concentration of 10 mM d-lyxose and d-mannose, 3.7 mM and 3.8 mM d-lyxose and d-glucose, respectively, were produced by two-step isomerization. This two-step isomerization is an easy method for in vitro catalysis and can be applied to industrial production.
INTRODUCTION
The characterization of hypothetical proteins can aid in selecting proteins suitable for industrial processes. Especially in this postgenomic era and with the availability of novel computational tools, this approach may provide clues to the functions of specific gene products. However, even with the ability to predict the type of reaction catalyzed by a specific gene product, the substrate used by the enzyme often still cannot be established. One area where this presents a problem is in predicting the catalytic functions of carbohydrate-active enzymes (10). Prokaryotic cells have an excellent ability to survive on different carbon sources and provide an interesting means of studying the functional properties of enzymes that are key aids in sugar catabolism. One of these enzymes is sugar isomerase, which carries out the interconversion of aldose to a ketose sugar form, making the carbohydrate available for catalysis through a metabolic pathway. Since this enzyme can utilize multiple substrates, there is a need to study its efficacy with different sugars, which also provides an opportunity to reveal commercially useful applications.
Monosaccharides have industrial and pharmaceutical importance, which has generated a great deal of interest in their production. They can be produced by chemical reactions, microbial fermentation, and enzymatic catalysis using epimerases, isomerases, and oxidoreductases (2, 4, 12, 17, 33). However, the chemical and fermentative methods are not suited for mass production, since they are complex, time-consuming, expensive, and laborious. Thus, significant attention has been paid to enzymes and their biotechnological applications for the biosynthesis of important carbohydrates. A number of sugar isomerases have been characterized and applied at the industrial level.
The major obstacle is limited substrate availability for mass production. d-Lyxose, which is used as starting material for antitumor and immunostimulating agents (24, 32), can be produced from xylulose by lyxose isomerase. Similarly, glucose, which is widely consumed in bioenergy and fermentative processes, can be produced from fructose by xylose (glucose) isomerase but xylulose and fructose are not readily available in nature.
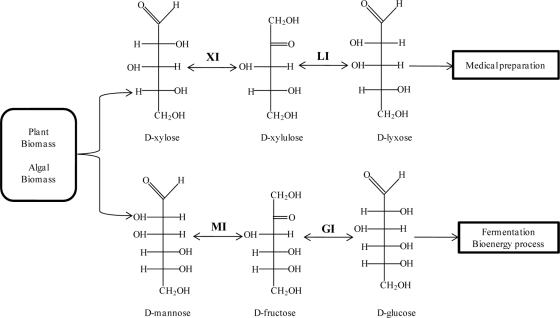
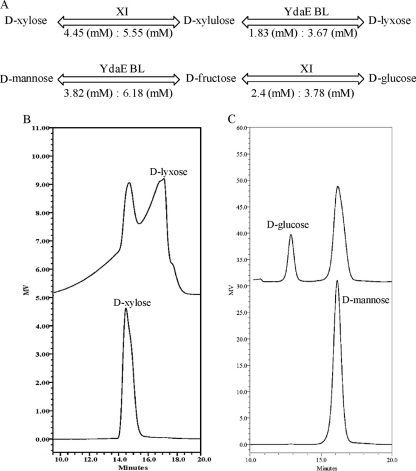
Two-step isomerization provides the solution for producing rare and industrially important sugars from available monosaccharides. With double isomerization, lyxose can be produced from xylose, one of the major constituents of plant biomass, by using xylose isomerase (EC 5.3.1.5) and lyxose isomerase (EC 5.3.1.15). Xylose isomerase converts xylose to xylulose, and lyxose isomerase extends the catalysis by converting xylulose to lyxose. Similarly, glucose, a sugar consumed in mass quantities in industrial fermentations and bioenergy processes, can be produced from naturally available algal and plant biomass by using glucose isomerase (EC 5.3.1.5) and mannose isomerase (EC 5.3.1.7) (Fig. 1). Extensive studies on the enzymatic transformations, structure, and catalytic mechanisms of xylose isomerase from different bacterial strains have been performed (7); however, scant information is currently available for lyxose isomerase.
Fig. 1.
Schematic representation of a two-step isomerization for the production of d-lyxose and d-glucose from d-xylose and d-mannose using two different isomerases. XI, xylose isomerase; LI, lyxose isomerase; MI, mannose isomerase; GI, glucose isomerase.
To date, only four lyxose isomerases, those from Providencia stuartii, Aerobacter aerogenes, Serratia proteamaculans, and Cohnella laevoribosii, have been characterized at the molecular level as targets for lyxose production. (5, 8, 23, 25). Mannose isomerases of Agrobacterium radiobacter and Mycobacterium smegmatis have been proposed for the catalysis of lyxose and mannose to xylulose and fructose, respectively, but their efficiency with lyxose is lower than that with mannose (14, 15). Phosphomannose isomerase also carries out the conversion of lyxose to xylulose, but it shows low specificity for lyxose (37). Available information about several uncharacterized genes present in prokaryotes has generated interest in the characterization of the encoded proteins and their use in bioproduction. The uncharacterized hypothetical protein ydaE (GenBank accession no. AAU22106) of Bacillus licheniformis shows 58% amino acid similarity to the thermophilic C. laevoribosii lyxose isomerase (8). Bacillus licheniformis, a nonpathogenic, spore-forming organism that has been utilized extensively in biotechnological applications (30), has an efficient extracellular polysaccharide-digesting capacity and is able to survive on a variety of carbon sources (36).
This article describes the isolation and characterization of a hypothetical protein encoded by ydaE of B. licheniformis and its use in the bioproduction of lyxose and glucose from xylose and mannose, respectively, by two-step isomerization, along with a previously characterized thermophilic xylose isomerase from Thermus thermophilus (11).
MATERIALS AND METHODS
Enzymes and materials.
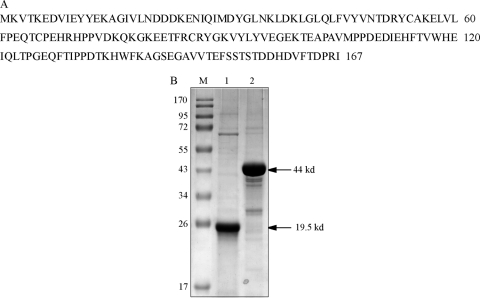
All molecular techniques were conducted according to methods described by Sambrook and Russell (29). ydaE of B. licheniformis (GenBank accession no. AAU22106), with 167 amino acids (Fig. 2 A) and a molecular mass of 19.5 kDa, and xylA (GenBank accession no. BAA14301) from T. thermus HB8, with 387 amino acids totaling 44 kDa, were explored for their action on d-xylose and d-mannose for the bioproduction of d-lyxose and d-glucose by two-step isomerization. The B. licheniformis DSM13 (KCTC 1918) and T. thermophilus HB8 (KACC 12317) strains were purchased from the Korean Culture Type Collection and the Korean Agriculture Culture Collection, respectively. All of the chemicals and media and the glucose estimation kit used (GAGO20) were procured from Sigma (St. Louis, MO), and DNA polymerase, restriction enzymes, and T4 DNA ligase were purchased from New England BioLabs (Ipswich, MA). The pRSET-A plasmid and Ni-nitrilotriacetic acid (NTA) agarose beads were purchased from Invitrogen (Carlsbad, CA). The pCOLD-I expression vector was procured from Takara (Ohtsu, Japan). Both pRSETA and pCOLDI encode a histidine tag to facilitate recombinant protein purification. High-performance liquid chromatography (HPLC; Waters, Bedford, MA) and a Rezex RPM-monosaccharide Pb+2 column (Phenomenex, Torrance, CA) were used to determine the sugar profile and concentrations.
Fig. 2.
(A) Amino acid sequence of ydaE of B. licheniformis. (B) SDS-PAGE analysis of purified proteins. Lanes: M, molecular size markers; 1, 19.5-kDa YdaE BL; 2, 44-kDa xylose isomerase. The values to the left are molecular sizes in kilodaltons.
Cloning and expression of ydaE BL and XI in Escherichia coli.
B. licheniformis was grown on brain heart infusion at 37°C overnight. DNA was isolated using lysozyme and proteinase K. Five hundred four base pairs of ydaE was amplified using primers 5′AGC GCT CTA BamHI ATG AAG GTG ACA AAG GAA GAT and 3′ GCA GTA AGC XhoI CTA AAT TCT CGG CTA TGT AAG. Template DNA (50 ng), 10 pmol of each primer, and a 52°C melting temperature (Tm) were used for the amplification of ydaE of B. licheniformis. An amplified fragment was restricted with BamHI and XhoI and eluted. The excised fragment was ligated into pRSET-A predigested with BamHI and XhoI. The construct was transformed into E. coli DH5α. Plasmid DNA was isolated, and the insert of ydaE was checked and sequenced (Genotech). After sequence confirmation, pRSET-A carrying ydaE was transformed into E. coli strain BL21(DE3). A single selected colony was inoculated into 5 ml LB broth containing 50 μg·ml−1 of ampicillin and incubated at 37oC overnight. The overgrown culture was inoculated into 400 ml LB broth and incubated at 37°C. Next, the cells were harvested, suspended in cell lysis buffer (pH 8.0), and lysed by sonication. The cell lysate was separated from the debris by centrifugation (13,000 rpm, 30 min) and loaded onto a Ni-NTA agarose bead column. After extensive washing with a wash buffer, the lysate was finally eluted with an elution buffer containing 250 mM imidazole (pH 8.0) and dialyzed against 50 mM Tris (pH 8.0) using a 10-kDa ultrafilter.
The gene xylA, encoding XI, was isolated from T. thermophilus HB08. Genomic DNA was extracted using lysozyme (1.0 mg ml−1) and proteinase K (0.5 mg ml−1) (19). xylA (1,164 bp) was amplified by PCR with primers 5′EcoRI ATGTACCAGCCCAAACCGG and 3′PstI CCCCCGCACCCCCAGGAGG. Template DNA (50 ng), 10 pmol of each primer, and a 60°C Tm were used for the specific amplification of xylA. The Tm was maintained at 60°C, and 50 cycles were performed. The xylA gene inserted into the pBluescript vector was amplified using T/A cloning, and the sequence was confirmed (Genotech). Next, xylA was excised from pBluescript using EcoRI and PstI and inserted into the pCOLDI expression vector (Takara), and the resulting plasmid, pCOLDI harboring xylA, was transformed into E. coli BL21(DE3). An isolated colony was inoculated into 5 ml LB broth containing 50 μg·ml−1 of ampicillin and incubated at 37°C overnight, and the overgrown culture was inoculated into 400 ml LB broth and incubated at 37°C until the optical density at 600 nm reached 0.4. The culture was transferred to 18°C for 24 h for cold shock induction. Cells were harvested after 24 h of expression, suspended in cell lysis buffer (pH 8.0), and lysed by sonication. The cell lysate was separated from the cellular debris by centrifugation (13,000 rpm, 30 min) and loaded onto Ni-NTA matrix. After extensive column washing, XI was eluted in an elution buffer containing 200 mM imidazole (pH 8.0) and dialyzed against 100 mM HEPES (pH 7.0). Finally, both proteins were quantified using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA) against bovine serum albumin as the standard. The purified protein was subjected to 12% SDS-PAGE and visualized with Coomassie brilliant blue R-250 staining.
Protein expression and purification levels were monitored by enzyme activity using the substrates d-mannose and d-fructose for YdaE BL and XI, respectively. One unit of activity was defined as the amount of enzyme required to produce 1 μmol of d-fructose/min for YdaE BL and d-glucose/min for XI under standard assay conditions. Activities were expressed in U/mg of protein.
Physicochemical characterization and thermal inactivation of YdaE BL.
To test the effects of pH and temperature on YdaE BL, the pH was varied from 5 to 9 using 50 mM phosphate buffer (pH 5.0 and 6.0), HEPES (pH 7.0), and Tris-Cl (pH 7.5 to 9.0) and the temperature was varied from 30°C to 65°C. Activation of YdaE BL by various divalent metals was performed by using 1 mM MnSO4·7H2O, ZnCl2·7H2O, CoCl2·6H2O, MgSO4·7H2O, CuSO4·7H2O, NiCl2·6H2O, and the control of apo enzyme in Tris-Cl (pH 8.0) with 10 mM d-mannose. The influence of temperature on YdaE BL was monitored in the range of 30°C to 50°C in 50 mM Tris-Cl (pH 8.0). At different time intervals, samples were withdrawn and the relative activity was determined after the reaction. The experimental data fit a first-order curve, and the half-life of the enzyme was calculated using SigmaPlot.
Catalytic efficiency of YdaE BL on lyxose and mannose and specificities for various sugars.
The efficiency of YdaE BL was analyzed at 40°C using a range of 1 mM to 300 mM lyxose and mannose. Each concentration was treated with enzyme containing 1 mM Mn2+ in 50 mM Tris-HCl, pH 8.0. Reactions were stopped after 10 min, and the reaction mixtures were analyzed for xylulose and fructose. Km (mM) and kcat (min−1) were determined by fitting the data to the Michaelis-Menten equation. Various sugars were analyzed for specificities toward YdaE BL. Ten millimolar d-xylose, d-lyxose, d-glucose, d-fructose, d-mannose, d-xylulose, l-rhamnose, or l-arabinose was treated with YdaE by adjustment (1.0 to 10 U·ml−1) under conditions optimized for a reaction time of 30 min. The isomerized product was checked either by resorcinol reagent or by HPLC analysis. Specific activity was defined as the amount of aldose or ketose produced per enzyme amount per reaction time.
Bioconversion of lyxose and mannose to xylulose and fructose by YdaE BL.
Ten millimolar lyxose or mannose was treated with 20 U·ml−1 of enzyme containing 1 mM Mn2+ in 50 mM Tris-HCl (pH 8.0). The reaction mixture was incubated at 40°C, and samples were taken at regular intervals and analyzed for xylulose and fructose production using resorcinol reagent (21).
Bioconversion of xylose and mannose to lyxose and glucose by two-step isomerization.
Bioconversion of xylose and mannose to lyxose and glucose was performed by using both the XI and YdaE BL enzymes in a sequential manner. Bioconversion of xylose to lyxose was performed using 50 U·ml−1 of XI treated with 10 mM xylose in the first catalysis in 50 mM Tris, pH 8.0, along with 1 mM Mn2+. The reaction mixture was incubated at 85°C, and xylulose formation was observed at regular intervals. At the end of saturation, 20 U·ml−1 of YdaE BL was added to the reaction mixture and the temperature was reduced to 40°C. Samples were drawn and checked for lyxose production by HPLC. In a similar fashion, 20 U·ml−1 YdaE BL was treated with 10 mM mannose under standard conditions and incubated at 40°C for 4.0 h. At the end of saturation of YdaE BL, 10 U·ml−1 of XI was added to the reaction mixture and the temperature was raised to 85°C. At regular intervals, samples were drawn and checked for glucose formation with a glucose estimation kit. Finally, the sugar mixture was checked by HPLC.
RESULTS
Cloning and expression of ydaE BL and XI in Escherichia coli.
Soluble forms of both recombinant proteins were obtained when they were expressed in E. coli strain BL21. SDS-PAGE analysis of Ni-NTA-purified proteins resulted in apparent molecular masses of 19.5 and 44 kDa for YdaE BL and XI, respectively (Fig. 2). The protein yield is summarized in Table 1; 10- and 32-fold purifications were achieved for YdaE BL (with mannose as a substrate) and XI (with fructose as a substrate), respectively. A total of 122 units of YdaE BL was produced from 400 ml of E. coli culture with 42 units of specific activity on mannose. The quantity of YdaE BL can be improved upon induction with isopropyl-β-d-thiogalactopyranoside (IPTG) or a different substrate as a carbon source. A total of 570 units of XI was expressed with a specific activity of 420 on fructose. XI from the thermophilic organism retained the correct folding when expressed under cold conditions in the mesophilic host.
Table 1.
Purification of YdaE BL and XIa
| Enzyme and purification step | Total units (μM·min−1) | Total protein (mg) | Sp act (U−1) | Yield (%) | Fold purification |
|---|---|---|---|---|---|
| YdaE BL | |||||
| Crude extract | 510 | 120 | 4.2 | 100 | 1 |
| Affinity column purification (pH 8.0) | 150 | 6.8 | 23 | 30 | 5.7 |
| Ultrafiltration (10 kDa, pH 8.0) | 130 | 3.0 | 42 | 24 | 10 |
| XI | |||||
| Crude extract | 1,800 | 130 | 13 | 100 | 1 |
| Affinity column purification (pH 8.0) | 1,400 | 5.2 | 270 | 78 | 20 |
| Ultrafiltration (10 kDa, pH 7.0) | 570 | 1.4 | 420 | 32 | 32 |
Results of recombinant YdaE BL and XI purification are shown. The activity of YdaE BL on lyxose was checked at pH 8.0 and 40oC in the presence of Mn2+, and XI activity was checked on a fructose substrate containing Mn2+ at pH 7.0 and 85oC.
Physicochemical characterization and thermal inactivation of YdaE BL.
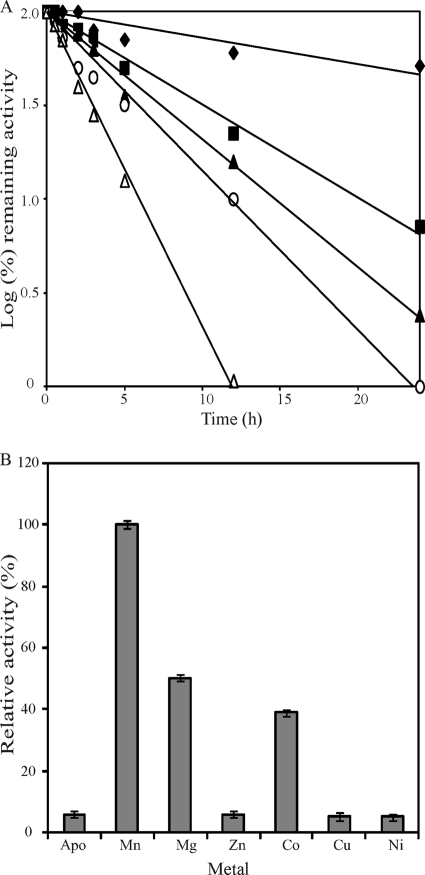
The characteristics of YdaE BL reveal that it is a mesophilic, alkaline, and metal-dependent enzyme. The optimal pH was found to be between pH 7.5 and 8.0. To determine the temperature at which the highest activity occurs, the temperature was varied from 30°C to 65°C. The enzyme activity increased as the temperature increased, showing higher activity at 40°C to 45°C, and was completely lost at temperatures greater than 65°C, an important feature of its two-step isomerization process. The residual activity does not interfere at elevated temperatures for a second isomerization reaction. Thermal inactivation followed first-order kinetics with half-lives of 140, 62, 30, 18, and 7 h at 30, 35, 40, 45, and 50°C, respectively.
Like most of the sugar isomerases, YdaE BL is also strictly activated in the presence of metal ions (Fig. 3 B) such as Mn2+, Mg2+, and Co2+, with Mn2+ causing maximum activation. Zn2+, Cu2+, and Ni2+ failed to activate YdaE BL. Various concentrations of Mn2+ and Mg2+ were tested to attain higher activity, and 1.0 mM Mn2+ produced the most activity (see the supplemental material).
Fig. 3.
(A) Thermal inactivation of YdaE BL at 30°C (filled diamonds), 35°C (filled squares), 40°C (filled triangles), 45°C (circles), and 50°C (triangles). (B) Effects of metal ions on the activity of YdaE BL. The reaction was run in 50 mM Tris-Cl (pH 8.0) containing 10 mM d-mannose at 40°C. Data are means of three experiments.
Determination of the kinetic parameters and substrate specificities of YdaE BL.
The Michaelis-Menten kinetic parameters (Km and Vmax) and catalytic efficiency (kcat) of YdaE BL were determined by plotting the reaction velocity (V0) against the substrate concentration ([S]). The affinities (Km) for lyxose and mannose were found to be 30.4 ± 0.7 mM and 26 ± 0.8 mM, respectively (Table 2). Lyxose showed a higher turnover number (kcat) and catalytic efficiency (kcat/Km), at 98 ± 0.2 (s−1) and 3.2 ± 0.1 (mM−1 s−1), respectively. Among the sugars tested for specificity for YdaE BL, d-xylulose showed the highest specific activity. YdaE BL showed no isomerization activity for d-glucose, d-xylose, l-arabinose, or l-rhamnose (Table 3).
Table 2.
Kinetic properties of YdaE BLa
| Substrate | Km (mM) | Vmax (U·mg−1) | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|---|
| d-Lyxose | 30.4 ± 0.7 | 902 ± 20 | 98 ± 0.2 | 3.2 ± 0.1 |
| d-Mannose | 26 ± 0.8 | 390 ± 1.2 | 43 ± 0.1 | 1.6 |
Kinetic properties of YdaE BL on d-lyxose and d-mannose. Data are means of three individual experiments.
Table 3.
Specific activities of YdaE BL
| Substrate | Product | Sp acta (μmol·min−1) | Equilibrium ratiob (aldose:ketose %) |
|---|---|---|---|
| d-Mannose | d-Fructose | 41 ± 1.3 | 62:38 |
| d-Fructose | d-Mannose | 12 ± 1.4 | 70:29 |
| d-Glucose | d-Fructose | ND | |
| d-Xylose | d-Xylulose | ND | |
| d-Xylulose | d-Lyxose | 78 ± 2.8 | 75:25 |
| d-Lyxose | d-Xylulose | 54 ± 1.2 | 65:35 |
| l-Arabinose | l-Ribulose | ND | |
| l-Rhamnose | l-Rhamulose | ND |
Data are means and standard deviations of three separate experiments ND, not detected. The reaction was run in 50 mM Tris-Cl (pH 8.0) containing 10 mM sugar and 1 mM Mn2+ for 30 min by treatment with at 1 to 10 U YdaE BL.
Equilibrium ratios were determined in 50 mM Tris-Cl (pH 8.0) containing 10 mM sugar, 10 U·ml−1 YdaE BL, and 1 mM Mn2+ at 40oC when an equilibrium between aldose and ketose was reached.
Bioconversion of lyxose and mannose to xylulose and fructose by YdaE BL.
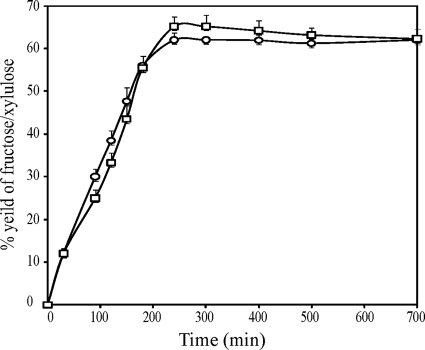
To investigate the conversion ratio, 10 mM lyxose and mannose was treated with 20 U·ml−1 of YdaE BL under optimized conditions of Mn2+, pH 8.0, and a 40°C temperature for different time intervals. d-Xylulose and d-fructose were quantified by resorcinol. After 300 min of incubation, saturation was observed for d-lyxose and d-xylulose. Sixty-five percent (6.5 mM) xylulose production was observed, leaving 35% (3.5 mM) d-lyxose. After 240 min of reaction time, 62% (6.2 mM) fructose production was observed, leaving 38% (3.8 mM) mannose (Fig. 4).
Fig. 4.
Bioconversion by YdaE BL of aldose d-mannose (circles) and d-lyxose (squares) to d-fructose and d-xylulose. Data represent three replicates.
Bioconversion of xylose and mannose to lyxose and glucose by two-step isomerization.
Figure 5 A shows the isomerization of d-xylose to d-lyxose and d-mannose to d-glucose after treatment with YdaE BL and XI. Initial 10 mM d-xylose underwent isomerization to d-xylulose by xylose isomerase and produced 5.5 mM after 7.0 h of incubation. The reaction was further extended by the addition of YdaE BL and produced 3.7 mM d-lyxose and left 1.8 mM d-xylulose. Similarly, 10 mM mannose was treated with 20 U·ml−1 of YdaE BL under optimized conditions and after 4 h of incubation, equilibrium between mannose and fructose was observed, and 6.2 mM mannose was isomerized to fructose. At the equilibrium point, 10 U·ml−1 of XI was added to the reaction mixture and the temperature rose to 85°C. At various time intervals, samples were analyzed for glucose formation and maximum glucose was observed after 90 min. The glucose yield was 3.8 mM, leaving 3.8 mM mannose and 2.4 mM fructose. The efficiency of XI was 12% lower at pH 8.0 than at pH 7.0. HPLC analysis clearly showed separate peaks of different sugars generated after two-step isomerization by two different isomerases (Fig. 5B and C).
Fig. 5.
Two-step isomerization process carried out by YdaE BL and XI for the production of d-lyxose and d-glucose from d-xylose and d-mannose, respectively. (A) Flow chart showing the saturation level observed at each reaction. At the end, 3.7 mM d-lyxose and 3.8 mM d-mannose were observed. Also shown are HPLC chromatograms of the bioconversion of d-xylose to d-lyxose (B) and d-mannose to d-glucose (C) at the end of the reaction. The retention times of lyxose and xylulose on this column are similar.
DISCUSSION
The diversity of prokaryotes is reflected by their great variation in modes of energy generation and metabolism, which allows them to flourish in all habitats suitable for life on Earth. The genomes of more than 380 microorganisms have been sequenced, and large numbers of hypothetical proteins have been identified by sequence homology. However, limited biochemical data are available regarding the functions of these hypothetical proteins (9, 35). Bacillus licheniformis, a soil bacterium with the ability to produce and secrete numerous hydrolytic enzymes that enable its cells to survive on a wide variety of substrates, has great industrial potential. The entire genome of B. licheniformis has been sequenced and found to contain two individual gene clusters dedicated to polysaccharide digestion (28). The ydaE gene is located after the alcohol dehydrogenase gene (ydaD) and is presumably activated by the transcriptional activator mtIR of the mannitol operon, suggesting that the primary function of ydaE is mannose isomerization to fructose, linking it to a central metabolic pathway. However, it is only through experimental work that the actual physiological function of these enzymes can be defined. Strain BL21 of E. coli harboring the gene ydaE is able to grow on d-lyxose as a carbon source, demonstrating the modification of a metabolic pathway to utilize d-lyxose (Fig. 6). Similar results have been obtained with an E. coli mutant that can utilize l-lyxose via the rhamnose pathway (6).
Fig. 6.
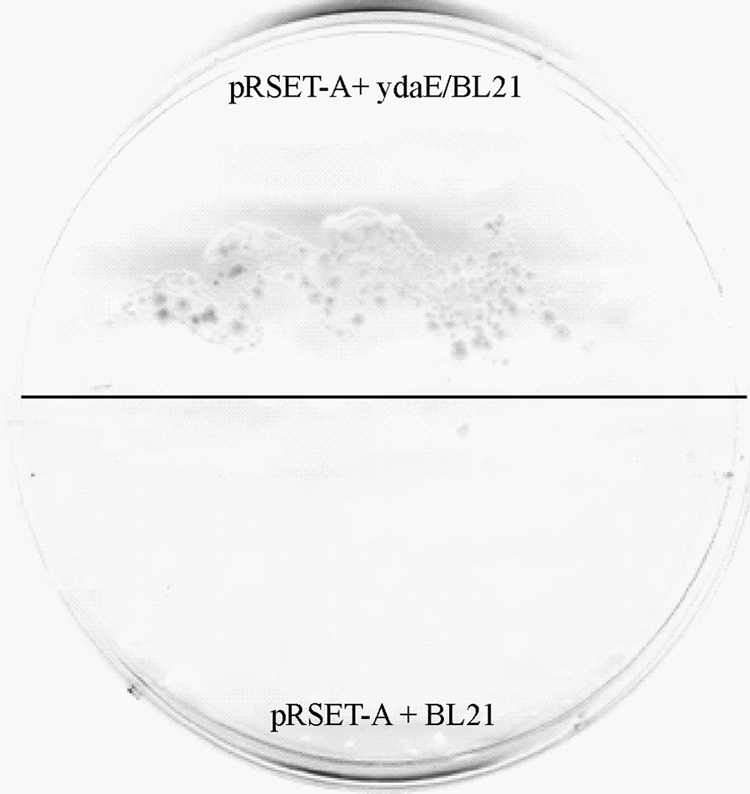
E. coli BL21 carrying ydaE of B. licheniformis is able to grow on d-lyxose as a sole carbon source in M9 salts medium, whereas empty-vector-containing E. coli BL21 does not initiate any growth on d-lyxose.
YdaE BL is a mesophilic, alkaline sugar isomerase that has characteristics similar to those of the d-lyxose isomerase of P. stuartii. The enzyme shows various levels of specificity for different sugars. The maximum specific activity was observed for d-xylulose, which points to YdaE BL as an attractive possible enzyme for d-lyxose production. However, this enzyme does not show activity for d-glucose, d-xylose, l-rhamnose, or l-arabinose, probably due to the positioning of the hydroxyl group on different carbons of these sugars (18). Typical of a sugar isomerase, YdaE BL requires a metal ion to initiate catalytic activity. However, the specific role of metals for mannose or lyxose isomerization remains to be clarified. The structural and catalytic mechanism of YihS of Salmonella spp. identified the responsibility of the histidine residue in the proton transfer between C1 and C2 of the d-mannose molecule (16). It is possible that YdaE BL uses the same mechanism of action, but its true function needs to be confirmed.
YdaE BL shows 58 and 76% similarity, respectively, to the characterized lyxose isomerase of C. laevoribosii (8) and an uncharacterized probable d-lyxose isomerase of Bacillus subtilis (GenBank accession no. BAA19258). An amino acid comparison indicates the great variations in their properties (Table 4). Thermal inactivation tests of YdaE BL show that mesophilic YdaE BL has a good capacity to withstand different temperatures. The higher thermal stability of YdaE BL can be explained by the higher content of the charged residues lysine, arginine, aspartic acid, and glutamic acid (KRED) (22). Table 4 shows the amino acid contents of YdaE BL, d-lyxose isomerase of C. laevoribosii, and the probable d-lyxose isomerase of B. subtilis. The d-lyxose isomerase of C. laevoribosii appears to be a thermophilic isomerase with a KRED content of 24.7%, the probable d-lyxose isomerase of B. subtilis shows 27.5% charged residues, and YdaE BL contains 31.1% charged residues. The N and Q content is generally lower in thermophilic proteins (1), decreasing their amydylation, and these proteins showed this pattern. The probable d-lyxose isomerase of B. subtilis has a higher N and Q content (7.8%) than YdaE BL (6.0%) and shows better biochemical properties than the sugar isomerase from B. subtilis. Although B. subtilis and B. licheniformis are closely related bacterial species that show 86.4% identity at the nucleotide level, they exhibit major differences in polysaccharide-digesting capacity. Two individual gene clusters for polysaccharide hydrolysis were identified in B. licheniformis for which there were no counterparts in B. subtilis (28). This indicates that the sugar-processing enzymes from B. licheniformis are superior in quality to those of B. subtilis.
Table 4.
Amino acid contents of B. licheniformis, C. laevoribosii, and B. subtilisa
| Organism | % Content of: |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ala | Cys | Asp | Glu | Phe | Gly | His | Ile | Lys | Leu | Met | Asn | Pro | Gln | Arg | Ser | Thr | Val | Trp | Tyr | |
| B. licheniformis | 3.6 | 1.8 | 9.0 | 10.8 | 4.8 | 5.4 | 3.6 | 4.8 | 8.4 | 6.0 | 1.8 | 2.4 | 6.6 | 3.6 | 3.0 | 2.4 | 7.8 | 8.14 | 1.2 | 4.8 |
| C. laevoribosii | 7.7 | 1.6 | 4.9 | 10.4 | 6.0 | 8.8 | 2.7 | 3.8 | 6.0 | 6.0 | 2.2 | 1.1 | 8.2 | 3.3 | 5.5 | 3.8 | 6.6 | 8.2 | 1.6 | 3.3 |
| B. subtilis | 1.8 | 1.8 | 5.4 | 11.4 | 3.0 | 8.4 | 3.6 | 4.2 | 6.6 | 6.6 | 1.8 | 1.8 | 7.2 | 6.0 | 4.2 | 3.0 | 7.8 | 8.4 | 1.2 | 6.0 |
Calculations were performed by using all of the open reading frames described in the genomic sequences present in GenBank.
The second major objective of the present investigation was to identify an efficient process for lyxose and glucose production by in vitro biocatalysis. Using two different isomerases and treating xylose and mannose in a sequential manner, we observed the bioproduction of lyxose, a medically important sugar, and glucose, which is widely utilized for bioenergy and fermentation processes. Production percentages of 37 and 38% were observed for lyxose and glucose, respectively.
Rare sugars are generally produced using microbial fermentations. Ahmed et al. (3) demonstrated lyxose production from glucose in a three-step process composed of two microbial fermentations and an enzymatic isomerization, and this accounted for 35% of the conversion ratio. The process is lengthy, and separation of the sugar from the fermentation medium is an obstacle. In contrast, two-step isomerizations under in vitro conditions have high potential because of their time- and cost-saving factors and allow for easy separations since there are only sugar mixtures in the catalytic reaction mixture. Helanto et al. (13) proposed an in vivo two-step isomerization in recombinant E. coli and Lactobacillus plantarum by introducing l-arabinose isomerase and achieved the bioconversion of l-arabinose to l-ribose, with l-ribulose as an intermediate product. Since recombinant protein expression and purification technology is a well-understood process, the same procedure can be performed quickly and easily under in vitro conditions by two-step isomerization. Structural rearrangement of sugars can also be achieved under subcritical water at 220°C, but the process is energy consuming and the production yield can vary from batch to batch (34).
The isomerization of mannose to glucose can enhance the fermentation process for bioenergy production. Although mannose and fructose are fermentable by Saccharomyces cerevisiae and other ethanol-producing organisms, the Km values for uptake by the various hexose transporters are much higher for mannose and fructose than for glucose (27). Furthermore, mannose and glucose compete for the same hexose transporters and the kinetics of utilization of a mixed substrate are determined by their relative and absolute concentrations in the sugar mixture. Keating et al. (19) reported that mannose was inferior to glucose for fermentation. The presence of equal amounts of glucose and mannose in the sugar mixture increases mannose utilization by organisms, and mannose may have a competitive advantage (31). Mannose and fructose are utilized according to the Kluyver rule (20), whereas glucose enters the glycolytic pathway directly. Using mannose as the sole carbon source inhibits the phosphomannose isomerase PMI40, which directs mannose into the glycolytic pathway (26); this constitutes another obstacle to the utilization of mannose for ethanol production.
In conclusion, a hypothetical protein encoded by ydaE of B. licheniformis was found to be a mesophilic, alkaline d-lyxose isomerase. It can be used in the industrial production of d-lyxose and d-mannose along with xylose isomerase. The two-step isomerization makes the process easy and convenient and can be applied at a commercial level to produce important sugars. Yields could be improved by protein engineering, enzyme immobilization, or isolating a better-performing sugar isomerase.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (project 2010-0020141) to H.-J. Bae and the World Class University project of the Ministry of Science and Technology of Korea (R31-2009-000-20025-0) to H.-J. Bae. Y.-H. Song is grateful for the BK21 Program provided by the Ministry of Education.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 18 March 2011.
REFERENCES
- 1. Ahern T. J., Klibanov A. M. 1985. The mechanisms of irreversible enzyme inactivation at 100°C. Science 228:1280–1284 [DOI] [PubMed] [Google Scholar]
- 2. Ahmed Z. 2001. Production of natural and rare pentoses using microorganisms and their enzymes. Electron. J. Biotechnol. 4:103–111 [Google Scholar]
- 3. Ahmed Z., et al. 1999. Production of d-lyxose from d-glucose by microbial and enzymatic methods. J. Biosci. Bioeng. 88:676–678 [DOI] [PubMed] [Google Scholar]
- 4. Akagi M., et al. 2002. A practical synthesis of l-ribose. Chem. Pharm. Bull. (Tokyo) 50:866–868 [DOI] [PubMed] [Google Scholar]
- 5. Anderson R. L., Allison D. P. 1965. Purification and characterization of d-lyxose isomerase. J. Biol. Chem. 240:2367–2372 [PubMed] [Google Scholar]
- 6. Badia J., et al. 1991. l-Lyxose metabolism employs the l-rhamnose pathway in mutant cells of Escherichia coli adapted to grow on l-lyxose. J. Bacteriol. 173:5144–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhosale S. H., Rao M. B., Deshpande V. V. 1996. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 60:280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho E. A., et al. 2007. Characterization of a novel d-lyxose isomerase from Cohnella laevoribosii RI-39 sp. nov. J. Bacteriol. 189:1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cordwell S. J. 1999. Microbial genomes and missing enzymes: redefining biochemical pathways. Arch. Microbiol. 172:269–279 [DOI] [PubMed] [Google Scholar]
- 10. Davies G. J., Henrissat B. 2002. Plant glyco-related genomics. Biochem. Soc. Trans. 30:292–297 [DOI] [PubMed] [Google Scholar]
- 11. Dekker K., Yamagata H., Sakaguchi K. K., Udaka S. 1991. Xylose (glucose) isomerase gene from the thermophile Thermus thermophilus: cloning, sequencing, and comparison with other thermostable xylose isomerases. J. Bacteriol. 173:3078–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granström T. B., Takata G., Tokuda M., Izumor K. 2004. Izumoring: a novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 97:89–94 [DOI] [PubMed] [Google Scholar]
- 13. Helanto M., Kiviharju K., Granström T., Leisola M., Nyyssölä A. 2009. Biotechnological production of l-ribose from l-arabinose. Appl. Microbiol. Biotechnol. 83:77–83 [DOI] [PubMed] [Google Scholar]
- 14. Hey-Ferguson A., Elbein A. D. 1970. Purification of a d-mannose isomerase from Mycobacterium smegmatis. J. Bacteriol. 101:777–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirose J., Maeda K., Yokoi H., Takasaki Y. 2001. Purification and characterization of mannose isomerase from Agrobacterium radiobacter M-1. Biosci. Biotechnol. Biochem. 65:658–661 [DOI] [PubMed] [Google Scholar]
- 16. Itoh T., Mikami B., Hashimoto W., Murata K. 2008. Crystal structure of YihS in complex with d-mannose: structural annotation of Escherichia coli and Salmonella enterica yihS-encoded proteins to an aldose-ketose isomerase. J. Mol. Biol. 377:1443–1459 [DOI] [PubMed] [Google Scholar]
- 17. Izumori k. 2002. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 89:120–124 [DOI] [PubMed] [Google Scholar]
- 18. Izumori K., Yamanaka K. 1977. Speculative studies on an anomeric specificity of inducers of d-lyxose isomerase. FEBS Lett. 77:133–135 [DOI] [PubMed] [Google Scholar]
- 19. Keating J. D., Robinson J., Cotta M. A., Saddler J. N., Mansfield S. D. 2004. An ethanologenic yeast exhibiting unusual metabolism in the fermentation of lignocellulosic hexose sugars. J. Ind. Microbiol. Biotechnol. 31:235–244 [DOI] [PubMed] [Google Scholar]
- 20. Kluyver A. J. 1914. Ph.D. thesis. Biochemische suikerbepalingen. Delft University of Technology, Delft, Netherlands [Google Scholar]
- 21. Kulka. R. G. 1956. Colorimetric estimation of ketopentoses and ketohexoses. Biochem. J. 63:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S., Tsai C. J., Nussinov R. 2000. Factors enhancing protein thermostability. Protein Eng. 13:179–191 [DOI] [PubMed] [Google Scholar]
- 23. Kwon H.-J., Yeom S.-J., Park C.-S., Oh D.-K. 2010. Substrate specificity of a recombinant d-lyxose isomerase from Providencia stuartii for monosaccharides. J. Biosci. Bioeng. 110:26–31 [DOI] [PubMed] [Google Scholar]
- 24. Morita M., et al. 1996. Practical total synthesis of (2S, 3S, 4R)-1-O-(α-d-galactopyranosyl)-N-hexacosanoyl-2-amino-1,3,4-octadecanetriol, the antitumoral and immunostimulatory α-galactosylceramide, KRN7000. Biosci. Biotechnol. Biochem. 60:288–292 [DOI] [PubMed] [Google Scholar]
- 25. Park C. S., Yeom S. J., Lim Y. R., Kim Y. S., Oh D. K. 2010. Substrate specificity of a recombinant d-lyxose isomerase from Serratia proteamaculans that produces d-lyxose and d-mannose. Lett. Appl. Microbiol. 51:343–350 [DOI] [PubMed] [Google Scholar]
- 26. Pitkänen J. P., et al. 2004. Excess mannose limits the growth of phosphomannose isomerase PMI40 deletion strain of Saccharomyces cerevisiae. J. Biol. Chem. 279:55737–55743 [DOI] [PubMed] [Google Scholar]
- 27. Reifenberger E., Boles E., Ciriacy M. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur. J. Biochem. 245:324–333 [DOI] [PubMed] [Google Scholar]
- 28. >Rey M. W., et al. 2004. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol. 5:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 30. Schallmey M., Singh A., Ward O. P. 2004. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 50:1–17 [DOI] [PubMed] [Google Scholar]
- 31. Stewart C. S., Duncan S. H., Richardson A. J., Calder A. G., Dewey P. J. S. 1995. The effect of the presence of glucose on the fermentation of mannose by anaerobic fungus Neocallimastix frontalis strain RE1. FEMS Microbiol. Lett. 127:57–63 [Google Scholar]
- 32. Takagi Y., Nakai K., Tsuchiya T., Takeuchi T. 1996. A 5′-(trifluoromethyl)anthracycline glycoside: synthesis of antitumor-active 7-O-(2,6-dideoxy-6,6,6-trifluoro-alpha-l-lyxo-hexopyranosyl)adriamycinone. J. Med. Chem. 39:1582–1588 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi H., Iwai Y., Hitomi Y., Ikegami S. 2002. Novel synthesis of l-ribose from d-mannono-1,4-lactone. Org. Lett. 4:2401–2403 [DOI] [PubMed] [Google Scholar]
- 34. Usuki C., Kimura Y., Adachi S. 2007. Isomerization of hexoses in subcritical water. Food Sci. Technol. Res. 13:205–209 [Google Scholar]
- 35. White R. H. 2006. The difficult road from sequence to function. J. Bacteriol. 188:3431–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamabhai M., et al. 2008. Secretion of recombinant Bacillus hydrolytic enzymes using Escherichia coli expression systems. J. Biotechnol. 133:50–57 [DOI] [PubMed] [Google Scholar]
- 37. Yeom S. J., Ji J. H., Kim N. H., Park C. S., Oh D. K. 2009. Substrate specificity of mannose-6-phosphate isomerase from Bacillus subtilis and its application in the production of l-ribose. Appl. Environ. Microbiol. 75:4705–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.