Abstract
The efficacy of Pseudomonas fluorescens EPS62e in the biocontrol of Erwinia amylovora was improved by a procedure of physiological adaptation to increase colonization and survival in the phytosphere of rosaceous plants. The procedure consisted of osmoadaptation (OA) and nutritional enhancement (NE). OA was based on saline stress and osmolyte amendment of the growth medium during inoculum preparation. NE consisted of addition of glycine and Tween 80 to the formulation. NE and OA increased the growth rate and carrying capacity of EPS62e under high-relative-humidity (RH) conditions and improved survival at low RH on flowers under controlled environmental conditions. NE did not promote growth or affect infection capacity of E. amylovora. The effect of both methods was tested in the field by following the population of EPS62e using quantitative PCR (Q-PCR) (total population) and CFU counting (culturable population) methods. Following field application, EPS62e colonized blossoms, but it was stressed, as indicated by a sharp decrease in culturable compared to total population levels. However, once established in flowers and at the end of bloom, almost all the total population was culturable. The physiological adaptation treatments increased population levels of EPS62e over those of nonadapted cells during the late stage of the flowering period. Control of fire blight infections in flowers and immature fruits was tested by field application of EPS62e and subsequent inoculation with E. amylovora under controlled-environment conditions. The efficacy of fire blight control increased significantly with the combination of nutritional enhancement and osmoadaptation, in comparison with the absence of physiological adaptation.
INTRODUCTION
Biological control is considered a promising method for the management of fire blight caused by Erwinia amylovora, but field evaluations indicate limitations due to variability in efficacy and consistency from trial to trial (14, 23, 30, 35). Several biological control agents are available commercially for the suppression of fire blight, such as Pseudomonas fluorescens A506 (38), Pantoea agglomerans C9-1 (12), P. agglomerans D325 (26), P. agglomerans Pc10 (36), Bacillus subtilis QST713 (1), and B. subtilis BD170 (5). Biological control agents of fire blight reduce or suppress E. amylovora on floral surfaces, particularly the stigmas, by competition for growth-limiting resources or antibiosis or by excluding the pathogen from infection sites (15, 37, 38). The research effort to identify new antagonists of E. amylovora in our laboratory led to the selection of P. fluorescens EPS62e for its high efficacy in controlling fire blight infections on different plant parts. The inhibition of E. amylovora by EPS62e relies on its superior fitness on pome fruit tree surfaces, due to a high efficiency in nutrient use, and direct cell-to-cell antagonistic interaction (6).
Because efficient fire blight control requires the establishment of the antagonistic bacterium on the surface of plant organs, prior to the arrival of the pathogen, an improvement of its ecological fitness may enhance its efficiency. However, field studies on fitness of biocontrol agents are difficult because of the need for methods to estimate population dynamics at strain level. The development of methods for the specific quantitative analysis of strain EPS62e by real-time quantitative PCR (Q-PCR) in combination with plate counting methods (24) provided a tool to analyze its population behavior in terms of the proportion of viable cells (24, 25). Using this approach, cell death of the biocontrol agent was observed immediately after field delivery due to the sharp change in the biological and physical environmental conditions from the optimal laboratory culture medium to the growth-limiting tree surface (25). It has also been reported that aerial surfaces of plants can be transiently inadequate for bacterial growth, because they are exposed to rapid and wide changes in water availability and temperature, UV light, and limitation in nutrients (18, 19, 32).
Stress tolerance in microorganisms can be induced by cultivation under suboptimal conditions. A procedure of osmoadaptation based on the combination of saline osmotic stress and osmolyte amendment of the growth medium has been used to increase drought stress tolerance and improve epiphytic survival and biocontrol efficacy of the apple blue mold biocontrol agent Pantoea agglomerans EPS125 (3) and the fire blight biocontrol agent P. fluorescens EPS62e (4).
Another strategy to increase the fitness of a biological control agent upon delivery to the field is the use of a formulation with nutrients that are more efficiently used by the biocontrol agent than by the pathogen. This method of nutritional enhancement has been reported to improve biocontrol of fungal rot in postharvest diseases (13). In the case of EPS62e, differences in nutrient use from that of E. amylovora have been observed (6), but nutritional enhancement has not been tested in fire blight biocontrol.
These findings suggest that the combination of osmoadaptation and nutritional enhancement may improve population levels of EPS62e on flowers and other plant surfaces, especially in the short period after application in the field and colonization of pathogen entry sites. However, a combination of the two methods of physiological adaptation of a biocontrol agent against fire blight has not been reported.
The aim of the present work was to determine which carbon sources are used for growth more efficiently by EPS62e than by E. amylovora strains, to study the effect of nutritional enhancement and its combination with osmoadaptation on fitness of EPS62e on pome fruit blossoms, and to determine the effect of these treatments on the efficacy of control of infections by E. amylovora.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. fluorescens EPS62e was cultured, for routine use, in Luria-Bertani (LB) broth to obtain standard cells or in glucose minimal medium (GMM) plus NaCl and glycine betaine (GB) to obtain osmoadapted cells as previously described (4). To monitor culturable populations of EPS62e in the field, a spontaneous mutant resistant to nalidixic acid (EPS62e Nalr) was selected on LB agar supplemented with 50 mg liter−1 of nalidixic acid. The resistant strain is similar to the parental strain in colony morphology, growth rate characteristics, and fitness in the phytosphere of rosaceous plants (data not shown). E. amylovora EPS101, isolated from an infected shoot of a Conference pear tree in Lleida (Spain), was used in pathogen inoculation experiments (7). In some experiments, E. amylovora CUCM273 (kindly provided by S. V. Beer), isolated from Malus sylvestris, and CFBP1430 (from the Collection Française de Bactèries Phytopathogènes), isolated from Crataegus sp., were used. Ultrafreeze-preserved cultures (−80°C) of the pathogen and EPS62e were grown overnight at 25°C in LB agar. Colonies were scraped from the agar surface and suspended in sterile distilled water. The cell culture was adjusted to an optical density (OD) corresponding to 1 × 108 CFU ml−1 and diluted with sterile distilled water to obtain an appropriate concentration.
Effect of carbon sources on growth.
Strains EPS62e, CUCM273, CFBP1430, and EPS101 were grown in GMM in which glucose was replaced by one of 16 carbon sources at 50 mM comprising sugars (l-arabinose, d-fructose, d-galactose, d-glucose, and d-xylose), amino acids (d-alanine, l-asparagine, l-cysteine, glycine, and l-leucine), organic acids (acetic acid, citric acid, malonic acid, and dl-β-hydroxybutyric acid), and other compounds (myoinositol and Tween 80 [used at 5 mM]). To confirm the ability of EPS62e to use selected nutrients for growth, the medium was inoculated with a 1% (vol/vol) stationary-phase preculture grown in GMM. Growth was monitored at 25°C for 48 h using a Bioscreen C microplate reader (Labsystems, Helsinki, Finland) by means of measurements of OD at 600 nm. A vibration shaking of 2 min was programmed before OD reading to prevent cell sedimentation and favor aeration. OD was measured at 10-min intervals for each medium composition condition and replicate. Maximum OD and specific growth rate (μ) were determined from OD measurements. Triplicates of each treatment were done, and two independent experiments were performed. The growth rate obtained for strains was classified as low (μ < 0.1 h−1) or high (μ ≥ 0.1 h−1). Also, carbon sources were considered better used by EPS62e than by E. amylovora when the corresponding carbon source growth ratio was >2.
Effect of nutritional enhancement and osmoadaptation on EPS62e fitness under controlled-environment conditions.
The survival of nutrient-enriched, osmoadapted, nutrient-enriched and osmoadapted, and standard EPS62e cells was determined on detached pear and apple flowers under high- and low-relative-humidity (RH) conditions. Open blossoms were collected from two commercial orchards and taken to the laboratory. Blossoms were treated with a hand sprayer to runoff point (5 ml per blossom) with a suspension of EPS62e Nalr cells prepared according to the standard procedure (STA; cultured in LB broth), nutrient enriched (NE; cultured in LB broth and enriched with glycine at 50 mM and Tween 80 at 5 mM), osmoadapted (OA; cultured in GMM plus 0.7 M NaCl and 0.1 mM GB), or nutrient enriched and osmoadapted (NEOA; cultured in GMM plus 0.7 M NaCl and 0.1 mM GB and enriched with glycine and Tween 80). To prepare the inoculum, cells were cultured for 24 h at 25°C, harvested by centrifugation, resuspended in water to 108 CFU ml−1, and (depending on the treatment) enriched with nutrients. The procedure used for inoculation of flowers was essentially as described by Pusey (26). The individual flowers were maintained with the cut peduncle submerged in 1 ml of a 5% sucrose solution in a single plastic Eppendorf vial of 1.5 ml. Vials containing flowers were placed in plastic tube racks in controlled-environment chambers at 20°C, in the dark. The treated flowers were split into two sets that were incubated under high (90%)- or low (50%)-RH conditions in controlled-environment chambers (PGR-15 [Conviron, Canada] and SGC097.PFX.F, Fitotron [Sanyo Gallenkamp PLC, United Kingdom]). Low RH was obtained by placing CaCl2 into the chamber as a humidity absorber. Sampling for monitoring population levels of EPS62e was performed at 0, 1, 2 or 4, and 5 or 6 days depending on the experiment. Samples of 5 flowers were taken from each replicate and sampling date. Flowers were homogenized in a sterile plastic bag with 20 ml of 0.05 M phosphate buffer (pH 7.0) and 0.1% peptone using a stomacher (Masticator; IUL Instruments, United Kingdom). Extracts obtained were serially diluted, and appropriate dilutions were seeded using a spiral plater system (Eddy Jet; IUL Instruments, Spain) onto LB agar plates. The LB plates were supplemented with 50 μg ml−1 of nalidixic acid to counterselect the strain inoculated and with 50 μg ml−1 of econazole nitrate salt to avoid fungal growth. Plates were incubated at 25°C for 48 h, and the colonies were counted using an automatic counter system (Countermat Flash; IUL Instruments, Spain). The population levels of EPS62e were expressed as log10 CFU per flower. Three replicates of 25 flowers per replicate were used for each treatment and incubation conditions. Two independent experiments were performed, at high RH using Passe Crassane and Abate Fetel pear flowers and at low RH using Golden apple and Doyenne du Comice pear flowers.
Effect of nutritional amendment on performance of EPS62e and on E. amylovora infections.
In spite of the nutrients glycine and Tween 80 being only slightly used by E. amylovora strains, they could have an effect on infections. For this reason, an experiment was done under controlled environmental conditions on detached flowers of Passe Crassane and Conference pear to determine the effect of nutrients on performance of EPS62e and on E. amylovora infections. Individual flowers were treated by spraying them with nutrients (glycine, Tween 80, and glycine plus Tween 80) or with water and also with EPS62e at 108 CFU ml−1 alone or amended with one of the nutrients or with their combination. Vials containing treated flowers were placed in plastic tube racks in controlled-environment chambers at 20°C and 90% RH in the dark. After 24 h, hypanthia of flowers were inoculated with 10 μl of a suspension of E. amylovora EPS101 at 107 CFU ml−1. The inoculated flowers were placed again in plastic boxes and incubated at 20°C and high relative humidity for 5 days. Three replicates of 8 flowers were used for each treatment. Infection incidence on flowers was evaluated for each replicate 5 days after pathogen inoculation.
Effect of nutritional enhancement and osmoadaptation on EPS62e fitness under field conditions.
Field experiments were performed at the Mas Badia Agricultural Experiment Station (Girona, Spain) in Doyenne du Comice pear and Modi and Golden apple tree orchard plots. During the experiments, weather conditions were measured by a meteorological station. Air temperature and RH were monitored with CR10 or CR10X data loggers (Campbell Scientific Ltd., Leicester, United Kingdom) connected to a combined temperature-RH sensor (model MP100 or HMP35AC), and rainfall was monitored with an ARG 100 sensor (Campbell Scientific Ltd., Leicester, United Kingdom). Temperature, RH, and rainfall were measured every 10 min, and means were recorded hourly.
The survival of NE, OA, NEOA, and STA treated EPS62e cells was determined on pear and apple blossoms under field conditions. In each experiment, treatments were distributed following a completely randomized block design with 3 replications of each treatment and 10 trees per replicate. In each tree, two branches containing blossoms were tagged. Treatments corresponded to suspensions of STA, NE, OA, and NEOA cells (see above). Open blossoms from tagged branches were spray inoculated until near runoff with the bacterial suspension using a hand-held 1-liter sprayer (5 ml per blossom). Treatments were applied to trees on 6 April 2009 (Doyenne du Comice pear) and 16 April 2009 (Golden and Modi apple).
For monitoring population levels, samples were taken at 0, 1, and 10 days after field application of EPS62e. Samples of two blossoms (each with 4 to 6 flowers and accompanying leaves) were taken from each replicate of the corresponding treatment and sampling date and were homogenized as described above. Two methods were used to estimate population levels of the biocontrol agent. To assess the total population size, a real-time PCR method was used, whereas the CFU-counting method was used to estimate the culturable population as described previously (24). The quantification of the total cell number was obtained by means of a standard curve specifically developed for each trial (apple or pear blossoms). An extract of each type of plant material, without biocontrol agent treatment, was mixed with several dilutions of known concentrations of an EPS62e cell suspension prior to DNA extraction. The concentrations used covered a 5-log range (from 105 to 109 CFU blossom−1). Three replicates of each concentration were used in the PCR to obtain the standard curve. The quantification of samples was obtained by plotting the threshold cycle (CT), i.e., the cycle in which the fluorescence emitted by an amplified product crosses over a threshold fixed at 0.03, against the concentration of known samples.
For CFU counting, dilutions of sample homogenates were plated in LB agar supplemented with 50 mg liter−1 of econazole nitrate salt and 50 mg liter−1 of nalidixic acid to select EPS62e colonies as described above.
Effect of nutritional enhancement and osmoadaptation on EPS62e efficacy of fire blight control.
Plant material (blossoms and immature fruits) was treated in the experimental orchard plots with NE, OA, NEOA, and STA EPS62e cells. Treated blossoms and immature fruits were collected 48 h after the treatment to inoculate the pathogen and perform efficacy assays in the laboratory under controlled-environment conditions. This was because the pathogen is a quarantine organism in the European Union and Spain is considered a protected zone. So, it is extremely difficult to conduct ideal experiments with trees in orchards because artificial inoculation of E. amylovora is not allowed. Efficacy assays were performed on Golden and Modi apple blossoms, Doyenne du Comice pear blossoms, and Conference immature pear fruits. In each assay, treatments were distributed in a completely randomized block design with 3 replications of each treatment and 10 trees per replicate. In each tree, two branches containing blossoms or immature fruits were tagged. Blossoms or immature fruits were sprayed to runoff point (5 ml of bacterial suspension per blossom or fruit). To prepare the treatment suspension, cells were prepared as described above, depending on the treatment. The individual flowers treated in the field were collected and prepared in the laboratory as described above in single Eppendorf vials (26). Treated immature fruits were also collected from the field and wounded with a flame-sterilized cork borer (four wounds per fruit) (5-mm depth, 3-mm diameter), placed in polystyrene tray packs into plastic boxes, and maintained overnight at 20°C and high RH to permit colonization of wounds by the surface population of EPS62e. Then, the hypanthia of flowers and the fruit wounds were inoculated with 10 μl of a suspension of E. amylovora EPS101 at 107 CFU ml−1. The inoculated plant material was placed again in plastic boxes and incubated at 20°C and high RH for 5 days. The experimental design consisted of three replicates of 8 flowers or 4 immature fruits per replicate for each treatment and sampling date. Nontreated controls inoculated with water or with the pathogen alone were included. Infection incidence on flowers and fruits was evaluated for each replicate after 5 days of pathogen inoculation.
Data analysis.
To test the significance of the effect of treatments (nutritional enhancement, osmoadaptation, or their combination) on the population level and biocontrol efficacy of EPS62e under different environmental conditions, a one-way analysis of variance was performed. Means were separated according to the Waller-Duncan test at P ≤ 0.05. The statistical analyses were performed using the GLM procedure of the PC-SAS (version 9.1; SAS Institute Inc., Cary, NC).
RESULTS
Growth of EPS62e on carbon sources compared to E. amylovora
EPS62e has a wider spectrum of carbon source utilization than do E. amylovora strains (Fig. 1). EPS62e grew at a high rate in minimal medium supplemented with d-fructose, d-glucose, d-xylose, l-arabinose, d-alanine, glycine, l-asparagine, l-cysteine, myoinositol, or Tween 80. E. amylovora strains showed a reduced capacity to grow on the assayed carbon sources, especially strains CUCM273 and CFBP1430, which used efficiently (high specific growth rate) only 2 and 3 carbon sources out of the 16 assayed, respectively. Strain EPS101 showed a wider range than the other two strains and used 7 out of the 16 carbon sources. EPS62e has a carbon source utilization ratio (see above) higher than 2 when cultured in minimal medium supplemented with d-glucose (2.2), l-arabinose (2.4), glycine (8), or Tween 80 (2.5). These compounds were suitable candidates as nutrient supplements because they were more efficiently used by EPS62e than by E. amylovora strains. Since sugars are used by many epiphytic microorganisms, glycine and Tween 80 were the compounds finally selected for the nutritional enhancement strategy.
Fig. 1.
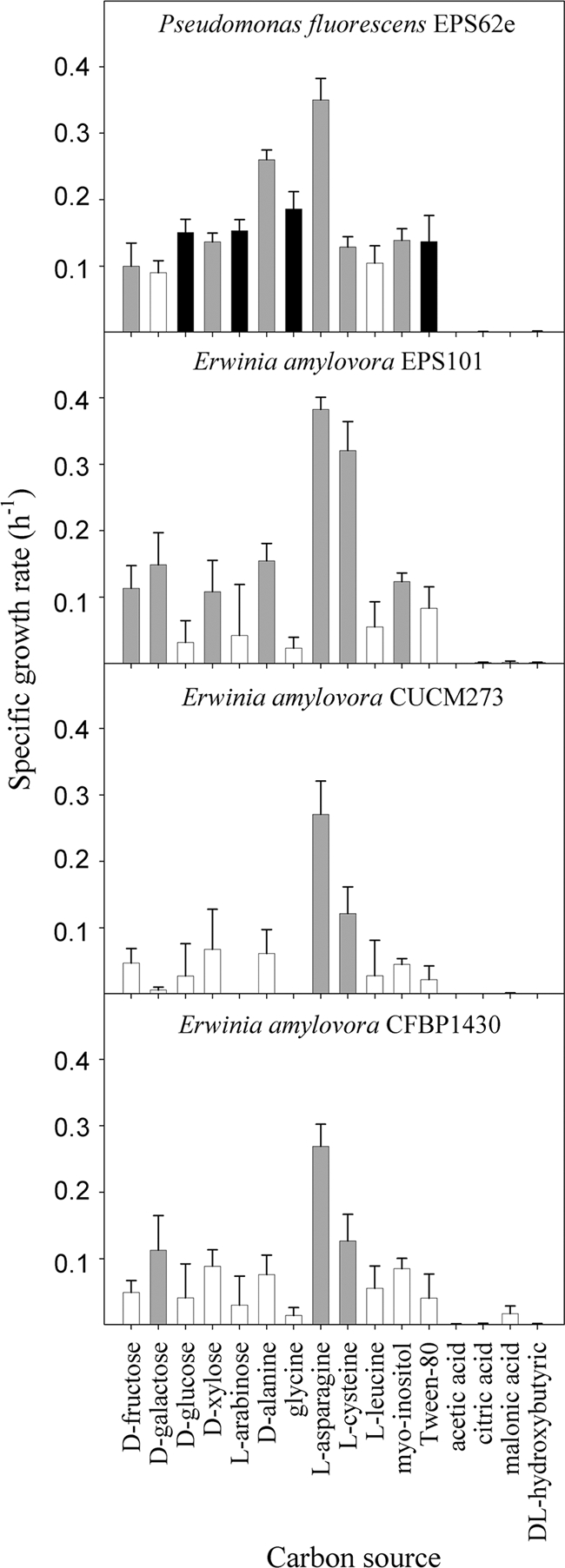
Growth rates of P. fluorescens EPS62e and Erwinia amylovora strains EPS101, CUCM273, and CFBP1430 in minimal medium supplemented with different carbon sources. Error bars represent the 95% confidence intervals. Growth intensity was classified as low (<0.1 h−1) (white bars) or high (>0.1 h−1) (gray or black bars). Black bars represent carbon sources that provided a ratio of the two rates (EPS62e/Ea strain) higher than 2.
Effect of osmoadaptation and nutritional enhancement under controlled-environment conditions.
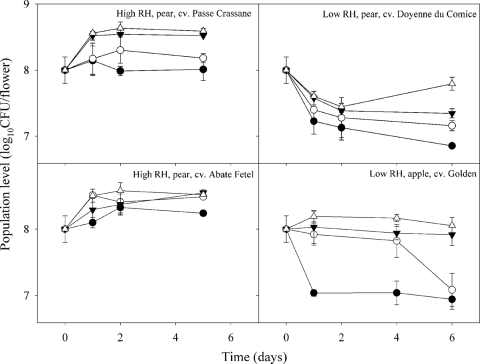
Under high-RH conditions, STA EPS62e cells (see Materials and Methods) maintained initial population levels or slightly increased on flowers, around 108 CFU flower−1, in Passe Crassane and Abate Fetel pear flowers (Fig. 2). However, a significant increase of the population was observed in the NE and OA treatments in both pear cultivars. The combined strategy produced the highest increase in both pear cultivars at all dates assessed. The increase attained 5 days after the treatment in the combined strategy was 0.5 (Passe Crassane) and 0.6 (Abate Fetel) log higher than that with the STA treatment.
Fig. 2.
Effect of nutritional enhancement, osmoadaptation, and their combination on survival of P. fluorescens EPS62e on apple and pear flowers at high (90%) and low (50%) relative humidity (RH) under controlled-environment conditions. Detached flowers were sprayed with standard EPS62e cells (cultured in LB) (•), nutrient-enriched cells (cultured in LB broth and amended with glycine and Tween 80) (○), osmoadapted cells (cultured in minimal medium with NaCl plus glycine betaine) (▾), or osmoadapted and nutrient-enriched cells (cultured in minimal medium with NaCl plus glycine betaine and enriched with glycine and Tween 80) (▵). Values are the means of three replicates. Error bars represent the 95% confidence intervals of the means.
Under low-RH conditions, the EPS62e behavior in both Golden apple and Doyenne du Comice pear detached flowers was clearly different from those at high RH, because population levels decreased from 108 to around 107 CFU flower−1 in the STA treatment. Globally, the combination of NE with OA had the highest effect on the improvement of survival in both pear and apple flowers. After 6 days, population levels were maintained within the initial values observed after inoculation, around 108 CFU flower−1, 1 log higher than that in the STA treatment. NE and OA treatments alone were less effective than NEOA, this effect not being significantly different for OA in Golden apple flowers.
Effect of the addition of nutrients on the infection capacity of E. amylovora.
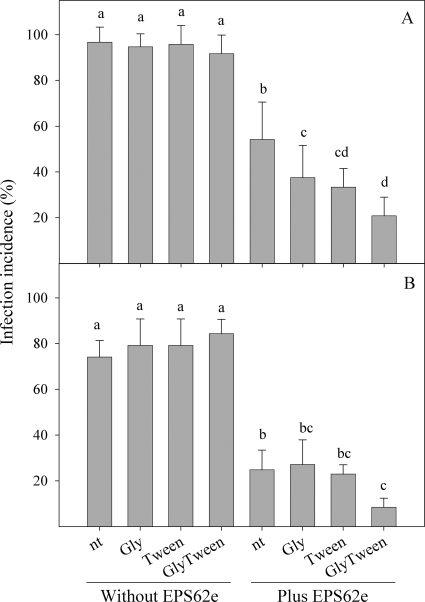
The incidence of infections by E. amylovora was not significantly affected by the treatment with the nutrients selected for the NE strategy (Fig. 3). Interestingly, the combination of EPS62e with glycine plus Tween 80 significantly decreased infection incidence compared to that with the strain alone in both pear cultivars. However, the amendment of EPS62e with a single carbon source (either Tween 80 or glycine) reduced infection levels significantly in Passe Crassane flowers but not in Conference flowers.
Fig. 3.
Effect of nutritional enrichment of P. fluorescens EPS62e on susceptibility of flowers to infection by E. amylovora. Detached flowers of Passe Crassane (A) and Conference (B) pear were treated with nutrients alone or with EPS62e (108 CFU ml−1) amended with glycine (Gly), Tween 80 (Tween), or glycine plus Tween (GlyTween) or treated with water (nt). Flowers were treated with the cell suspension and after 24 h inoculated with E. amylovora at 107 CFU ml−1. Values are the means of three replicates. Error bars represent the 95% confidence intervals of the means. Bars with the same letter in the same panel do not differ significantly (P < 0.05) according to the Waller-Duncan test.
Effect of osmoadaptation and nutritional enhancement on the survival of EPS62e under field conditions.
Weather conditions during field experiments were characterized by frequent but slight rainfalls of less than 15 mm (data not shown), and the temperature ranged from 10 to 18°C during the bloom period (mean, 12.1°C). The experiment was conducted in a relatively wet period with a fluctuation of the wetness duration of between 8 and 15 h per day, except for a dry period which happened on day 7 in the apple assay and on days 8, 9, and 11 in the pear assay. The relative humidity was around 75% in the pear assay, whereas in the apple trial it decreased to 50% on days 8 and 9. In general, weather conditions were suitable for EPS62e establishment in blossoms, except for the dry warmer periods, in which RH was lower than 70% without wetness.
Colonization of blossoms by EPS62e was evaluated by both real-time PCR (total population) and plate counting (culturable population) methods (Table 1). Using both techniques simultaneously, we were able to determine the degree of activity of the introduced strain in the field. Immediately after field application, the total populations (T) and culturable levels (C) were similar (data not shown). Mean values were 8.05 log10 CFU/blossom (Modi apple, 8.13 log10 CFU/blossom; Golden apple, 7.96 log10 CFU/blossom; Doyenne du Comice pear, 8.03 log10 CFU/blossom). One day after the field application, T were around 10 times higher than C, indicating that there was a considerable stress of the cells due to the transition from pure culture in synthetic media to the plant surface under field conditions. Interestingly, T values assessed after 10 days from delivery of EPS62e were similar to C, indicating that practically all cells were active because the population of the biocontrol agent was established on trees. Globally, from days 1 to 10, T values decreased around 1 to 2 log, especially in the STA cells, whereas C values slightly decreased in the STA cells but remained stable or even increased slightly in the treatments.
Table 1.
Total and culturable population levels of P. fluorescens EPS62e in blossoms at 1 and 10 days after inoculation in orchard plots of apple and pear cultivars, depending on the treatment
| Apple or pear cultivar | Timea (days) | Treatmentb | Total cells (T)c,d (log10 cells/blossom) | Culturable cells (C)d,e (log10 CFU/blossom) | Significance of differences between T and C (P < F)f |
|---|---|---|---|---|---|
| Modi apple | 1 | STA | 8.2 a | 6.8 ab | 0.0082 |
| NE | 7.4 b | 6.7 b | 0.0068 | ||
| OA | 8.3 a | 7.0 ab | 0.0056 | ||
| NEOA | 7.7 ab | 7.2 a | 0.0118 | ||
| 10 | STA | 6.2 b | 6.4 b | 0.6862 | |
| NE | 7.1 a | 7.3 a | 0.5750 | ||
| OA | 7.3 a | 7.3 a | 0.8464 | ||
| NEOA | 7.5 a | 7.5 a | 0.8954 | ||
| Golden apple | 1 | STA | 7.6 ab | 6.4 c | 0.0079 |
| NE | 7.2 b | 6.6 bc | 0.1077 | ||
| OA | 7.6 ab | 6.8 ab | 0.0085 | ||
| NEOA | 7.8 a | 7.1 a | 0.0003 | ||
| 10 | STA | 6.1 b | 6.1 c | 0.3592 | |
| NE | 6.7 ab | 6.9 b | 0.4350 | ||
| OA | 6.9 a | 7.0 ab | 0.8989 | ||
| NEOA | 7.3 a | 7.3 a | 0.9495 | ||
| Doyenne du Comice pear | 1 | STA | 8.2 a | 7.0 a | 0.0006 |
| NE | 7.6 ab | 6.9 a | 0.0453 | ||
| OA | 7.8 ab | 7.1 a | 0.1017 | ||
| NEOA | 7.4 b | 7.4 a | 0.6902 | ||
| 10 | STA | 6.8 a | 5.9 b | 0.0298 | |
| NE | 6.7 a | 6.9 a | 0.4429 | ||
| OA | 7.1 a | 7.3 a | 0.6368 | ||
| NEOA | 7.2 a | 7.3 a | 0.6503 |
Number of days after treatment.
Blossoms were treated in the field with EPS62e cells (STA) cultured in LB, nutrient-enriched cells (NE) cultured in LB and enriched with glycine and Tween 80, osmoadapted cells (OA) cultured in minimal medium with NaCl plus glycine betaine, or osmoadapted and nutrient-enriched cells (NEOA) cultured in minimal medium with NaCl plus glycine betaine and enriched with glycine and Tween 80.
Total population level determined by Q-PCR.
Values are the means of three replicates. Different letters within each sampling day and cultivar indicate significant differences between treatments according to a Waller-Duncan test (P < 0.05).
Culturable population level determined by plate counting method.
P < F, probability of the estimator F.
When treatments were compared with STA, the following patterns were observed depending on the sampling time and the method of analysis of the population levels.
At 1 day after inoculation and in the case of T values, there were differences between NE and STA in Modi apple and between NEOA and STA in Doyenne du Comice pear. In the case of C, only values in OA and NEOA were higher than those in STA in Golden apple. At 10 days after inoculation, T values were higher than those in STA in all treatments in Modi apple and in NEOA and OA treatments in Golden apple and C values were higher than those in STA in all treatments in both apple and pear cultivars.
Comparisons between treatments indicated that for C levels at 1 day after inoculation of EPS62e, there were differences between NEOA and NE in Modi and Golden apple but not in Doyenne du Comice pear. Ten days after inoculation, no significant differences were observed between treatments in Modi apple and Doyenne du Comice pear, but there were differences between NEOA and NE in Golden apple.
Effect of nutritional enhancement and osmoadaptation on EPS62e efficacy of fire blight biocontrol.
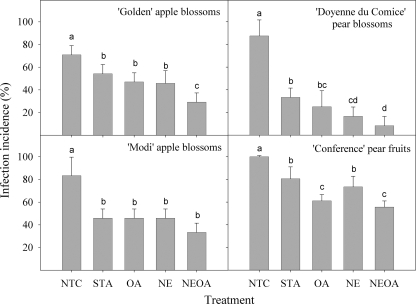
The effect of field application of EPS62e alone (STA) or formulated (NE, OA, and NEOA) on the incidence of infections of E. amylovora is shown in Fig. 4. In this experiment the biocontrol agent was applied on the tree blossoms or immature fruits, and after 48 h, the plant material was transported to the laboratory and inoculated with E. amylovora and the infection process was developed under controlled-environment conditions. In the four trials performed, infection incidence significantly decreased by EPS62e treatment (STA). In the single treatments, OA differed significantly from STA in the immature fruit in Conference pear and NE differed from STA in the Doyenne du Comice pear blossoms. However, the treatment consisting of the combination of OA and NE was better than STA in 3 out of the 4 trials (except in Modi apple blossoms). Also, the combined treatment differed significantly from both single treatments in Golden apple blossoms. Thus, the efficacy for the STA treatment ranged from 19.4 to 62.0%, whereas in the combined treatment the efficacy ranged from 44.5 to 90.5%.
Fig. 4.
Effect of field treatments with physiologically improved P. fluorescens EPS62e on susceptibility of flowers and immature fruits to infection by E. amylovora. Blossoms of apple and pear or pear immature fruits were treated under field conditions. The treatments correspond to standard EPS62e cells (STA), nutrient-enriched cells (NE), osmoadapted cells (OA), osmoadapted and nutrient-enriched cells (NEOA), or no treatment (NTC). At 48 h after the field treatment, blossoms or immature fruits were collected, transported to the laboratory, inoculated with a cell suspension at 107 CFU ml−1 of E. amylovora, and incubated under controlled-environment conditions. Values are the means of three replicates. Error bars represent the 95% confidence intervals of the means. Bars with the same letter in the same panel do not differ significantly (P < 0.05) according to the Waller-Duncan test.
DISCUSSION
In the present study, we developed a strategy to improve fitness and efficacy of P. fluorescens EPS62e to fight fire blight based on a combination of nutritional enhancement and osmoadaptation. Inconsistency of control encountered under field conditions is a major obstacle for implementing biological control of fire blight. This can be due to many reasons, including the performance of the biocontrol agent (33), pathogen aggressiveness (7), type (species and cultivar) and phenological state (prebloom, bloom, harvest, etc.) of the host, or environmental conditions (climate and location) (33). Then, to counteract partially the risk of inconsistent results, we covered a wide range of host conditions using pear and apple as model plant species, different apple (Modi and Golden) and pear (Doyenne du Comice, Abate Fetel, Passe Crassane, and Conference) cultivars, and different plant organs (flowers and immature fruit).
In previous studies, we have reported that osmoadaptation increased drought stress tolerance and improved epiphytic survival of the apple blue mold biocontrol agent Pantoea agglomerans EPS125 (3) and the fire blight biocontrol agent P. fluorescens EPS62e (4). Nutritional enhancement of the biocontrol agent cell suspension with nutrients before delivery to the plant environment has been reported to enhance survival and adaptability (9) as well as biocontrol efficacy in several fungal plant pathogens (8, 11, 13, 22). However, nutrients have to be carefully chosen for each particular biocontrol agent/pathogen system, because nutritional enhancement may have unexpected effects, like potentiation of the pathogen activity. To select the most suitable nutrients, we have taken into account a previous study on nutrient use by EPS62e and E. amylovora performed with the Biolog system (6). Data on growth potential on a given nutrient source are more relevant for colonization (21) than simply oxidation as determined by the Biolog system. Thus, in the present study, we demonstrate that EPS62e cells proliferate on glycine and Tween 80, whereas growth of E. amylovora on these carbon sources is poor. Interestingly, we further confirmed that there was no effect of either carbon substrate on E. amylovora infection potential and that the addition of these compounds to EPS62e cell suspensions significantly improved fire blight biocontrol in detached apple and pear flowers. Additionally, these nutrients have several advantages. Glycine is an amino acid acting as a carbon and nitrogen source which is detected in small amounts in stigma exudates of pear and apple flowers (10, 29). Moreover, Tween 80 is a detergent and enhances bacterial dispersion in plant surfaces and helps to reduce surface tension at the liquid-solid interface, facilitating air distribution. Notably, Tween 80 is used as an adjuvant in the formulation of several biopesticides (2, 17, 31).
Following a single field application during bloom, EPS62e colonized blossoms and reached population levels near the carrying capacity of apple and pear flowers (6, 24, 25); this behavior on blossoms is similar to that of other biocontrol agents of fire blight (16, 27, 37) that efficiently colonize blossoms when phenoclimatic conditions during spring are optimal (14). However, under limiting environmental and/or nutritional conditions, growth and colonization of biocontrol agents are not optimal. Also, during delivery of the biocontrol agents to the orchard a sharp change in the environment conditions from laboratory to field occurs, affecting survival and colonization (25). The monitoring of the population of the introduced EPS62e strain into orchards using real-time PCR and CFU-counting methods provided information on the population viability. In the present work, we have observed 10-times-higher quantitative PCR (Q-PCR) values than culturable values in the immediate period following field introduction, indicating that a large part of the cells were dead or not culturable. However, at the end of the bloom period, the population levels estimated by Q-PCR and C were similar, indicating that practically all cells at this time were viable. This is in agreement with our previous reports on the fitness of this biocontrol agent (24, 25). In addition, the fact that T values decreased progressively from days 1 to 10, until they approached levels similar to C, is probably an indication that stressed cells finally die and lyse, especially DNA, which is the target of Q-PCR.
Survival and colonization of EPS62e on flowers were significantly improved by physiological adaptation, and the beneficial effect was more significant under limiting RH than under high RH. Under field conditions, the beneficial effect was important compared to the STA procedure, especially upon establishment of the population at the end of the bloom period, but differences in survival between NE, OA, and combined treatments were generally not significant. The effect of the treatments seemed to be stronger when some limitation occurred on blossoms, such as low availability of nutrients and/or unfavorable environmental conditions. Because the shelf life of apple and pear flowers is between 1 and 2 weeks (depending on cultivar and environmental conditions), it is assumed that the nutrients on flowers become limiting as soon as the bloom period resumes (20, 28, 34). This happened in our study at the end of the bloom period; growth of EPS62e on flowers was presumably affected by limiting environmental conditions, such as a warmer dry period. We believe that in this period both nutrient limitation and unfavorable weather conditions have decreased populations of EPS62e on flowers, and this effect was minimized with osmoadaptation and nutritional enhancement.
The combined strategy of physiological adaptation based on NE and OA improved biocontrol of E. amylovora in both blossoms and immature fruits. This beneficial effect is related to the increase of the growth capacity and survival of EPS62e on apple and pear tree surfaces. However, there are other possible effects. Particularly, nutritional enhancement could trigger natural protective processes such as favoring the development of beneficial microorganisms or inducing antimicrobial production by other microorganisms.
A ready-to-use formulation with the physiologically adapted biocontrol agent can be easily prepared. This can be done by growing the biocontrol agent in a bioreactor with a suitable broth culture amended with salt and an osmolyte (osmoadaptation), and the harvested cells formulated with the specific nutrient (nutritional enhancement) can be processed to make a liquid or dried formulation (23). It is expected that this methodology could be applied to improve commercial agents of biocontrol not only of fire blight but also of other plant diseases. However, there are many aspects that remain to be studied, such as the effect of nutritional enhancement on the normal microbiota or in other pathogens different from E. amylovora (controlled or even stimulated) or a further refinement of the osmoadaptation and nutritional enhancement procedures.
ACKNOWLEDGMENTS
Funding was provided by Spain MICINN (project AGL2006-13564-c02-01/AGR and AGL2009-13255-c02-01). The research group is under accreditation by SGR 2009-0812, XaRTA, and TECNIO Net from Catalonia.
We thank P. Vilardell for technical assistance and the Mas Badia Agricultural Experiment Station for providing access to experimental orchards.
Footnotes
Published ahead of print on 25 March 2011.
REFERENCES
- 1. Aldwinckle H. S., Bhaskara Reddy M. V., Norelli J. L. 2002. Evaluation of control of fire blight infection of apple blossoms and shoots with SAR inducers, biological agents, a growth regulator, copper compounds, and other materials. Acta Hortic. 590:325–331 [Google Scholar]
- 2. Bailey K. L., et al. 2007. Effect of spraying adjuvants with the biocontrol fungus Microsphaeropsis ochracea at different water volumes on the colonization of apple leaves. Biocontrol Sci. Technol. 17:1021–1036 [Google Scholar]
- 3. Bonaterra A., Camps J., Montesinos E. 2005. Osmotically induced trehalose and glycine betaine accumulation improves tolerance to desiccation, survival and efficacy of the postharvest biocontrol agent Pantoea agglomerans EPS125. FEMS Microbiol. Lett. 250:1–8 [DOI] [PubMed] [Google Scholar]
- 4. Bonaterra A., Cabrefiga J., Camps J., Montesinos E. 2007. Increasing survival and efficacy of a biocontrol agent of fire blight of rosaceous plants by means of osmoadaptation. FEMS Microbiol. Ecol. 61:185–195 [DOI] [PubMed] [Google Scholar]
- 5. Broggini-Schärer G. A. L., et al. 2005. Detection of the fire blight biocontrol agent Bacillus subtilis BD170 (Biopro) in a Swiss apple orchard. Eur. J. Plant Pathol. 111:93–100 [Google Scholar]
- 6. Cabrefiga J., Bonaterra A., Montesinos E. 2007. Mechanisms of antagonism of Pseudomonas fluorescens EPS62e against Erwinia amylovora, the causal agent of fire blight. Int. Microbiol. 10:123–132 [PubMed] [Google Scholar]
- 7. Cabrefiga J., Montesinos E. 2005. Analysis of aggressiveness of Erwinia amylovora using disease-dose and time relationships. Phytopathology 95:1430–1437 [DOI] [PubMed] [Google Scholar]
- 8. Druvefors U. A., Passoth A., Schnürer J. 2005. Nutrient effects on biocontrol of Penicillium roqueforti by Pichia anomala J121 during airtight storage of wheat. Appl. Environ. Microbiol. 71:1865–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fouilleux G., Revellin C., Hartmann A., Catroux G. 1996. Increase of Bradyrhizobium japonicum numbers in soils and enhanced nodulation of soybean (Glycine max (L) merr.) using granular inoculants amended with nutrients. FEMS Microbiol. Ecol. 20:173–183 [Google Scholar]
- 10. Grassi G., Millard P., Wendler R., Minotta G., Tagliavini M. 2002. Measurement of xylem sap amino acid concentrations in conjunction with whole tree transpiration estimates spring N remobilization by cherry (Prunus avium L.) trees. Plant Cell Environ. 25:689–1699 [Google Scholar]
- 11. Guetsky R., Elad Y., Shtienberg D., Dinoor A. 2002. Improved biocontrol of Botrytis cinerea on detached strawberry leaves by adding nutritional supplements to a mixture of Pichia guilermondii and Bacillus mycoides. Biocontrol Sci. Technol. 12:625–630 [Google Scholar]
- 12. Ishimaru C. A., Klos E. J., Brubaker R. R. 1988. Multiple antibiotic production by Erwinia herbicola. Phytopathology 78:746–750 [Google Scholar]
- 13. Janisiewicz W. J., Usall J., Bors B. 1992. Nutritional enhancement of biocontrol of blue mold on apples. Phytopathology 82:1364–1370 [Google Scholar]
- 14. Johnson K. B., Stockwell V. O. 1998. Management of fire blight: a case study in microbial ecology. Annu. Rev. Phytopathol. 36:227–248 [DOI] [PubMed] [Google Scholar]
- 15. Johnson K. B., et al. 1993. Effect of antagonistic bacteria on establishment of honey bee-dispersed Erwinia amylovora in pear blossoms and on fire blight control. Phytopathology 83:995–1002 [Google Scholar]
- 16. Johnson K. B., Stockwell V. O., Sawyer T. L., Sugar D. 2000. Assessment of environmental factors influencing growth and spread of Pantoea agglomerans on and among blossoms of pear and apple. Phytopathology 90:1285–1294 [DOI] [PubMed] [Google Scholar]
- 17. Jones K. A., Burges H. D. 1998. Technology of formulation and application. p. 7–30 In Burges H. D. (ed.), Formulation of microbial biopesticides. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 18. Leben C. 1988. Relative humidity and the survival of epiphytic bacteria with buds and leaves of cucumber plants. Phytopathology 78:179–185 [Google Scholar]
- 19. Lindow S. E., Brandl M. T. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindow S. E., Suslow T. V. 2003. Temporal dynamics of the biocontrol agent Pseudomonas fluorescens strain A506 in flowers in inoculated pear trees. Phytopathology 93:727–737 [DOI] [PubMed] [Google Scholar]
- 21. Lugtenberg B. J. J., Kravchenko L. V., Simons M. 1999. Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ. Microbiol. 1:439–446 [DOI] [PubMed] [Google Scholar]
- 22. Moënne-Loccoz Y., et al. 1999. Effect of inoculum preparation and formulation on survival and biocontrol efficacy of Pseudomonas fluorescens F113. J. Appl. Microbiol. 86:108–116 [Google Scholar]
- 23. Montesinos E., Bonaterra A. 2009. Microbial pesticides, p. 110–120 In Schaechter M. (ed.), Encyclopedia of microbiology, 3rd ed Elsevier Inc., New York, NY [Google Scholar]
- 24. Pujol M., Badosa E., Manceau C., Montesinos E. 2006. Assessment of the environmental fate of the biological control agent of fire blight, Pseudomonas fluorescens EPS62e, on apple by culturable and real-time PCR methods. Appl. Environ. Microbiol. 72:2421–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pujol M., Badosa E., Montesinos E. 2007. Epiphytic fitness of a biological control agent of fire blight in apple and pear orchards under Mediterranean weather conditions. FEMS Microbiol. Ecol. 59:186–193 [DOI] [PubMed] [Google Scholar]
- 26. Pusey P. L. 1997. Crab apple blossoms as a model for research on biological control of fire blight. Phytopathology 87:1096–1102 [DOI] [PubMed] [Google Scholar]
- 27. Pusey P. L. 2002. Biological control agents for fire blight of apple compared under conditions limiting natural dispersal. Plant Dis. 86:639–644 [DOI] [PubMed] [Google Scholar]
- 28. Pusey P. L., Curry E. A. 2004. Temperature and pomaceous flower age related to colonization by Erwinia amylovora and antagonists. Phytopathology 94:901–911 [DOI] [PubMed] [Google Scholar]
- 29. Pusey P. L., Rudell D. R., Curry E. A., Mattheis J. P. 2008. Characterization of stigma exudates in aqueous extracts from apple and pear flowers. Hortscience 43:1471–1478 [Google Scholar]
- 30. Pusey P. L., Stockwell V. O., Mazzola M. 2009. Epiphytic bacteria and yeasts on apple blossoms and their potential as antagonists of Erwinia amylovora. Phytopathology 99:571–581 [DOI] [PubMed] [Google Scholar]
- 31. Sabuquillo P., De Cal A., Melgarejo P. 2005. Dispersal improvement of a powder formulation of Penicillium oxalicum, a biocontrol agent of tomato wilt. Plant Dis. 89:1317–1323 [DOI] [PubMed] [Google Scholar]
- 32. Stockwell V. O., Johnson K. B., Loper J. E. 1998. Establishment of bacterial antagonists of Erwinia amylovora on pear and apple blossoms as influenced by inoculum preparation. Phytopathology 88:506–513 [DOI] [PubMed] [Google Scholar]
- 33. Stockwell V. O., Johnson K. B., Sugar D., Loper J. E. 2011. Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 101:113–123 [DOI] [PubMed] [Google Scholar]
- 34. Stockwell V. O., et al. 1999. Epiphytic colonization of pear stigmas and hypanthia by bacteria during primary bloom. Phytopathology 89:1162–1168 [DOI] [PubMed] [Google Scholar]
- 35. Sundin G. W., Werner N. A., Yoder K. S., Aldwinckle H. S. 2009. Field evaluation of biological control of fire blight in the eastern United States. Plant Dis. 93:386–394 [DOI] [PubMed] [Google Scholar]
- 36. Vanneste J. L., Cornish D. A., Yu J., Voyle M. D. 2002. P10c: a new biological control agent for control of fire blight which can be sprayed or distributed using honey bees. Acta Hortic. 590:231–236 [Google Scholar]
- 37. Wilson M., Epton H. A. S., Sigee D. C. 1992. Biological control of fire blight of hawthorn (Crataegus monogyna) with fluorescent Pseudomonas spp. under protected conditions. J. Phytopathol. 136:16–26 [Google Scholar]
- 38. Wilson M., Lindow S. E. 1993. Interaction between the biological control agent Pseudomonas fluorescens A506 and Erwinia amylovora in pear blossoms. Phytopathology 83:117–123 [Google Scholar]