Abstract
The dominant genus of sulfate-reducing bacteria (SRB) in humans is Desulfovibrio, and quantitative PCR (QPCR) targeting the 16S rRNA gene is often used in assays. We show that the 16S rRNA gene assay overestimated SRB abundance in feces from 24 adults compared to QPCR assays using primers targeting two genes involved in SRB energy metabolism.
INTRODUCTION
There is growing interest in the quantification of sulfate-reducing bacteria (SRB) in humans because of reports suggesting SRB to be involved in the etiology of gastrointestinal diseases (10, 18). The principal byproduct of SRB metabolism is hydrogen sulfide (H2S), which can be toxic to epithelial cells in the colon, where it is mainly produced. At higher levels, H2S can inhibit butyrate oxidation (20) and phagocytosis and bacterial killing (11) and induces hyperproliferation and metabolic abnormalities in epithelial cells (5). H2S is also produced endogenously by colonocytes (21) and is physiologically active in the brain, heart, vasculature, urogenital system, and gastrointestinal tract at nontoxic levels (8, 25, 26). Ulcerative colitis patients were reported to have higher levels of H2S (19) and a higher abundance of SRB (12) in their feces. Use of the Desulfovibrio 16S rRNA gene for detection of SRB in human feces has revealed a higher abundance in elderly people compared to healthy adults (9), a positive correlation between Desulfovibrio abundance and smoking (14), and no difference (2) or a decrease (22) in Desulfovibrio levels in colorectal cancer patients compared to healthy individuals. Therefore, to determine the role of SRB in gastrointestinal health, an accurate estimate of the abundance of SRB is vital.
For quantification of SRB in environmental samples, three genes, the 16S rRNA, adenosine-5′-phosphosulfate reductase (aps), and dissimilatory (bi)sulfite (dsrA) genes, are generally targeted (3, 24). The aps and dsrA genes are involved in the energy metabolism of SRB and have been identified as reliable gene markers for SRB (24). The 16S rRNA gene is, however, considered inadequate for determinations involving environmental samples, because SRB are found in different phyla in the phylogenetic tree (4, 24). Therefore, the 16S rRNA gene assay cannot cover all the different phyla and underestimates the SRB abundance in environmental samples (3, 24). This may not be the case, however, for human fecal samples, as SRB of the genus Desulfovibrio can occur in large numbers in the gut and because Desulfovibrio has been identified as the main genus of SRB in humans (9, 12).
In this study, we used quantitative real-time PCR (QPCR) to examine the accuracy of use of the 16S rRNA gene to quantify SRB in human feces by comparing an assay employing the widely used 16S rRNA primer pair that targets Desulfovibrio (9) with two QPCR assays targeting the functional aps and dsrA SRB genes that have been found to give reliable quantifications of SRB in environmental samples.
Human fecal samples were collected from a group of 24 healthy individuals (14 males and 10 females) with an average age of 53.7 years (range, 33 to 67) who had not had any antibiotic treatment during the past 6 months. DNA was extracted from these samples using the repeated beat-beading and column cleanup method described by Yu and Morrison (27). Desulfovibrio numbers, aps and dsrA, and the total number of bacteria in human fecal samples were quantified using QPCR. Quantifications were performed using 1× Ssofast Evagreen Supermix fluorescent nucleic acid dye (Bio-Rad Laboratories, Hercules, CA) and 0.4 μl bovine serum albumin (BSA) (Promega, Madison, WI). The primers were as follows: for total bacteria (concentration, 175 nM), primers 1114f (5′-CGGCAACGAGCGCAACCC-3′) and 1275r (5′-CCATTGTAGCACGTGTGTAGCC-3′) (6); for aps (400 nM), primers aps3f (5′-TGGCAGATCATGWTYAAYGG-3′) and aps2r (5′-GGGCCGTAACCRTCYTTRAA-3′) (modified from reference 7); for dsrA (400 nM), primers Dsr1F (5′-ACSCACTGGAAGCACGGCGG-3′) and Dsr1R (5′-GTGGMRCCTGCAKRTTGG-3′) (16); and for the Desulfovibrio 16S rRNA assay (300 nM), primers DSV691F (5′-CCGTAGATATCTGGAGGAACATCAG-3′) and DSV826R (5′-ACATCTAGCATCCATCGTTTACAGC-3′) (9). For quantification, a total of 10 to 30 ng of template DNA was used and cycling was performed using a Chromo-4 thermocycler (MJ Research Inc., Waltham, MA). Reaction mixtures for aps assays contained 1 μl dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) instead of BSA. The PCR cycling conditions were as follows: 98°C for 4 min followed by 35 cycles of 98°C for 30 s, 58 to 65°C for 15 to 30 s (for total bacteria, 60°C for 20 s; for aps, 58°C for 30 s; for dsrA, 65°C for 15 s; and for Desulfovibrio spp., 62°C for 30 s), and 72°C for 30 s. Elongation was followed by fluorescence acquisition; however, a further elongation step at 83°C for 15 s was performed before fluorescence acquisition for dsrA, and a final melt-curve analysis was performed after completion of all cycles, with fluorescence acquired at 0.5°C intervals between 55 and 95°C to verify that only the expected fragment was amplified. PCR products were also visualized on a 1.5% agarose gel. Nontemplate controls were included, and assays were performed in technical triplicate experiments by analyzing the same DNA sample in 3 independent reactions. A series of eight 10-fold dilutions of a sample derived-plasmid construct (Topo chemical competent cells; Invitrogen) containing the target amplicon were analyzed in parallel with DNA samples for estimations of absolute abundance and PCR efficiency for all assays. Results were analyzed using Opticon Monitor software (version 3.1; Bio-Rad Laboratories) for absolute abundance estimates. All calculations were done using an assay specific for PCR efficiency. Using a clone library, a specificity test was performed on the 16S rRNA gene primers. Twenty-four clones were sequenced using a 96-capillary 3730xl DNA analyzer and putatively identified using the Basic Local Alignment Search Tool (1). Statistical analysis was performed using the Primer 6 with Permanova+ package (PRIMER-E Ltd., Plymouth, United Kingdom). Natural-logarithm-normalized data were used for statistical analysis of absolute quantities.
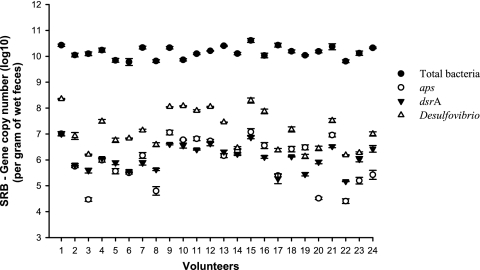
The absolute quantification of SRB in human feces by use of the 3 primer sets showed the following average abundance values (no. of copies × gram wet feces−1 [range]): for aps, 3.26 × 106 (2.54 × 104 to 1.19 × 107); for dsrA, 2.21 × 106 (1.48 × 105 to 1.01 × 107); and for Desulfovibrio spp., 4.53 × 107 (1.36 × 106 to 2.26 × 108). These abundances revealed a significantly higher absolute abundance (Fig. 1) for Desulfovibrio compared to aps (P = 0.00005) and dsrA (P = 0.00001), but no significant differences were observed between aps and dsrA results. Similar differences relative to the total numbers of bacteria were found when data were analyzed using Qbase+ (13, 23) (data not shown). The PCR efficiency values for the assays were as follows: for total bacteria, 103%; for Desulfovibrio, 103%; for dsrA, 105%; and for aps, 99%. The efficiency results were similar for plasmid-derived DNA and stool-derived DNA. The clone library revealed that 18 clones (75%) were 98 to 100% similar and 4 clones (17%) were between 92% and 97% similar to known Desulfovibrio sequences, whereas 2 clones (8%) were putatively identified as Papillibacter cinnamivorans. It was also noted that all 24 healthy volunteers had detectable numbers of SRB.
Fig. 1.
Abundance of SRB (mean ± SD) in feces from 24 healthy volunteers measured with QPCR using primers that target the Desulfovibrio 16S rRNA gene (Δ), the adenosine-5′-phosphosulfate reductase (aps) gene (○), and the dissimilatory (bi)sulfite (dsrA) gene (▾). QPCR estimates of total number of bacteria are also represented (•).
This report supports previous evidence indicating that Desulfovibrio is the dominant genus of SRB found in stool samples from humans on the basis of the clone library and suggests that using the Desulfovibrio 16S rRNA gene overestimates the abundance of SRB in human feces compared to SRB abundances estimated using the aps or the dsrA gene. Hence, caution has to be exercised in analyzing and reporting SRB abundances when quantifications are performed using the 16S rRNA gene. However, the abundances of aps and dsrA also differ between individuals (Fig. 1) because some SRB may carry both genes and some only one (3). According to other studies, both aps and dsrA are genes suitable for reliable quantification of SRB populations and are specific for the SRB energy metabolism. The absolute quantities of Desulfovibrio spp., as determined in this study by use of the 16S rRNA primers, are similar to the findings of Fite et al. (9), and, given the results showing PCR efficiencies of around 100% (99% to 105%), we are confident that the 16S rRNA gene primers used in this study overestimated the abundance of SRB in human feces. Those results differ from what is found in experiments using environmental samples but are in line with the general idea that 16S rRNA primers do often overestimate abundances due to a higher copy number of the 16S rRNA gene compared to aps and dsrA copy numbers. According to the rRNA Operon Copy Number Database (15, 17), members of the genus Desulfovibrio have, on average, 4.5 copies of the 16S rRNA gene. Another reason for the overestimation of the abundance of Desulfovibrio spp. by 16S rRNA gene primers is the specificity of the primers. The clone library showed that the primers did amplify two fragments that did not match Desulfovibrio sequences, even though in silico tests showed the 16S rRNA gene primers to have several mismatches. In conclusion, the overestimation of abundance observed when using 16S rRNA gene primers compared to aps and dsrA primers in this study was almost certainly due to unspecific priming of the 16S rRNA gene primers and a higher copy number of the 16S rRNA gene compared to the aps and dsrA gene.
Footnotes
Published ahead of print on 1 April 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balamurugan R., Rajendiran E., George S., Samuel G. V., Ramakrishna B. S. 2008. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J. Gastroenterol. Hepatol. 23:1298–1303 [DOI] [PubMed] [Google Scholar]
- 3. Barton L. L., Fauque G. D. 2009. Biochemistry, physiology and biotechnology of sulphate-reduring bacteria. Adv. Appl. Microbiol. 68:41–98 [DOI] [PubMed] [Google Scholar]
- 4. Castro H. F., Williams N. H., Ogram A. 2000. Phylogeny of sulfate-reducing bacteria. FEMS Microbiol. Ecol. 31:1–9 [DOI] [PubMed] [Google Scholar]
- 5. Christl S. U., Eisner H.-D., Dusel G., Kasper H., Scheppach W. 1996. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa. Dig. Dis. Sci. 41:2477–2481 [DOI] [PubMed] [Google Scholar]
- 6. Denman S. E., McSweeney C. S. 2006. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 58:572–582 [DOI] [PubMed] [Google Scholar]
- 7. Deplancke B., et al. 2000. Molecular ecological analysis of the succession and diversity of sulfate-reducing bacteria in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 66:2166–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiorucci S., Distrutti E., Cirino G., Wallace J. L. 2006. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology 131:259–271 [DOI] [PubMed] [Google Scholar]
- 9. Fite A., et al. 2004. Identification and quantitation of mucosal and faecal Desulfovibrios using real time polymerase chain reaction. Gut 53:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frank D. N., et al. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U. S. A. 104:13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gardiner K. R., et al. 1995. Significance of systemic endotoxemia in inflammatory bowel disease. Gut 36:897–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gibson G. R., Cummings J. H., Macfarlane G. T. 1991. Growth and activities of sulfate-reducing bacteria in the gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol. Ecol. 9:103–111 [Google Scholar]
- 13. Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato I., et al. 2010. Smoking and other personal characteristics as potential predictors for fecal bacteria populations in humans. Med. Sci. Monit. 16:CR1–CR7 [PMC free article] [PubMed] [Google Scholar]
- 15. Klappenbach J. A., Saxman P. R., Cole J. R., Schmidt T. M. 2001. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 29:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondo R., Nedwell D. B., Purdy K. J., Silva S. Q. 2004. Detection and enumeration of sulphate-reducing bacteria in estuarine sediments by competitive PCR. Geomicrobiol. J. 21:145–157 [Google Scholar]
- 17. Lee Z. M. P., Bussema C., Schmidt T. M. 2009. rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res. 37:D489–D493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malinen E., et al. 2005. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 100:373–382 [DOI] [PubMed] [Google Scholar]
- 19. Pitcher M. C. L., Beatty E. R., Cummings J. H. 2000. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut 46:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roediger W. E., Duncan A., Kapaniris O., Millard S. 1993. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology 104:802–809 [DOI] [PubMed] [Google Scholar]
- 21. Rowan F. E., Docherty N. G., Coffey J. C., O'Connell P. R. 2009. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br. J. Surg. 96:151–158 [DOI] [PubMed] [Google Scholar]
- 22. Scanlan P. D., Shanahan F., Marchesi J. R. 2009. Culture-independent analysis of Desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol. Ecol. 69:213–221 [DOI] [PubMed] [Google Scholar]
- 23. Vandesompele J., et al. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:R0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagner M., et al. 2005. Functional marker genes for identification of sulfate-reducing prokaryotes. Methods Enzymol. 397:469–489 [DOI] [PubMed] [Google Scholar]
- 25. Wallace J. L. 2010. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid. Redox. Signal. 12:1125–1133 [DOI] [PubMed] [Google Scholar]
- 26. Wang R. 2002. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16:1792–1798 [DOI] [PubMed] [Google Scholar]
- 27. Yu Z. T., Morrison M. 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812 [DOI] [PubMed] [Google Scholar]