Abstract
Production of N-acetyl-d-neuraminic acid (Neu5Ac) via biocatalysis is traditionally conducted using isolated enzymes or whole cells. The use of isolated enzymes is restricted by the time-consuming purification process, whereas the application of whole cells is limited by the permeability barrier presented by the microbial cell membrane. In this study, a novel type of biocatalyst, Neu5Ac aldolase presented on the surface of Bacillus subtilis spores, was used for the production of Neu5Ac. Under optimal conditions, Neu5Ac at a high concentration (54.7 g liter−1) and a high yield (90.2%) was obtained under a 5-fold excess of pyruvate over N-acetyl-d-mannosamine. The novel biocatalyst system, which is able to express and immobilize the target enzyme simultaneously on the surface of B. subtilis spores, represents a suitable alternative for value-added chemical production.
INTRODUCTION
N-Acetyl-d-neuraminic acid (Neu5Ac), a major representative of sialic acid, has attracted researchers' attention because of its versatile biological functions and considerable contribution in the therapeutics field (25, 35). Neu5Ac is traditionally prepared by extraction from natural sources and chemical hydrolysis of colominic acid (a homopolymer of Neu5Ac) (25). These methods are limited by the low yield and unsatisfactory stereoselectivity (25, 38). In the past decades, biocatalysis has emerged as an important tool for large-scale synthesis of Neu5Ac (29, 30), because of its high application potential and environment-friendly properties.
Production of Neu5Ac via biocatalysis can be conducted using purified enzymes or whole cells. The use of isolated enzymes requires an extraction and a time-consuming purification or partial purification procedure. Although this problem can be overcome through the use of whole cells, the mass transfer limitation caused by the microbial cell membrane restricts the application of whole cells (20). Thus, a novel biocatalyst which excludes the permeability barrier and the time-consuming enzyme purification procedure would represent a suitable alternative for Neu5Ac production.
Surface display, a powerful technique that uses different microbial components to express heterologous peptides or proteins, can effectively display the bioactive molecules on the surfaces of cells (16, 32). Target proteins anchored on the outside of cells play a role in biocatalysis without the need for any purification or permeation procedure. Among the numerous systems that have been employed in surface display, bacterial spores offer considerable advantages because of their resistance to heat, radiation, and chemicals in a harsh environment (27). Enzymatic transgalactosylation by using the spore-displayed enzyme as a catalyst has been reported (20).
Neu5Ac synthase (EC 4.1.3.19) and Neu5Ac aldolase (NanA, EC 4.1.3.3) have been used in the production of Neu5Ac. NanA is preferred because its substrate (pyruvate) is much more readily available than that of Neu5Ac synthase (phosphoenolpyruvate) (28, 33). In the present study, a Bacillus subtilis surface display system was constructed. After active NanA was successfully observed on the surface of B. subtilis spores, the potential of recombinant spores in the production of Neu5Ac was confirmed.
MATERIALS AND METHODS
Chemicals.
N-Acetyl-d-glucosamine (GlcNAc) (≥97% purity) and sodium pyruvate were purchased from Shandong Dongying Marine Bio-chemical Co. Ltd. All restriction enzymes used for DNA manipulations were supplied by Takara (Dalian, People's Republic of China). Trypsin and proteinase K were purchased from Sigma. N-Acetyl-d-mannosamine (ManNAc) (>90% purity) was prepared from GlcNAc in our laboratory by using alkaline epimerization (38).
Bacteria, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. B. subtilis strains were cultivated in glucose-yeast extract-salt (GYS) medium consisting of (NH4)2SO4, 2 g liter−1; yeast extract, 2 g liter−1; K2HPO4, 0.5 g liter−1; glucose, 1 g liter−1; MgSO4, 0.41 g liter−1; CaCl2·H2O, 0.08 g liter−1; and MnSO4·5H2O, 0.07 g liter−1 at 37°C and 250 rpm for 24 h (20). Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C.
Table 1.
Strains, plasmids, and primers used in this work
| Strain, plasmid, or primer | Description | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli Mach1 | F− φ80 (lacZ) ΔM15 ΔlacX74 hsdR(rK− mK+) ΔrecA1398 endA1 tonA | Transgene |
| Bacillus subtilis 168 | Type strain | Bacillus Genetic Stock Center (BGSC) |
| B. subtilis WB600 | trpC2 ΔnprE ΔaprE Δepr Δbpf Δmpr ΔnprB | Bacillus Genetic Stock Center (BGSC) |
| E. coli K-12 | Type strain | Laboratory stock |
| Plasmids | ||
| pEASY-T3 | Cloning vector | Transgene |
| pEASY-T3-cotG-nanA | pEASY-T3 with fusion gene cotG-nanA | This study |
| pHP13 | Low-copy-number E. coli-B. subtilis shuttle vector | Alan D. Grossman |
| pHP13-cotG-nanA | pHP13 with fusion gene cotG-nanA | This study |
| pDG1728 | E. coli-B. subtilis shuttle vector with spectinomycin resistance gene | 7 |
| pGJ103 | High-copy-number E. coli-B. subtilis shuttle vector | 39 |
| pEB03 | High-copy-number E. coli-B. subtilis shuttle vector | This study |
| pEB03-cotG-nanA | pEB03 with fusion gene cotG-nanA | This study |
| Primersa | ||
| P1 | 5′-GCCTTTGAATTCAGTGTCCCTAGCTCCGAG-3′ | This study |
| P2b | 5′-CTATTGACTAGTTGAACCCCCACCTCCTTTGTATTTCTTTTTGACTA-3′ | This study |
| P3 | 5′-AGACTAGTATGGCAACGAATTTAC-3′ | This study |
| P4 | 5′-TCCTGCAGTCACCCGCGCTCTT-3′ | This study |
| Spc-up | 5′-TTGGGCCCGAATGGCGATTTTCGTTC-3′ | This study |
| Spc-down | 5′-TGCGCTCGAGCCAATTAGAATGAATATTTC-3′ | This study |
The italicized letters indicate the introduction of restriction sites.
The underlined letters indicate the introduction of a flexible linker at the C terminus of the cotG structural gene product.
Plasmids and strain construction.
The spore coat, a multilayered structure surrounding the spore, is composed of more than 25 highly cross-linked polypeptide species and renders the spore resistant to treatment with chloroform or lysozyme (2). Many outer coat proteins of the spores, including CotB (7), CotC (26, 42), and CotG (13, 14, 20), have successfully been used for spore surface display of either antigens or bioactive molecules. To display NanA on the surface of B. subtilis spores, we constructed a genetic fusion of cotG from B. subtilis and nanA from E. coli K-12. The DNA fragment with the cotG promoter and structure gene was amplified using primers P1 and P2 from the B. subtilis 168 genome, digested with EcoRI and SpeI, and ligated into pEASY-T3 cloning vector to generate the plasmid pEASY-T3-cotG (a flexible linker [Gly-Gly-Gly-Gly-Ser] was inserted at the C terminus of the cotG structural gene product). The nanA gene, amplified using primers P3 and P4 and E. coli K-12 chromosome as a template, was digested with SpeI and PstI and cloned into the same restriction endonuclease sites of the plasmid pEASY-T3-cotG to yield an intermediate plasmid, pEASY-T3-cotG-nanA.
The fusion gene cotG-nanA was cleaved with EcoRI and PstI and inserted into the shuttle vector pHP13, treated with the same enzymes, to yield recombinant plasmid pHP13-cotG-nanA (1). The plasmid was transformed into the multiple-protease-deficient B. subtilis WB600 by electroporation transformation as described previously (1). Chloramphenicol (100 μg ml−1) was added for the selection of recombinant B. subtilis harboring the shuttle plasmid pHP13-cotG-nanA.
The spectinomycin resistance gene was amplified using pDG1728 as a template (7), with Spc-up and Spc-down as primers. The fragment was digested using ApaI and XhoI and inserted into the high-copy-number vector pGJ103, treated with the same enzymes (39), to yield the high-copy-number E. coli-B. subtilis shuttle vector pEB03. Plasmid pEB03 was further digested and ligated in the same manner as was pHP13, to create the plasmid pEB03-cotG-nanA. The plasmid pEB03-cotG-nanA was then transformed into B. subtilis WB600 (37). Spectinomycin (100 μg ml−1) was added for the selection of recombinant B. subtilis harboring the shuttle plasmid pEB03-cotG-nanA (see Fig. S1 in the supplemental material).
Spore purification.
After cultivation in the GYS medium at 37°C for 24 h, spores and sporangial cells of B. subtilis WB600 with recombinant plasmids were harvested by centrifugation and resuspended in 67 mM phosphate buffer (pH 7.4). The suspension was lysed by 0.5% lysozyme at 37°C for 1 h and then centrifuged for 30 min at 12,000 rpm. After the resulting pellets were washed with 1 M NaCl, 1 M KCl, and phosphate buffer, the purified spores were obtained and resuspended in phosphate buffer at 4°C. Phenylmethylsulfonyl fluoride (1 mM) was added throughout the process to inhibit proteolysis.
NanA activity assay.
Activity of NanA was determined at 37°C in 1 ml of 67 mM phosphate buffer (pH 7.4), 4 mM Neu5Ac, and the purified spore suspension. After a 10-min reaction, 500 μl of 1 M NaOH was added and the resulting mixture was subjected to high-performance liquid chromatography (HPLC). One unit of enzyme activity is defined as the amount of NanA required to catalyze the production of 1 μmol pyruvate per min (38).
Analytical procedures.
Pyruvate, ManNAc, and Neu5Ac were determined by HPLC (Agilent 1100 series; Hewlett-Packard), using a Bio-Rad Aminex HPX-87H column (300 by 7.8 mm) as described by Xu et al. (38). The number of spores was calculated by direct counting with a Burker chamber under an optical microscope.
Biotransformation by spores with NanA activity.
For the optimization of biotransformation conditions, 20 ml of the reaction mixture in a 100-ml Erlenmeyer flask was used with variations as follows. The NanA concentrations ranged from 0.075 to 1.2 U ml−1. Temperatures ranged from 30 to 70°C. The ratio of [pyruvate]/[ManNAc] was 1 to 10. After addition of 1 M NaOH to stop the reaction, the resulting mixture was centrifuged. The concentrations of Neu5Ac were analyzed by HPLC as described above.
RESULTS
Construction of surface display system.
CotG, a 23.9-kDa outer spore coat protein, was used as the anchoring protein for the display of NanA on the surface of B. subtilis spores (20). A flexible linker composed of 5 amino acids (Gly-Gly-Gly-Gly-Ser) was inserted at the C terminus of the cotG gene product and the N terminus of the nanA gene product to obtain the cotG-linker-nanA fusion gene (see Fig. S1 in the supplemental material). A cotG-specific promoter was employed in the surface display system to initiate expression of NanA and sporulation of B. subtilis synchronously.
Since proteases may destabilize target enzymes, the multiple-protease-deficient strain B. subtilis WB600 was used for the display of NanA (37). The recombinant vectors pHP13-cotG-nanA and pEB03-cotG-nanA were constructed and transformed into B. subtilis WB600. The NanA activities of B. subtilis spores were assayed. As shown in Fig. 1, spores not harboring the recombinant plasmid had very low NanA activity. The activity of NanA obtained using pEB03 as the expression vector was 6-fold higher than that obtained using pHP13 as the expression vector. The pEB03 vector used in the present study contained the pMB1 replicon used in E. coli, as well as the pGDV1 replicon used in B. subtilis. This enabled the recombinant plasmid to have a high copy number not only in E. coli but also in B. subtilis (39). This may explain the higher activity of NanA in the system in which plasmid pEB03 was used as the expression vector.
Fig. 1.
Activities of N-acetyl-d-neuraminic acid (Neu5Ac) aldolase (NanA) displayed on spore surface. ▤, intact spores; ▧, spores treated with 0.1% trypsin for 1 h; ▨, spores treated with 0.1% proteinase K for 1 h.
The expression of NanA on the external surface of B. subtilis spores was verified by protease treatment. Purified spores of WB600(pEB03-cotG-nanA) and WB600(pHP13-cotG-nanA) were suspended in 67 mM phosphate buffer (pH 7.4) containing 0.1% trypsin or 0.1% proteinase K for 1 h. A control sample with the spores was also prepared in phosphate buffer without protease. As shown in Fig. 1, spores treated with protease had lower NanA activity than did intact spores. For exogenously added protease that cannot penetrate through the spore wall, this result means that the NanA is located on the surface of spores (14).
Optimization of biocatalysis conditions.
ManNAc is condensed with pyruvate by NanA to produce Neu5Ac without the need for cofactors. Therefore, the synthesis of Neu5Ac from ManNAc and pyruvate in a simple biocatalysis system, using spores with NanA activities as the biocatalyst, should be feasible. Initially, the reaction conditions for the efficient production of Neu5Ac were optimized.
To obtain the maximum Neu5Ac yield, we studied the effect of the amount of NanA on bioconversion. The reaction mixture contained 0.5 M pyruvate and 0.2 M ManNAc as the substrates and different concentrations of spore-displayed NanA as the biocatalyst. After 24-h biotransformation at 30°C, the optimum concentration of NanA was confirmed to be 0.3 U ml−1, corresponding to an approximately 82% yield of Neu5Ac (see Fig. S2 in the supplemental material). The influence of the reaction temperature on Neu5Ac production was studied at temperatures ranging from 30 to 70°C. The highest Neu5Ac production was detected at 50°C after 16 h (data not shown).
In a dilute solution, the reaction catalyzed by NanA is the cleavage of Neu5Ac. The equilibrium lies on the side of pyruvate and ManNAc. Thus, for the production of Neu5Ac, excess pyruvate and ManNAc are needed to achieve a high yield (23). Because ManNAc is very expensive and not readily available in large quantities, an excess of pyruvate over ManNAc is generally used. The ratio of [pyruvate]/[ManNAc] had a considerable effect on the yield of Neu5Ac (see Fig. S3 in the supplemental material). The use of a 5-fold ratio of pyruvate to ManNAc gave the highest Neu5Ac yield.
Biotransformation under optimal conditions.
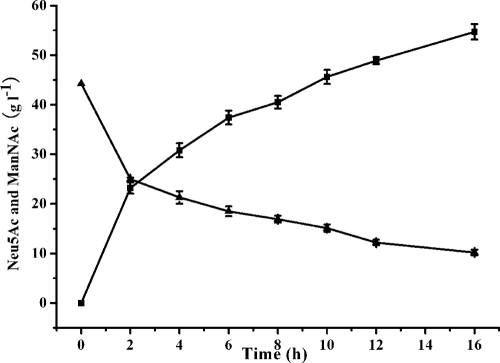
A biotransformation system was developed using a combination of the optimal catalysis conditions described above. The preparation of Neu5Ac was carried out under the following optimal conditions: spore-displayed NanA, 0.3 U ml−1; 67 mM phosphate buffer, pH 7.4; temperature, 50°C; pyruvate, 1.0 M; and ManNAc, 0.2 M. As shown in Fig. 2, 54.7 g liter−1 Neu5Ac, representing a yield of 90.2%, was produced after 16 h of biotransformation.
Fig. 2.
Time course of production of Neu5Ac under optimum conditions. ▴, N-acetyl-d-mannosamine (ManNAc); ▪, Neu5Ac.
Spores have the characteristic of easy purification by centrifugation or filtration, and therefore, the recombinant spores were reused for the Neu5Ac production. All reactions were conducted at 50°C for 16 h with 1 M pyruvate and 0.2 M ManNAc as the substrates. After 1 reaction cycle, the spores were isolated by centrifugation at 10,000 × g for 10 min and washed twice with 67 mM phosphate buffer. The recombinant spores could be used for up to 3 reaction cycles without a significant decrease in the conversion rate (Fig. 3).
Fig. 3.
Reuse of the spore-displayed NanA. The reaction mixture contained 1 M pyruvate and 0.2 M ManNAc, and the spore-displayed enzyme was incubated at 50°C for 16 h. ▪, specific activity of NanA; ▧, yield of Neu5Ac.
As shown in Fig. 3, the specific activity of NanA in the spores of WB600(pEB03-cotG-nanA) was not affected throughout the reutilization process. However, the amount of WB600(pEB03-cotG-nanA) spores recycled decreased after the biocatalysis process (see Fig. S4 in the supplemental material). As mentioned above, addition of excess pyruvate is needed to achieve a high yield of Neu5Ac. Falcone and Bresciani (4) reported that pyruvate caused germination of B. subtilis spores. Nisin, a 34-amino-acid peptide produced by Lactococcus lactis subsp. lactis (8), can inhibit the germination of Bacillus spores (Fig. 4). Addition of nisin to the biocatalytic system might result in a higher reutilization potential of the surface-displayed NanA (see Fig. S5 in the supplemental material).
Fig. 4.
Effects of pyruvate and nisin on the spore-displayed NanA. ▨, residual NanA activity; ▧, residual amount of spores. Purified spores of WB600(pEB03-cotG-nanA) were suspended in phosphate buffer (pH 7.4) containing 1 M pyruvate and different concentrations of nisin for 24 h. A control sample with the spores was also made in phosphate buffer (pH 7.4) without pyruvate.
DISCUSSION
Enzymatic synthesis of Neu5Ac from ManNAc and pyruvate by using NanA has been widely studied. In previous studies, free NanA, immobilized NanA, and whole cells containing NanA were used for Neu5Ac production (34). In recent years, biological surface display has been used in various areas such as screening of polypeptide libraries, production of live recombinant bacterial vaccines, and industrial and environmental utilization of whole-cell adsorbents (5). In this study, we introduced a novel system using surface-displayed NanA for the enzymatic synthesis of Neu5Ac.
Bacterial spores constitute a metabolically dormant form of an organism's life cycle, developed in response to nutrient starvation. Bacterial spores have been reported to be good carriers of target proteins. Spores of Bacillus species, including B. subtilis, B. clausii, B. coagulans, B. cereus, and B. natto, are generally recognized as safe and are employed as additives in human and animal food, as well as used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders (6, 9). Among the various Bacillus species, B. subtilis attracts considerable attention because of its advantages of easy molecular genetic manipulation (31) and detailed genetic and structural information (3, 12, 19). These attributes, which facilitate the construction of recombinant spores, make B. subtilis an attractive vehicle for displaying heterogenous proteins on its surface.
Coat proteins are mainly used as anchors for spore surface display (2). It has been reported that the absence of some coat structural proteins such as CotA, CotB, CotC, CotD, CotF, and CotG does not alter spore properties of viability, resistance, and germination (2). CotG, a 23.9-kDa outer coat protein, was a convincing example of a coat protein that resulted in the display of functional enzymes (15, 20, 40). Thus, in this study, we used CotG as the anchoring protein for the display of NanA on the surface of B. subtilis spores. The fusion gene cotG-nanA, constructed using the cotG transcriptional and translational signal, was inserted into the expression vectors pEB03 and pHP13, to ensure expression of the enzyme during the sporulation process.
Most strategies of genetic construction to display the heterogenous protein on spores utilize low-copy-number expression vectors (13, 14, 42) or chromosomal integration (11, 20, 26) with characteristic stable expression. The expression vector pEB03 constructed in our study has a high copy number in E. coli and also in B. subtilis. This advantage resulted in higher NanA activity (Fig. 1) than that obtained using pHP13 as the expression vector. Thus, the recombinant spores harboring the pEB03-cotG-nanA plasmid were used as biocatalysts for the successive biotransformation process.
After optimization of the procedure, recombinant spores harboring the pEB03-cotG-nanA plasmid were able to catalyze 0.2 M ManNAc into 0.18 M (54.70 g liter−1) Neu5Ac in 16 h. In previous studies, NanA was purified and immobilized to understand the conversion reaction and circular catalysis (10, 23, 36). In our study, we were able to easily separate the recombinant spores from the catalysis system and subject them to circular catalysis. Compared with previous studies, the immobilized Neu5Ac on the surface of the recombinant spores represents a promising alternative for Neu5Ac production, because it excludes the purification and immobilization process. The pyruvate in the biocatalytic system caused germination of spores and decrease of NanA activities. Addition of nisin could inhibit the germination of Bacillus spores (Fig. 4). Introducing a mutation that could not germinate in the presence of pyruvate might also avoid the germination of spores and enhance the reutilization potential of surface-displayed NanA. On the other hand, ManNAc is very expensive and not readily available in large quantities. It is generally prepared from the relatively cheaper substrate GlcNAc by epimerization. By coupling of the GlcNAc 2-epimerase (AGE, EC 5.3.1.8) with NanA, a 2-step enzymatic approach was developed for Neu5Ac production (21, 22, 41). This procedure is regarded as the most cost-effective enzymatic method for Neu5Ac production on an industrial scale (17, 18, 24, 25). Further improvement of its application potential, by simultaneously displaying GlcNAc 2-epimerase and NanA on the spore surface, represents a new strategy for the enzymatic production of Neu5Ac.
Other than simple purification, the natural robustness of spores in the presence of heat, solvents, pH, oxidizing agents, and salts has been reported (27). Surface-displayed β-galactosidase and NADPH-cytochrome P450 oxidoreductase showed higher thermostability than the respective native forms (20, 40). The stability of surface-displayed β-galactosidase in the presence of various organic solvents has enabled it to be used as a biocatalyst for transgalactosylation in water-solvent biphasic reaction systems (20). In this study, we introduced a value-added chemical production process by using surface-displayed NanA in an aqueous system. The spore-displayed enzymes could help to meet the ever-increasing industrial demand for preparation and stabilization of biocatalysts and may be generally applicable to numerous biocatalytic reactions.
Supplementary Material
ACKNOWLEDGMENTS
This work was partially supported by grants from the Major Basic Research Development Program of China (2007CB714303) and the National Natural Science Foundation of China (31000014 and 31070062).
We also thank Alan D. Grossman (Massachusetts Institute of Technology) for the kind gift of plasmid pHP13.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 25 March 2011.
REFERENCES
- 1. Bron S. 1990. Plasmids, p. 75–174 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 2. Driks A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Driks A. 2004. The Bacillus spore coat. Phytopathology 94:1249–1251 [DOI] [PubMed] [Google Scholar]
- 4. Falcone G., Bresciani F. 1963. On the mechanism of spore germination in B. subtilis. Permeability and germination by pyruvate. Cell. Mol. Life Sci. 19:152–154 [Google Scholar]
- 5. Georgiou G., et al. 1997. Display of heterologous proteins on the surface of microorganisms: from the screening of combinational libraries to live recombinant vaccines. Nat. Biotechnol. 15:29–34 [DOI] [PubMed] [Google Scholar]
- 6. Green D. H., et al. 1999. Characterization of two Bacillus probiotics. Appl. Environ. Microbiol. 65:4288–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guérout-Fleury A. M., Frandsen N., Stragier P. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61 [DOI] [PubMed] [Google Scholar]
- 8. Gut I. M., Prouty A. M., Ballard J. D., van der Donk W. A., Blanke S. R. 2008. Inhibition of Bacillus anthracis spore outgrowth by nisin. Antimicrob. Agents Chemother. 52:4281–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoa N. T., et al. 2000. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl. Environ. Microbiol. 66:5241–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu S., et al. 2010. Coupled bioconversion for preparation of N-acetyl-d-neuraminic acid using immobilized N-acetyl-d-glucosamine-2-epimerase and N-acetyl-d-neuraminic acid lyase. Appl. Microbiol. Biotechnol. 85:1383–1391 [DOI] [PubMed] [Google Scholar]
- 11. Isticato R., et al. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 183:6294–6301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isticato R., et al. 2004. Assembly of multiple CotC forms into the Bacillus subtilis spore coat. J. Bacteriol. 186:1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim J. H., et al. 2007. Bacterial surface display of GFPuv on Bacillus subtilis spores. J. Microbiol. Biotechnol. 17:677–680 [PubMed] [Google Scholar]
- 14. Kim J. H., Lee C. S., Kim B. G. 2005. Spore-displayed streptavidin: a live diagnostic tool in biotechnology. Biochem. Biophys. Res. Commun. 331:210–214 [DOI] [PubMed] [Google Scholar]
- 15. Kim J. H., et al. December 2001. Methods for expression of proteins on spore surface. International patent WO2002046388. [Google Scholar]
- 16. Kim J., Schumann W. 2009. Display of proteins on Bacillus subtilis endospores. Cell. Mol. Life Sci. 66:3127–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim M. J., Hennen W. J., Sweers H. M., Wong C. H. 1988. Enzymes in carbohydrate synthesis: N-acetylneuraminic acid aldolase catalyzed-reactions and preparation of N-acetyl-2-deoxy-d-neuraminic acid-derivatives. J. Am. Chem. Soc. 110:6481–6486 [Google Scholar]
- 18. Kragl U., Gygax D., Ghisalba O., Wandrey C. 1991. Enzymatic 2-step synthesis of N-acetylneuraminic acid in the enzyme membrane reactor. Angew. Chem. 30:827–828 [Google Scholar]
- 19. Kunst F., et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249–256 [DOI] [PubMed] [Google Scholar]
- 20. Kwon S. J., Jung H. C., Pan J. G. 2007. Transgalactosylation in a water-solvent biphasic reaction system with beta-galactosidase displayed on the surfaces of Bacillus subtilis spores. Appl. Environ. Microbiol. 73:2251–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J. O., Yi J. K., Lee S. G., Takahashi S., Kim B. G. 2004. Production of N-acetylneuraminic acid from N-acetylglucosamine and pyruvate using recombinant human renin binding protein and sialic acid aldolase in one pot. Enzyme Microb. Technol. 35:121–125 [Google Scholar]
- 22. Lee Y. C., Chien H. C., Hsu W. H. 2007. Production of N-acetyl-d-neuraminic acid by recombinant whole cells expressing Anabaena sp. CH1 N-acetyl-d-glucosamine 2-epimerase and Escherichia coli N-acetyl-d-neuraminic acid lyase. J. Biotechnol. 129:453–460 [DOI] [PubMed] [Google Scholar]
- 23. Mahmoudian M., et al. 1997. An efficient process for production of N-acetylneuraminic acid using N-acetylneuraminic acid aldolase. Enzyme Microb. Technol. 20:393–400 [DOI] [PubMed] [Google Scholar]
- 24. Maru I., Ohnishi J., Ohta Y., Tsukada Y. 1998. Simple and large-scale production of N-acetylneuraminic acid from N-acetyl-d-glucosamine and pyruvate using N-acyl-d-glucosamine 2-epimerase and N-acetylneuraminate lyase. Carbohydr. Res. 306:575–578 [DOI] [PubMed] [Google Scholar]
- 25. Maru I., Ohnishi J., Ohta Y., Tsukada Y. 2002. Why is sialic acid attracting interest now?Complete enzymatic synthesis of sialic acid with N-acylglucosamine 2-epimerase. J. Biosci. Bioeng. 93:258–265 [DOI] [PubMed] [Google Scholar]
- 26. Mauriello E. M., Duc le H., et al. 2004. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 22:1177–1187 [DOI] [PubMed] [Google Scholar]
- 27. Nicholson W. L., Munakata N., Horneck G., Melosh H. J., Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez-Aparicio L. B., Ferrero M. A., Reglero A. 1995. N-Acetyl-d-neuraminic acid synthesis in Escherichia coli K1 occurs through condensation of N-acetyl-d-mannosamine and pyruvate. Biochem. J. 308:501–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmid A., et al. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258–268 [DOI] [PubMed] [Google Scholar]
- 30. Schoemaker H. E., Mink D., Wubbolts M. G. 2003. Dispelling the myths—biocatalysis in industrial synthesis. Science 299:1694–1697 [DOI] [PubMed] [Google Scholar]
- 31. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 32. Stahl S., Uhlen M. 1997. Bacterial surface display: trends and progress. Trends Biotechnol. 15:185–192 [DOI] [PubMed] [Google Scholar]
- 33. Tabata K., Koizumi S., Endo T., Ozaki A. 2002. Production of N-acetyl-d-neuraminic acid by coupling bacteria expressing N-acetyl-d-glucosamine 2-epimerase and N-acetyl-d-neuraminic acid synthetase. Enzyme Microb. Technol. 30:327–333 [Google Scholar]
- 34. Tao F., Zhang Y. N., Ma C. Q., Xu P. 2010. Biotechnological production and applications of N-acetyl-d-neuraminic acid: current state and perspectives. Appl. Microbiol. Biotechnol. 87:1281–1289 [DOI] [PubMed] [Google Scholar]
- 35. von Itzstein M., et al. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423 [DOI] [PubMed] [Google Scholar]
- 36. Wang T. H., et al. 2009. Production of N-acetyl-d-neuraminic acid using two sequential enzymes overexpressed as double-tagged fusion proteins. BMC Biotechnol. 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu X.-C., Lee W., Tran L., Wong S.-L. 1991. Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J. Bacteriol. 173:4952–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu P., et al. 2007. Efficient whole-cell biocatalytic synthesis of N-acetyl-d-neuraminic acid. Adv. Synth. Catal. 349:1614–1618 [Google Scholar]
- 39. Yang M. M., Zhang W. W., Zhang X. F., Cen P. L. 2006. Construction and characterization of a novel maltose inducible expression vector in Bacillus subtilis. Biotechnol. Lett. 28:1713–1718 [DOI] [PubMed] [Google Scholar]
- 40. Yim S. K., Jung H. C., Yun C. H., Pan J. G. 2009. Functional expression in Bacillus subtilis of mammalian NADPH-cytochrome P450 oxidoreductase and its spore-display. Protein Expr. Purif. 63:5–11 [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y. N., et al. 2010. An efficient method for N-acetyl-d-neuraminic acid production using coupled bacteria cells with a safe temperature-induced system. Appl. Microbiol. Biotechnol. 86:481–489 [DOI] [PubMed] [Google Scholar]
- 42. Zhou Z., et al. 2008. Immunogenicity of recombinant Bacillus subtilis spores expressing Clonorchis sinensis tegumental protein. Parasitol. Res. 102:293–297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.