Abstract
Pantoea agglomerans is a common soil bacterium used in the biocontrol of fungi and bacteria but is also an opportunistic human pathogen. It has been described extensively in this context, but knowledge of bacteriophages infecting this species is limited. Bacteriophages LIMEzero and LIMElight of P. agglomerans are lytic phages, isolated from soil samples, belonging to the Podoviridae and are the first Pantoea phages of this family to be described. The double-stranded DNA (dsDNA) genomes (43,032 bp and 44,546 bp, respectively) encode 57 and 55 open reading frames (ORFs). Based on the presence of an RNA polymerase in their genomes and their overall genome architecture, these phages should be classified in the subfamily of the Autographivirinae, within the genus of the “phiKMV-like viruses.” Phylogenetic analysis of all the sequenced members of the Autographivirinae supports the classification of phages LIMElight and LIMEzero as members of the “phiKMV-like viruses” and corroborates the subdivision into the different genera. These data expand the knowledge of Pantoea phages and illustrate the wide host diversity of phages within the “phiKMV-like viruses.”
INTRODUCTION
Pantoea agglomerans bacteria are Gram-negative, nonsporulating, facultatively anaerobic rods that belong to the family of the Enterobacteriaceae. Synonyms of this species name are Enterobacter agglomerans, Erwinia herbicola, and Erwinia milletiae (6, 19). The genus name Pantoea is derived from the Greek word pantoios, which means “of all sorts and sources,” a descriptor well reflected in the habitat, since species of the genus have been isolated from plant surfaces, seeds, water, soil, and even humans and animals (3, 19, 41). Pantoea agglomerans is known in plant production as a biocontrol agent. It is used in postharvest control of a number of fungi (for instance, Penicillium expansum, Rhizopus stolonifer, Monilinia laxa, and Botrytis cinerea) on a wide variety of fruits (18, 44, 45, 54). Some strains of P. agglomerans or E. herbicola have been reported to produce an antibiotic compound against Erwinia amylovora, the cause of fire blight on apple and pear trees, and can be used in orchards (24, 26, 47). However, P. agglomerans has a malevolent side as well, as indicated by reports of clinical isolates that are opportunistic human pathogens (10). In most cases, the bacteria cause soft tissue or bone/joint infections (septic arthritis) after penetration of the skin by vegetation, e.g., thorns or wood slivers (11, 14, 17, 29, 38, 55, 57). The potential pathogenicity of the bacterium prevents its use in biocontrol in Europe, but recently a single marker characteristic that may be useful in biosafety determinations and regulation has been discovered in the biocontrol strains (48).
Bacteriophages (also referred to simply as phages) can be used as a means to control bacteria. For P. agglomerans, phage therapy can focus on different aspects, such as reduction of bacterial residues on apples and pears, sanitation of appliances used in agriculture, or use as an alternative for antibiotics in human disease control (40, 52, 53). Lehman (2007) has reported the use of P. agglomerans strains as carriers for phages in the biological control of fire blight (36). In this case, phages infecting both P. agglomerans and Erwinia amylovora are applied on orchards together with a nonpathogenic P. agglomerans strain in order to support a population of phages during bloom.
To date, only one genome sequence of a phage infecting P. agglomerans is publicly available: Erwinia phage phiEa21-4 (RefSeq accession no. NC_011811) has a genome of 84,576 bp and is a member of the family Myoviridae (37). In this paper, we report on the isolation and characterization of two bacteriophages of Pantoea agglomerans, called LIMElight and LIMEzero (Leuven ILVO Merelbeke). Genome analysis of these phages shows them to be members of the “phiKMV-like viruses.” This recently established phage genus is classified within the Autographivirinae subfamily of the Podoviridae along with the “T7-like viruses” and “SP6-like viruses.” It groups phages with a common transcriptional scheme, able to infect a wide range of environmentally important bacteria, such as Vibrio spp., Pseudomonas spp., and Ralstonia solanacearum (5, 8, 25, 30, 31, 32, 33, 46).
MATERIALS AND METHODS
Bacterial strains and growth.
Pantoea agglomerans strain GBBC 2043 was isolated from potato fields in Merelbeke, Belgium (Institute for Agricultural and Fisheries Research [ILVO]) and was classified by sequencing of the 16S rRNA gene and the additional core genes gyrB and rpoB (EMBL accession no. FR832419, FR832420, and FR832421, respectively) according to the work of Brady et al. (7). All strains used in this paper (Table 1) were grown at 30°C in standard LB medium. LB was supplemented with 1.5% agar for plating and with 0.7% agar for agar overlays.
Table 1.
Bacterial strains used in this study
| Species and strain | Yr of isolation | Location | Biological origin |
|---|---|---|---|
| Pantoea agglomerans | |||
| GBBC 2043 | 2008 | Belgium | Soil |
| LMG 2660 | 1970 | Japan | Wisteria floribunda |
| LMG 2570 | 1980 | United States | Sorbus sp. |
| Pantoea stewartii | |||
| LMG 2712 | 1957 | United States | Zea mays |
| LMG 2713 | 1963 | United States | Zea mays |
| LMG 2714 | 1932 | United States | Zea mays |
| LMG 2717 | 1954 | United States | Beetle |
| LMG 2718 | 1963 | United States | Unknown |
| LMG 2719 | 1975 | United States | Corn flea beetle |
| Erwinia amylovora | |||
| LMG 2024 | 1959 | United Kingdom | Pyrus communis |
| GBBC 403 | 2000 | Belgium | Crataegus sp. |
| Erwinia mallotivora | |||
| LMG 1271 | 1975 | Japan | Mallotus japonicus |
Bacteriophage isolation, amplification, and purification.
Phages LIMElight and LIMEzero were isolated from 20-g soil samples taken from the same potato fields from which the bacterial strain was isolated, shaken for 30 min in sterile, demineralized water, and filtered over a 0.45-μm-pore-size membrane (Millipore). The filtrate was centrifuged for 90 min at 28,000 × g (Sigma 3K30 centrifuge; fixed-angle rotor, type 12156-H [B. Braun Biotech]), and the pellet was resuspended in phage buffer (10 mM Tris-HCl [pH 7.5], 10 mM MgSO4, 150 mM NaCl). This suspension was spotted onto a plate with a soft agar overlay of a stationary Pantoea agglomerans culture. Lysis zones were picked up, and three successive single-plaque isolations were performed using the standard agar overlay method (2). Phage were amplified by plating out 105 PFU per plate, scraping off the soft agar layer after overnight incubation, and suspending it in phage buffer. Polyethylene glycol 8000 (PEG 8000) containing 1 M NaCl was added to the suspension to a final concentration of 8%, followed by incubation for a minimum of 3 h at 4°C. Phage particles were then precipitated by centrifuging the solution for 30 min at 8,000 × g in a Sorvall Legend RT Plus centrifuge with a swing-out 4-place rotor, type 75006445 (Thermo Scientific, Waltham, MA), and were resuspended in 15 ml phage buffer. The phage suspension was subsequently layered on a CsCl step gradient (1.33, 1.45, 1.50, and 1.70 g/cm3) and was centrifuged at 140,000 × g for 3 h with a Beckman Coulter (Brea, CA) Optima LE-80K ultracentrifuge (rotor type, SW28). LIMElight and LIMEzero were both collected at the interface between the gradients of 1.45 and 1.50 g/cm3 and were dialyzed three times for 30 min against 300 volumes of phage buffer.
Electron microscopy.
Phage particles were pelleted by centrifugation for 1 h at 25,000 × g and were washed twice in 0.1 M ammonium acetate (pH 7.0) using a Beckman (Palo Alto, CA) high-speed centrifuge and a JA-18.1 fixed-angle rotor. The pellets were then deposited on copper grids with carbon-coated Formvar films, stained with 2% (wt/vol) potassium phosphotungstate (pH 7.0), and examined with a Philips EM 300 electron microscope (1).
Host range and infection assays.
The host range of the bacteriophages was determined by spotting 10 μl of phage suspension (106 PFU/ml) on plates with a bacterial lawn and by the standard agar overlay where a phage suspension was added to the bacterial lawn. Phages were tested on 12 strains, summarized in Table 1. In adsorption experiments, the host strain GBBC 2043 was grown to an optical density at 600 nm of 0.4 and was infected with phages at a multiplicity of infection (MOI) of 0.001. After infection, 100-μl samples were taken immediately and every minute. Samples were transferred to 850 μl LB medium supplemented with 50 μl CHCl3. These mixtures were shaken gently for 15 min to lyse any remaining bacteria. The supernatant was titrated to determine the amount of nonadsorbed or reversibly adsorbed phages.
DNA isolation and sequencing.
DNA was isolated according to the method of Sambrook and Russell (2001) (50). Approximately 1 μg of DNA was sheared by sonication, and fragments of 1 to 2 kb were isolated. An end repair reaction was performed with T4 and Klenow polymerase, and the fragments were ligated in the pJET1.2 blunt vector (Fermentas), which was transformed into Escherichia coli XL1-Blue cells. Individual colonies were selected, and the vectors were isolated and sequenced using vector primers on an ABI 3130 capillary sequencer. Contigs were aligned with Sequencher software (version 4.1; Gene Codes).
In silico analysis.
The genomes of LIMElight and LIMEzero were scanned for potential open reading frames (ORFs) with ORF Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/) and GeneMark.hmm software (39). Shine-Dalgarno sequences were verified manually upstream of each annotated ORF. Functional bioinformatic annotation was carried out by comparing translated ORFs in a BLASTP (20) analysis against the nonredundant GenBank protein database and by using the HHPred prediction software (51). The presence of transmembrane domains was verified with TMHMM software (43), and signal peptides were identified with SignalP (16). Host promoter regions were identified using the Nostradamus (21) prediction program. Potential phage promoter sites were scanned for using MEME (4) and PHIRE (34) software. Palindromic repeat regions were identified by TransTerm (28) and Mfold (59). Terminators were defined as palindromic sequences followed by a U-rich stretch and a stable secondary structure as calculated by Mfold. Nucleotide similarity between phages was compared using the Stretcher algorithm (42).
Comparative genomics.
Phylogenetic trees were constructed using Phylogeny.fr (12). The nucleic acid sequences of the phages as found on NCBI were loaded in a FASTA file onto the server using the “One Click” option. Multiple alignment was carried out with the MUSCLE algorithm (15) without a curation step. Trees were constructed with PhyML (22) using the maximum-likelihood method and were drawn with TreeDyn.
RESULTS AND DISCUSSION
Phage isolation and morphological characteristics.
Bacteriophages LIMEzero and LIMElight were isolated from soil samples of potato trial fields of the Institute for Agricultural and Fisheries Research (ILVO), Merelbeke, Belgium. Both formed clear plaques on Pantoea agglomerans strain GBBC 2043, an isolate from the same field soil. Two distinct plaque morphologies were observed: a clear zone surrounded by a turbid halo with a diameter of 2 mm for LIMEzero and a small clear plaque of 1 mm in diameter for LIMElight. The host range of phage LIMEzero was limited to the P. agglomerans strain on which it was originally isolated. LIMElight also infected P. agglomerans strain LMG 2660, as confirmed by a plaque assay and spot tests. In the spot tests, the phage showed an inhibitory effect, the formation of a turbid halo without lysis, on Erwinia amylovora strain GBBC 403, Erwinia mallotivora strain LMG 1271, and Pantoea stewartii strains LMG 2717 and LMG 2719, suggesting the presence of an exopolysaccharide (EPS) depolymerase (23). No plaques were formed on these strains.
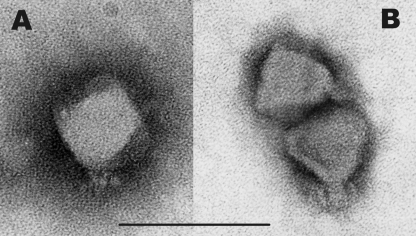
Electron microscopy revealed the phages to be members of the Podoviridae, with a morphotype identical to that of phiKMV (Fig. 1). The particles are indistinguishable from each other, with icosahedral heads of approximately 60 nm in diameter, a short, stubby tail 12 nm long, and a number of tail spikes (10 by 3 nm). The infection processes of LIMElight and LIMEzero were compared by infection and adsorption assays. New phage particles were released after 30 min for LIMEzero and after 270 min for LIMElight. The adsorption constants were 8.2 × 10−9 ml/min and 1.2 × 10−9 ml/min, respectively.
Fig. 1.
Electron microscopic images of phage LIMEzero (A) and phage LIMElight (B). Bar, 100 nm. Phages are colored with 2% phosphotungstate.
Genome analysis.
The genomic sequences of phages LIMElight and LIMEzero were determined using DNA extracted from purified phage particles by shotgun sequencing and primer walking. For LIMElight, this resulted in a sequence of 44,546 bp delineated by direct terminal repeats (DTRs) of 277 bp. These DTRs were determined by direct sequencing with outward-directed primers located in and right outside the repeats. LIMEzero has a genome of 43,032 bp, but we were not able to perform primer walking for the determination of DTRs in this phage, although they are expected. The GC contents of LIMElight and LIMEzero were 54.0% and 55.4%, respectively.
The genome of LIMEzero consisted of 57 ORFs, 18 of which were similar to those of members of the “phiKMV-like viruses” at the amino acid level (see Table S1 in the supplemental material). For LIMElight, 55 ORFs were predicted, also with 18 ORFs similar to those of the “phiKMV-like viruses” (see Table S2 in the supplemental material). No marked DNA similarity exists between the two phages.
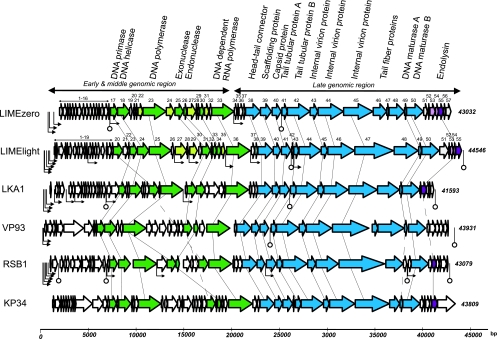
Phages of the Autographivirinae (35) share a genome organization and make use of both the host RNA polymerase and a phage-encoded RNA polymerase. In contrast to the “T7-like viruses,” these phages depend on the host RNA polymerase for the transcription of both early and middle genes. Therefore, the phages should encode both host σ70 promoters and phage-specific promoter sites (Fig. 2 and Table 2). In LIMElight, seven σ70 promoters were identified in intergenic regions; five of these are situated upstream of ORF1. One is located in front of the RNA polymerase gene and a final one in front of the sequence coding for tail protein A. A search for conserved intergenic motifs yielded four putative phage promoter sites: one in the early region, two in the DNA metabolism region, and one at the start of the predicted structural region (Fig. 2; Table 2). Two factor-independent terminators were located, one directly behind the capsid protein gene and one at the end of the genome, in front of the right DTR. In LIMEzero, only three potential host promoter sites were identified, all upstream of the first ORF. Putative phage-specific promoters were found in front of ORF17 in the beginning of the DNA metabolism region and in front of the RNA polymerase. Two terminators were identified; the first was located behind ORF16, in front of the first phage-specific promoter, and the second was located behind the capsid protein gene.
Fig. 2.
Comparison of the genomes of Pantoea phages LIMEzero and LIMElight with those of Pseudomonas phage LKA1, Vibrio phage VP93, Ralstonia phage RSB1, and Klebsiella phage KP34. The predicted open reading frames are indicated by arrows. ORFs of unknown function in the Pantoea phages are in light gray, predicted DNA metabolism genes in green, structural genes in blue, and lysis genes in purple. (ORFs in lighter shades have no equivalents in the other phages.) Functionally equivalent genes are connected by thin lines, and putative functions are designated. Host promoters are indicated by bent arrows with triangular points and phage-specific promoters by smaller bent arrows; factor-independent terminators are shown with stem-loop structures.
Table 2.
Regulatory elements in the genomes of LIMEzero and LIMElight
| Position | ORFa | Regulatory element sequenceb |
|---|---|---|
| Host promoters | ||
| LIMEzero | ||
| 801_847 | 1 | TAGTGCTTGTCAGATGGTTCGGATTGTTGTGTAATTCTCATCACCGA |
| 928_974 | 1 | CGCTCTTTATACAATCTGGTCTGGTTCTGTGATAGTCCACCAATCAA |
| 1399_1445 | 1 | AAGTACTTGACGGTTACAACGCAGTATGATGTAATTCGTCTCAAGCA |
| LIMElight | ||
| 500_546 | 1 | GTTATTTGTTATTTCGTTCGTCGCCTCACTCTATAACGTCAATCATA |
| 549_595 | 1 | TATCTTTGAACGTAATCCGTTTATTGATAGGTAAAACCTTGTGTACA |
| 805_849 | 1 | AGTGCTTGCAATCTCTAAATCATTCATC---TAGTATTCAATCCATC |
| 967_1010 | 1 | AGTGTTTGACAAGTGAATCCAGAAGGAT---TAGATTAGTTAGCAAC |
| 1097_1140 | 1 | AACGCTTGACAAGTTGAACGATAACGAT---TAACTTAGATAGCAAG |
| 19347_19391 | 36 | TCGTCTTGATAAAGCCGAGTGAGTGGTGT--TATAATCAATGGACAT |
| 26470_26513 | 43 | ATATTTTCAGTGTTCCTACGGGAGCACT---TATAAATACCATTCAC |
| Consensus promoter, E. coli | ------TTGACA(−35)--------------TATAAT(−10)----- | |
| Phage promoters | ||
| LIMEzero | ||
| 7294_7313 | 17 | TTTAAAGGCAATCACTAAGG |
| 20330_20349 | 34 | TATAAAGGCAAGCACTATGA |
| LIMElight | ||
| 4898_4922 | 12 | AATGGTGATGCACGTTATAGACCGA |
| 14061_14085 | 27 | ATTAAAGAGTGACGTTATAGAGAGA |
| 15535_15559 | 29 | TTTGAAGATACACGTTATAGAGAGC |
| 22024_22048 | 37 | AATGATGATGTACGTTATAGAGAGA |
| Factor-independent terminators | ||
| LIMEzero | ||
| 7255_7284 | 17 | AAGCCCATTTAAATAAGTGGGCTTTATTAT |
| 25202_25236 | 41 | AGCCCTGGACTCGTCCAGGGTTTGATTATGTTTT |
| LIMElight | ||
| 26239_26279 | 42 | TCTAAGGGAGCCTTTCGAGGTTCCCTTATTTGCC |
| 44216_44250 | DTR | GCAGCTGGTGTAATCCAGCACCTGAAATTTATT |
Number of the ORF downstream of the element.
Underlined sequences represent the −35 and −10 boxes for the host promoters and the palindromic sequence for the terminators.
Interestingly, direct genomic sequencing of LIMElight led to a sudden drop in the signal strength of the chromatograms at four specific locations on the noncoding strand, suggesting the presence of single-stranded breaks or nicks at these locations. A consensus sequence was found at the nick site (Table 3). In contrast, no nicks and no consensus sequence were identified in the genome of LIMEzero. The presence of such conserved nicks has also been observed in phages T5 (58) and phikF77 (31), but their consensus sequences bore no similarity to that of LIMElight (Table 3). The assumption has been made that these nick consensus sites function as phage-specific promoters (31), but for phage LIMElight, these promoters are clearly separate entities, with a different location and a different consensus sequence (Table 2). To date, no suitable explanation for the presence of these nicks has been offered. However, it should be noted that the presence of these nicks is conserved across different host species, but not within the phage genus or species.
Table 3.
Nicks in the genome of LIMElight
| Nick | Position | Sequence (5′–3′)a |
|---|---|---|
| n1 | 7422 | CAGGGCCGTGCGCTATGCT▾CAAGCAGAACAAATT |
| n2 | 9551 | GCCGCGTGTGCAGCCTGCA▾CCAGCAGACGTAATC |
| n3 | 15580 | GGAGCTGGTGCCGCTTACA▾CCAGCACAACAAAAC |
| n4 | 20424 | CGGATGGGTGCAGGCTGCA▾CGAGCACAAGGTATC |
| Consensus | GTGC-N4-TRCW▾CVAGCA | |
| T5 consensus | CCCT▾GCGC | |
| phikF77 consensus | CGAC-N5-CCTA▾CTCCG |
Underlined sequences mark the consensus site.
Genome architecture.
Two distinct genomic regions, marked by the location of the RNA polymerase gene, can be delineated in the genomes of the “phiKMV-like viruses.” A typical characteristic of these phages is that the early and middle region ends with the DNA-dependent RNA polymerase and encompasses the host conversion and DNA replication genes. The late region comprises the genes coding for structural and lysis proteins.
The genome architecture of all proposed “phiKMV-like viruses” (Table 4) is conserved despite the low DNA homology between a number of phages. Pseudomonas phages phiKMV, LKD16, LUZ19, phikF77, PT2, and PT5 show strong DNA similarity and can be considered the same species (with phiKMV as the type phage), whereas the other phages are each clearly distinct species. Phage KP34, not yet officially assigned to any genus, shows a similar genome architecture and is therefore included in this study.
Table 4.
Summary of the proposed “phiKMV-like viruses”
| Phage | Host | Genome (bp) | % DNA homology with: |
GenBank accession no. | Source or reference(s) | |
|---|---|---|---|---|---|---|
| φKMV | LKA1 | |||||
| phiKMV | Pseudomonas aeruginosa | 42,519 | 100 | 53.0 | NC_005045 | 33 |
| LKD16 | P. aeruginosa | 43,200 | 84.1 | 53.1 | NC_009935 | 8 |
| LUZ19 | P. aeruginosa | 43,548 | 89.1 | 52.8 | NC_010326 | 32 |
| phikF77 | P. aeruginosa | 43,152 | 86.5 | 52.9 | NC_012418 | 30, 31 |
| PT2 | P. aeruginosa | 42,961 | 96.7 | 52.9 | NC_011107 | Unpublished |
| PT5 | P. aeruginosa | 42,954 | 95.2 | 53.0 | NC_011105 | Unpublished |
| LKA1 | P. aeruginosa | 41,593 | 53.0 | 100 | NC_009936 | 8 |
| Phi-2 | P. fluorescens | 43,144 | 52.5 | 52.7 | NC_013638 | 46 |
| VP93 | Vibrio parahaemolyticus | 43,931 | 47.6 | 47.5 | NC_012662 | 5 |
| RSB1 | R. solanacearum | 43,079 | 50.2 | 49.7 | NC_011201 | 25 |
| KP34 | Klebsiella pneumoniae | 43,593 | 48.1 | 48.2 | NC_013649 | 13 |
| LIMElight | Pantoea agglomerans | 44,546 | 49.7 | 49.2 | FR687252 | This study |
| LIMEzero | Pantoea agglomerans | 43,032 | 50.1 | 49.5 | FR751545 | This study |
Early and middle region.
The early and middle region comprises the early genes and the DNA metabolism genes (Fig. 2). In LIMEzero and LIMElight there are, respectively, 16 and 19 small, early genes, which show no similarity to each other and have no counterpart in any public database. These genes are transcribed immediately after infection and are involved in host conversion. Recently, Roucourt and Lavigne (49) have shown that these proteins protect the bacteriophage from bacterial defense mechanisms or adapt the host cell mechanism (49). The strong divergence within the “phiKMV-like viruses” may thus be explained in part by the fact that they infect a different range of host bacteria or utilize different defense/adaptation mechanisms.
The first genes of the DNA replication region are the DNA primase (ORF17) in LIMEzero and the DNA ligase (ORF20) in LIMElight. In LIMEzero, no ligase could be identified by either BLAST or HHPred. Next is the DNA polymerase, followed by a 5′–3′ exonuclease, an endonuclease, and a polynucleotide 5′ kinase/3′ phosphatase, in the same order in both phages. This region ends with the DNA-dependent RNA polymerase. This gene order is preserved among most “phiKMV-like viruses.” Phage VP93 lacks a discernible DNA ligase, just like LIMEzero, whereas the DNA ligase in phage RSB1 is situated directly in front of the RNA polymerase instead of at the beginning of the region. In the latter, no predicted endonuclease has been found.
RNA polymerase and phage-specific promoters.
The DNA-dependent RNA polymerases of phages LIMEzero and LIMElight are very similar (BlastP expectancy values, 10e−90) and are also conserved throughout the genomes of all the “phiKMV-like viruses” (summarized in Table 4), with BlastP expectancy values between 10e−59 and zero. Conservation of the catalytic residues Tyr639, Lys631, Asp537, and Asp812 of the T7 RNA polymerase (9) was observed for LIMEzero and LIMElight. No homology was detected with either the recognition loop (amino acids 93 to 101) or the specificity loop (amino acids 739 to 770) of the T7 RNA polymerase, suggesting divergence in phage-specific promoter sequences. Indeed, no T7-like phage-specific promoter sequences were identified in LIMEzero or LIMElight. As mentioned above, two and four phage-specific promoters were found in LIMEzero and LIMElight, respectively (Fig. 2). The location of these promoters does not seem to be conserved within the “phiKMV-like viruses,” although both LIMEzero and LIMElight have one at the start of the structural region.
Structural region.
The structural region starts downstream of the RNA polymerase gene with several small genes, 4 in LIMEzero and 2 in LIMElight, followed by the first functionally identified gene, the head-tail connector (Fig. 2). The two small genes of LIMElight encode a conserved hypothetical protein (Gp37) and a conserved structural protein (Gp38) and are related to Gp36 and Gp37 of LIMEzero. These show similarity to the proteins of unknown function of phiKMV, Gp28 and Gp29, respectively, located in front of the phiKMV head-tail connector gene. In both LIMEzero and LIMElight, the scaffolding protein gene was found next, followed by the genes for the major capsid protein and tubular tail proteins A and B. This gene order was observed in all “phiKMV-like viruses.” Three internal virion proteins were identified in the Pantoea phages, one of which has a lysozyme domain as predicted by HHPred. Similar internal virion proteins have been confirmed by mass spectrometry for LKD16 and LKA1 (8), but they have not been identified in other phages. However, three genes of the same size are present in the same location in the other phages (Fig. 2). On this basis, it can be assumed that these are also internal virion protein genes, despite the lack of sequence or amino acid homology. The tail fiber proteins were the most strongly divergent proteins between LIMEzero and LIMElight. The former has two tail fiber proteins and a tail spike protein, like phiKMV; the latter encodes a single tail fiber protein with a predicted EPS depolymerase. This EPS depolymerase showed strong homology to counterparts in Erwinia amylovora phages phi-Ea1h (27) and Era103 (56), explaining the results of the host screen assay and suggesting lateral acquisition of this gene. Phages LKA1, RSB1, and KP34 also have only one tail fiber gene each, showing little homology with each other (8, 13, 25, 34). VP93 is the only phage with two tail fiber genes (5). The last two functionally annotated structural protein genes code for DNA maturase A and B and are well conserved among all “phiKMV-like viruses.”
Lysis genes.
Phages LIMEzero and LIMElight both encoded endolysin proteins (Gp55 in both phages), but only LIMElight had a clearly definable holin (Gp54). In LIMEzero, a presumptive holin gene was identified 5′ of the endolysin gene, based on the presence of a transmembrane domain and the overlap of the ORF with the endolysin. The holin-lysin mechanism for lysis of bacteria has been proposed for phages phiKMV and LKD16 (8, 33), and based on the amino acid homology of their holins and endolysins, it can be considered a common mechanism for the entire phiKMV species. Between the other “phiKMV-like viruses” there is limited protein similarity in the lysis genes. LKA1 does encode a presumed holin (based on the presence of two hydrophobic domains) and an endolysin (8). For the other phages in this group, no holins have been identified, only endolysins, except for VP93, for which neither has been found. The internally overlapping Rz/Rz1 proteins, generally encoded downstream of the endolysin gene, have been located in phiKMV, LKA1, and LKD16 (8) but not in any of the non-Pseudomonas phages. LIMEzero and LIMElight do not seem to encode such proteins downstream of their endolysin but have an interesting feature in front of the presumed holin. In both phages, ORF53 has a signal peptide and a transmembrane domain, suggesting a function in the lysis mechanism.
Comparative genome analysis.
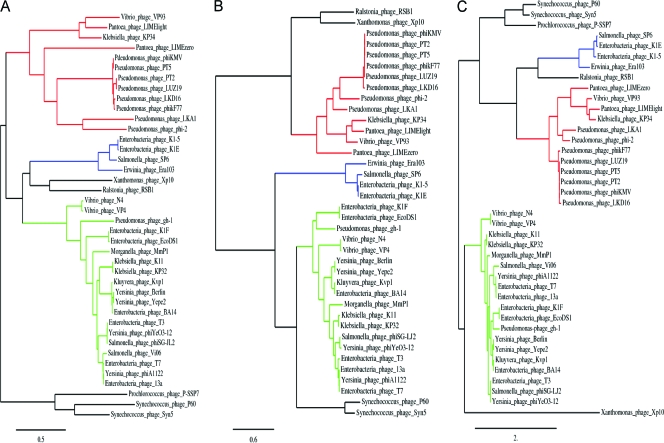
A hallmark of the genus of the “phiKMV-like viruses” is the presence of a DNA-dependent RNA polymerase located directly in front of the genes encoding the structural proteins. The other members of the Autographivirinae subfamily also encode an RNA polymerase, but in a different location in the genome, leading to two other genera, the “T7-like viruses” and the “SP6-like viruses.” Phylogenetic analysis of the RNA polymerase, DNA polymerase, and major capsid protein genes of all Autographivirinae with available sequences was carried out to investigate the clustering of the phages in these genera. The novel Pantoea phages LIMEzero and LIMElight were also included, as was Klebsiella phage KP34. The Siphovirus phage Xp10, infecting Xanthomonas, was also added because of the presence of a T7-like RNA polymerase.
The three phylogenetic trees (Fig. 3) supported the current genus subdivision within the Autographivirinae. As expected, the “T7-like viruses” all clustered within the same group, supplemented by the unclassified T7-like phages. The “SP6-like viruses” also formed a distinct clade, showing more relatedness to the “T7-like viruses” according to the RNA and DNA polymerase trees (Fig. 3A and B) but clustering closer to the “phiKMV-like viruses” according to the major capsid protein gene tree (Fig. 3C). The last genus is the most diverse, with all but one of its phages clustering together. Ralstonia phage RSB1 does not fit in with any genus. Instead, it clusters together with Xp10 for the polymerases and with the “SP6-like viruses” for the major capsid protein genes. The new Pantoea phages were assigned to the “phiKMV-like viruses” cluster, and their presence in this genus is thereby confirmed. The unassigned members of the Autographivirinae, Prochlorococcus phage P-SSP7 and Synechococcus phages P60 and Syn5, formed a separate clade, suggesting that they all belong to a new genus.
Fig. 3.
PhyML phylogenetic trees of the RNA polymerases (A), DNA polymerases (B), and major capsid genes (C) of the phages of the Autographivirinae. Red lines indicate the clade of “phiKMV-like viruses”; blue lines, the “SP6-like viruses”; and green lines, the “T7-like viruses.” The unassigned Autographivirinae are indicated by black lines, as are Siphovirus phage Xp10 and Ralstonia phage RSB1, which do not cluster in any of the previous groups.
Conclusion.
In this paper we studied two newly isolated Pantoea agglomerans phages, LIMEzero and LIMElight. They are the first members of the Podoviridae infecting this host species whose genomes have been fully sequenced. Based on their overall genome architecture, they are classified within the subfamily Autographivirinae, in the genus of the “phiKMV-like viruses.”
The “phiKMV-like viruses” grouping was first individualized by Ceyssens et al. in 2006 with the discovery of Pseudomonas phages LKD16 and LKA1 (8), which showed marked similarity to another Pseudomonas phage, phiKMV (33). The genus was extended with other Pseudomonas phages (PT2, PT5, phikF77, and LUZ19) (30, 31, 32), Vibrio phage VP93 (5), and Ralstonia phage RSB1 (25). Recently, Pseudomonas fluorescens phage phi-2 has been proposed as a member of this genus (46). In addition, Klebsiella phage KP34 (13), currently assigned to the unclassified Podoviridae, also shares the typical genome architecture. In this paper we were able to extend the genus “phiKMV-like viruses” with the novel Pantoea phages LIMEzero and LIMElight.
The known “phiKMV-like viruses” encapsulate a genome of approximately 42 kb; LIMElight had the largest genome, at 44.5 kb. Within this genus, all the phages have relatively long direct terminal repeats of 277 to 488 bp.
In the previously described “phiKMV-like viruses” LKD16, LUZ19, phikF77, PT2, PT5, and phiKMV, the overall gene sequences are highly conserved, with the exception of the early region and the tail fiber genes. They show more than 84% DNA homology with phiKMV and can be thus considered members of the same species, with phiKMV as the type phage. Bacteriophages LKA1, phi-2, VP93, RSB1, and KP34, and the new phages LIMElight and LIMEzero, show little DNA homology with phiKMV or with each other but display a conserved genome organization and gene order (Fig. 2).
We conclude that the genus “phiKMV-like viruses” is still expanding and is ubiquitously distributed, since isolates have been collected from all over the world (e.g., in Russia, Belgium, Chile, and Japan) and infect a range of different hosts.
Supplementary Material
ACKNOWLEDGMENT
P.-J.C. holds a postdoctoral fellowship from the “FWO Vlaanderen” at the K.U. Leuven.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 18 March 2011.
REFERENCES
- 1. Ackermann H. W. 2009. Basic phage electron microscopy, p. 113–126 In Clokie M. R. J., Kropinski A. M. (ed.), Bacteriophages: methods and protocols, vol. 1 Isolation, characterization, and interactions. Humana Press, Clifton, NJ: [DOI] [PubMed] [Google Scholar]
- 2. Adams M. H. 1959. Bacteriophages, p. 14–15 Interscience Publishers, Inc., New York, NY [Google Scholar]
- 3. Andersson A. M., Weiss N., Rainey F., Salkinoja-Salonen M. S. 1999. Dust-borne bacteria in animal sheds, schools and children's day care centres. J. Appl. Microbiol. 86:622–634 [DOI] [PubMed] [Google Scholar]
- 4. Bailey T. L., Elkan C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers, p. 28–36 In Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. AAAI Press, Menlo Park, CA: [PubMed] [Google Scholar]
- 5. Bastías R., Higuera G., Sierralta W., Espejo R. T. 2010. A new group of cosmopolitan bacteriophages induce a carrier state in the pandemic strain of Vibrio parahaemolyticus. Environ. Microbiol. 12:990–1000 [DOI] [PubMed] [Google Scholar]
- 6. Beji A., et al. 1988. Subjective synonymy of Erwinia herbicola, Erwinia milletiae and Enterobacter agglomerans and redefinition of the taxon by genotypic and phenotypic data. Int. J. Syst. Bacteriol. 38:77–88 [Google Scholar]
- 7. Brady C., et al. 2008. Phylogeny and identification of Pantoea species associated with plants, humans and the natural environment based on multilocus sequence analysis (MLSA). Syst. Appl. Microbiol. 31:447–460 [DOI] [PubMed] [Google Scholar]
- 8. Ceyssens P. J., et al. 2006. Genomic analysis of Pseudomonas aeruginosa phages LKD16 and LKA1: establishment of the phiKMV subgroup within the T7 supergroup. J. Bacteriol. 188:6924–6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheetham G. M., Steitz T. A. 2000. Insights into transcription: structure and function of single-subunit DNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 10:117–123 [DOI] [PubMed] [Google Scholar]
- 10. Cruz A. T., Cazacu A. C., Allen C. H. 2007. Pantoea agglomerans, a plant pathogen causing human disease. J. Clin. Microbiol. 45:1989–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Champs C., et al. 2000. Isolation of Pantoea agglomerans in two cases of septic mono-arthritis after plant thorn and wood sliver injuries. J. Clin. Microbiol. 38:460–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dereeper A., et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36(Web Server issue):W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drulis-Kawa Z., et al. 2011. Isolation and characterization of KP34—a novel φKMV-like bacteriophage for Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. doi:10.1007/s00253-011-3149-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duerinckx J. F. H. 2008. Case report: subacute synovitis of the knee after a rose thorn injury: unusual clinical picture. Clin. Orthop. Relat. Res. 466:3138–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Emanuelsson O., Brunak S., von Heijne G., Nielsen H. 2007. Locating proteins in the cell using TargetP, SignalP, and related tools. Nat. Protoc. 2:953–971 [DOI] [PubMed] [Google Scholar]
- 17. Flatauer F. E., Kahn M. A. 1978. Septic arthritis caused by Enterobacter agglomerans. Arch. Intern. Med. 138:788. [PubMed] [Google Scholar]
- 18. Francés J., et al. 2006. Pathogen aggressiveness and postharvest biocontrol efficiency in Pantoea agglomerans. Postharvest Biol. Technol. 39:299–307 [Google Scholar]
- 19. Gavini F., et al. 1989. Transfer of Enterobacter agglomerans (Beijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and description of Pantoea dispersa sp. nov. Int. J. Syst. Bacteriol. 39:337–345 [Google Scholar]
- 20. Gertz E. M. 16 March 2005, posting date. Blast scoring parameters. http://www.ice.mbt.cuhk.edu.hk/download/bio%204330/References/blastscoring.pdf
- 21. Gordon L., Chervonenkis A., Gammerman A. J., Shahmuradov I. A., Sovolyev V. 2003. Sequence Alignment Kernel for recognition of promoter regions. Bioinformatics 19:1964–1971 [DOI] [PubMed] [Google Scholar]
- 22. Guindon S., Gascuel O. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 23. Hartung J. S., Fulbright D. W., Klos E. J. 1988. Cloning of a bacteriophage polysaccharide depolymerase gene and its expression in Erwinia amylovora. Mol. Plant Microbe Interact. 1:87–93 [Google Scholar]
- 24. Ishimaru C. A., Klos E. J., Brubaker R. R. 1988. Multiple antibiotic production by Erwinia herbicola. Phytopathology 78:746–750 [Google Scholar]
- 25. Kawasaki T., et al. 2009. Genomic characterization of Ralstonia solanacearum phage phiRSB1, a T7-like wide-host-range phage. J. Bacteriol. 191:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kearns L. P., Hale C. N. 1996. Partial characterization of an inhibitory strain of Erwinia herbicola with potential as a biocontrol agent for Erwinia amylovora, the fire blight pathogen. J. Appl. Microbiol. 81:369–374 [Google Scholar]
- 27. Kim W. S., Geider K. 2000. Characterization of a viral EPS depolymerase, a potential tool for control of fire blight. Phytopathology 90:1263–1268 [DOI] [PubMed] [Google Scholar]
- 28. Kingsford C. L., Ayanbule K., Salzberg S. L. 2007. Rapid, accurate, computational discovery of Rho-independent transcription terminators illuminates their relationship to DNA uptake. Genome Biol. 8:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kratz A., Greenberg D., Barki Y., Cohen E., Lifshitz M. 2003. Pantoea agglomerans as a cause of septic arthritis after palm tree thorn injury; case report and literature review. Arch. Dis. Child. 88:542–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kulakov L. A., Ksenzenko V. N., Kochetkov V. V., Mazepa V. N., Boronin A. M. 1985. DNA homology and adsorption specificity of Pseudomonas aeruginosa virulent bacteriophages. Mol. Gen. Genet. 200:123–127 [DOI] [PubMed] [Google Scholar]
- 31. Kulakov L. A., et al. 2009. Genomes of “phiKMV-like viruses” of Pseudomonas aeruginosa contain localized single-strand interruptions. Virology 391:1–4 [DOI] [PubMed] [Google Scholar]
- 32. Lammens E., et al. 2009. Representational Difference Analysis (RDA) of bacteriophage genomes. J. Microbiol. Methods 77:207–213 [DOI] [PubMed] [Google Scholar]
- 33. Lavigne R., et al. 2003. The genome of bacteriophage phiKMV, a T7-like virus infecting Pseudomonas aeruginosa. Virology 312:49–59 [DOI] [PubMed] [Google Scholar]
- 34. Lavigne R., Sun W. D., Volckaert G. 2004. PHIRE, a deterministic approach to reveal regulatory elements in bacteriophage genomes. Bioinformatics 20:629–635 [DOI] [PubMed] [Google Scholar]
- 35. Lavigne R., Seto D., Mahadevan P., Ackermann H. W., Kropinski A. M. 2008. Unifying classical and molecular taxonomic classification: analysis of the Podoviridae using BLASTP-based tools. Res. Microbiol. 159:406–414 [DOI] [PubMed] [Google Scholar]
- 36. Lehman S. M. 2007. Development of a bacteriophage-based biopesticide for fire blight. Ph.D. thesis. Brock University, St. Catharines, Ontario, Canada [Google Scholar]
- 37. Lehman S. M., Kropinski A. M., Castle A. J., Svircev A. M. 2009. Complete genome of the broad-host-range Erwinia amylovora phage phiEa21-4 and its relationship to Salmonella phage Felix O1. Appl. Environ. Microbiol. 75:2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liberto M. C., et al. 2009. Six cases of sepsis caused by Pantoea agglomerans in a teaching hospital. New Microbiol. 32:119–123 [PubMed] [Google Scholar]
- 39. Lukashin A., Borodovsky M. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Merril C. R., Scholl D., Adhya S. 2006. Phage therapy, p. 725–741 In Calendar R. (ed.), Bacteriophages, 2nd ed. Oxford University Press, New York, NY [Google Scholar]
- 41. Monier J. M., Lindow S. E. 2005. Aggregates of resident bacteria facilitate survival of immigrant bacteria on leaf surfaces. Microb. Ecol. 49:343–352 [DOI] [PubMed] [Google Scholar]
- 42. Myers E. W., Miller W. 1988. Optimal alignments in linear space. Comput. Appl. Biosci. 4:11–17 [DOI] [PubMed] [Google Scholar]
- 43. Nielsen H., Engelbrecht J., Brunak S., von Heijne G. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1–6 [DOI] [PubMed] [Google Scholar]
- 44. Nunes C., Usall J., Teixidó N., Viñas 2001. Biological control of postharvest pear diseases using a bacterium, Pantoea agglomerans CPA-2. Int. J. Food Microbiol. 70:53–61 [DOI] [PubMed] [Google Scholar]
- 45. Nunes C., Usall J., Teixidó N., Fons E., Viñas I. 2002. Post-harvest biological control by Pantoea agglomerans (CPA-2) on Golden Delicious apples. J. Appl. Microbiol. 92:247–255 [DOI] [PubMed] [Google Scholar]
- 46. Paterson S., et al. 2010. Antagonistic coevolution accelerates molecular evolution. Nature 464:275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pusey P. L., Rudell D. R., Stockwell V. O. 2008. Mechanisms of Pantoea agglomerans strain E325 as antagonist of Erwinia amylovora. Acta Hort. 793:457–460 [DOI] [PubMed] [Google Scholar]
- 48. Rezzonico F., Smits T. H. M., Montesinos E., Frey J. E., Duffy B. 2009. Genotypic comparison of Pantoea agglomerans plant and clinical strains. BMC Microbiol. 9:204–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roucourt B., Lavigne R. 2009. The role of interactions between phage and bacterial proteins within the infected cell: a diverse and puzzling interactome. Environ. Microbiol. 11:2789–2805 [DOI] [PubMed] [Google Scholar]
- 50. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 1, p. 2.56–2.58 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 51. Söding J., Biegert A., Lupas A. N. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33(Web Server issue):W244–W248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sulakvelidze A., Barrow P. 2005. Phage therapy in animals and agribusiness, p. 335–380 In Kutter E., Sulakvelidze A. (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 53. Sulakvelidze A., Kutter E. 2005. Bacteriophage therapy in humans, p. 381–436 In Kutter E., Sulakvelidze A. (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 54. Trotel-Aziz P., Couderchet M., Biagianti S., Aziz A. 2008. Characterization of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas spp. mediating grapevine resistance against Botrytis cinerea. Environ. Exp. Bot. 64:21–32 [Google Scholar]
- 55. Ulloa-Gutierrez R., Moya T., Avila-Aguera M. L. 2004. Pantoea agglomerans and thorn-associated suppurative arthritis. Pediatr. Infect. Dis. J. 23:690. [DOI] [PubMed] [Google Scholar]
- 56. Vandenbergh P. A., Cole R. L. 1986. Cloning and expression in Escherichia coli of the polysaccharide depolymerase associated with bacteriophage-infected Erwinia amylovora. Appl. Environ. Microbiol. 51:862–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vincent K., Szabo R. M. 1988. Enterobacter agglomerans osteomyelitis of the hand from a rose thorn. A case report. Orthopedics 11:465–467 [DOI] [PubMed] [Google Scholar]
- 58. Wang J., et al. 2005. Complete genome sequence of bacteriophage T5. Virology 332:45–65 [DOI] [PubMed] [Google Scholar]
- 59. Zuker M., Mathews D. H., Turner D. H. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide in RNA biochemistry and biotechnology, p. 11–43 In Barciszewski J., Clark B. F. C. (ed.), RNA biochemistry and biotechnology. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.