Abstract
Major histocompatibility complex (MHC) molecules expressed on the surface of human immunodeficiency virus (HIV) are potential targets for neutralizing antibodies. Since MHC molecules are polymorphic, nonself MHC can also be immunogenic. We have used combinations of novel recombinant HLA class I and II and HIV/simian immunodeficiency virus (SIV) antigens, all linked to dextran, to investigate whether they can elicit protective immunity against heterologous simian/human immunodeficiency virus (SHIV) challenge in rhesus macaques. Three groups of animals were immunized with HLA (group 1, n = 8), trimeric YU2 HIV type 1 (HIV-1) gp140 and SIV p27 (HIV/SIV antigens; group 2, n = 8), or HLA plus HIV/SIV antigens (group 3, n = 8), all with Hsp70 and TiterMax Gold adjuvant. Another group (group 4, n = 6) received the same vaccine as group 3 without TiterMax Gold. Two of eight macaques in group 3 were completely protected against intravenous challenge with 18 50% animal infective doses (AID50) of SHIV-SF162P4/C grown in human cells expressing HLA class I and II lineages represented in the vaccine, while the remaining six macaques showed decreased viral loads compared to those in unimmunized animals. Complement-dependent neutralizing activity in serum and high levels of anti-HLA antibodies were elicited in groups 1 and 3, and both were inversely correlated with the plasma viral load at 2 weeks postchallenge. Antibody-mediated protection was strongly supported by the fact that transfer of pooled serum from the two challenged but uninfected animals protected two naïve animals against repeated low-dose challenge with the same SHIV stock. This study demonstrates that immunization with recombinant HLA in combination with HIV-1 antigens might be developed into an alternative strategy for a future AIDS vaccine.
INTRODUCTION
The need for a prophylactic human immunodeficiency virus (HIV) vaccine remains urgent, especially in the developing world. It is widely believed that an effective vaccine against HIV/AIDS will have to induce responses of both the cellular and humoral arms of the immune system. However, despite many years of intense research, attempts to elicit potent and broadly neutralizing antibodies against HIV have so far been unsuccessful. This is largely due to the enormous diversity of the HIV-encoded targets for neutralizing antibodies, i.e., the surface and transmembrane Env glycoproteins, gp120 and gp41, respectively, which enables virus variants to escape antibody recognition. In addition, these viral glycoproteins have other mechanisms to protect themselves from neutralizing antibodies, such as shedding of gp120, glycan shielding, and masking of conserved structures (reviewed in reference 14). Alternative strategies for inducing effective humoral immune responses against HIV are therefore desirable.
When a nascent HIV virus particle buds from an infected cell, it incorporates several host cell proteins into its envelope membrane. Among these proteins are the major histocompatibility complex (MHC) class I and II molecules (reviewed in reference 25). It has been repeatedly shown that xenoimmunization of macaques, with either human cells alone, purified human leukocyte antigen (HLA) class I or class II proteins, or, more efficiently, fixed simian immunodeficiency virus (SIV)-infected human cells or inactivated SIV grown in human cells can provide sterilizing immunity against subsequent challenge with SIV (1, 5, 16, 31, 39). Anti-HLA antibodies are likely to be involved in this protection, by direct neutralization, complement-dependent inhibition, or some other mechanism (5), but cellular and/or innate immune responses may also play a role (45).
The HLA molecules are the most polymorphic human proteins, and as nearly every individual has a unique suite of HLA antigens, exposure to foreign HLA can result in an alloimmune response against the variable regions of the HLA molecules. Thus, the mechanism of protection against SIV by xenoimmunization may also be effective in alloimmunization against HIV. So far this possibility has not been extensively studied, but several observations support the concept. Whole-cell alloimmunization of women with their partner's leukocytes elicited resistance to HIV replication in vitro (46), and their sera neutralized HIV grown in their partner's peripheral blood mononuclear cells (PBMC) (18). There were no adverse effects, and since similar alloimmunization procedures have been carried out with over 1,000 women, this practice appears to be safe. Patients receiving multiple blood transfusions also develop alloresponses, including anti-HLA antibodies, and these antibodies neutralized HIV grown in cells expressing the corresponding HLA lineages (35). Furthermore, the observation that mother-child HLA class I concordance (20) is associated with an increased perinatal HIV type 1 (HIV-1) transmission rate suggests that allogeneic immune responses may have a protective effect. In a clinical trial with a fixed inactivated HIV-1 vaccine, participants developed anti-HLA antibody responses concordant with the host cell line used for vaccine production, showing the potential for alloimmunization (29). In a potential future prophylactic allovaccine against HIV, a sufficient number of different HLA lineages would have to be included to elicit immune responses, and thereby protection, in >90% of the population. In, e.g., a sub-Saharan population, four HLA class I alleles (HLA-A*01:01, -A*02:01, -A*03:01, and -A*29:01) would be sufficient to obtain such HLA coverage (unpublished data).
To explore xenovaccination as an alternative strategy for eliciting protective immunity against SHIV challenge in a proof-of-concept study, we immunized macaques with recombinant HLA class I and II molecules, with or without trimeric HIV-1 gp140 and SIV p27 proteins and the TiterMax Gold adjuvant, and challenged them intravenously (i.v.) with heterologous R5 simian/human immunodeficiency virus (SHIV). Complete or partial protection was achieved in macaques immunized with all vaccine components. Transfer of serum from two completely protected animals to naïve macaques protected these from subsequent SHIV challenge. The HLA-immunized macaques displayed complement-dependent neutralization activity in serum and anti-HLA antibodies that correlated inversely with the viral load at 2 weeks after challenge.
MATERIALS AND METHODS
Vaccine preparation.
Four HLA class I alleles and one HLA class II allele were selected, as they are common in the Caucasian population (8), their peptide folding is known, and they are likely to stimulate an alloantibody response in the majority of humans (shown together with their respective folding peptide in parentheses): (i) HLA-A*01:01(IVDCLTEMY), (ii) HLA-A*02:01(GLIQLVEGV), (iii) HLA-A*03:01(RIAAWMATY), (iv) HLA-A*11:01(VTDFSVIK), and (v) HLA-DRB1*04:01. Preparation of recombinant HLA class I heavy chains and β2 microglobulin (β2 M) was described in detail elsewhere (32). Briefly, synthetic genes encoding the protein-coding sequence minus the signal peptide and transmembrane regions of the human MHC class I alleles were created by PCR of overlapping DNA primers based on GenBank sequence information. Recombinant HLA class I and β2 M were produced by fermentation, isolated, and purified. The HLA heavy chain was biotinylated before in vitro folding together with β2 M and peptide into functional peptide-MHC complexes. HLA-DRB1*04:01 was purchased from Benaroya Research Institute, Seattle, WA. Briefly, HLA-DRB1*04:01-leucine zipper-biotinylation site expression vectors made using the PCR-mediated splicing overlap technique together with the plasmid pUChsneo, which carries the neomycin resistance marker, were cotransfected into Schneider cells S-2 by standard calcium phosphate transfection techniques. The DRB1*04:01 molecules were purified by affinity chromatography using L243 as described previously (38), biotinylated, and then loaded with peptide by incubation for 72 h at 37°C.
TheYU2 HIV-1 gp140-foldon trimers were genetically engineered by PCR-mediated cloning to encode a C-terminal, site-specific biotinylation motif known as an AviTag sequence (GeneCopoeia, Rockville, MD). The C terminus of the gp140 trimeric glycoproteins thereby ended with the HIV-1-encoded gp41 sequence at residue 683 K (HXBc2 numbering), followed by the foldon motif (as previously described [47]), followed by a His6 purification tag and finally the AviTag sequence. The recombinant proteins were expressed in Freestyle 293F cells, purified, and concentrated, and the trimeric fraction was isolated as previously described (23). Following purification, the trimers were monobiotinylated using biotin and biotin ligase as per the manufacturer's instructions (GeneCopoeia).
Monobiotinylated SIV p27 was produced following subcloning and excision of the glutathione S-transferase (GST) fusion partner from the original clone (SIV p27-gst) (21). The C-terminal fragment of Mycobacterium tuberculosis Hsp70 (positions 359 to 609) (2) was subcloned to enable in vitro biotinylation. A primer of 76 bases for PCR that allowed fusion of the recognition site for in vivo biotinylation in Escherichia coli to the C-terminal ends of SIV p27 and Hsp70359-609 was designed. The proteins were expressed and purified from recombinant BL21(DE3). To achieve a higher purity, the protein(s) underwent two further purification steps by anion-exchange and size exclusion chromatography.
For preparation of the dextramer vaccine complexes, the biotinylated peptide-MHC class I and class II complexes, trimeric HIVgp140, SIVp27, and Hsp70359-609 were linked to a streptavidin-coated, divinyl sulfone acid-activated dextran backbone (32).
Animals and immunizations.
The study was carried out in compliance with the provisions and general guidelines of the Swedish Animal Welfare Agency, and all procedures were approved by the Ethical Committee on Animal Experiments of North Stockholm (permit number N90/06). Thirty-eight female rhesus macaques (Macaca mulatta) of Chinese origin, 3 to 5 years old at the start of the study, were housed in the Astrid Fagraeus laboratory at the Swedish Institute for Communicable Disease Control. Immunizations and blood sampling were performed under sedation with ketamine at 10 mg/kg intramuscularly (i.m.) (Ketaminol, 100 mg/ml; Intervet, Sweden). Before entering the study, all animals were confirmed to be negative for simian immunodeficiency virus (SIV), simian T-cell lymphotropic virus, and simian retrovirus type D. The MHC class I alleles were determined for all animals. RNA isolation, reverse transcription-PCR (RT-PCR), and cloning and sequencing of macaque class I A and B alleles were described elsewhere (26, 27). Unreported alleles, of which at least three full-length clones were present, were submitted to the EMBL-EBI database and also to the nonhuman primate MHC section of the Immuno Polymorphism Database (for official designations, see reference 28).
Groups of eight animals were immunized with HLA class I (A*01:01, A*02:01, A*03:01, and A*11:01; 100 μg of each/full dose) and class II (DRB1*04:01; 113.5 μg/full dose) (group 1), trimeric YU2 HIV-1 gp140 and SIV Gag p27 (HIV/SIV antigens; 152.4 and 37.1 μg/full dose, respectively) (group 2), or both (group 3), all together with Hsp70biopep3 (3 × 25.8 μg/full dose) and coupled to dextran backbones (162.2 to 648.8 μg/full dose, depending on the number of attached components). Group 1 to 3 animals received a full vaccine dose at the first immunization and half doses at the subsequent immunizations. The vaccines for groups 1 to 3 were given subcutaneously (s.c.) formulated as an emulsion with the TiterMax Gold adjuvant (0.5 ml/full dose; Sigma-Aldrich, St. Louis, MO). Six additional animals (group 4) received the same vaccine as group 3 but without TiterMax Gold, i.m., and with full vaccine doses at all immunizations. Group 5 control animals were unvaccinated (Table 1).
Table 1.
Immunizations and challengea
| Group | n | Dextramer immunogens | Adjuvant |
|---|---|---|---|
| 1 | 8 | HLA-I/II + Hsp70 + dextran | TiterMax Gold |
| 2 | 8 | HIV-1 gp140/SIV p27 + Hsp70 + dextran | TiterMax Gold |
| 3 | 8 | HLA-I/II + HIV-1 gp140/SIV p27 + Hsp70 + dextran | TiterMax Gold |
| 4 | 6 | HLA-I/II + HIV-1 gp140/SIV p27 + Hsp70 + dextran | None |
| 5 | 8 | None | None |
The animals in groups 1 to 4 were immunized four times, at weeks 0, 4, 8, and 16. Four weeks after the last immunization, all vaccinated animals plus eight naïve animals (group 5) were challenged i.v. with 18 AID50 of SHIV-SF162P4/C. For details, see Materials and Methods.
SHIV challenge.
SHIV-SF162P4, kindly provided by Nancy Miller at NIAID, NIH, was propagated in the human T-cell line C8166-CCR5 (11), which expresses HLA-A*01 and -DRB1*04. This cell line is engineered to express CCR5, which allowed productive infection by the R5 SHIV strain used here. The cell line-propagated virus stock was designated SHIV-SF162P4/C. A limited in vivo titration was performed with nine rhesus macaques of Chinese origin. Three of three, 2/3, and 0/3 animals became infected after i.v. inoculation with 1 ml of 1/1,000, 1/100,000, and 1/1,000,000 dilutions, respectively. The approximate 50% animal infective dose (AID50) of the virus stock was determined by the Reed-Muench method to be 1.8 × 105/ml. At week 20, i.e., 4 weeks after the fourth immunization, group 1, 2, 3, and 4 animals plus eight naïve animals (group 5) were challenged i.v. with 1 ml of a 1/10,000 dilution (corresponding to 18 animal infective doses [AID50], 5.8 × 104 RNA equivalents [RNA eq]/ml, and 22.4 and 0.3 50% tissue culture infective doses [TCID50] as measured on C8166-CCR5 cells and rhesus PBMC, respectively) of SHIV-SF162P4/C. Viral load was monitored as reverse transcriptase activity in plasma using the ExaVir Load version 3 kit (Cavidi Tech AB, Uppsala, Sweden) as described previously (7) and translated to RNA equivalents/ml. Areas under the curve (AUCs) were calculated on log10-transformed values using the GraphPad Prism statistical software (GraphPad Software Inc., La Jolla, CA).
Neutralization activity.
Macaque sera were tested for their neutralization activity against SHIV-SF162P4, propagated in C8166-CCR5 cells (SHIV-SF162P4/C), using a TZM-bl based assay. Inhibitory activity in serum was analyzed both in the presence of complement, using serum from a healthy AB+ blood donor as a source of complement, and in the absence of complement, using heat-inactivated AB+ serum. To inhibit replication of the virus in TZM-bl cells, a final concentration of 1 μM indinavir (AIDS reagent program 8145) was added to Iscove's modified Dulbecco medium (IMDM) supplemented with 10% AB+ serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and DEAE-dextran (37.5 μg/ml). To control for possible cytotoxic effects by serum anti-HLA antibodies, HIV pseudotyped with vesicular stomatitis virus G protein (VSV-G), obtained by cotransfection of 293T cells with a pNL4-3 plasmid containing a stop codon in env and a VSV-G plasmid, was used. HLA expressed by the 293T cells did not match the vaccine alleles.
TZM-bl cells were seeded 1 day in advance at 4,000 cells per well. A final inoculum of 20 TCID50 SHIV-SF162P4/C or VSV-G-pseudotyped HIV in a volume of 50 μl was incubated for 1 h at 37°C with 2-fold serial dilutions of macaque serum (range, 1:40 to 1:1280) and was then added to the TZM-bl cells. For peptide competition assays, serum dilutions were preincubated with BaL V3 peptide (TRPNNNTRKSIHIGPGRAFYTTG), whose sequence closely matches that of YU2 (GPGRAF versus GPGRAL crown of V3 loop), or scrambled peptide (NKGTHNIPTARNIYGFPTSRRGT) at a final concentration of 20 μg/ml for 30 min at 37°C before the addition of virus. After 4 h of incubation at 37°C, TZM-bl cells were washed once with 150 μl phosphate-buffered saline (PBS) and subsequently replenished in 200 μl IMDM supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and DEAE-dextran (37.5 μg/ml). After 48 h, the cells were again washed in 150 μl PBS. Next, 25 μl freshly prepared luciferase substrate (0.83 mM ATP, 0.83 mM d-luciferin [Duchefa Biochemie B.V., Haarlem, Netherlands], 18.7 mM MgCl2, 0.78 μM Na2H2P2O7, 38.9 mM Tris [pH 7.8], 0.39% [vol/vol] glycerol, 0.03% [vol/vol] Triton X-100, and 2.6 μM dithiothreitol) was added and luminescence was measured for 1 s per well. Experiments were performed in triplicate. For calculations, the background luciferase expression was subtracted from the relative light units (RLU) of the test wells. The percent neutralization was calculated by determining the reduction in luciferase expression in the presence of neutralizing agent compared to the cultures with virus only. Fifty percent inhibitory dilutions (ID50) were determined by linear regression. For calculations, viruses with ID50 of <40 or >1,280 were assigned a value of 20 or 1,280, respectively.
Serum neutralization was measured in parallel in two different laboratories. The second laboratory used essentially the same protocol as described above with some minor differences (22). Instead of preseeding the TZM-bl cells, freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 75 μg/ml DEAE-dextran) were added to each well. For readout, 100 μl of cells was transferred to a 96-well black solid plate (Costar) for measurements of luminescence using the Britelite luminescence reporter gene assay system (Perkin-Elmer Life Sciences). The ID50 values presented are mean values from the two laboratories.
Anti-HLA antibody measurement.
The Luminex Labscreen mixed-HLA antibody method (One Lambda Inc., Canoga Park, CA) was used to detect HLA class I and class II binding antibodies, and Labscreen single antigen beads (One Lambda Inc.) were used to show the HLA antigen specificity. Assays were carried out according to the manufacturer's instructions.
Anti-C8166-CCR5 antibody measurement.
Serially diluted heat-inactivated sera (50 μl) were incubated with 1.25 × 105 C8166-CCR5 cells for 30 min at +4°C. After being washed, the cells were incubated for 30 min with polyclonal rabbit anti-human IgG-fluorescein isothiocyanate (FITC) (F0202; DakoCytomation, Glostrup, Denmark), washed, and fixed with 4% paraformaldehyde (PFA) for 1 h at room temperature. The median fluorescence intensity (MFI) was measured with a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA).
Anti-gp120 antibody measurement.
For determination of Env-specific IgG responses, enzyme-linked immunosorbent assay (ELISA) was carried out as described previously (24), using insect cell (S2)-produced YU2 gp120. Optical density (OD) titration curves were created using the variable-slope sigmoidal dose-response model in GraphPad Prism software. Endpoint reciprocal titers were determined using a cutoff defined as the OD value of an immune control serum at a 1/12,500 dilution, which was chosen for being in the linear range of the titration curve.
Fluorospot assay.
For simultaneous measurement of gamma interferon (IFN-γ) and interleukin-2 (IL-2) production in response to HIV/SIV-specific stimulation, the novel monkey IFN-γ/IL-2 Fluorospot assay (Mabtech AB, Nacka, Sweden) was employed, mostly according to the manufacturer's instructions. Briefly, frozen macaque PBMC were thawed and rested overnight, and then 125,000 cells/well were added in duplicate to 96-well IFPL plates (Millipore, Bedford, MA) which had been coated with anti-IFN-γ and -IL-2 MAbs (10 μg/ml each) and blocked with 10% fetal calf serum (FCS). The cell cultures were incubated in 5% CO2 at 37°C for 20 h in culture medium supplemented with anti-CD3 MAb CD3-1 (Mabtech; 10 ng/ml), with one of four 15-mer overlapping peptide pools (2.5 μg/peptide/ml) representing SIVmac239 Gag or HIV-1YU2 Env (two pools of each; New England Peptide LLC, Gardner, MA), or with 0.5% dimethyl sulfoxide (DMSO) (to equalize the DMSO concentration to the peptide wells). RPMI 1640 supplemented with penicillin (50 IU/ml), streptomycin (50 μg/ml), 10% FCS, and anti-CD28 MAb (0.1 μg/ml) was used as the culture medium. Secreted and captured cytokines were detected using FITC-conjugated anti-IFN-γ and biotinylated anti-IL-2 MAbs, followed by anti-FITC-green and streptavidin-red detection reagents. Plates were washed with PBS after each step (except after blocking) and were finally incubated with a fluorescence enhancer and left to dry at room temperature. Spots were counted using an automated AID iSpot reader (AID GmbH, Strassberg, Germany). Responses to each protein were obtained by addition of the two peptide pools and were expressed as number of spot-forming cells (SFC) per 106 PBMC.
Passive immunization and repeated low-dose SHIV challenge.
The two protected animals in group 3, F42 and F44, were given two booster immunizations (half doses) at weeks 44 and 56. They were sacrificed at week 58 and serum was collected. After heat inactivation the sera were pooled and given intraperitoneally (i.p.) to two naïve animals, F101 and F102, at approximately 110 ml/animal (36 ml/kg). Two control animals, F118 and F119, were given serum from naïve rhesus macaques. Starting the following day, all four animals were challenged i.v. six times, twice a week, with a low dose of SHIV-SF162P4/C (approximately 1/30 of the challenge dose used in the original experiment, i.e., approximately 0.6 AID50 and 7 and 0.01 TCID50 as measured on C8166-CCR5 cells and rhesus PBMC, respectively). Plasma viral load and anti-C8166-CCR5 antibodies were monitored throughout the experiment until the animals were sacrificed at 11 weeks after the passive immunization.
Depletion of anti-C8166 antibodies.
Heat-inactivated pooled serum from animals F42 and F44 taken 2 weeks after the fifth immunization was diluted 1/10 in PBS supplemented with 0.1% FCS and incubated with or without 6 × 107 C8166 cells/ml with end-over-end rotation at +4°C. After 20 h, the cells were removed by centrifugation and the depleted serum was harvested; >95% of the C8166 and C8166-CCR5 binding IgG was removed from the serum pool.
Virion lysis assay.
Sera from immunized rhesus macaques immunized with HLA antigen were obtained at the time of challenge and analyzed for the ability to induce complement-mediated lysis of the challenge virus. An aliquot of 200 μl of the SHIV-SF162P4/C stock diluted 1/10 in medium, corresponding to approximately 1 × 106 virus copies or 4,500 TCID50 (according to titration on C8166-CCR5 cells), 200 μl of rhesus serum diluted 1/10 or 1/50 in medium, and 10% of complement source (45 μl of human serum pooled from four donors) were mixed and incubated at 37°C for 1 h. Virus was incubated in parallel with normal rhesus serum, medium, or heat-inactivated (56°C, 60 min) complement source. Nonlysed virus was pelleted after incubation by high-speed centrifugation for 90 min at 8°C. Subsequently, the SHIV core antigen content in supernatant (i.e., lysed virus) was analyzed by Vironostika HIV-1 antigen ELISA (bioMérieux, Boxtel, Netherlands), which is cross-reactive for SIV antigen, according to the manufacturer's instructions.
Statistical analyses.
The nonparametric Mann-Whitney and Spearman tests were used, with Dunn's correction for multiple tests where appropriate. GraphPad Prism software was used for statistical analysis.
Nucleotide sequence accession numbers.
The sequences of the new alleles determined in this study have been submitted to the EMBL-EBI database and to the nonhuman primate MHC section of the Immuno Polymorphism Database under accession numbers FN985452 to FN985480 and FN995437.
RESULTS
Protection against SHIV-SF162P4/C by immunization with recombinant HLA and HIV/SIV antigens.
To explore xenoimmunization as a strategy for eliciting protective immunity against SHIV challenge, four groups of macaques were immunized at weeks 0, 4, 8, and 16, as summarized in Table 1, with vaccine components coupled to dextran backbones, forming so-called dextramers. Group 1 (n = 8) was immunized with HLA, group 2 (n = 8) with trimeric YU2 HIV-1 gp140 and SIV p27 (HIV/SIV antigens), and groups 3 (n = 8) and 4 (n = 6) with both HLA and HIV/SIV antigens. All groups also received Hsp70, and all groups except group 4 were given the TiterMax Gold adjuvant. Four weeks after the last immunization, all vaccinated animals plus eight naïve animals (group 5) were challenged with 18 AID50 of SHIV-SF162P4/C. This virus stock was grown in C8166-CCR5 cells, a T-cell line expressing HLA-A*01 and -DRB1*04; both lineages are part of the HLA component of the vaccine. The virus particles should therefore express on their surface both HLA class I and class II lineages matching the HLA component of the vaccine.
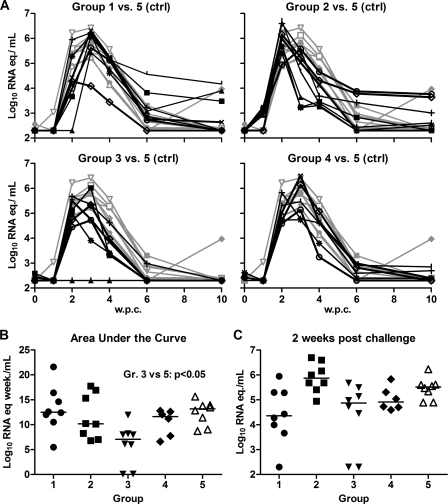
In the unvaccinated group 5, all macaques became infected, with the median peak viremia occurring after 3 weeks (Fig. 1A). The median peak plasma viral load was 5.8 log10 RNA copy equivalents (RNA eq)/ml (range, 5.2 to 6.4), and at 10 weeks postchallenge the viral load had dropped to undetectable levels except in one animal, which had 4.0 log10 RNA eq/ml. The median area under the curve (AUC), a measure of cumulative virus production during 10 weeks after challenge, was 13.2 log10 RNA eq · week/ml (Fig. 1B). In contrast, in group 3, which received all vaccine components, two of eight macaques remained nonviremic until they were sacrificed at 38 weeks postchallenge. Furthermore, the six infected animals showed a significantly lower median AUC viral load (8.0 log10 RNA eq · week/ml) than the naïve control animals (P < 0.05) (Fig. 1B). In group 4, which received the same immunogens as group 3 but without TiterMax Gold, all animals became viremic and no significant reduction in viral load was observed.
Fig. 1.
Plasma viral load after i.v. SHIV-SF162P4/C challenge in immunized animals (groups 1, 2, 3, and 4) and control animals (group 5). (A) Individual viral load kinetics at 0 to 10 weeks postchallenge (w.p.c.). Black lines, group 1 to 4 animals; gray lines, group 5 control animals. (B) Area-under-the-curve (AUC) viral load, representing cumulative viral load over 10 weeks. The infected macaques in group 3 had a significantly lower median AUC than the macaques in group 5 (P < 0.05). (C) Viral load at 2 w.p.c. Horizontal bars indicate medians.
In groups 1 and 2, all animals became infected and showed no improved control of viral replication in comparison to the naïve controls. Nevertheless, in group 1 animals the median viral load at 2 weeks postchallenge was more than 1 log10 unit lower than that in the naïve controls, 4.4 versus 5.5 log10 RNA eq/ml (Fig. 1C), suggesting a delay in virus replication in group 1. Indeed, preliminary in vivo titration studies with the same SHIV stock indicated an inverse correlation between the size of the virus inoculum and the time from challenge to peak viremia (data not shown). Surprisingly, the median time to peak viremia in group 2, immunized with HIV/SIV antigens, was significantly shorter than that for the unvaccinated animals (2 compared with 3 weeks, respectively; P < 0.01). Mamu typing revealed that none of the reported protective Mamu class I lineages, A*01, B*08, or B*17 (reviewed in reference 13), was present in any animal.
In summary, protection from infection or control of viral replication was achieved in animals that had been immunized with both HLA and HIV/SIV antigens and the TiterMax Gold adjuvant.
SHIV-neutralizing activity in serum.
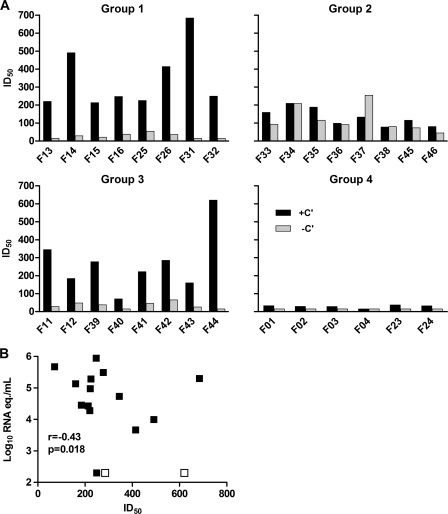
In order to study correlates of vaccine-induced protective immunity, macaque sera obtained at time points before, during, and after immunization were tested for their relative in vitro neutralizing activities against the challenge virus SHIV-SF162P4/C. The neutralizing activities of macaque sera were analyzed in both the absence and presence of complement. Indeed, in sera from group 1 and group 3 macaques, both of which were immunized with HLA, neutralizing activity was observed only in the presence of complement (Fig. 2A). In contrast, sera from group 2 animals, immunized with HIV/SIV antigens but not HLA, yielded significant neutralizing activity both with and without complement, but the former was at a reduced level compared with groups 1 and 3. Neutralizing activity in serum became detectable after the second immunization and was enhanced in some but not all animals after the third and/or fourth immunization (see Fig. S1A in the supplemental material). No inhibition of a VSV-G-pseudotyped HIV variant by group 1 and group 3 sera in the presence of complement was observed (data not shown), suggesting that the observed neutralizing activity in group 1 and 3 sera was not the result of an effect of these sera on the viability of the target cells in the neutralization assay. Furthermore, neutralizing activity of sera from 5/8 group 2 animals was inhibited in the presence of a peptide representing the BaL gp120 V3 region (see Fig. S1B in the supplemental material), confirming that the activity in these sera was directed against the virally encoded envelope glycoproteins. In contrast, the neutralizing activity in sera from group 3 animals was not reduced in the presence of BaL V3 peptide. Neutralization in the presence of complement by sera from group 4 animals was only slightly enhanced compared to that by sera from the control animals (Fig. 2A), suggesting that the TiterMax Gold adjuvant was essential for the development of a potent neutralizing activity. There was an inverse correlation between neutralizing activity in the presence of complement and plasma viral load at 2 weeks postchallenge in the immunized macaques (r = −0.43; P = 0.018) (Fig. 2B).
Fig. 2.
Serum neutralizing activity in immunized animals (groups 1, 2, 3, and 4) at the time of challenge, presented as 50% inhibitory dilution (ID50). (A) Neutralizing activity in the presence or absence of complement (+C′ or −C′, respectively). (B) Neutralizing activity in the presence of complement versus plasma viral load at 2 w.p.c. The protected animals (F42 and F44) are denoted by empty symbols.
Antibodies directed against HLA classes I and II and the C8166-CCR5 cell line.
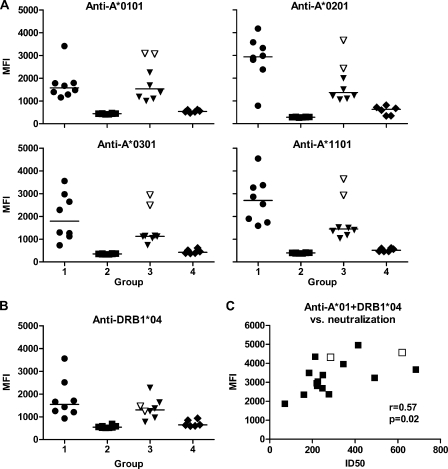
High antibody levels, detectable at a 1/1,000 dilution, were elicited in groups 1 and 3 to all four HLA class I recombinant antigens and one class II recombinant antigen of the vaccine, whereas in group 4 only weak anti-HLA antibody responses were detected (Fig. 3A and B). HLA binding antibodies were absent in all preimmunization sera and were not detected in group 2 (not immunized with HLA antigens) or in the unimmunized group 5 animals.
Fig. 3.
Serum anti-HLA IgG levels in immunized animals (groups 1, 2, 3, and 4) at the time of challenge, measured by Luminex at a 1/1,000 dilution. The protected animals (F42 and F44) are denoted by empty symbols. (A) IgG antibodies directed against the vaccine HLA class I antigens. (B) IgG antibodies directed against the vaccine HLA class II antigen. (C) Correlation between anti-A*01 and -DRB1*04 IgG and neutralizing antibodies, in the presence of complement, in groups 1 and 3.
In the HLA-immunized macaques in groups 1 and 3 (both of which received the TiterMax Gold adjuvant), there was a correlation between the level of antibodies against HLA-A*01 and HLA-DRB1*04, the two immunizing HLA lineages expressed on the surface of the challenge virus, and serum neutralizing activity in the presence (r = 0.57; P = 0.02) (Fig. 3C), but not in the absence (r = −0.02; P = 0.95), of complement. Moreover, anti-A*01 and -DRB1*04 antibodies also correlated inversely with the viral load at 2 weeks postchallenge (r = −0.57; P = 0.005) in the immunized animals, as did anti-A*01 (r = −0.51; P = 0.016) but not anti-DRB1*04 (r = −0.36; P = 0.099) antibodies (data not shown).
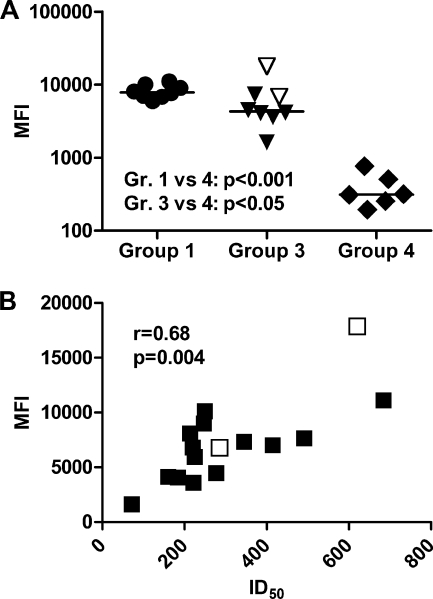
Measuring antibodies against the C8166-CCR5 cell line provided a method for detection of antibodies recognizing the challenge virus-matched HLA molecules in their native conformation. High levels of anti-C8166-CCR5 IgG antibodies were elicited in groups 1 and 3, while only weak responses were seen in group 4 (Fig. 4A). At the time of challenge, anti-C8166-CCR5 antibody responses within groups 1 and 3 correlated with the cumulative anti-A*01 and -DRB1*04 antibody responses as measured by Luminex (r = 0.56; P = 0.023), both lineages expressed by C8166-CCR5. Furthermore, in these macaques the anti-C8166-CCR5 antibody levels correlated strongly with neutralization in the presence (r = 0.68; P = 0.0036) but not in the absence (r = −0.44; P = 0.086) of complement (Fig. 4B).
Fig. 4.
Serum anti-C8166-CCR5 IgG in immunized animals that received HLA-containing vaccine (groups 1, 3, and 4). Anti-C8166-CCR5 IgG was detected by FITC-conjugated anti-human IgG secondary antibody, and the mean fluorescence intensity (MFI) was measured. (A) Anti-C8166-CCR5 at the time of challenge. (B) Correlation between anti-C8166-CCR5 IgG and neutralizing antibodies, in the presence of complement, in groups 1 and 3. The protected animals F42 and F44 are denoted with empty symbols.
Anti-gp120 antibodies.
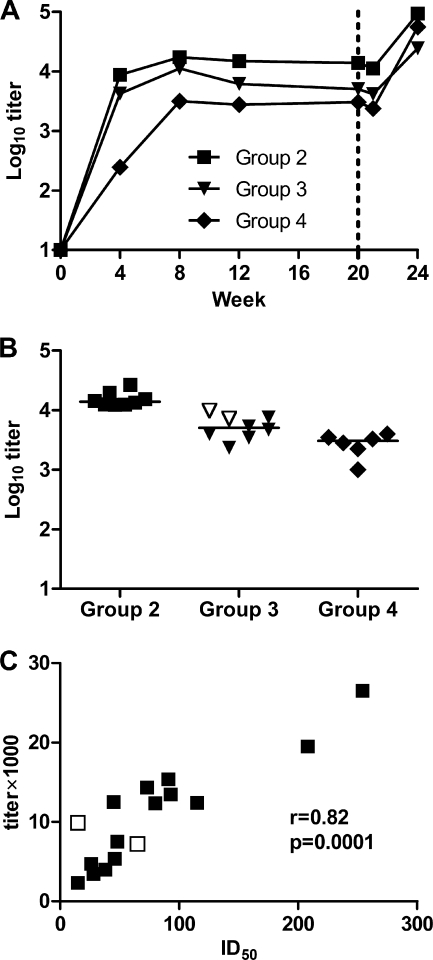
Vaccine-induced anti-gp120 antibodies were elicited in all animals in groups 2, 3, and 4 (see Fig. S2 in the supplemental material), with median titers reaching plateau levels after the second immunization (Fig. 5A). The highest titers at the time of challenge were detected in group 2, immunized with HIV/SIV antigens plus TiterMax Gold, with a median titer of 13,889, which was significantly higher than those in group 3 (5,042) and 4 (3,048) (P < 0.05 and P < 0.001, respectively) (Fig. 5B). Within groups 2 and 3, both of which received HIV/SIV antigens together with TiterMax Gold, anti-gp120 antibodies correlated strongly with neutralization in the absence (r = 0.82; P < 0.0001) but not in the presence (r = −0.30; P = 0.26) of complement (Fig. 5C). There was no correlation between the anti-gp120 antibody levels at the time of challenge and plasma viral load. All macaques in groups 2, 3, and 4 showed anamnestic anti-gp120 antibody responses at 4 weeks after challenge, except the protected macaques F42 and F44 (see Fig. S2 in the supplemental material).
Fig. 5.
Serum anti-gp120 IgG in immunized animals that received HIV/SIV antigen-containing vaccine (groups 2, 3, and 4). (A) Median endpoint titers over time. The dashed line indicates the time of challenge. (B) Individual endpoint titers at the time of challenge. (C) Correlation between anti-gp120 IgG levels (log10 titer) and neutralizing activity in the absence of complement (ID50) at the time of challenge in groups 2 and 3. The protected animals (F42 and F44) are denoted with empty symbols.
Env- and Gag-specific T-cell responses.
Since Env and Gag proteins were included in the vaccines given to groups 2, 3, and 4 we tested the T-cell responses against these proteins to see if they were contributing to the control of virus replication. Simultaneous measurement of IFN-γ and IL-2 secretion after stimulation with vaccine-homologous HIV Env or SIV Gag overlapping peptide pools was carried out using the novel Fluorospot assay. Frozen PBMC from the time of challenge and 1 week after challenge were tested. Substantial anti-Env and -Gag responses were observed in groups 2 and 3, while responses in group 4 were weak or absent (see Fig. S3 in the supplemental material). However, we found no correlation between the T-cell responses and viral load.
Protection against SHIV infection by passive immunization.
To investigate whether the complete protection observed in F42 and F44 was mediated by antibodies, a passive immunization experiment was performed. After being given two additional booster immunizations, these two animals were sacrificed and serum was collected. Pooled and heat-inactivated serum (36 ml/kg of body weight) was administered i.p. to two naïve animals, F101 and F102. Since only 2/8 animals were protected against infection in group 3, we reasoned that the challenge virus dose might have been too high to reveal the full protective potential of the vaccine. As we were also uncertain whether binding/neutralizing serum antibody titers of the same magnitude would be reached in the passively immunized animals, we decided to challenge the macaques repeatedly with a lower virus dose. F101 and F102 as well as two control animals, which received normal rhesus serum, were therefore challenged i.v. with 0.6 AID50 of SHIV-SF162P4/C twice weekly for 3 weeks, starting 24 h after the serum transfer (day 1).
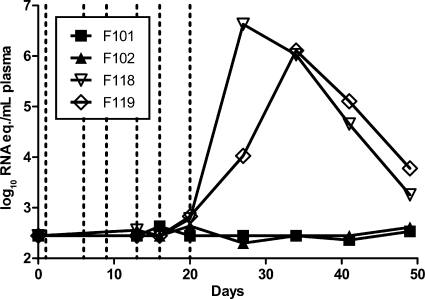
The anti-C8166-CCR5 IgG MFI in the immune serum pool was 21,151, and at day 1 the serum titers in F101 and F102 were 6,951 and 5,742, respectively (see Fig. S4A in the supplemental material). At the time of the last virus challenge, day 20, the titers had decreased to 2,668 and 2,292, respectively (half-life [t1/2], 14.0 and 14.1 days, respectively). For comparison, the serum antibody titers to C8166-CCR5 of F42 and F44 at the time of challenge in the original experiment were 4,443 and 11,971, respectively. The neutralizing activities against the challenge virus 1 day after the serum transfer were 388 and 455 ID50 in F101 and F102, respectively, and by the time of the last challenge they had decreased to 97 and 136 ID50, respectively (see Fig. S4B in the supplemental material). The two control macaques, F118 and F119, became viremic at day 20, with peak viremias of 6.6 and 6.1 log10 RNA eq/ml plasma reached at days 27 and 34, respectively (Fig. 6). In contrast, F101 and F102 remained nonviremic until they were sacrificed at day 77, showing that the protection seen in group 3 could be transferred by passive immunization with serum. None of the reported protective Mamu class I lineages (A*01, B*08, or B*17) was present in these four animals.
Fig. 6.
Plasma viral load in passively immunized macaques after repeated i.v. SHIV-SF162P4/C challenge. Immune sera from protected animals F42 and F44 were transferred to naïve animals F101 and F102, while control serum was given to F118 and F119. Starting the following day, all animals were challenged i.v. twice weekly for 3 weeks with 0.6 AID50 of SHIV-SF162P4/C (dashed lines).
Specificity and mechanism of complement-dependent neutralization.
To determine the specificity of the complement-dependent neutralization, sera from F42 and F44 taken at 2 weeks after the fifth immunization were pooled and mixed with C8166 cells in order to deplete antibodies specific for cell surface molecules present on these cells. By this method >95% of both C8166 and C8166-CCR5 binding antibodies were depleted. When tested for neutralization in the presence of complement, all neutralizing activity was found to be lost in the depleted sera (Table 2).
Table 2.
Serum neutralization titers after cell adsorptiona
| Serum | ID50 |
|
|---|---|---|
| Without complement | With complement | |
| Nonadsorbed | <40 | >1,280 |
| C8166 adsorbed | <40 | <40 |
Pooled sera from animals F42 and F44 taken after the fifth immunization were preadsorbed with C8166 cells and then tested for neutralizing activity against SHIV-SF162P4/C in the absence or presence of complement. For details, see Materials and Methods.
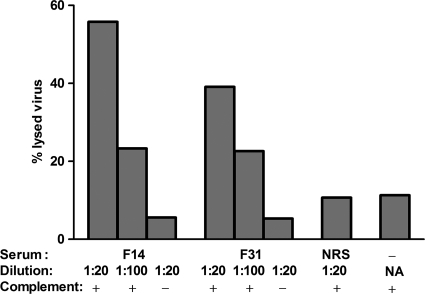
In order to elucidate whether the complement-dependent neutralizing activity mediated by anti-HLA antibodies could be linked to lysis of virions, a virion lysis assay was set up. The challenge virus, SHIV-SF162P4/C, was incubated with sera obtained after HLA immunization, at the time of challenge, from group 1 animals displaying the strongest complement-dependent neutralizing activity (F14 and F31). Indeed, sera from these animals lysed the challenge virus in a dose-dependent fashion in the presence of active complement (Fig. 7); at the lowest serum dilution tested (1/20), 56 and 39% were lysed, respectively, while using inactivated complement resulted in only 6 and 5% lysis, respectively. Normal rhesus serum or medium did not induce any substantial amount of virion lysis in the presence of active complement. Taken together, these results suggest that virion lysis contributes significantly to complement-dependent neutralizing activity in sera from macaques immunized with HLA.
Fig. 7.
Anti-HLA antibody- and complement-mediated virion lysis. Lysis of SHIV-SF162P4/C virions in the presence of complement and immune sera from HLA immunized animals is shown. Sera from group 1 animals (F14 and F31), normal rhesus serum (NRS) or medium only (−) was incubated with SHIV-SF162P4/C in the presence (+) or absence (−) of complement. NA, not applicable.
DISCUSSION
In this study we present evidence that immunization with a combination of recombinant HLA class I and II proteins and HIV/SIV antigens (Env and Gag proteins) can protect macaques against i.v. challenge with heterologous SHIV. The protection was complete in two of eight macaques and resulted in decreased viral loads in the remaining six immunized macaques. A passive immunization experiment revealed that the protective immunity could be transferred to naïve animals using immune serum from the two protected animals.
Using, for the first time, recombinant HLA proteins and thereby eliminating the potential risk of incompletely inactivated viruses or transmission of other cell-associated viruses, this investigation confirms and extends previous studies where protection against SIV grown in human cells was achieved by immunization with fixed SIV-infected human cells or with inactivated SIV grown in human cells (16, 31, 41). However, whereas protection after immunization with uninfected human cells (39), mouse cells expressing HLA class II (40), or purified HLA class I or II proteins (1, 5) has been reported, we did not observe any protection after recombinant HLA immunization in the absence of HIV/SIV antigens (Env and Gag). The reason for this is not clear, but the observation is in line with previous studies which revealed that immunization with fixed SIV-infected human cells or inactivated SIV grown in human cells gave greater and more consistent protection (16) than immunization with uninfected human cells, although the latter studies are few and included small numbers of animals (39).
The protection elicited by transfer of immune serum confirms previous observations (40) and for the first time demonstrates that protection against heterologous SHIV challenge can be achieved by passive immunization with sera from animals immunized with HLA molecules in combination with HIV/SIV antigens in the absence of other cell components. This offers further evidence that antibodies mediated the protection. Indeed, potent complement-dependent neutralizing activity was observed in sera from the protected group 3 macaques, which were immunized with all components, and it correlated inversely with viral load at 2 weeks postchallenge.
As protection was achieved in macaques that were immunized with both HLA class I and II and HIV/SIV antigens (containing HIV-1 Env; group 3), the specificity of the putative protective antibodies could potentially involve any one of these. Anti-HLA class I antibodies appeared to be important, as the levels of these were highest in the two completely protected animals and they correlated inversely with viral load levels at 2 weeks postchallenge. In contrast, no such correlations were found with anti-Env antibodies. Furthermore, macaques immunized with Env but without HLA (group 2) were not protected. On the other hand, neither was there any protection in macaques immunized with HLA but without Env (group 1), except for a trend toward a delayed viremia. These results suggest that in this heterologous SHIV infection model, anti-HLA antibodies were crucial but not sufficient for protection. As noted above, previous studies have indicated that immunization with fixed SIV-infected human cells or inactivated SIV grown in human cells results in more efficient protection than HLA immunization in the absence of viral antigens, and our results are consistent with these findings. The mechanism, however, is not clear. In fact, it was not reflected in the serum neutralization capacity, as neutralization titers at the time of challenge were comparable in groups 1 and 3. It is possible that the additional effect of anti-Env antibodies may be mediated by a different mechanism than classical (or complement-dependent) neutralization (reviewed in references 10 and 15). Furthermore, combining HLA and Env antigens may result in a synergistic effect, as we observed no signs of protection in macaques immunized with Env without HLA antigens (group 2).
Both anti-HLA and -C8166-CCR5 antibodies correlated with the complement-dependent neutralization activity in sera of group 1 and 3 animals, which in turn could be abolished by adsorption with C8166 cells. These findings suggest that anti-HLA antibodies were responsible for the complement-dependent neutralization. This is consistent with previous reports that HIV-1 neutralization by human alloimmune sera is dependent on or greatly enhanced by the presence of active complement and that the neutralization activity is likely to be mediated by HLA-directed antibodies (33, 35). However, while in those studies other polymorphic cell surface molecules, such as ICAM-1 or LFA-1, could not be ruled out as targets for complement-dependent neutralization, here the only cell membrane molecules used for immunization were HLA class I and II. It is also noteworthy that the anti-gp120 antibody levels in groups 2 and 3, the two groups immunized with Env proteins and TiterMax Gold adjuvant, correlated with neutralization in the absence of complement, suggesting that the anti-Env antibodies mediated complement-independent neutralization. The minimal neutralizing activity in the absence of complement and the lack of neutralization inhibition by BaL V3 peptide indicate that the immunization regime in group 3 animals has failed to elicit anti-Env antibodies with neutralizing capacity.
The mechanism of complement-mediated inhibition of HIV-1 infection could be either by virion lysis through antibody-mediated activation of the classical complement pathway (34) or by steric hindrance of viral entry as a consequence of dense coating of the virus particle with antibody plus complement (42). Other possible antibody-dependent complement-mediated antiviral mechanisms are phagocytosis (3) and regulation of adaptive immunity (4). Here we demonstrate that incubation of SHIV particles bearing HLA class I and II matching the vaccine with serum from HLA-immunized macaques and active complement caused more than 50% virion lysis at the lowest serum dilution tested. This suggests that a major part of the neutralizing activity in group 1 and 3 macaques was mediated by HLA-directed antibody-dependent complement-mediated virion lysis, and it is in agreement with studies on alloimmune human serum neutralization of HIV-1 (33, 35). We cannot, however, exclude the possibility that steric hindrance by antibody and complement coating of the virions may play a role.
We hypothesize that in the two completely protected macaques of group 3 and in the two passively immunized macaques, the challenge virus was blocked in the preentry phase of the infection, by anti-HLA antibodies or by a combination of anti-HLA and -Env antibodies. We postulate that in the remaining group 3 macaques, which became infected but displayed significantly lower levels of virus replication, the inoculated virus was incompletely neutralized, leaving a reduced virus inoculum, which delayed the replication of the virus and thereby allowed a better control by the virus-specific adaptive immunity. A trend toward delayed viremia in the macaques immunized with HLA only (group 1) was observed, and in preceding in vivo titrations of the challenge virus stock we observed an inverse correlation between the size of the virus inoculum and the time to peak viremia. There was, however, no delayed viremia in the HLA- and HIV/SIV antigen-immunized group 3 animals. Since in the macaques immunized with HIV/SIV antigen only (group 2) the median peak viremia occurred significantly earlier than in the control animals, it may be that the forces driving and slowing the viral replication in groups 2 and 1, respectively, counteract each other in the HLA- and HIV/SIV antigen-immunized group 3 animals. The earlier peak viremia in group 2 animals was unexpected. It can be speculated that the HIV/SIV antigen-specific cellular responses provided an expanded pool of CD4+ target cells for the virus. Similar findings have been reported after vaccination against both SIV and feline immunodeficiency virus (FIV) (17, 36), and SHIV- and HIV-1-specific CD4+ T cells were shown to be preferentially infected in macaques and humans, respectively (9, 37).
As demonstrated above, immunization with HLA, especially in combination with viral antigens, can protect macaques against challenge with SIV or SHIV grown in human cells, i.e., protection by xenoimmunization. Together with observations in humans, indicating that alloimmune responses may confer protection against HIV (18, 20, 29, 35), this provides support for the hypothesis that this vaccination strategy could be transferred to humans and HIV. However, this would require alloimmunization with MHC class I and II antigens. To our knowledge, only two macaque studies testing alloimmunization against SIV infection have been published; one showed protection (40), and another failed to show protection (30). This discrepancy can be accounted for by a number of parameters such as differences in vaccine production, challenge dose, adjuvant, and route of immunization. Clearly, further studies are required.
The present study has used a multicomponent vaccine strategy, and further investigation is needed to identify the critical components for protection. It is clear that a strong adjuvant, such as TiterMax Gold, was an essential component of the vaccine, but we cannot from our results determine the necessity of including Hsp70 or linking the vaccine components with dextran. Mycobacterial Hsp70 binds to CD40 (43) and has been shown to possess adjuvant properties when fused or linked to an antigen (12, 19, 44). The C-terminal fragment (amino acids 359 to 609) was chosen for this study, as it has been shown to be essential for the adjuvant effect (19, 44). We were not able to compare immunizations with and without Hsp70, but its effect on the humoral and cellular immune responses appeared to be modest, especially compared to the additional effect of TiterMax Gold. Similarly, we need to determine whether both HIV-1 gp140 and SIV p27 are critical for protection. Besides the effect of anti-Env antibodies discussed above, it is possible that anti-Env and/or -Gag cellular responses may play a role in the increased control of viral replication in group 3 macaques, although no correlation between IFN-γ or IL-2 secretion and viral load was found in these animals. Neither can we conclude that both HLA class I and II are essential for protection. Previous reports are contradictory, perhaps because different cell lines with different HLA expression patterns have been used for immunization or for propagating virus for immunization and challenge. In our study anti-HLA class I antibodies correlated best with protection, and this is in agreement with other studies where the same cell line, C8166, has been used for propagation of the challenge virus (5, 6).
We have demonstrated that protection against i.v. challenge with heterologous SHIV in macaques can be achieved by immunization with a combination of recombinant HLA and HIV/SIV antigens. The protection appears to be mediated by antibodies, likely directed against HLA class I and HIV-1 Env. Further investigations are needed to establish which components are critical for protection and whether similar protection can be achieved by alloimmunization with MHC and against mucosal challenge.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Wehlin, E. Hansson-Pihlainen, L. Bergqvist, I. Silhammar, O. Shaw, T. Seidl, R. Spallek, C. Halgreen, and L. Brix for excellent technical assistance. We also thank veterinarian M. Spångberg and the staff at Astrid Fagreus laboratory for excellent animal care. We greatly appreciate N. Miller providing SHIV-SF162P4, the advice received from W. W. Kwok, and N. Ahlborg at Mabtech AB providing Fluorospot material and reagents.
This study was funded by Bill & Melinda Gates Foundation grant 38608 as part of the Collaboration for AIDS Vaccine Discovery (CAVD). Development and production of trimeric HIV-1 gp140 was supported by the NIH intramural research program.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 13 April 2011.
REFERENCES
- 1. Arthur L. O., et al. 1995. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J. Virol. 69:3117–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babaahmady K., Oehlmann W., Singh M., Lehner T. 2007. Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407-426. J. Virol. 81:3354–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown E. J. 1991. Complement receptors and phagocytosis. Curr. Opin. Immunol. 3:76–82 [DOI] [PubMed] [Google Scholar]
- 4. Carroll M. C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986 [DOI] [PubMed] [Google Scholar]
- 5. Chan W. L., et al. 1995. Immunization with class I human histocompatibility leukocyte antigen can protect macaques against challenge infection with SIVmac-32H. AIDS 9:223–228 [PubMed] [Google Scholar]
- 6. Chan W. L., et al. 1992. Protection in simian immunodeficiency virus-vaccinated monkeys correlates with anti-HLA class I antibody response. J. Exp. Med. 176:1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corrigan G. E., et al. 2006. Reverse transcriptase viral load correlates with RNA in SIV/SHIV-infected macaques. AIDS Res. Hum. Retroviruses 22:917–923 [DOI] [PubMed] [Google Scholar]
- 8. Dawson D. V., Ozgur M., Sari K., Ghanayem M., Kostyu D. D. 2001. Ramifications of HLA class I polymorphism and population genetics for vaccine development. Genet. Epidemiol. 20:87–106 [DOI] [PubMed] [Google Scholar]
- 9. Douek D. C., et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95–98 [DOI] [PubMed] [Google Scholar]
- 10. Forthal D. N., Moog C. 2009. Fc receptor-mediated antiviral antibodies. Curr. Opin. HIV AIDS 4:388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fouchier R. A., Meyer B. E., Simon J. H., Fischer U., Malim M. H. 1997. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 16:4531–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ge F. F., Qiu Y. F., Yang Y. W., Chen P. Y. 2007. An hsp70 fusion protein vaccine potentiates the immune response against Japanese encephalitis virus. Arch. Virol. 152:125–135 [DOI] [PubMed] [Google Scholar]
- 13. Goulder P. J., Watkins D. I. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haynes B. F., Montefiori D. C. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5:579–595 [DOI] [PubMed] [Google Scholar]
- 15. Huber M., Trkola A. 2007. Humoral immunity to HIV-1: neutralization and beyond. J. Intern. Med. 262:5–25 [DOI] [PubMed] [Google Scholar]
- 16. Hunsmann G., et al. 1995. Protection of macaques against simian immunodeficiency virus infection with inactivated vaccines: comparison of adjuvants, doses and challenge viruses. The European Concerted Action on Macaque Models for AIDS Research. Vaccine 13:295–300 [PubMed] [Google Scholar]
- 17. Klonjkowski B., et al. 2009. Gag-specific immune enhancement of lentiviral infection after vaccination with an adenoviral vector in an animal model of AIDS. Vaccine 27:928–939 [DOI] [PubMed] [Google Scholar]
- 18. Leith J. G., et al. 2003. Assessing human alloimmunization as a strategy for inducing HIV type 1 neutralizing anti-HLA responses. AIDS Res. Hum. Retroviruses 19:957–965 [DOI] [PubMed] [Google Scholar]
- 19. Li X., Yang X., Li L., Liu H., Liu J. 2006. A truncated C-terminal fragment of Mycobacterium tuberculosis HSP70 gene enhanced potency of HBV DNA vaccine. Vaccine 24:3321–3331 [DOI] [PubMed] [Google Scholar]
- 20. MacDonald K. S., et al. 1998. Mother-child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J. Infect. Dis. 177:551–556 [DOI] [PubMed] [Google Scholar]
- 21. Mills K. H., et al. 1992. Protection against SIV infection in macaques by immunization with inactivated virus from the BK28 molecular clone, but not with BK28-derived recombinant env and gag proteins. J. Med. Primatol. 21:50–58 [PubMed] [Google Scholar]
- 22. Montefiori D. C. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. Chapter 12:Unit 12.11 [DOI] [PubMed] [Google Scholar]
- 23. Mörner A., et al. 2009. Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J. Virol. 83:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nilsson C., et al. 1995. Protection against monkey-cell grown cell-free HIV-2 challenge in macaques immunized with native HIV-2 envelope glycoprotein gp125. Vaccine Res. 4:165–175 [Google Scholar]
- 25. Ott D. E. 1997. Cellular proteins in HIV virions. Rev. Med. Virol. 7:167–180 [DOI] [PubMed] [Google Scholar]
- 26. Otting N., et al. 2007. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics 59:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Otting N., Doxiadis G. G., Bontrop R. E. 2009. Definition of Mafa-A and -B haplotypes in pedigreed cynomolgus macaques (Macaca fascicularis). Immunogenetics 61:745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otting N., Morner A., Bontrop R. E. 2011. Novel major histocompatibility complex class I alleles extracted from two rhesus macaque populations. Tissue Antigens 77:79–80 [DOI] [PubMed] [Google Scholar]
- 29. Page M., et al. 2007. Specificity of anti-human leukocyte antigen antibody responses after immunization with Remune, an inactivated HIV-1 vaccine. AIDS 21:375–377 [DOI] [PubMed] [Google Scholar]
- 30. Polyanskaya N., et al. 1997. Anti-major histocompatibility complex antibody responses to simian B cells do not protect macaques against SIVmac infection. AIDS Res. Hum. Retroviruses 13:923–931 [DOI] [PubMed] [Google Scholar]
- 31. Putkonen P., et al. 1992. A formalin inactivated whole SIVmac vaccine in Ribi adjuvant protects against homologous and heterologous SIV challenge. J. Med. Primatol. 21:108–112 [PubMed] [Google Scholar]
- 32. Scholler J., et al. 2010. A recombinant human HLA-class I antigen linked to dextran elicits innate and adaptive immune responses. J. Immunol. Methods 360:1–9 [DOI] [PubMed] [Google Scholar]
- 33. Spear G. T., Olinger G. G., Saifuddin M., Gebel H. M. 2001. Human antibodies to major histocompatibility complex alloantigens mediate lysis and neutralization of HIV-1 primary isolate virions in the presence of complement. J. Acquir. Immune Defic. Syndr. 26:103–110 [DOI] [PubMed] [Google Scholar]
- 34. Spear G. T., Sullivan B. L., Landay A. L., Lint T. F. 1990. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J. Virol. 64:5869–5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spruth M., Stoiber H., Kacani L., Schonitzer D., Dierich M. P. 1999. Neutralization of HIV type 1 by alloimmune sera derived from polytransfused patients. AIDS Res. Hum. Retroviruses 15:533–543 [DOI] [PubMed] [Google Scholar]
- 36. Staprans S. I., et al. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. U. S. A. 101:13026–13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steger K. K., Waterman P. M., Pauza C. D. 1999. Acute effects of pathogenic simian-human immunodeficiency virus challenge on vaccine-induced cellular and humoral immune responses to Gag in rhesus macaques. J. Virol. 73:1853–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stern L. J., Wiley D. C. 1992. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell 68:465–477 [DOI] [PubMed] [Google Scholar]
- 39. Stott E. J. 1991. Anti-cell antibody in macaques. Nature 353:393. [DOI] [PubMed] [Google Scholar]
- 40. Stott E. J. 1994. Protection against simian immunodeficiency virus infection of macaques by cellular or viral antigens. Neuvieme Colloque Cent Gardes 1994:219–224 [Google Scholar]
- 41. Stott E. J., et al. 1990. Preliminary report: protection of cynomolgus macaques against simian immunodeficiency virus by fixed infected-cell vaccine. Lancet 336:1538–1541 [DOI] [PubMed] [Google Scholar]
- 42. Sullivan B. L., Takefman D. M., Spear G. T. 1998. Complement can neutralize HIV-1 plasma virus by a C5-independent mechanism. Virology 248:173–181 [DOI] [PubMed] [Google Scholar]
- 43. Wang Y., et al. 2001. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15:971–983 [DOI] [PubMed] [Google Scholar]
- 44. Wang Y., et al. 2002. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J. Immunol. 169:2422–2429 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y., et al. 1998. Generation of CD8 suppressor factor and beta chemokines, induced by xenogeneic immunization, in the prevention of simian immunodeficiency virus infection in macaques. Proc. Natl. Acad. Sci. U. S. A. 95:5223–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y., et al. 1999. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat. Med. 5:1004–1009 [DOI] [PubMed] [Google Scholar]
- 47. Yang X., Wyatt R., Sodroski J. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 75:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.