Abstract
Infectious hematopoietic necrosis virus (IHNV) is an important fish pathogen that infects both wild and cultured salmonids. As a species of the genus Novirhabdovirus, IHNV is a valuable model system for exploring the host entry mechanisms of rhabdoviruses. In this study, quantum dots (QDs) were used as fluorescent labels for sensitive, long-term tracking of IHNV entry. Using live-cell fluorescence microscopy, we found that IHNV is internalized through clathrin-coated pits after the virus binds to host cell membranes. Pretreatment of host cells with chlorpromazine, a drug that blocks clathrin-mediated endocytosis, and clathrin light chain (LCa) depletion using RNA interference both resulted in a marked reduction in viral entry. We also visualized transport of the virus via the cytoskeleton (i.e., actin filaments and microtubules) in real time. Actin polymerization is involved in the transport of endocytic vesicles into the cytosol, whereas microtubules are required for the trafficking of clathrin-coated vesicles to early endosomes, late endosomes, and lysosomes. Disrupting the host cell cytoskeleton with cytochalasin D or nocodazole significantly impaired IHNV infectivity. Furthermore, infection was significantly affected by pretreating the host cells with bafilomycin A1, a compound that inhibits the acidification of endosomes and lysosomes. Strong colocalizations of IHNV with endosomes indicated that the virus is internalized into these membrane-bound compartments. This is the first report in which QD labeling is used to visualize the dynamic interactions between viruses and endocytic structures; the results presented demonstrate that IHNV enters host cells via clathrin-mediated endocytic, cytoskeleton-dependent, and low-pH-dependent pathways.
INTRODUCTION
The infectious hematopoietic necrosis virus (IHNV) is one of the most important rhabdoviral fish pathogens of both wild and cultured salmon and trout and causes extensive economic losses in fish culture facilities (1, 16, 49). The first reported IHNV epidemics occurred during the 1950s in the United States at fish hatcheries in Washington and Oregon (20, 40). IHNV is an enveloped, negative-sense, single-stranded RNA virus that belongs to the Novirhabdovirus genus within the Rhabdoviridae family. Like other Rhabdoviridae family members, the gene order of IHNV is 3′-leader-N-P-M-G-NV-L-trailer-5′, with a coding capacity for five structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), RNA polymerase (L), and a nonstructural protein (NV) (1, 19, 36).
Recently, there has been much interest in the cellular mechanisms underlying the entry of viruses into host cells. Many viruses infect their host cells through endocytosis. Clathrin-mediated endocytosis is a well-described process that has been extensively studied. Many viruses are internalized via clathrin-coated pits (CCPs) after the virus binds to specific receptors on the cell membrane; after the virus binds to the host cell, the coated pits invaginate and pinch off from the plasma membrane to form intracellular clathrin-coated vesicles (CCVs). The released vesicles rapidly lose their clathrin coats and fuse with early endosomes that then fuse with each other to form late endosomes that finally fuse with lysosomes (9, 33, 41).
Studies have shown that fibronectin may be a primary receptor of IHNV that can mediate the attachment and entry of IHNV into host cells (4, 24). However, the exact trafficking pathway utilized by IHNV to enter host cells remains unclear. In this study, we used quantum dots (QDs) to visualize the endocytosis of IHNV from the point of attachment to the transport of internalized virus particles via the cytoskeleton. Typically, the visualization of viruses requires the use of dye molecules or expression of fused fluorescent proteins (21, 26, 29). However, to achieve sensitive long-term tracking, we chose to use QDs as fluorescent labels. QDs are light-emitting particles on the nanometer scale that have unique optical and electronic properties, including size-tunable light emission, superior signal brightness, photostability, resistance to photobleaching, and broad absorption spectra for simultaneous excitation of multiple fluorescence colors, unlike the single colors of dye molecules and fluorescent proteins (32, 44). Several studies have utilized QDs to image viral behaviors in host cells (14, 15, 18, 23, 25). We expect to establish the QD labeling method as a standard cellular entry assay, and our systematic study showed that IHNV enters host cells via clathrin-mediated endocytic, cytoskeleton-dependent, and low-pH-dependent pathways.
MATERIALS AND METHODS
Cells.
The IHNV-susceptible epithelioma papulosum cyprini (EPC) cell line used in this study was kindly provided by Q. Y. Zhang, Institute of Hydrobiology, Chinese Academy of Sciences. A total of 15 ml from a suspension of roughly 106 cells/ml was pipetted into 75-cm2 tissue culture flasks. These flasks were then incubated overnight at 25°C. Cells were grown in minimum essential medium (MEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and buffered to pH 7.2 with sodium bicarbonate (NaHCO3; 44 mM) and HEPES (25 mM).
Purification and quantum dot labeling of IHNVs.
IHNV was kindly provided by L. B. Zeng, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences. The virus was propagated in monolayer cultures of EPC cells in 100-mm by 20-mm plastic tissue culture flasks at 18°C in the presence of 5% FBS. The cytopathic effect (CPE) of the virus was assessed at 3 to 5 days postinfection (p.i.) prior to removal of cell debris by centrifugation (2,000 × g for 15 min) at 4°C. Roughly 60 ml of supernatant was subsequently ultracentrifuged for 2 h at 50,000 × g using an SW40 Ti rotor in a Beckman Optima LE-80K Ultracentrifuge at 4°C. The pellet was resuspended in phosphate-buffered saline (PBS) and layered onto preformed 10 to 50% sucrose gradients before an additional ultracentrifugation at 50,000 × g for 60 min at 4°C. The virus band was collected and concentrated by ultracentrifugation in PBS at 50,000 × g for 2 h at 4°C. The pellet was then resuspended in 0.5 ml of PBS and stored at −70°C until further use.
To label the viruses, biotin was first introduced to the viral surface. Due to the tight interaction between biotin and streptavidin, the addition of streptavidin-conjugated QDs allows the specific labeling of viral particles with QDs (14, 15). Purified IHNV was labeled with sulfo-NHS-LC-biotin (sulfosuccinimidyl-6-biotinamido-hexanoate; Thermo) according to the manufacturer's instructions. Monolayers of EPC cells that were grown in microplates were incubated with biotin-IHNV for 20 min at 4°C and then washed twice with prechilled PBS. The plates were then incubated with Qdots Streptavidin Conjugate (SA-QDs; Wuhan Jiayuan Quantum Dots Co.., Ltd.) for 30 min at 4°C. After two additional washes with PBS, the QD-labeled IHNVs were used for analysis.
Antiserum production and antibodies.
The cDNA sequences of fragmented (from 54 to 278 amino acids [aa]) and full-length IHNV glycoproteins were amplified by reverse transcription-PCR (RT-PCR). IHNV proteins were expressed in Escherichia coli BL21 cells using pET30a expression vectors; the expressed glycoproteins were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein bands were freeze-dried and milled prior to injection into New Zealand White rabbits. Three series of intradermal injections, each containing 300 μg of purified protein, were performed at 2-week intervals. Antiserum was used at a 1:1,000 or 1:2,000 dilution for Western blot experiments and at a 1:100 or 1:200 dilution for immunofluorescence experiments. Clathrin light chain (LCa) antibody (ab77542) was purchased from Abcam. All secondary antibodies were from Thermo.
Real-time qPCR analysis.
The virus genome was amplified by real-time quantitative PCR. First, RNA was extracted from EPC cells at 24 h p.i. using TRIzol reagent (Invitrogen). Next, first-strand cDNA was synthesized using a RevertAid First Stand cDNA Synthesis Kit (Fermentas). For quantitative PCRs (qPCRs), each sample contained 12.5 μl of SYBR green real-time PCR Master Mix (Toyobo), forward and reverse primers (each at a final concentration of 0.2 μM), and 1 μl of cDNA; ultrapure water was added to bring the final volume of each sample to 25 μl. The cycling conditions were as follows: an initial hold at 95°C for 1 min, followed by 40 cycles consisting of 94°C for 15 s, 60°C for 15 s, and 72°C for 45 s. Following amplification, DNA melting curve analysis was performed to confirm the specificity of the PCR products. Melting curves were acquired on the SYBR channel using a ramping rate of 1°C/60 s for 60 to 99°C. 18S rRNA was used as an internal control. The relative expression ratios were calculated using a mathematical model for relative quantification. The primers used for the real-time PCRs include the following: virus genome forward primer 5′-TGACGGTCGCTGTGGACGAGGTA-3′ and reverse primer 5′-CAGGTCTGGAATAATGGTGGTGTT-3′ (product length, 147 bp); 18S gene forward primer 5′-AAACGGCTACCACATCCAAG-3′ and reverse primer 5′-TTACAGGGCCTCGAAAGAGA-3′ (product length, 109 bp).
One-step growth curve.
EPC cells were infected with IHNV, biotin-IHNV, and QD-labeled IHNV at a multiplicity of infection (MOI) of 5, ensuring that increases in virus titer in the supernatant would reflect amplification from a single round of infection. Medium supernatant was taken at 4, 8, 12, 24, 36, 48, 72 h after infection, and all samples were stored at −70°C until titer determinations. Titers of the aliquots were determined in duplicate on EPC cells. Initially, virus dilutions were prepared in MEM at dilutions ranging from 1:10 to 1:10,000. Inoculum was introduced directly onto cell monolayers for 45 min at 18°C. Infected monolayers were washed three times with PBS and incubated at 18°C in MEM supplemented with 5% FBS. Cell monolayers were rinsed twice with PBS, fixed with 4% paraformaldehyde at 48 h p.i., and prepared immediately for immunofluorescence staining and immunofluorescence focus counting. Foci were treated as immunofluorescent focus units (IFU), and 10 microscopic fields were examined for each sample. The number of IFU of virus per milliliter was calculated as follows: the average number of IFU per field × the number of fields per well × reciprocal of the dilution of the virus inoculum × a volume factor (for conversion to milliliters).
Virus entry assay and drug treatments.
Inhibitors of various cellular entry pathways were used to investigate the entry mechanisms of the IHNV. EPC cell monolayers were grown in 24-well microplates in MEM supplemented with 10% FBS; the cells were pretreated with drugs as listed below. After pretreatment, cells were washed with PBS and incubated with IHNV at an MOI of 5 in the absence of drugs for 45 min at 18°C. Infected monolayers were washed three times with PBS and incubated at 18°C in MEM supplemented with 5% FBS. Cells were processed either for qPCR analysis at 24 h p.i. or for immunofluorescence and Western blot analysis 48 h p.i. At least two independent experiments, repeated three times for each sample, were performed.
The treatment conditions for the drugs were as follows: for chlorpromazine (5 to 35 μM), nocodazole (5 to 70 μM), cytochalasin D (1 to 50 μM), and bafilomycin A1 (1 to 50 nM), cells were pretreated in MEM without FBS for 30 min at 25°C; for nystatin (20 to 200 μM), cells were pretreated in MEM without FBS for 1 h at 25°C.
Plasmids, small interfering RNAs (siRNAs), and transfections.
Full-length LCa was amplified by RT-PCR from EPC cells and inserted into pEGFP-C1 (where EGFP is enhanced green fluorescent protein) vector (Clontech), resulting in a pEGFP-LCa construct. GFP-mouse talin (GFP-mTn) and GFP–microtubule-associated protein 4 (GFP-MAP4) constructs were kindly provided by Nam-Hai Chua, Rockefeller University, New York, NY; the fusion proteins were transferred into pcDNA3.1(+), resulting in pcDNA3.1-GFP-mTn and pcDNA3.1-GFP-MAP4 constructs. Full-length Rab5 and Rab7 were amplified by RT-PCR from a zebrafish and inserted into pEGFP-C1 vector, resulting in pEGFP-Rab5 and pEGFP-Rab7 constructs.
The LCa siRNAs (oligonucleotide 1, GAGCAGUGAAUGGAGAUCUTT; oligonucleotide 2, GGAAGGAGAAAGCAAAGCUTT; oligonucleotide 3, GCGCCUUUGUGAUUUCAAUTT) and a negative-control siRNA (UUCUCCGAACGUGUCACGUTT) were designed and synthesized by Shanghai GenePharma Co., Ltd.
EPC cells grown to 70% confluence in 24-well microplates were transfected with each construct or siRNA using Lipofectamine 2000 reagent (Invitrogen). Briefly, 0.8 μg of each construct or 20 pmol of each siRNA was diluted in 50 μl of OptiMEM and combined with 50 μl of OptiMEM containing 2 μl or 1 μl of Lipofectamine. After a 20-min incubation at room temperature, the DNA-liposome complexes were added to the cells, and cultures were incubated for 6 h at 25°C. After this incubation, the medium was replaced with fresh MEM, and the cells were incubated for an additional 24 to 48 h before virus entry assays were performed.
Live-cell fluorescence microscopy.
EPC cells were plated in 35-mm glass-bottom culture dishes (MatTek catalogue item P35G-1.5-14-C) and grown to 70% confluence prior to transfection. Then, 24 h after transfection, the cells were incubated with biotin-IHNV for 20 min at 4°C and subsequently washed twice with prechilled PBS. SA-QDs were incubated for 30 min at 4°C and washed twice. The cultures were observed using a Revolution XD confocal microscope (Andor) at 18°C. The GFP signal was excited using a 488-nm helium-neon laser and a 525 ± 25-nm band-pass filter for GFP fluorescent emission. QDs were excited using a 488-nm laser, and a 605 ± 35-nm band-pass filter was used for emission. The fluorescent emission was collected using an oil immersion objective and imaged on an iXon DV885K Single Photon Detector charge-coupled-device (CCD) camera (Andor). Differential interference contrast (DIC) optics (Olympus, JPN) were used to obtain cell images before and after fluorescence imaging. The movement and speed of the virus as well as the fluorescence intensity and colocalization of the virus with the various cellular structures were analyzed by Andor iQ.
EM.
EPC cells were incubated with IHNV at an MOI of 20 for 20 min at 4°C to allow the virus to attach to the plasma membrane. Cells were then incubated for 0 min, 5 min, 15 min, 30 min, and 2 h at 18°C. These cells were then fixed in 2% glutaraldehyde in PBS. Ultrathin sections of these cells were examined by FEI Tecnai G2 electron microscopy (EM).
RESULTS
IHNV enters EPC cells via clathrin-mediated endocytosis.
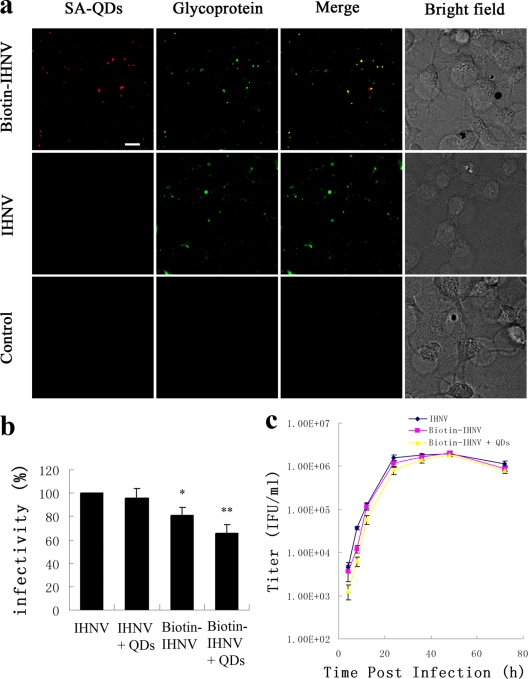
To study the role of clathrin-mediated endocytosis in IHNV entry, we engineered an LCa and enhanced green fluorescent (EGFP) fusion protein. This fusion protein construct was transfected into EPC cells; because the functional integrity of clathrin is not compromised by fluorescent protein labels, the EGFP-LCa clusters revealed all CCPs and CCVs (9, 11, 41). We chose QDs as a fluorescent label for sensitive, long-term tracking of virus movement. QD-labeled particles were immunolabeled with antiserum against IHNV glycoprotein; subsequently, we added fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG. Though 8% of IHNV particles (green) were not labeled by QDs, nearly all of the QD-labeled particles colocalized with glycoprotein, suggesting that all of the QD images revealed IHNV particles. Native IHNV and QD controls showed negligible fluorescence in all cases, ruling out interference from nonspecific binding (Fig. 1a). The infectivity of biotin-IHNV was calculated by qPCR analysis at 24 h p.i., and the results showed that viral infectivity remained at 80% after biotinylation and at 65% after conjunction to QDs (Fig. 1b). The QD-labeled IHNV was delayed in inducing CPE in EPC cells. It also showed a slight delay in initial virus production but reached a maximum titer similar to that of unmodified virus (Fig. 1c).
Fig. 1.
Labeling of IHNVs with QDs. EPC cells were incubated with biotin-IHNV, IHNV, or no virus (control) for 20 min at 4°C. (a) Immunofluorescence staining was performed using antiserum (green) and IHNV particles that were also incubated with SA-QDs (red). Scale bar, 10 μm. (b) The same quantities of biotin-IHNV and IHNV were used at each group, and then cells were either incubated with SA-QDs or left untreated. Viral infectivity was calculated by qPCR analysis at 24 h p.i. Asterisks denote a statistically significant change in infectivity compared to native IHNV (*, P < 0.05; **, P < 0.01). The data shown represent the mean values and standard deviations of the results. Three independent experiments, repeated three times for each sample, were performed. (c) One-step growth curve of IHNV, biotin-IHNV, and QD-labeled IHNV in EPC cells. Initial infections were done at an MOI of 5. Titers in the supernatants at each time of collection were determined in EPC cells.
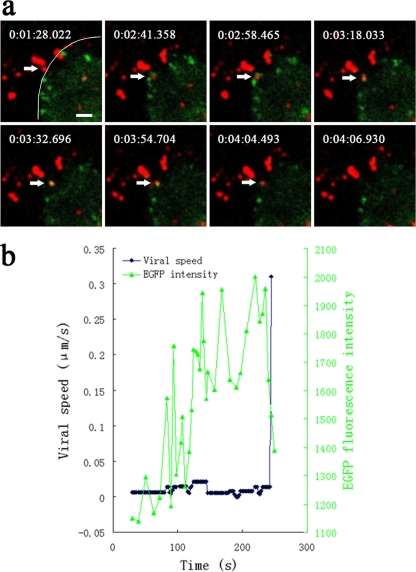
We then simultaneously imaged individual viruses and clathrin-coated structures. We found that only 16% of the viruses colocalized with preexisting CCPs. The other viruses were internalized through de novo formation of CCPs at the virus binding sites. These viruses initially bound to sites without an EGFP-LCa cluster; subsequently, a fluorescent EGFP spot appeared and formed a cluster. The EGFP fluorescence intensity gradually increased before rapidly disappearing, suggesting that the viruses were internalized via CCPs, followed by rapid uncoating of CCVs. We observed that the formation of CCPs occurred initiated roughly 2 min after the virus bound to the cell membrane; these CCPs remained visible for roughly 120 s while the uncoating of CCVs took only about 15 s (Fig. 2a and b). Subsequently, we observed a rapid movement of the particle, with a speed of 0.310 μm/s after disassembly of the clathrin coat (Fig. 2b).
Fig. 2.
Internalization of IHNV particles through clathrin-mediated endocytosis. (a) Snapshots of an IHNV particle (indicated by an arrow) being internalized through de novo formation of a CCP. A movie depicting this process is also available (see Video S1 in the supplemental material). EPC cells were transfected with pEGFP-LCa prior to incubation with QD-labeled IHNVs. The border of the cell is indicated by a curve. The time at which each snapshot was taken is indicated in white at the top of each frame. Scale bar, 2 μm. (b) The time trajectories of an IHNV particle internalized through de novo formation of a CCP. Black symbols represent the velocity-time trajectories of the virus. Green symbols represent the integrated fluorescence intensity of EGFP-LCa associated with the particle. The EGFP fluorescence intensity gradually increased and then rapidly disappeared (corresponding to disassembly of the clathrin coat), followed by rapid movement of the particle.
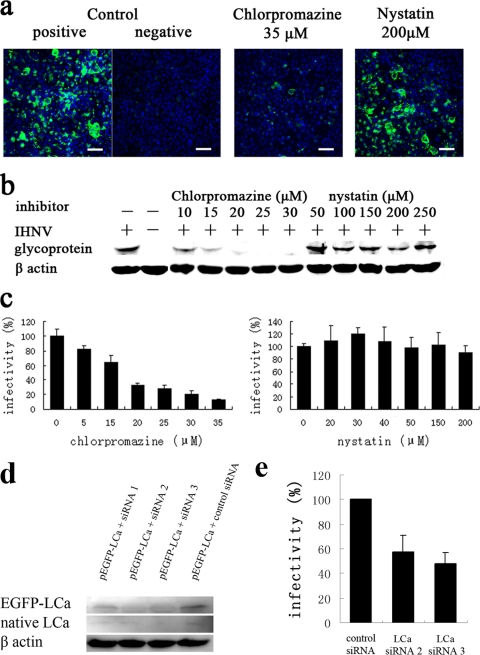
To determine whether IHNV enters cells via clathrin-mediated endocytosis, EPC cells were pretreated with drugs that inhibit clathrin-mediated endocytosis (chlorpromazine, a cationic, amphiphilic molecule that prevents the assembly and disassembly of clathrin lattices at cell surfaces and on endosomes) (47) and caveola-mediated endocytosis (nystatin, a sterol-binding drug that disrupts the cholesterol-rich caveola-containing membrane microdomains by sequestering cholesterol) (2, 35). Initially, we assayed a range of concentrations of each drug to determine the effective concentrations and avoid potential toxic effects (data not shown). We then tested the effect of these two inhibitors on the expression of IHNV glycoprotein using an indirect immunofluorescence assay (using an FITC-goat anti-rabbit IgG) at 48 h p.i. Untreated cells that had been infected with the same amount of virus were included as a positive control, and uninfected cells were included as a negative control. Cells that were pretreated with 35 μM chlorpromazine prior to inoculation with IHNV exhibited significantly low IHNV infection levels. In contrast, IHNV infection was not inhibited by nystatin, even when cells were preincubated with very high concentrations of the drug (Fig. 3a). In addition, glycoprotein expression was semiquantitated by Western blot analysis. As shown in Fig. 3b, pretreatment of EPC cells with chlorpromazine prior to inoculation with IHNV significantly inhibited viral infection in a dose-dependent manner while pretreatment of EPC with nystatin had no effect. Finally, using a qPCR assay, we observed that pretreatment of EPC cells with 35 μM chlorpromazine reduced viral infectivity to 12.5% of that observed in control cells (Fig. 3c).
Fig. 3.
Effect of endocytosis inhibitors on infection of EPC cells by IHNV. (a) Cells, either untreated (positive control) or pretreated with either 35 μM chlorpromazine or 200 μM nystatin, were infected with IHNV. Uninfected cells (negative control) were also included in the experiment. At 48 h p.i., immunofluorescence staining was performed using antiserum (green) and Hoechst nuclear stain (blue). Scale bar, 50 μm. (b and c) Cells were treated with various concentrations of chlorpromazine and nystatin prior to infection with IHNV. Viral infection was quantified by Western blot analysis at 48 h p.i. (b) and by qPCR analysis at 24 h p.i. (c). Viral infectivity was quantified as the percentage of amplified viral RNA of drug-treated cells relative to untreated control cells. (d) Cells were cotransfected with siRNAs and pEGFP-LCa, and expression of LCa was detected by Western blot analysis after 48 h. (e) Cells were infected by IHNV after 48 h of transfection of siRNA, and viral infectivity was calculated by qPCR analysis at 24 h p.i. The data shown represent the mean values and standard deviations of the results. At least two independent experiments, repeated three times for each sample, were performed.
To deplete the expression of the LCa, we selected three siRNA duplexes that target its open reading frame. We tested fibronectin mRNA expression in LCa depletion cells, and these siRNAs did not affect the viral receptor expression (see supplemental material). Cells were cotransfected with siRNAs and pEGFP-LCa, and we quantitated the reduction in LCa expression by immunoblotting with antibody directed against the LCa after 48 h of incubation. The LCa signals of cells transfected with siRNA 2 and 3 were strongly reduced, whereas the actin signals had not changed (Fig. 3d). So we choose these two for gene silencing, and virus entry assays were performed after 48 h of transfection. Using a qPCR assay, we observed that viral infectivities of both siRNAs were reduced to about 50% (Fig. 3e). Taken together, these results show that IHNVs enter EPC cells via clathrin-mediated endocytosis.
Cytoskeleton-dependent pathway.
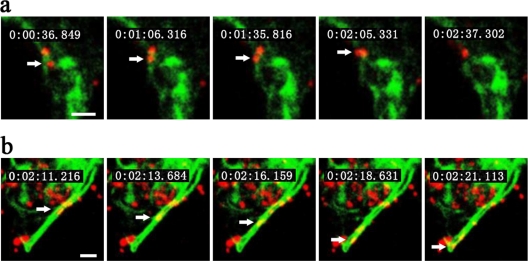
To visualize cytoskeleton-dependent pathways of IHNV internalization in EPC cells, we used two fluorescent fusion proteins: GFP-mTn and GFP-MAP4. Transient or stable expression of GFP-mTn (GFP fused to the N terminus of mouse talin) allows the visualization of the actin cytoskeleton (17) while transient or stable expression of GFP-MAP4 (GFP fused to the microtubule-binding domain of mammalian microtubule-associated protein 4) fusion protein reveals the localization of microtubules and has been used in both mammalian and plant cells (28, 34). Analyzing the virus trajectories in GFP-mTn-expressing EPC cells, we observed a slow movement of virus particles in the actin-enriched cell periphery. The average speed of this movement was roughly 0.023 ± 0.002 μm/s, and the displacement was typically within 2 μm of the site of initial binding (Fig. 4a). This movement likely represents the transport of virus-containing endocytic vesicles into the EPC cells propelled by actin filaments (3, 7, 31, 37, 50). We next characterized the virus trajectories in EPC cells expressing the GFP-MAP4 fusion protein; in these cells, we observed a rapid movement along microtubules that typically lasted for several seconds, with an instantaneous speed of 0.593 ± 0.273 μm/s (Fig. 4b). This value is consistent with the velocity of dynein movement along microtubules (27). This transport represents the movement of virus-containing endosomes.
Fig. 4.
Host cytoskeleton-dependent IHNV entry pathway. Snapshots of IHNV particle (indicated by an arrow) transport via the cytoskeleton (actin filament in panel a and microtubule in panel b). Movies depicting these processes are available (see Videos S2 and S3 in the supplemental material). EPC cells were transfected with pcDNA3.1-GFP-mTn and pcDNA3.1-GFP-MAP4 prior to incubation with QD-labeled IHNVs. The time at which each snapshot was taken is indicated in white at the top of each frame. Scale bar, 2 μm.
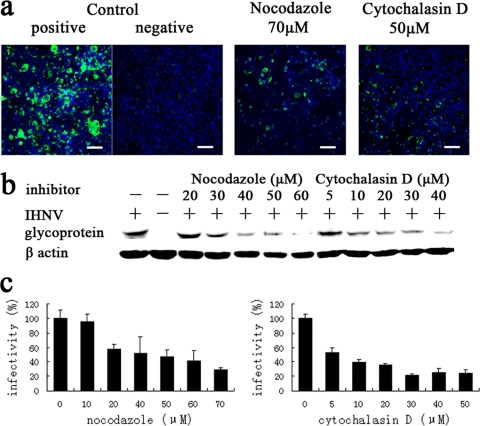
To further investigate the role of the host cytoskeleton in IHNV entry into EPC cells, cells were pretreated with cytochalasin D and nocodazole. Cytochalasin D inhibits actin filament elongation by binding to high-affinity sites located at the polymerization end of the filaments (10). Nocodazole disrupts microtubules by binding to β-tubulin and preventing formation of one of the two interchain disulfide linkages, thus inhibiting microtubule dynamics (6, 46). We tested a range of concentrations of each drug to determine the effective concentrations to avoid potential toxic effects (data not shown). The results of indirect immunofluorescence and Western blot analyses showed that pretreatment of EPC cells with these two drugs prior to inoculation with IHNV significantly inhibited virus infection in a dose-dependent manner (Fig. 5a and b). We also observed that virus infectivity was reduced to 24% of that observed in control cells in EPC cells treated with 50 μM cytochalasin D and to 29% in cells treated with 70 μM nocodazole, as determined with a qPCR assay (Fig. 5c).
Fig. 5.
The effects of cytoskeleton-disrupting drugs on the infection of EPC cells by IHNV. (a) Cells, either untreated (positive control) or treated with 70 μM nocodazole or 50 μM cytochalasin D, were infected with IHNV. Uninfected cells (negative control) were also included in the experiment. At 48 h p.i., immunofluorescence staining was performed as described in the legend of Fig. 3a. Scale bar, 50 μm. (b and c) Cells were treated with various concentrations of nocodazole and cytochalasin D prior to infection with IHNV. Viral infectivity was quantified as described in the legends of Fig. 3b and c.
Low-pH-dependent pathway.
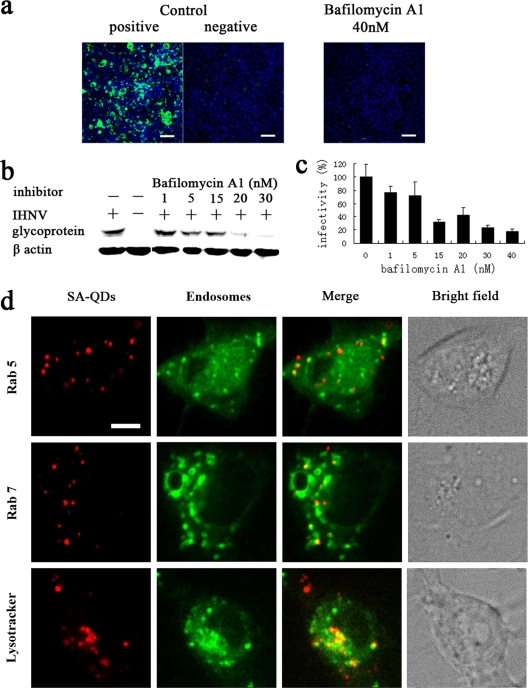
Most enveloped viruses that enter host cells via endocytosis require a low-pH step to trigger fusion of the viral envelope with the endosome membrane and release their genome into the cytosol for replication. To investigate the role of a low-pH step in IHNV entry, EPC cells were pretreated with the potent and specific inhibitor of endosome proton ATPases, bafilomycin A1, which inhibits the acidification of endosomes and lysosomes (51). We tested a range of concentrations to determine its effective concentration and avoid toxicity-related effects (data not shown). The results of indirect immunofluorescence analysis showed that the expression of glycoproteins was almost completely blocked when EPC cells were pretreated with bafilomycin A1 prior to inoculation with IHNV (Fig. 6a); furthermore, Western blot analysis revealed that bafilomycin A1 significantly inhibited virus infection in a dose-dependent manner (Fig. 6b). Using a qPCR assay, we observed that pretreatment of EPC cells with 40 nM bafilomycin A1 reduced viral infectivity to 18% of that observed in untreated control cells (Fig. 6c). To determine whether the internalized IHNV particles migrate into endosomes, we expressed the GFP-tagged endosomal markers (Rab5 for early endosome and Rab7 for late endosome) in EPC cells (5, 30, 42). Also, late endosomes and lysosomes were stained with LysoTracker (a late endosome and lysosome-specific dye [Invitrogen]). At 5 min p.i. IHNV particles had fused with early endosomes, and at 30 min p.i. IHNV strongly colocalized with late endosomes (or lysosomes), suggesting that the viral particles were transported to late endosomes after endocytosis (Fig. 6d). These results also indicated that the fusion of IHNV particles with the membranes of endocytotic vesicles occurred after the particles migrated into late endosomes.
Fig. 6.
Low-pH-dependent entry pathway. (a) Cells, either untreated (positive control) or treated with 40 nM bafilomycin A1, were infected with IHNV. Uninfected cells (negative control) were also included in the experiment. At 48 h p.i., immunofluorescence staining was performed as described in the legend of Fig. 3a. Scale bar, 50 μm. (b and c) Cells were treated with various concentrations of bafilomycin A1 prior to infection with IHNV. Viral infectivity was quantified as described in the legends of Fig. 3b and c. (d) EPC cells were transfected with pEGFP-Rab5 and pEGFP-Rab7 prior to incubation with QD-labeled IHNVs. Colocalizations of IHNV with Rab5 and Rab7 were imaged at 5 and 30 min p.i., respectively. Fusion of IHNV particles with early endosomes is also shown in Video S4 in the supplemental material. Cells were inoculated with QD-labeled IHNV. At 30 min p.i. cells were treated with LysoTracker for 5 min. Scale bar, 5 μm.
Ultrastructural analysis of viral entry.
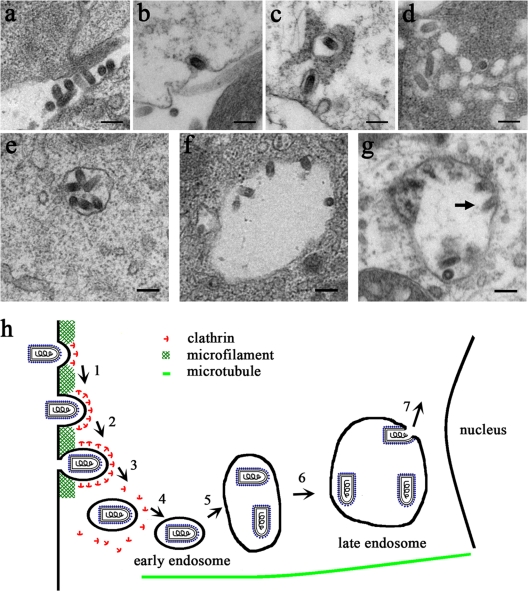
We used electron microscopy (EM) to analyze IHNV entry into EPC cells. To visualize synchronized entry of IHNV, viral particles were allowed to bind to cells at 4°C for 20 min. Low-temperature treatment allows IHNV to bind to cell surface receptors but prevents the internalization of virus particles into the cells. At 0 min p.i., we observed the attachment of roughly 200-nm-long IHNV particles to the surfaces of EPC cells (Fig. 7a). At 5 min p.i., IHNV particles were found within invaginations in the plasma membrane; these invaginations resembled CCPs (Fig. 7b). At 15 min p.i., the IHNV-containing pits had detached from the cell surface (Fig. 7c). At 30 min p.i., most of the viral particles observed were localized within endocytic vesicles, each of which contained a single virus particle (Fig. 7c and d). Vesicles that contained viral particles appeared to fuse together (Fig. 7d), resulting in the formation of large vesicles that were roughly 400 to 1,000 nm in diameter and contained several viral particles (Fig. 7e and f). At 2 h p.i., the viral envelopes had loosened and fused with the membranes of endocytic vesicles (Fig. 7g); the membrane fusion of IHNVs appeared to have occurred in the endosomes.
Fig. 7.
Ultrastructural analysis of the entry of IHNVs into EPC cells using electron microscopy. (a) Binding of IHNV particles on the plasma membrane of EPC cells. (b) Uptake of an IHNV particle by a CCP. (c) Internalization of single IHNV particles within CCVs. (d) CCVs containing IHNV particles appear to fuse. (e and f) Large vesicles containing several virus particles were observed. (g) Loosening of the IHNV envelope and fusion with the membrane of an endocytic vesicle membrane (indicated by an arrow). Scale bar, 200 nm. (h) A model of the entry pathway of IHNV into EPC cells. Steps 1 to 3 represent actin filament-dependent active transport: attachment of IHNV to the surfaces of EPC cells and the early stages of clathrin assembly (1), internalization of the viruses via CCPs (2), and the rapid uncoating of CCVs (3). Steps 4 to 6 represent microtubule-dependent movement: fusion with early endosomes (4), fusion of early endosomes with each other (5), and migration into late endosomes and lysosomes (6). Step 7 represents the fusion of IHNV particles with the membranes of endocytotic vesicles and release of its genome into the cytosol for replication.
DISCUSSION
Recently, many studies have focused on the cellular mechanisms responsible for the entry of viruses into host cells. To investigate the mechanisms of IHNV entry into EPC cells, we visualized the dynamic behavior of the virus particles using QDs. Initially, we demonstrated that the infectious properties of QD-labeled IHNV and unmodified virus are similar. Using live-cell fluorescence microscopy, we imaged the dynamic interactions between IHNVs and clathrin-coated structures in living cells. We found that the viruses are internalized predominantly through the de novo formation of CCPs around the viruses. We also observed that the formation of CCPs took roughly 120 s and that the uncoating of CCVs is completed in roughly 15 s. This result is consistent with the entry of influenza viruses into BS-C-1 cells, and the rapid translocation of IHNV particles after the clathrin coat disassembly may occur through the microtubules (41). To confirm the role of clathrin-mediated endocytosis, pretreatment with several different endocytosis-inhibiting drugs and depletion of LCa were carried out prior to the addition of virus. All the results showed that IHNV enters EPC cells via clathrin-mediated endocytosis.
This conclusion is consistent with entry pathways used by other rhabdoviruses. Vesicular stomatitis virus (VSV) and rabies virus (RV), both members of the Rhabdoviridae family, enter host cells via receptor/clathrin-mediated endocytosis (8, 13, 22, 33). VSV binds to cells via a phosphatidylserine receptor (43), and the rabies virus binds to cells via acetylcholine and neural cell adhesion molecule (NCAM) receptors (22, 45). Therefore, we propose that receptor/clathrin-mediated endocytosis may be a main entry pathway utilized by Rhabdoviridae.
Previously published evidence showed that actin is closely associated with CCPs; after CCPs are pinched off from the plasma membrane, actin polymerization may be involved in the transport of endocytic vesicles into the cytosol. Microtubules are required for trafficking CCVs to early endosomes, late endosomes, and lysosomes. Therefore, we investigated whether the host cytoskeleton is involved in IHNV entry by visualizing cytoskeleton-dependent pathways of IHNV entry in living cells. We observed a slow movement of virus in the actin-enriched cell periphery, with an average speed of 0.023 ± 0.002 μm/s and a displacement of 2 μm. We also observed a rapid movement along microtubules; this movement typically persisted for several seconds, with an instantaneous speed of 0.593 ± 0.273 μm/s. These two movement patterns are consistent with those described by previous reports (21, 27). Furthermore, IHNV entry is significantly inhibited after disruption of the host cell cytoskeleton with cytochalasin D or nocodazole. Taken together, these results indicate that the host cell cytoskeleton plays a critical role in the IHNV entry process.
Because CCVs fuse with acidic early endosomes after uncoating, we also investigated whether a low-pH-dependent compartment was necessary for IHNV entry. Pretreatment of EPC cells with bafilomycin A1 significantly inhibited IHNV infection. Recent evidence showed that the conformational change of rhabdovirus glycoprotein (fusion protein) is triggered by low-pH conditions; the prefusion form is shaped like a tripod with exposed fusion loops that face the viral membrane and induce membrane merging (12, 38, 39). IHNV glycoproteins may utilize the same mechanism for inducing membrane fusion. Because microtubule disruption impaired IHNV infectivity in EPC cells, we hypothesized that IHNV particles are transported from early to late endosomes for the pH-dependent fusion between the viral envelope and the endosomal membrane. This hypothesis was proven by the strong colocalization of IHNV and late endosomes (or lysosomes) observed in our live-cell studies. These results indicated that low-pH compartments are necessary for IHNV infection.
Finally, we performed EM experiments. IHNV particles were always found inside CCPs and vesicles. The results of our ultrastructural analysis are similar to observations of other viruses that use clathrin-mediated endocytosis (6, 13, 48). In summary, the results of electron microscopy, real-time fluorescence microscopy, and experiments using various inhibitors revealed that IHNV enters EPC cells via clathrin-mediated endocytic, cytoskeleton-dependent, and low-pH-dependent pathways. These results also suggested that viral transport is a multistep process. The first step is actin filament-dependent active transport in the cell periphery with the process of de novo formation of a CCP in about 120 s and rapid uncoating of a CCV in about 15 s. The second step is a rapid and microtubule-dependent movement toward the perinuclear region with the process of fusion with early endosomes, which fuse with each other to form late endosomes (or further fuse with lysosomes). The third step is the fusion of IHNV particles with the membranes of endocytotic vesicles, which occurs after the particles migrate into late endosomes. As a result, IHNV releases its genome into the cytosol for replication (Fig. 7 h).
To our knowledge, this is first time that the entry of IHNV into host cells via clathrin-mediated endocytic, cytoskeleton-dependent, and low-pH-dependent pathways has been demonstrated. In addition, this is the first study to use QD labeling to visualize the dynamic interactions between viruses and host endocytic structures.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Key Scientific Program (973)-Nanoscience and Nanotechnology (2011CB933600), the 100 Talents Program of the Chinese Academy of Sciences (KSCX2-YW-R-147), the Science Fund for Creative Research Groups of NSFC (20621502 and 20921062), and the National Natural Science Foundation of China (20833006).
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 27 April 2011.
REFERENCES
- 1. Ammayappan A., LaPatra S. E., Vakharia V. N. 2010. Molecular characterization of the virulent infectious hematopoietic necrosis virus (IHNV) strain 220-90. Virol. J. 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson H. A., Chen Y., Norkin L. C. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apodaca G. 2001. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2:149–159 [DOI] [PubMed] [Google Scholar]
- 4. Bearzotti M., et al. 1999. Fish rhabdovirus cell entry is mediated by fibronectin. J. Virol. 73:7703–7709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bucci C., et al. 1992. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70:715–728 [DOI] [PubMed] [Google Scholar]
- 6. Chu J. J., Ng M. L. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543–10555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coller K. E., et al. 2009. RNA interference and single particle tracking analysis of hepatitis C virus endocytosis. PLoS Pathog. 5:e1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cureton D. K., Massol R. H., Saffarian S., Kirchhausen T. L., Whelan S. P. 2009. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5:e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehrlich M., et al. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591–605 [DOI] [PubMed] [Google Scholar]
- 10. Flanagan M. D., Lin S. 1980. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J. Biol. Chem. 255:835–838 [PubMed] [Google Scholar]
- 11. Gaidarov I., Santini F., Warren R. A., Keen J. H. 1999. Spatial control of coated-pit dynamics in living cells. Nat. Cell Biol. 1:1–7 [DOI] [PubMed] [Google Scholar]
- 12. Gaudin Y. 2000. Reversibility in fusion protein conformational changes. The intriguing case of rhabdovirus-induced membrane fusion. Subcell. Biochem. 34:379–408 [DOI] [PubMed] [Google Scholar]
- 13. Johannsdottir H. K., Mancini R., Kartenbeck J., Amato L., Helenius A. 2009. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 83:440–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joo K. I., et al. 2008. Site-specific labeling of enveloped viruses with quantum dots for single virus tracking. ACS Nano 2:1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kampani K., et al. 2007. A novel high throughput quantum dot-based fluorescence assay for quantitation of virus binding and attachment. J. Virol. Methods 141:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolodziejek J., Schachner O., Durrwald R., Latif M., Nowotny N. 2008. “Mid-G” region sequences of the glycoprotein gene of Austrian infectious hematopoietic necrosis virus isolates form two lineages within European isolates and are distinct from American and Asian lineages. J. Clin. Microbiol. 46:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kost B., Spielhofer P., Chua N. H. 1998. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16:393–401 [DOI] [PubMed] [Google Scholar]
- 18. Kukura P., et al. 2009. High-speed nanoscopic tracking of the position and orientation of a single virus. Nat. Methods 6:923–927 [DOI] [PubMed] [Google Scholar]
- 19. Kurath G., Ahern K. G., Pearson G. D., Leong J. C. 1985. Molecular cloning of the six mRNA species of infectious hematopoietic necrosis virus, a fish rhabdovirus, and gene order determination by R-loop mapping. J. Virol. 53:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurath G., et al. 2003. Phylogeography of infectious haematopoietic necrosis virus in North America. J. Gen. Virol. 84:803–814 [DOI] [PubMed] [Google Scholar]
- 21. Lakadamyali M., Rust M. J., Babcock H. P., Zhuang X. 2003. Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 100:9280–9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis P., Fu Y., Lentz T. L. 1998. Rabies virus entry into endosomes in IMR-32 human neuroblastoma cells. Exp. Neurol. 153:65–73 [DOI] [PubMed] [Google Scholar]
- 23. Li F., et al. 2009. Imaging viral behavior in Mammalian cells with self-assembled capsid-quantum-dot hybrid particles. Small 5:718–726 [DOI] [PubMed] [Google Scholar]
- 24. Liu X., Collodi P. 2002. Novel form of fibronectin from zebrafish mediates infectious hematopoietic necrosis virus infection. J. Virol. 76:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo K., et al. 2010. Real-time visualization of prion transport in single live cells using quantum dots. Biochem. Biophys. Res. Commun. 394:493–497 [DOI] [PubMed] [Google Scholar]
- 26. Lux K., et al. 2005. Green fluorescent protein-tagged adeno-associated virus particles allow the study of cytosolic and nuclear trafficking. J. Virol. 79:11776–11787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma S., Chisholm R. L. 2002. Cytoplasmic dynein-associated structures move bidirectionally in vivo. J. Cell Sci. 115:1453–1460 [DOI] [PubMed] [Google Scholar]
- 28. Mathur J., Chua N. H. 2000. Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell 12:465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDonald D., et al. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159:441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meresse S., Gorvel J. P., Chavrier P. 1995. The Rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 108:3349–3358 [DOI] [PubMed] [Google Scholar]
- 31. Merrifield C. J., Feldman M. E., Wan L., Almers W. 2002. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4:691–698 [DOI] [PubMed] [Google Scholar]
- 32. Michalet X., et al. 2005. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mudhakir D., Harashima H. 2009. Learning from the viral journey: how to enter cells and how to overcome intracellular barriers to reach the nucleus. AAPS J. 11:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olson K. R., McIntosh J. R., Olmsted J. B. 1995. Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J. Cell Biol. 130:639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pelkmans L., Kartenbeck J., Helenius A. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473–483 [DOI] [PubMed] [Google Scholar]
- 36. Penaranda M. M., Purcell M. K., Kurath G. 2009. Differential virulence mechanisms of infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss) include host entry and virus replication kinetics. J. Gen. Virol. 90:2172–2182 [DOI] [PubMed] [Google Scholar]
- 37. Qualmann B., Kessels M. M. 2002. Endocytosis and the cytoskeleton. Int. Rev. Cytol. 220:93–144 [DOI] [PubMed] [Google Scholar]
- 38. Roche S., Bressanelli S., Rey F. A., Gaudin Y. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187–191 [DOI] [PubMed] [Google Scholar]
- 39. Roche S., Rey F. A., Gaudin Y., Bressanelli S. 2007. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science 315:843–848 [DOI] [PubMed] [Google Scholar]
- 40. Rucker R. R., Whipple W. J., Parvin J. R., Evans C. A. 1953. A contagious disease of salmon possibly of virus origin. U.S. Fish Wildl. Serv. Fish. Bull. 54:35–46 [Google Scholar]
- 41. Rust M. J., Lakadamyali M., Zhang F., Zhuang X. 2004. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 11:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schimmoller F., Riezman H. 1993. Involvement of Ypt7p, a small GTPase, in traffic from late endosome to the vacuole in yeast. J. Cell Sci. 106:823–830 [DOI] [PubMed] [Google Scholar]
- 43. Schlegel R., Tralka T. S., Willingham M. C., Pastan I. 1983. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell 32:639–646 [DOI] [PubMed] [Google Scholar]
- 44. Smith A. M., Duan H., Mohs A. M., Nie S. 2008. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv Rev. 60:1226–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thoulouze M. I., et al. 1998. The neural cell adhesion molecule is a receptor for rabies virus. J. Virol. 72:7181–7190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vasquez R. J., Howell B., Yvon A. M., Wadsworth P., Cassimeris L. 1997. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8:973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang L. H., Rothberg K. G., Anderson R. G. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei T., Chen H., Ichiki-Uehara T., Hibino H., Omura T. 2007. Entry of Rice dwarf virus into cultured cells of its insect vector involves clathrin-mediated endocytosis. J. Virol. 81:7811–7815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Winton J. R. 1991. Recent advances in detection and control of infectious hematopoietic necrosis virus in aquaculture. Annu. Rev. Fish Dis. 1:83–93 [Google Scholar]
- 50. Yarar D., Waterman-Storer C. M., Schmid S. L. 2005. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell 16:964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. 1991. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266:17707–17712 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.