Abstract
Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) establish latency and express the latency-associated transcript (LAT) preferentially in different murine sensory neuron populations, with most HSV-1 LAT expression in A5+ neurons and most HSV-2 LAT expression in KH10+ neurons. To study the mechanisms regulating the establishment of HSV latency in specific subtypes of neurons, cultured dissociated adult murine trigeminal ganglion (TG) neurons were assessed for relative permissiveness for productive infection. In contrast to that for neonatal TG, the relative distribution of A5+ and KH10+ neurons in cultured adult TG was similar to that seen in vivo. Productive infection with HSV was restricted, and only 45% of cultured neurons could be productively infected with either HSV-1 or HSV-2. A5+ neurons supported productive infection with HSV-2 but were selectively nonpermissive for productive infection with HSV-1, a phenomenon that was not due to restricted viral entry or DNA uncoating, since HSV-1 expressing β-galactosidase under the control of the neurofilament promoter was detected in ∼90% of cultured neurons, with no preference for any neuronal subtype. Infection with HSV-1 reporter viruses expressing enhanced green fluorescent protein (EGFP) from immediate early (IE), early, and late gene promoters indicated that the block to productive infection occurred before IE gene expression. Trichostatin A treatment of quiescently infected neurons induced productive infection preferentially from non-A5+ neurons, demonstrating that the nonpermissive neuronal subtype is also nonpermissive for reactivation. Thus, HSV-1 is capable of entering the majority of sensory neurons in vitro; productive infection occurs within a subset of these neurons; and this differential distribution of productive infection is determined at or before the expression of the viral IE genes.
INTRODUCTION
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) replication at the periphery is accompanied by infection of neuronal axons and subsequent retrograde axonal transport to cell bodies of primary sensory neurons, where infection may follow either a productive or a latent pathway. For some neurons, lytic gene expression and progeny virus production are thought to result in cell death, while in other neurons, the productive cycle fails and the virus establishes a latent infection. The factors that determine whether HSV progresses through a productive cycle or establishes latency are not clear. It is suspected that different neuronal subtypes and/or the presence or absence of certain host factors may be critical in determining the outcome of infection.
Primary sensory neurons are a diverse population of cells that are classified according to cellular morphology, physiological response properties, and patterns of gene expression. We have demonstrated previously that HSV-1 and HSV-2 preferentially establish latency and express the latency-associated transcript (LAT) in different populations of neurons, identified by the A5 and KH10 markers, within sensory ganglia (21, 28, 52). Although all neuronal populations are thought to be capable of supporting productive HSV infection, some neuronal populations of the trigeminal ganglion (TG) appear to be more permissive for productive infection than others, and the permissiveness of neuronal subtypes differs for HSV-1 and HSV-2. Based on in situ hybridization for the LAT, the neuronal population identified by monoclonal antibody (MAb) A5 is the principal reservoir for latent HSV-1 infection in mouse models of both ocular and footpad infection. In contrast, HSV-2 establishes a latent infection in very few A5+ neurons in the same models.
In mouse sensory ganglia, the A5 and KH10 markers identify functionally distinct nociceptive neuronal populations. A5+ neurons are nerve growth factor (NGF) responsive, are immunoreactive for the calcitonin gene-related peptide (CGRP), and project Aδ and C fibers to laminae I and II (outer) of the dorsal horn (14). KH10+ neurons are colabeled with Bandeiraea simplicifolia isolectin B4 (BSL-IB4) and are small-diameter, RET-positive neurons that express the ATP-gated ion channel P2X3 and receptors for glial-cell-derived neurotrophic factor (GDNF) and neurturin (4, 14, 28, 33, 41, 54). They project C fibers to lamina II (inner) of the dorsal horn (14).
Animal models of infection and latency have been valuable in the study of HSV pathogenesis but have limitations for studying mechanisms that regulate the establishment and maintenance of viral latency. These limitations include the relatively small proportion of ganglionic neurons in which latency is established, the asynchronicity of events, the very small number of neurons that can be induced to reactivate, and the difficulty of manipulating the molecular state of infected neurons. In vitro models overcome some of these limitations, allowing for synchronized infection of a large number of neurons, as well as coordinated pharmacological manipulation of these cells, but suffer the drawback of not modeling neuronal populations in adult sensory ganglia. An in vitro model using NGF-differentiated PC12 cells has been detailed, but the transformed cells take on some, but not all, of the characteristics of autonomic neurons, not sensory neurons. Furthermore, in vitro models with embryonic or neonatal sensory neurons do not reflect the mature heterogeneous populations of neurons in the adult sensory ganglia. To examine the neuronal mechanisms that regulate the establishment of HSV latency in specific neuronal subtypes, we developed an in vitro model of HSV infection using dissociated adult murine trigeminal ganglion neurons. This in vitro model closely mimics results seen previously with in vivo mouse models, with HSV causing a productive infection in some neurons and a quiescent infection in others. Using this in vitro model, we determined that A5+ trigeminal ganglion neurons are relatively nonpermissive for productive HSV-1 infection compared to other populations of trigeminal ganglion neurons. In this model we also determined that preferential permissiveness for productive infection is regulated at, or before, the level of immediate early (IE) viral gene expression.
MATERIALS AND METHODS
Neuronal cultures.
Six-week-old female Swiss Webster mice (Simonsen Labs, Gilroy, CA) were euthanized by CO2, followed by transcardial perfusion with cold, calcium- and magnesium-free (CMF) phosphate-buffered saline (PBS). Trigeminal ganglia (TG) were removed, incubated at 37°C for 20 min in papain (25 mg) (Worthington, Lakewood, NJ) reconstituted with 5 ml Neurobasal A medium (Invitrogen) and for 20 min in Hanks balanced salt solution (HBSS) containing dispase (4.67 mg/ml) and collagenase (4 mg/ml) (Sigma) on a rotator, and mechanically dissociated by triturating with a 1,000-μl pipette. The resultant cell suspension was layered on a 5-step OptiPrep (Sigma) gradient. OptiPrep was first diluted with 0.8% sodium chloride (50.5:49.5) to make a working solution and was then further diluted with Neurobasal A medium to make gradient steps as follows: 150 μl of OptiPrep working solution and 850 μl of Neurobasal A, 250 and 750 μl, 300 and 700 μl, 350 and 650 μl, and 400 and 600 μl, respectively. The cell suspension was layered on top of the gradient and was centrifuged for 20 min at 800 × g. The lower end of the centrifuged gradient (∼3.5 ml), minus the pellet, was then transferred to a new tube and was washed twice with Neurobasal A medium supplemented with 2% B27 supplement (Invitrogen) and 1% penicillin-streptomycin (PS). Neurons were counted and plated on poly-d-lysine- and laminin-coated 8-well chamber slides (BD Biosciences) at a density of 3,000 per well. Neuronal cultures were maintained with complete neuronal medium, consisting of Neurobasal A medium supplemented with 2% B27 supplement, 1% PS, l-glutamine (500 μM), nerve growth factor (NGF; 50 ng/ml), glial-cell-derived neurotrophic factor (GDNF; 50 ng/ml), and the mitotic inhibitors fluorodeoxyuridine (40 μM) and aphidicolin (16.6 μg/ml) for the first 3 days. The medium was then replaced with fresh medium without fluorodeoxyuridine and aphidicolin (growth factors were from R&D Systems, and other supplements were from Sigma). Neonatal trigeminal ganglia were cultured using identical methods and conditions. Adult and neonatal superior cervical ganglia (SCG) were cultured using the same methods, but without gradient centrifugation.
Viruses.
The wild-type HSV-1 strains KOS, RE, and 17syn+ and the wild-type HSV-2 strain 333, as well as all mutant virus strains, were propagated in rabbit skin cells (52). HSV1-VP26-GFP and HSV2-VP26-GFP were generated in Vero cells by cotransfection and homologous recombination of plasmid pK26GFP (kindly provided by Prashant Desai, Johns Hopkins University) with purified viral DNA from either HSV-1 strain 17+ or HSV-2 strain 333 by using previously described methods (6). The VP26-GFP viruses express a fusion protein of VP26 and green fluorescent protein (GFP) (13). KOS/58, an HSV-1-based virus expressing lacZ under the control of the neurofilament light (NFL) promoter at the gC locus, and KOS/62, an HSV-1-based virus expressing lacZ inserted between SacII and the second HpaI sites downstream of the LAT promoter, have been described previously (29). The RE-pICP0-EGFP, RE-pgB-EGFP, and RE-pgC-EGFP reporter viruses, which are based on HSV-1 strain RE, express enhanced GFP (EGFP) under the control of the ICP0, gB, and gC promoters inserted at the gC locus, in a manner similar to that described previously (11, 37). RE-pgC-EGFP and RE-pICP0-EGFP have been described previously (11). To develop RE-pICP4-EGFP, plasmid pK1-2 (a kind gift from Neil Deluca, University of Pittsburgh) was digested with HindIII and BamHI to release the ICP4 promoter, which was then cloned into the polylinker upstream of EGFP in plasmid gC-EGFP to drive EGFP expression, in a manner similar to that detailed previously (11). To develop the RE-pICP27-EGFP virus, the upstream portion of ICP27 from 37 bp upstream of the ICP27 ATG to a position approximately 1,000 bp further upstream was amplified by PCR using primers 5′-gcagatctGTCGGATATGGCCTCTGGTGTGGCGCA and 5′-gagtaagcttCCTACACGAAAATTACCCGCCT (lowercase sequences encode restriction sites for cloning). The resulting fragment was then digested with BglII and HindIII and was cloned into the HindIIII BamHI sites in the polylinker of gC-EGFP to drive EGFP expression. Viruses were derived and purified by selection for EGFP-positive plaques. RE-gB-EGFP, in which the gB promoter drives EGFP expression, was constructed using primers that amplify approximately 500 bp of sequence upstream of the gB coding sequences as described previously (37). The gB promoter amplification product was placed into the gC-EGFP plasmid to drive EGFP and then was linearized and used to derive and purify fluorescent virus. All viruses were confirmed for correct insertion by Southern blotting. Viral titers were determined by standard viral plaque assays on Vero cells.
Viral infections.
Neuronal cultures were infected with viruses at various multiplicities of infection (MOI) from 1 to 100 PFU/cell, achieved by dilution in Neurobasal A medium. After a 1-h adsorption period, virus was removed and replaced with complete neuronal medium (without fluorodeoxyuridine and aphidicolin). For infections lasting longer than 15 h, pooled human IgG was added to the medium to inhibit viral spread through the medium after the first productive cycle. IgG was removed for assays of infectious virus and viral reactivation. For immunofluorescent (IF) assays, paraformaldehyde (PFA) was added directly to the medium of the cultured cells after the time periods designated in the figure legends, to a final concentration of 2%, for 5 to 10 min. The fixative was then removed; the cultures were immunostained for the antigens designated in the figure legends; and neurons were counted using a Nikon Microphot fluorescent microscope. For infectious virus assays, 150 μl of the medium was transferred to a cryotube; cells were scraped and suspended in the remaining 150 μl of medium in each well before being transferred to a second cryotube; and the samples were frozen at −80°C. Samples were freeze-thawed to release infectious virus, and titers of the medium and homogenate were determined on Vero cells using a standard plaque assay. All experiments were carried out in parallel with uninfected cultures, which served as negative controls.
Immunofluorescence.
Infected cultures were immunostained with IgM MAb A5 (Developmental Studies Hybridoma Bank), followed by incubation with fluorescein isothiocyanate (FITC)- or rhodamine-labeled anti-IgM secondary antibodies (Santa Cruz). Neurons were initially differentiated from satellite glial cells (SGCs) by anti-NeuN antibody staining (Santa Cruz), and subsequently by morphology. GFP expression by VP26-GFP-expressing viruses was not immunohistochemically amplified. Since neuronal cells are slightly autofluorescent in the GFP spectral range, neurons in infected cultures were compared to neurons in uninfected cultures for determination of the presence of GFP expression. EGFP expression by immediate early (IE), early (E), and late (L) gene reporter viruses was amplified by incubation with a rabbit anti-GFP antibody (Santa Cruz) followed by FITC-labeled anti-rabbit IgG (Santa Cruz). 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining or rabbit IgG against β-galactosidase (β-gal; Abcam) as the primary antibody with FITC-labeled anti-rabbit IgG (Abcam) as the secondary antibody was used to visualize β-galactosidase. Immunolabeled neuronal cultures were evaluated by fluorescence microscopy.
RESULTS
Cultured adult murine trigeminal neurons mimic in vivo neuron characteristics.
In previous studies, we used combined fluorescent in situ hybridization (FISH) for the LAT in conjunction with immunofluorescence (IF) for neuronal markers to demonstrate that HSV-1 preferentially establishes latent infection of murine sensory ganglia in A5-positive neurons while HSV-2 preferentially establishes latent infection in KH10-positive neurons after ocular or footpad infection of mice (21, 28). One mechanism that could account for these findings is differential permissiveness of different types of sensory neurons for productive infection with HSV-1 and HSV-2. To test this hypothesis directly, we developed an in vitro system for studying direct viral infection of dissociated adult murine trigeminal ganglion neurons, thus minimizing the confounding roles of the immune system and the variability of the efficiency of viral delivery to the axons in vivo. Cultures of adult murine trigeminal neurons, generated as described in Materials and Methods, contained an average of 9.3% A5+ neurons and 13.8% KH10+ neurons, proportions similar to those of these neurons in tissue sections of murine trigeminal ganglia (Fig. 1) (21, 28, 52). In the adult neuron cultures, the A5 marker colocalized with immunoreactivity for the calcitonin gene-related peptide (CGRP) but not for staining with the lectin BSL-IB4. In contrast, 98.6% of the KH10-positive neurons costained with the lectin BSL-IB4, and only 3.4% expressed the CGRP (data not shown). These results demonstrate that our in vitro cultures maintain neuronal heterogeneity and some of the well-established in vivo features of adult murine trigeminal neurons that we have reported previously (28).
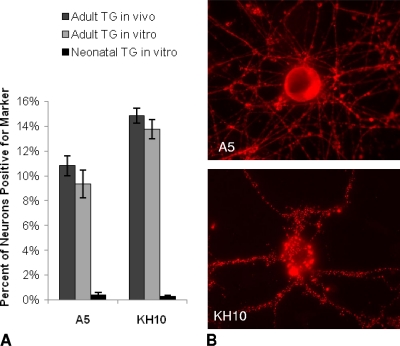
Fig. 1.
A5+ and KH10+ neuron distribution in uninfected neuron cultures. (A) Percentages of A5+ and KH10+ adult murine trigeminal neurons in vivo (tissue sections), dissociated adult trigeminal neurons in cultures in vitro, and dissociated neonatal trigeminal neurons in cultures in vitro. (B) Representative fluorescent microscopy images of cultured adult trigeminal neurons positive for the A5 (top) and KH10 (bottom) markers, revealed by use of monoclonal antibodies and a rhodamine-labeled secondary antibody.
Since neonatal neuron cultures are routinely used by others for studies of HSV infection, we also cultured and evaluated neonatal trigeminal neurons (postnatal day 1) for the A5 and KH10 markers, using the same methods as for the adult trigeminal ganglia. After an equivalent time in vitro, <1% of the cultured neonatal trigeminal ganglion neurons were positive for either the A5 (0.38%) or the KH10 (0.29%) marker (Fig. 1). Cultures of adult and neonatal superior cervical ganglia (SCG) were also negative for both the A5 and KH10 markers under the same conditions (data not shown). This suggests that the A5 and KH10 sensory neuronal markers are largely adult-specific markers.
Adult trigeminal sensory neurons are nonpermissive for productive infection with HSV-1 and HSV-2.
In previous in vivo studies, we observed that many ganglionic neurons that projected to the site of inoculation (eye or footpad) failed to become productively infected. Some neurons simply did not become infected, while others were apparently infected but exhibited little or no expression of productive-cycle viral genes. To determine the percentage of neurons that are productively infected when exposed to virus in vitro, cultured dissociated adult trigeminal neurons were infected with HSV1-VP26-GFP or HSV2-VP26-GFP, both of which express the virion nucleocapsid protein VP26 as a GFP fusion protein and incorporate VP26-GFP into the nucleocapsid during active viral replication and assembly. During late gene expression (10 to 12 h postinfection [hpi]), we observed VP26-GFP localizing to distinct regions of the nucleus in a punctate pattern corresponding to capsid assembly compartments, as previously described (13). Later in infection, VP26-GFP displayed a more diffuse nuclear fluorescence, with the appearance of fluorescence at the cell membrane, correlating with the detection of maturing infectious viral progeny by standard plaque assays. We used the detection of GFP during infection with HSV1-VP26-GFP or HSV2-VP26-GFP as an indicator of productive viral infection.
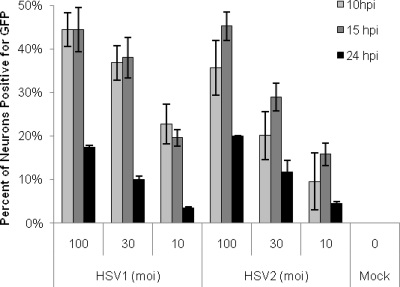
Cultured neurons were found to be relatively resistant to productive infection and GFP expression with both HSV-1 and HSV-2. At an MOI of 10, a maximum of only 22% of neurons in cultures infected with HSV1-VP26-GFP and 16% of neurons in cultures infected with HSV2-VP26-GFP showed evidence of productive infection, as assayed by GFP expression. Even at an MOI of 100, only 45% of all of the neurons in the culture became productively infected with HSV2-VP26-GFP or HSV1-VP26-GFP (Fig. 2). The percentages of neurons productively infected with HSV1-VP26-GFP were similar at 10 and 15 hpi, but HSV2-VP26-GFP demonstrated slightly slower kinetics, peaking at 15 hpi. To verify that these results were indicative of productive HSV infection rather than an idiosyncrasy of our VP26-GFP constructs, we repeated these studies using wild-type HSV-1 (strains KOS and 17+) and HSV-2 (strain 333), followed by detection of expressed productive-cycle viral gene products with polyclonal anti-HSV antisera. Once again, we found that a significant fraction of the neurons failed to be productively infected (data not shown). At an MOI of 10, wild-type HSV-1 was detected in 17 to 32% of cultured neurons and HSV-2 was detected in 17 to 20% of cultured neurons, proportions similar to those observed with the viruses expressing VP26-GFP. Standard plaque assays verified the production of comparable quantities of infectious virus in neuronal cultures following inoculation with either wild-type or HSV VP26-GFP viruses, with infectious virus detected as early as 10 h postinoculation and increasing to reach a peak at 48 h postinoculation. At the peak, mean viral titers were 136,000 PFU/ml for KOS, 161,600 PFU/ml for 17+, 152,200 PFU/ml for HSV1-VP26-GFP, and 140,400 PFU/ml for HSV2-VP26-GFP (data not shown). We thus conclude that a subpopulation of the cultured neurons was unable to support productive replication by either HSV-1 or HSV-2.
Fig. 2.
Percentages of neurons expressing GFP following infection with HSV1-VP26-GFP or HSV2-VP26-GFP. Neuronal cultures were infected with HSV1-VP26-GFP or HSV2-VP26-GFP (at an MOI of 100, 30, or 10) for 10, 15, or 24 h and were assayed for GFP expression as a marker of productive infection. Both viral constructs express a VP26-GFP fusion protein during active viral assembly, permitting GFP visualization during productive infection. Results reflect data from six separate experiments for each virus for the 10- and 15-h time points and from two experiments for each virus for the 24-h time point. For each time point, 2,636 to 12,027 neurons were assayed.
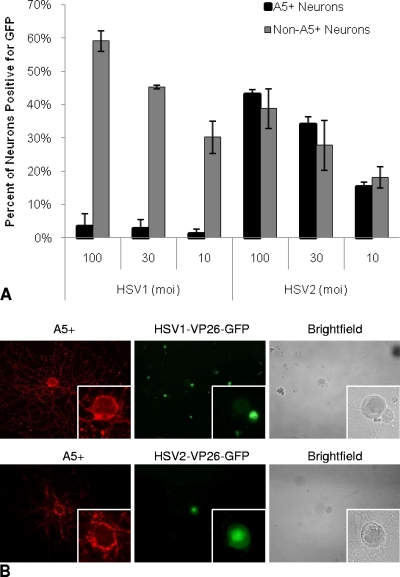
Based on our previous in vivo findings that HSV-1 preferentially established latent infection and expressed the LAT in A5+ neurons, we hypothesized that A5+ neurons in vitro would be relatively nonpermissive for productive infection with HSV-1, compared to the other neurons in the ganglion. To test this hypothesis, we infected neuronal cultures with HSV1-VP26-GFP and evaluated GFP expression in neurons that were colabeled with MAb A5 (Fig. 3). Even at an MOI of 100, <4% of A5+ neurons were positive for productive infection with HSV1-VP26-GFP. At an MOI of 10, only about 1% of A5+ neurons scored positive for productive viral infection. In comparison, relatively large percentages of non-A5+ neurons became productively infected under the same conditions (56.1%, 45.9%, and 25.4% of non-A5+ neurons at MOI of 100, 30, and 10, respectively) (Fig. 3A). Furthermore, productively infected non-A5+ neurons were frequently found immediately adjacent to A5+ neurons in which there was no evidence of VP26-GFP expression, despite a high multiplicity of infection (Fig. 3B). Similar results were observed when we infected cultures with wild-type HSV-1 (KOS and 17+) and visualized productive infection by assaying for HSV antigen expression with polyclonal antisera (data not shown). Although it is possible that some A5+ neurons may undergo a low level of productive HSV-1 viral infection, below the threshold of our assays, productive infection was not detected either by GFP expression in cultures infected with HSV1-VP26-GFP or by viral antigen expression in cultures infected with wild-type viruses. When we infected adult neuronal cultures with HSV2-VP26-GFP, in contrast to our observations with HSV-1, HSV2-VP26-GFP productively infected A5+ and non-A5+ neurons to similar extents (Fig. 3A), and GFP expression was frequently found in A5+ neurons (Fig. 3B). This result indicates that the nonpermissiveness of A5+ neurons for productive infection is specific for HSV-1 and does not apply to HSV in general.
Fig. 3.
(A) Percentages of A5+ and non-A5+ neurons expressing GFP following infection with HSV1-VP26-GFP or HSV2-VP26-GFP. Neuronal cultures were infected with HSV1-VP26-GFP or HSV2-VP26-GFP. A5+ and non-A5+ neurons were evaluated for GFP expression as a marker of productive infection at 10 h postinfection. Results reflect data from two separate experiments for each MOI. A minimum of 3,520 total neurons were assayed for each MOI. (B) Fluorescent microscope images of A5+ neurons (red) with HSV1-VP26-GFP (top) or HSV2-VP26-GFP (bottom) productive infection (green). (Insets) Higher-magnification images of A5+ neurons. HSV1-VP26-GFP productive infection, indicated by GFP expression, was frequently found in non-A5+ neurons adjacent to nonproductively infected A5+ neurons, while HSV2-VP26-GFP was found in A5+ neurons as well as in non-A5+ neurons.
The nonpermissiveness of A5+ neurons for productive HSV-1 infection is not due to preferential viral entry.
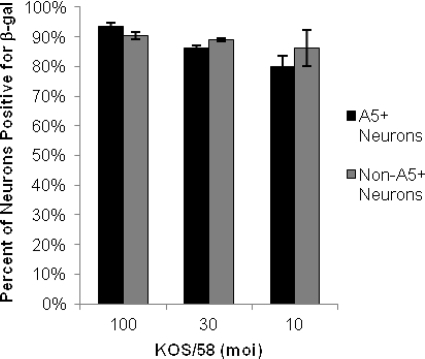
To determine if the absence of productive HSV-1 infection in A5+ neurons was due to a failure of HSV-1 to enter these neurons, we assayed neuronal cultures that had been infected with KOS/58, which expresses β-galactosidase (β-gal) under the control of the neurofilament light (NFL) promoter at the gC locus (29). We predicted that this virus should express β-gal in all infected neurons, whether productively or quiescently infected, since the neurofilament light promoter is constitutively expressed in all sensory neurons. By use of dual immunofluorescence for β-gal and the A5 neuronal marker, β-gal expression was detected in 86 to 90% of all cultured neurons following infection with KOS/58 at MOI of 10, 30, and 100 (Fig. 4). Furthermore, β-gal expression was detected equivalently in A5+ and non-A5+ neurons, regardless of the MOI of the inoculum. These results indicate that the limited productive infection in A5+ neurons that we observed with HSV1-VP26-GFP or wild-type HSV-1 strains KOS or 17+ was a consequence of the relatively nonpermissive nature of these neurons for productive infection rather than of restricted viral entry and DNA release.
Fig. 4.
Percentage of neurons expressing β-galactosidase following infection with KOS/58. Expression of β-gal, driven by the neurofilament promoter at the HSV-1 gC locus, was detected in A5+ and non-A5+ neurons by immunofluorescence with MAb A5 and an anti-β-gal polyclonal antibody. Experiments were carried out in duplicate, and a minimum of 3,726 neurons were assayed for each MOI.
Regulation of permissiveness for productive infection occurs at or before the level of IE gene expression.
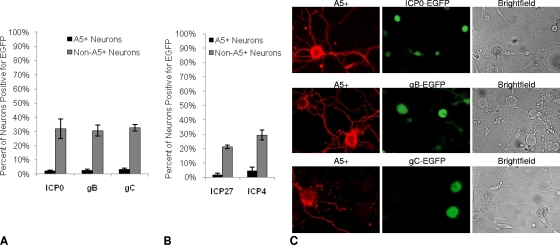
Productive-cycle gene expression during HSV infection occurs in a characteristic temporal cascade. Upon entry, viral tegument protein VP16 forms a complex with cellular Oct-1 and host cell factor 1 (HCF-1) to initiate the expression of immediate early (IE) genes and the production of IE proteins, which then sequentially initiate early gene expression, DNA replication, and late gene expression and viral assembly. To determine the level at which productive HSV-1 infection was blocked in A5+ neurons, we examined the expression of productive-cycle viral genes using several HSV-1 recombinants that express the fluorescent reporter protein EGFP under the control of IE, early (E), and late (L) gene promoters inserted at the gC locus of HSV-1. At an MOI of 30, the peak IE, E, and L gene promoter activities occurred at 6, 8, and 10 hpi, respectively. Immediate early gene promoter activity was assessed using the reporter virus RE-pICP0-EGFP (11), which expresses EGFP under the control of the ICP0 promoter; early gene promoter activity was assessed using the RE-pgB-EGFP virus, expressing EGFP under the control of the gB promoter, in a manner similar to that described previously (37); and late gene promoter activity was assessed using the reporter virus RE-pgC-EGFP, which expresses EGFP under the control of the gC promoter (11). As summarized in Fig. 5A, we found ICP0 promoter activity (as reported by EGFP expression) in 31.8% of the non-A5+ neurons but only 2.1% of the A5+ neurons. Likewise, early gene promoter activity was found in 30.6% of the non-A5+ neurons and 2.4% of the A5+ neurons, and late gene promoter activity was found in 32.8% of the non-A5+ neurons and 3.2% of the A5+ neurons. These data strongly suggest that the block to productive HSV-1 infection in A5+ neurons occurs at or before the level of viral IE gene expression.
Fig. 5.
Viral gene expression in A5+ and non-A5+ neurons. Neuronal cultures were infected at an MOI of 30 with viral constructs expressing EGFP under the control of immediate early (ICP0), early (gB), and late (gC) gene promoters, inserted at the gC locus. A5+ and non-A5+ neurons were evaluated for EGFP expression. (A) EGFP expression was determined for RE-pICP0-EGFP at 6 h, for RE-pgB-EGFP at 8 h, and for RE-pgC-EGFP at 10 h. Results reflect data from five separate experiments with ∼4,100 to 9,300 neurons assayed for each virus studied. (B) EGFP expression was determined for RE-pICP27-EGFP and RE-pICP4-EGFP at 6 h. Results reflect data from three separate experiments with ∼4,100 to 4,200 neurons assayed for each virus studied. (C) Fluorescent microscope images of A5+ neurons (red) with EGFP expression (green) after infection with RE-pICP0-EGFP (top), RE-pgB-EGFP (center), or RE-pgC-EGFP (bottom). Corresponding bright-field images are shown on the right.
To determine whether the expression of immediate early gene promoters other than ICP0 was similarly restricted in A5+ neurons, we also evaluated the expression of the ICP27 and ICP4 promoters in HSV-1 constructs that were made similarly to RE-pICP0-EGFP, but in which the ICP27 and ICP4 promoters were used to express EGFP at the gC locus of HSV-1 strain RE. As summarized in Fig. 5B, ICP27 promoter activity was seen in 21.3% of the non-A5+ neurons but only in 1.5% of A5+ neurons, and ICP4 promoter activity was found in 29.4% of non-A5+ and 4.5% of A5+ neurons. Figure 5C illustrates the typical appearance of dually stained neurons in these studies. We conclude that A5-positive neurons are highly refractory to HSV-1 gene expression.
LAT promoter activity is present in neurons quiescently infected with HSV in vitro, and quiescent HSV can be reactivated.
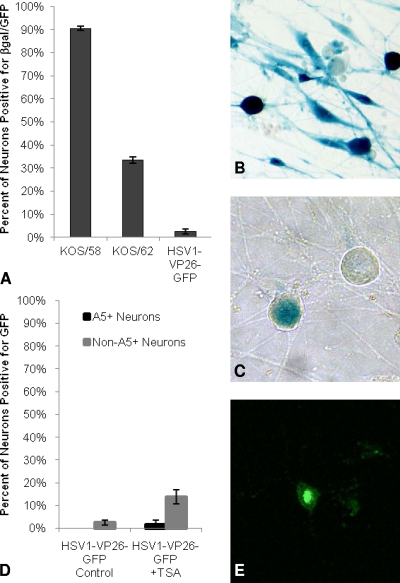
The absence of lytic gene expression, the expression of the LAT, and the ability to reactivate from a quiescent state are the hallmarks of latent HSV infection. We next sought to determine whether these were features of quiescent HSV-1 infection of adult sensory neurons in vitro. Adult trigeminal ganglion neuronal cultures were infected with KOS/58, KOS/62, or HSV1-VP26-GFP (Fig. 6). After infection with HSV1-VP26-GFP, GFP expression and infectious virus levels increased for 48 h and then decreased steadily until day 5, at which time no infectious virus could be detected in the medium and minimal GFP expression was observed in the cultures. These observations are consistent with a recent report showing that HSV lytic activity is essentially complete before day 5 postinfection in cultured porcine trigeminal neurons (12). Five days after infection with KOS/58, β-gal expression from the neurofilament light promoter was detected in 90.5% of the cultured neurons, demonstrating that nearly all of the surviving neurons were infected with HSV-1 (Fig. 6A and B). However, no infectious KOS/58 virus was detected by a plaque assay in the medium at this time point, and only a mean of 75 PFU was detected in the cell homogenate from each culture well. We detected LAT promoter activity in 33.4% of the surviving cultured neurons 5 days following infection with KOS/62, as revealed by β-gal expression under the control of the LAT promoter at the LAT locus (Fig. 6A and B). These findings correlate well with reports of in vivo latency where LAT expression can be detected in about one-third of latently infected neurons (8, 30). Five days after infection with HSV1-VP26-GFP, we observed GFP from the VP26-GFP fusion protein in only 2.5% of surviving neurons, demonstrating that the vast majority of infected neurons had no evidence of productive infection at that time point, as assessed by GFP expression (Fig. 6A). Furthermore, when VP26-GFP expression was observed, it occurred in discrete focal plaques (with an average of 1 to 2 plaques per culture well of approximately 2,000 neurons), involving one or more neurons and suggestive of focal spontaneous reactivation with local spread. Thus, at 5 days postinfection, the vast majority of cultured adult trigeminal ganglion neurons were quiescently infected with HSV-1, and approximately one-third of the infected neurons demonstrated LAT promoter activity. However, it should be noted that the level of LAT reporter activity in KOS/62 may underestimate the abundant accumulation of the LAT in wild-type HSV-1, since there is partial disruption of the LAT enhancer in KOS/62 (27).
Fig. 6.
Viral gene expression from KOS/58, KOS/62, and HSV1-VP26-GFP. (A) Neuronal cultures were infected with either KOS/58, KOS/62, or HSV1-VP26-GFP (at an MOI of 10) and were evaluated for β-gal or GFP expression 5 days postinoculation. (B) β-gal-positive neurons (blue) 5 days after infection with KOS/58 (β-gal from the neurofilament light promoter). (C) β-gal-positive neurons (blue) 5 days after infection with KOS/62 (β-gal from the LAT promoter). (D) Five days after infection with HSV1-VP26-GFP (at an MOI of 10), cultures were treated with TSA or with a control medium containing no TSA; 24 h later, A5+ and non-A5+ neurons were evaluated for GFP expression. (E) GFP expression in a cultured neuron 24 h after TSA treatment of a culture quiescently infected with HSV1-VP26-GFP. Results reflect data collected from two or more separate experiments with a minimum of 1,503 total neurons assayed for each virus.
To test for the competence of HSV reactivation from quiescently infected neurons in vitro, we infected cultures with HSV1-VP26-GFP, and 5 days later, cultures that showed little or no GFP expression were treated with trichostatin A (TSA), a histone deacetylase inhibitor that has been used previously to reactivate quiescent virus in differentiated PC12 cells (10, 31). TSA has also been shown to activate viral gene expression from quiescent viral genomes in cultured neonatal dorsal root ganglia (DRG) (1). As shown in Fig. 6D, we detected GFP activity from HSV1-VP26-GFP in 2.5% of all neurons in control cultures 24 h after treatment with fresh medium that did not contain TSA on day 5 postinoculation. No VP26-GFP expression was detected in A5-positive neurons in these control cultures. In contrast, in cultures that received fresh medium containing TSA, GFP expression was observed in 13.6% of total neurons 24 h after treatment. However, GFP expression was detected primarily in non-A5+ neurons after TSA treatment (Fig. 6D), suggesting that the A5+ neurons are less permissive for reactivation than the non-A5+ neurons, a notion that correlates with our observation that A5+ neurons are less permissive for productive infection during the initial infection with HSV-1. Figure 6E illustrates a typical VP26-GFP-positive neuron after TSA treatment, displaying strong nuclear GFP expression. To verify that we were observing productive infection rather than just VP26 gene activation after TSA treatment, neuronal cultures quiescently infected with HSV1-VP26-GFP at an MOI of 10 were treated with TSA 5 days after infection, and virus production was assessed 24 h later. We found a mean viral titer of 1,560 PFU/well in homogenates of TSA-treated cultures compared to a mean viral titer of 84 PFU/well from control cultures. Similar results were observed following TSA treatment of neuronal cultures quiescently infected with wild-type HSV-1 strain 17+. In these cultures, we observed a mean increase of 1,062 PFU/well in the level of infectious virus over that in controls that did not receive TSA. Therefore, treatment with TSA induced quiescent virus to reactivate and produce viral progeny. In total, these findings strongly suggest that neurons labeled with the A5 marker are highly refractory to HSV-1 lytic cycle gene expression and productive infection, both during initial infection and during reactivation.
DISCUSSION
In previous studies, we demonstrated that HSV-1 preferentially establishes latency in neurons recognized by MAb A5, while HSV-2 preferentially establishes latent infection in neurons recognized by MAb KH10 (21, 28). In the studies described in the present work, we report that dissociated adult trigeminal neurons are relatively nonpermissive for productive infection with HSV. At an MOI of 10, only about 20% of the cultured neurons became productively infected; even at an MOI of 100, only 45% of the cultured neurons supported productive viral infection. Data from studies with KOS/58 indicate that this was not a consequence of limited viral entry or uncoating of the genome. Furthermore, we show that neuronal subtypes were not equally permissive for productive infection; when we specifically evaluated the ability of A5+ neurons to support productive infection, <5% were permissive at an MOI of 100, an inoculum presumably much greater than biologically relevant levels. Forty percent of the non-A5+ population also failed to support productive HSV-1 infection at this high MOI, indicating that other neuronal populations are also refractory to productive infection. These results correlate well with our previous in vivo reports indicating that HSV-1 preferentially establishes latent infection in A5+ neurons, with approximately 50% of HSV-1 latent sites located in A5+ neurons, although only about 11% of the neurons in the trigeminal ganglion are A5+ (52). This would suggest that HSV-1 enters a quiescent or latent state in A5+ neurons because these neurons cannot support productive HSV-1 infection. In contrast, our work demonstrates that A5+ neurons and non-A5+ neurons support productive HSV-2 infection equivalently, suggesting that the restriction by A5+ neurons for productive infection is specific for HSV-1. This result also correlates with our previous finding that latent HSV-2 infection is found infrequently in A5+ neurons (28). Thus, productive infections with HSV-1 and HSV-2 are regulated differently in different types of neurons in the trigeminal ganglion.
The neuronal composition of the trigeminal and dorsal root ganglia is heterogeneous, and these ganglia undergo significant change during embryogenesis, as well during the first several weeks of life. During embryonic development, approximately 80% of rodent ganglionic neurons require NGF for survival. Postnatally, a population of small neurons that is selectively labeled by the lectin IB4 and is identical to the KH10+ population stops expressing TrkA (the high-affinity NGF receptor) and starts expressing Ret and GDNF receptor alpha (GFRα), switching their dependence from NGF to GDNF (3, 33). This postnatal reduction in the percentage of TrkA+ neurons occurs gradually over 3 weeks (postnatal day 1 [P1] to P21), coinciding with the critical period during which alteration of neurotrophins can permanently alter the physiology of neonatal dorsal root and trigeminal ganglion neurons (19, 25). Downregulation of TrkA by IB4-binding neurons results in differences in expression profiles and signaling cascades in response to certain classes of stimuli (33, 39, 53). Between P1 and P6, the neuronal expression of somatostatin, FRAP, P2X3, and oligosaccharide conjugates (which include lactoseries carbohydrates recognized by MAbs A5 and KH10) changes dramatically in response to GDNF (18, 48). These differences are significant because A5+ neurons, which are nonpermissive for productive HSV-1 infection in vitro and maintain the latent HSV reservoir in vivo, are mostly TrkA positive, while the IB4-labeled, KH10+ neurons, which support productive infection with HSV-1, express the GDNF receptor. Neonatal and adult sensory neurons also differ in their responses to injury. IB4-binding neurons are selectively vulnerable to neonatal axotomy (49), and adult neurons are more resistant than neonatal neurons to apoptotic stimuli, including phosphatidylinositol 3-kinase (PI3K) inhibition and NGF withdrawal (47). Finally, although adult ganglionic neurons differ in size (both in vitro and in vivo), cultured neonatal neurons are homogeneous in size (19). Thus, neonatal cultures may lack specific neuronal populations as well as important regulatory signaling cascades and functional properties critical to the regulation of HSV infection in the fully developed nervous system.
During productive infection, HSV undergoes tightly regulated temporal expression of productive-cycle genes. Assuming that acute infection of neuronal cultures follows a similar cycle, our work strongly suggests that the block to productive HSV-1 infection in A5-positive neurons occurs at or before the level of viral IE gene expression. It is clear that many cellular factors regulate the expression of HSV-1 immediate early genes, including Oct-1, Oct-2, and HCF, and it is likely that specific cellular factors present in the biochemically distinct neuronal populations of the sensory ganglia play a role in initiating or repressing HSV immediate early genes, which in turn regulate productive versus latent infection within specific neuronal cell types. Mechanistically, the most widely accepted view of latency establishment is a failure of IE gene activation (16, 17, 22), which we observed in the A5+ neurons in the TG cultures after infection with HSV-1. Chromatin modulation, regulatory microRNAs, and cellular localization of specific neuronal factors are proposed mechanisms that could differentially regulate IE gene transcription in different types of sensory neurons (7, 23). Chromatin structure modulation plays an important role in transcriptional regulation in neurons (36, 38), and proposed mechanisms of HSV chromatin modulation include host cell factor 1 (HCF-1)-mediated recruitment of lysine-specific demethylase 1 (LSD-1) to viral immediate early promoters (26, 34) and HSV IE promoter repression by Nab2 (42), early growth response gene 1 (Egr-1) (2), or the repressor element 1 silencing transcription factor (REST)/neuronal restrictive silencer factor (NRSF)/corepressor for element 1 silencing transcription factor (CoREST) complex (15, 35). MicroRNAs also play an important role in the transcriptional regulation of neurons (24, 44) and have been proposed to regulate productive HSV infection by silencing the expression of ICP0 (43, 45) and/or ICP4 (40, 45).
In the current study, we also demonstrated that HSV-1 established a quiescent infection with LAT expression in cultured adult murine trigeminal ganglion neurons and that the quiescent infection, like latent infection in vivo, represented a reactivation-competent state. Of interest, only about one-third of the quiescently infected neurons had detectable expression from the viral LAT promoter. These data are consistent with those of previously reported studies in which approximately one-third of the infected cells expressed the LAT at latent time points (8, 30). We have shown previously that the LAT region, specifically exon 1, appears to regulate the neuron type-specific establishment of latency (5), and it is well known that a number of different host cell factors, including Oct-1, HCF-1, Sp1, CREB, GRB-2, EGR-1, AP2, and AP1, regulate gene expression from this region of the viral genome (reviewed by Millhouse and Wigdahl in 2000 [32]). We were able to reactivate HSV-1 from its quiescent state by treating the cultures with TSA, a histone deacetylase inhibitor. However, HSV-1 was not reactivated equivalently from all types of neurons in response to the TSA treatment. A5+ neurons, which were nonpermissive for productive infection at earlier time points, were also largely nonpermissive for reactivation induced by the histone deacetylase inhibitor, indicating that either A5+ neurons may be completely nonpermissive for HSV-1 replication and reactivation in general or mechanisms other than those involving histone deacetylases are responsible for inducing reactivation in A5+ neurons. Our studies did not, however, address the possibility that the reactivating cell population derives from a proportion of neurons that survived initial lytic infection, and they did not determine whether this population initially established quiescence immediately upon infection. Since the LAT is not expressed equally in all latently infected neurons and the virus is not reactivated equally from all neuronal populations, dissociated adult trigeminal neurons that maintain neuronal heterogeneity in culture will likely be useful for determining the specific mechanisms regulating LAT expression and the role it plays in the establishment of latency and reactivation from a latent state.
Although previous in vitro models of HSV neuronal infection have been valuable for studying several aspects of HSV pathogenesis, most are limited or restricted in their use for investigating the mechanisms regulating the preferential establishment of latency in different neuronal subtypes. We believe that our in vitro model of HSV infection detailed here using dissociated adult murine trigeminal neuron cultures is superior in many respects. These cultures maintain neuronal heterogeneity with proportions of A5+ and KH10+ neurons nearly identical to those found in vivo. Furthermore, HSV infection of these neuronal cultures leads to a heterogeneous outcome, with productive infection in some neurons and quiescent infection in others, without the use of acyclovir to suppress lytic growth. Thus, the system that we have developed models a number of in vivo characteristics of HSV infection not modeled by PC12 cells, sympathetic neurons, or embryonic/neonatal sensory neurons. However, the neuronal culture system we have described for studying HSV infection is not without its limitations. First, we were unable to maintain quiescently infected neurons for extended periods at higher MOI, as has been reported for embryonic cultures (50, 51) or differentiated PC12 cells (9). However, in contrast to these prior studies, we did not attempt to use acyclovir to induce or maintain a quiescent state. Second, by 5 days postinoculation, the vast majority of infected neurons no longer expressed productive-cycle genes, a finding similar to those in previous reports of in vitro infection of neonatal DRG and TG (1, 12). However, we found substantial well-to-well variability, and GFP-positive plaque-like formations occurred periodically after day 5 postinoculation. The relatively low frequency, and clustering, of GFP-positive neurons at these later time points in cultures previously negative for GFP expression likely represents spontaneous-reactivation events, similar to those that occur in vivo. These spontaneous reactivations appeared to originate from an individual GFP-positive neuron and to spread to adjacent neurons, with the spread presumably limited by the pooled immunoglobulin in the culture medium. Third, we observed that satellite glial cells (SGCs) became infected early and were transformed into phagocytic cells, significantly reducing neuronal cell survival in cultures infected at high MOI. While SGCs are not the focus of this report, these observations suggest that SGCs play an important role in clearing virus in the ganglia but may also exacerbate neuronal damage in response to viral infection, consistent with previous reports (20, 46). However, resident SGCs could not be removed from the cultures entirely without jeopardizing the health of the neurons.
In summary, we have shown that in cultured dissociated adult sensory neurons, A5+ neurons are relatively nonpermissive for productive infection with HSV-1, a finding that correlates with the selective establishment of HSV-1 latency in A5+ neurons in vivo. Using the model culture system described above, we are now poised to efficiently dissect the mechanisms that regulate preferential productive or latent infection in specific types of neurons.
ACKNOWLEDGMENTS
The K26-GFP plasmid was a kind gift from Prashant Desai, Johns Hopkins University. Monoclonal antibodies FE-A5, developed by B. Fenderson, and KH10, developed by T. M. Jessell and J. Dodd, were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa Department of Biological Sciences.
This work was supported by the Littlefield Trust, That Man May See, the Ralph and Sophie Heintz Laboratory Fund, NIH grants EY02162, EY015291 (to P.R.K.), and EY08098 (to P.R.K.), and unrestricted funds from the Eye & Ear Foundation of Pittsburgh and Research to Prevent Blindness Inc.
The authors have no competing financial interest in the research presented.
Footnotes
Published ahead of print on 20 April 2011.
REFERENCES
- 1. Arthur J. L., et al. 2001. Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro. J. Virol. 75:3885–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bedadala G. R., Pinnoji R. C., Hsia S. C. 2007. Early growth response gene 1 (Egr-1) regulates HSV-1 ICP4 and ICP22 gene expression. Cell Res. 17:546–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett D. L., Averill S., Clary D. O., Priestley J. V., McMahon S. B. 1996. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur. J. Neurosci. 8:2204–2208 [DOI] [PubMed] [Google Scholar]
- 4. Bennett D. L., et al. 1998. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 18:3059–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertke A. S., et al. 2009. Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J. Virol. 83:10007–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertke A. S., Patel A., Krause P. R. 2007. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J. Virol. 81:6605–6613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloom D. C., Giordani N. V., Kwiatkowski D. L. Epigenetic regulation of latent HSV-1 gene expression. Biochim. Biophys. Acta 1799:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X. P., Mata M., Kelley M., Glorioso J. C., Fink D. J. 2002. The relationship of herpes simplex virus latency associated transcript expression to genome copy number: a quantitative study using laser capture microdissection. J. Neurovirol. 8:204–210 [DOI] [PubMed] [Google Scholar]
- 9. Danaher R. J., Jacob R. J., Miller C. S. 1999. Establishment of a quiescent herpes simplex virus type 1 infection in neurally-differentiated PC12 cells. J. Neurovirol. 5:258–267 [DOI] [PubMed] [Google Scholar]
- 10. Danaher R. J., et al. 2005. Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript-independent manner in neuronal cells. J. Neurovirol. 11:306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Decman V., Kinchington P. R., Harvey S. A., Hendricks R. L. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Regge N., Van Opdenbosch N., Nauwynck H. J., Efstathiou S., Favoreel H. W. 2010. Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro. PLoS One 5:e13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desai P., Person S. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563–7568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodd J., Jessell T. M. 1985. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J. Neurosci. 5:3278–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du T., Zhou G., Khan S., Gu H., Roizman B. 2010. Disruption of HDAC/CoREST/REST repressor by dnREST reduces genome silencing and increases virulence of herpes simplex virus. Proc. Natl. Acad. Sci. U. S. A. 107:15904–15909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Efstathiou S., Preston C. M. 2005. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 111:108–119 [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Blanco M. A., Cullen B. R. 1991. Molecular basis of latency in pathogenic human viruses. Science 254:815–820 [DOI] [PubMed] [Google Scholar]
- 18. Groves M. J., Martinian L., An S. F., Scaravilli F. 1999. Expression of three oligosaccharide conjugates by neonatal rat dorsal root ganglion neurons: comparison with CGRP and GAP43 immunoreactivity. J. Anat. 195(Pt 2):271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall A. K., et al. 1997. The generation of neuronal heterogeneity in a rat sensory ganglion. J. Neurosci. 17:2775–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanani M. 2005. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Brain Res. Rev. 48:457–476 [DOI] [PubMed] [Google Scholar]
- 21. Imai Y., Apakupakul K., Krause P. R., Halford W. P., Margolis T. P. 2009. Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia. J. Virol. 83:7873–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knipe D. M., Cliffe A. 2008. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 6:211–221 [DOI] [PubMed] [Google Scholar]
- 23. Kristie T. M., Liang Y., Vogel J. L. 2010. Control of alpha-herpesvirus IE gene expression by HCF-1 coupled chromatin modification activities. Biochim. Biophys. Acta 1799:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lau P., Hudson L. D. 2010. MicroRNAs in neural cell differentiation. Brain Res. 1338:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewin G. R., Ritter A. M., Mendell L. M. 1992. On the role of nerve growth factor in the development of myelinated nociceptors. J. Neurosci. 12:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang Y., Vogel J. L., Narayanan A., Peng H., Kristie T. M. 2009. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat. Med. 15:1312–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margolis T. P., Bloom D. C., Dobson A. T., Feldman L. T., Stevens J. G. 1993. Decreased reporter gene expression during latent infection with HSV LAT promoter constructs. Virology 197:585–592 [DOI] [PubMed] [Google Scholar]
- 28. Margolis T. P., Imai Y., Yang L., Vallas V., Krause P. R. 2007. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J. Virol. 81:1872–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Margolis T. P., Sedarati F., Dobson A. T., Feldman L. T., Stevens J. G. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150–160 [DOI] [PubMed] [Google Scholar]
- 30. Mehta A., et al. 1995. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology 206:633–640 [DOI] [PubMed] [Google Scholar]
- 31. Miller C. S., Danaher R. J., Jacob R. J. 2006. ICP0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells. J. Virol. 80:3360–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Millhouse S., Wigdahl B. 2000. Molecular circuitry regulating herpes simplex virus type 1 latency in neurons. J. Neurovirol. 6:6–24 [DOI] [PubMed] [Google Scholar]
- 33. Molliver D. C., et al. 1997. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19:849–861 [DOI] [PubMed] [Google Scholar]
- 34. Peng H., Nogueira M. L., Vogel J. L., Kristie T. M. 2010. Transcriptional coactivator HCF-1 couples the histone chaperone Asf1b to HSV-1 DNA replication components. Proc. Natl. Acad. Sci. U. S. A. 107:2461–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinnoji R. C., et al. 2007. Repressor element-1 silencing transcription factor/neuronal restrictive silencer factor (REST/NRSF) can regulate HSV-1 immediate-early transcription via histone modification. Virol. J. 4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qureshi I. A., Mehler M. F. 2010. Impact of nuclear organization and dynamics on epigenetic regulation in the central nervous system: implications for neurological disease states. Ann. N. Y. Acad. Sci. 1204(Suppl.):E20–E37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramachandran S., Knickelbein J. E., Ferko C., Hendricks R. L., Kinchington P. R. 2008. Development and pathogenic evaluation of recombinant herpes simplex virus type 1 expressing two fluorescent reporter genes from different lytic promoters. Virology 378:254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riccio A. 2010. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat. Neurosci. 13:1330–1337 [DOI] [PubMed] [Google Scholar]
- 39. Salvarezza S. B., Lopez H. S., Masco D. H. 2003. The same cellular signaling pathways mediate survival in sensory neurons that switch their trophic requirements during development. J. Neurochem. 85:1347–1358 [DOI] [PubMed] [Google Scholar]
- 40. Shen W., et al. 2009. Two small RNAs encoded within the first 1.5 kilobases of the herpes simplex virus type 1 latency-associated transcript can inhibit productive infection and cooperate to inhibit apoptosis. J. Virol. 83:9131–9139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silverman J. D., Kruger L. 1990. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J. Neurocytol. 19:789–801 [DOI] [PubMed] [Google Scholar]
- 42. Srinivasan R., Mager G. M., Ward R. M., Mayer J., Svaren J. 2006. NAB2 represses transcription by interacting with the CHD4 subunit of the nucleosome remodeling and deacetylase (NuRD) complex. J. Biol. Chem. 281:15129–15137 [DOI] [PubMed] [Google Scholar]
- 43. Tang S., Patel A., Krause P. R. 2009. Novel less-abundant viral microRNAs encoded by herpes simplex virus 2 latency-associated transcript and their roles in regulating ICP34.5 and ICP0 mRNAs. J. Virol. 83:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trivedi S., Ramakrishna G. 2009. miRNA and neurons. Int. J. Neurosci. 119:1995–2016 [DOI] [PubMed] [Google Scholar]
- 45. Umbach J. L., et al. 2008. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Velzen M., et al. 2009. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J. Immunol. 183:2456–2461 [DOI] [PubMed] [Google Scholar]
- 47. Walsh G. S., Orike N., Kaplan D. R., Miller F. D. 2004. The invulnerability of adult neurons: a critical role for p73. J. Neurosci. 24:9638–9647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang R., et al. 2003. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience 121:815–824 [DOI] [PubMed] [Google Scholar]
- 49. White F. A., et al. 1990. Neonatal infraorbital nerve transection in the rat: comparison of effects on substance P immunoreactive primary afferents and those recognized by the lectin Bandierea simplicifolia-I. J. Comp. Neurol. 300:249–262 [DOI] [PubMed] [Google Scholar]
- 50. Wilcox C. L., Johnson E. M., Jr 1987. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J. Virol. 61:2311–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilcox C. L., Smith R. L., Freed C. R., Johnson E. M., Jr 1990. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J. Neurosci. 10:1268–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang L., Voytek C. C., Margolis T. P. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 74:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu W., Galoyan S. M., Petruska J. C., Oxford G. S., Mendell L. M. 2004. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J. Neurophysiol. 92:3148–3152 [DOI] [PubMed] [Google Scholar]
- 54. Zwick M., et al. 2002. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J. Neurosci. 22:4057–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]