Abstract
NF-κB plays a key role in innate and acquired immunity. Its activity is regulated through intricate signaling networks. Persistent or excessive activation of NF-κB induces diseases, such as autoimmune disorders and malignant neoplasms. Infection by human T cell leukemia virus type 1 (HTLV-1) causes a fatal hematopoietic malignancy termed adult T cell leukemia (ATL). The HTLV-1 viral oncoprotein Tax functions pivotally in leukemogenesis through its potent activation of NF-κB. Recent findings suggest that protein ubiquitination is crucial for proper regulation of NF-κB signaling and for Tax activity. Here, we report that ubiquitin-specific peptidase USP20 deubiquitinates TRAF6 and Tax and suppresses interleukin 1β (IL-1β)- and Tax-induced NF-κB activation. Our results point to USP20 as a key negative regulator of Tax-induced NF-κB signaling.
INTRODUCTION
Protein ubiquitination is an essential posttranslational modification that is implicated in many biological processes (14, 52). Ubiquitin is a small protein composed of 76 amino acids. It contains 7 lysine residues (K6, K11, K27, K29, K33, K48, and K63). Multiple ubiquitin monomers can become covalently linked, and polyubiquitin molecules linked through the lysine 48 residue (K48) are known to modulate protein degradation. In contrast, polyubiquitin molecules linked through the lysine 63 (K63) residue do not induce degradation but influence protein localization, protein-protein interaction, protein functional activation, and other activities (14, 44). The addition of ubiquitin to or the removal of ubiquitin from protein substrates can reversibly and dynamically change protein functions, and these reactions are executed by ubiquitin ligases and deubiquitinases (44). Accordingly, the ubiquitination process is mediated by the serial actions of E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligase (14, 52), and deubiquitination is through deubiquitining enzymes (DUBs) that directly remove ubiquitin molecules from their substrates (32). Based on sequence analyses, approximately 90 DUB genes have been identified in the human genome. These DUBs are divided into 5 subclasses according to their protein sequences: the ubiquitin C-terminal hydrolases (UCHs), ubiquitin-specific proteases (USPs), Machado-Joseph disease protein domain proteases (MJDs), ovarian tumor proteases (OTUs), and JAMM motif proteases (32). The specificities and actions of these various deubiquitinases remain to be fully characterized.
In mammals, NF-κB signaling plays key roles in inflammation, cell proliferation, and apoptosis (36). Like phosphorylation, ubiquitination is an important posttranslational modification that can regulate NF-κB activity (43). For example, the ubiquitination of a regulatory subunit in the IKK complex, IKKγ which is also known as NEMO, has a central role in signal transduction. It has been shown that K63-linked linear conjugation of ubiquitin to IKKγ positively regulates NF-κB signaling (47, 53). In addition, the ubiquitination of tumor necrosis factor receptor-associated factors (TRAFs) is also important for IKK activation. TRAF6 is an E3 ubiquitin ligase, and it performs self-ubiquitination through K63-linked chains upon cellular activation through Toll-like receptors (TLRs) and cytokine receptors (8). Moreover, in the canonical NF-κB pathway, IκBα, which sequesters NF-κB proteins in the cytoplasm in an inactive state, is conjugated with K48-linked polyubiquitin chains and is proteasomally degraded when cells are stimulated to activate NF-κB (4). Similarly, cellular activation induces the ubiquitination and proteosomal processing of NF-κB2 p100 to p52, allowing NF-κB RelB/p52 dimers to translocate into the nucleus.
The role of DUBs in the NF-κB pathway has also been studied. For example, the familial cylindromatosis tumor suppressor CYLD is one of the DUBs that have been found to suppress NF-κB activity. CYLD has been shown to bind IKKγ and to reduce the ubiquitination of TRAF2, TRAF6, and IKKγ (3, 24, 48). A20 is a second well-studied DUB that negatively regulates NF-κB activation by reducing the ubiquitination of TRAF2, TRAF6, and RIP1 (17, 20, 41). A20 has dual activities in ubiquitination and deubiquitination. Hence, A20 with TAX1BP1 as a cofactor promotes the cleavage of K63-linked polyubiquitin chains on RIP1, and A20 with E3 ligase Itch can conjugate K48-linked chains on RIP for proteosomal degradation (39, 51). Recently, it was also found that A20 can inhibit the ubiquitination of TRAF2 and TRAF6 by dissociating complexes composed of TRAFs and E2 ubiquitin-conjugating enzymes (41).
Infection by human T cell leukemia virus type 1 (HTLV-1) causes a fatal hematopoietic malignancy, adult T cell leukemia (ATL) (29), and one of its key regulatory proteins, Tax, plays important roles in viral pathogenesis (2, 10, 11, 26, 37). Tax (29) can potently activate NF-κB through both the canonical and noncanonical pathways (15, 42, 45). Tax was recently found to inhibit A20 function by disrupting its interaction with TAX1BP1 and Itch (39). Currently, how other DUBs may contribute to Tax-induced NF-κB signaling has not been studied. Here, we report on the characterization of USP20 for its regulation of Tax- and TRAF6-mediated activation of NF-κB.
MATERIALS AND METHODS
Cells and reagents.
Human embryonic kidney cell line HEK293T and human cervical cancer cell line HeLa were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and antibiotics. Human T cell lines MT1, MT2, MT4, ATL2, Jurkat, CEM, and H9 were maintained in RPMI 1640 with 10% FBS. Recombinant human interleukin-1β (IL-1β) (Peprotech) was used.
Plasmids and siRNAs.
The κB-luc reporter plasmid was described previously (19). Human USP20 coding sequences were amplified by PCR and subcloned into the pcDNA3 (Invitrogen) and the pCAG-HA (50) expression vectors. A human USP33 cDNA clone (I.M.A.G.E. 3874822) was purchased from ATCC, and the coding region was inserted into pCAG-HA. For ectopic expression of Tax, Hpx-Tax (18) and pCAG-Flag-Tax were used. Flag-TRAF6 has been described elsewhere (20). The pcDNA-HA-ubiquitin expression vector was used in immunoblotting experiments to detect ubiquitinated Tax and TRAF6. For the knockdown of USP20, synthesized small interfering RNAs (siRNAs) (Qiagen) were used. The target sequence of USP20 is 5′-TCGAGTGACACGGATGAGAAA-3′. Sequence-nonspecific siRNA was purchased from Qiagen and used as a negative control.
Antibodies.
Mouse monoclonal anti-Tax (NIH AIDS Research and Reference Reagent Program) was used to detect Tax protein in immunoblotting. Anti-Flag monoclonal antibody (M2; Sigma), anti-Flag polyclonal antibody (Sigma), antihemagglutinin (anti-HA) monoclonal antibody (HA-7; Sigma), anti-HA polyclonal antibody (Sigma), anti-USP20 polyclonal antibody (Bethyl Laboratories), anti-USP33 polyclonal antibody (Bethyl Laboratories), anti-tubulin monoclonal antibody (DM1A; Sigma), and anti-actin monoclonal antibody (AC-40; Sigma) were purchased.
Luciferase assay.
Expression plasmids or siRNAs were transfected into cells using Lipofectamine 2000 (Invitrogen). Cells were transfected with Tax and USP expression vectors or were transfected with siRNAs first, followed 48 h later with κB-Luc and pCMV-β-galactosidase (pCMV-β-Gal) plasmids. At 24 h after transfection of the reporters, cell lysates were subjected to luciferase assay. Total amounts of DNA and RNA to be transfected were adjusted by the addition of empty vectors or nonspecific siRNAs. To detect luciferase and β-Gal activity, luciferase substrate (Promega) and the Galacto-Star assay system (Applied Biosystems) were used. Relative values of luciferase activity were calculated using β-Gal activity as an internal control for transfection.
Coimmunoprecipitation assay.
At 48 h after transfection, cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, 0.3% NP-40, 150 mM NaCl, 2 mM EDTA, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM sodium fluoride, 0.5 mM dithiothreitol [DTT], and 1× protease inhibitor cocktail from Roche). Cell extracts were subjected to immunoprecipitation with anti-Flag or anti-HA antibodies, and the captured proteins were detected by immunoblotting using the indicated antibodies.
Immunofluorescence.
Cells were cultured on glass coverslips, and fixed in 4% paraformaldehyde at 24 h after transfection. After blocking of nonspecific reactions with 1% bovine serum albumin (BSA), cells were then incubated with the indicated primary antibodies, followed by a subsequent incubation with the secondary antibodies conjugated with Alexa Fluor 488 or 594 (Molecular Probes). DNA was counterstained with 0.1 μg/ml Hoechst 33342. Coverslips were mounted in Prolong Antifade (Molecular Probes), and cells were visualized with a Leica TCS SP2 confocal microscope.
Real-time PCR.
USP20 transcripts were quantified by real-time PCR. The sequences of the primers for USP20 were 5′-TCACAGAAGTCCACGAGACG-3′ (sense) and 5′-TTGTCCTTCCCCTTGACGAA-3′ (antisense). To compare the relative expression levels between samples, we quantified mRNA levels of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as the internal control using the primers 5′-GCTCACTGGCATGGCCTTCCGTGT-3′ (sense) and 5′-TGGAGGAGTGGGTGTCGCTGTTGA-3′ (antisense). Power SYBR green PCR Master Mix (Applied Biosystems) was used for preparation of the PCR mixtures, and the reaction was carried out with an Applied Biosystems 7500 real-time PCR system. All samples were measured in triplicate and analyzed with ΔΔCT method.
Cell proliferation assay.
ATL2 cells were transfected with USP20, USP33, or control expression vector using Amaxa Nucleofector II (Human T Cell Nucleofector kit, protocol X-001). Using a green fluorescent protein (GFP)-expressing plasmid, we analyzed the transfection efficiency of ATL2 cells with this method and found that routinely ∼60% of cells were visibly GFP positive at 48 h after transfection. Cell proliferation was evaluated with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay.
RESULTS
USP20 suppresses HTLV-1 Tax- and cytokine-induced NF-κB activation.
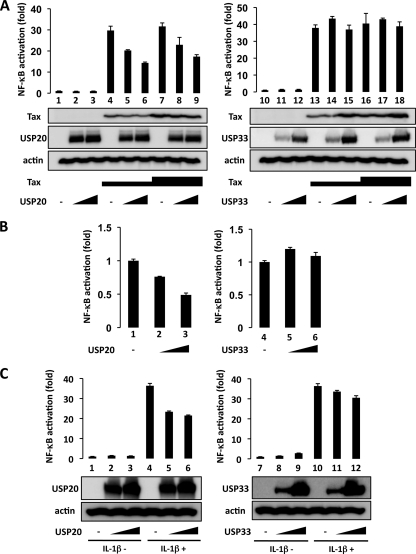
Activation of NF-κB contributes to cellular transformation by HTLV-1, and Tax is a potent activator of NF-κB (15, 29, 42). Protein ubiquitination plays an important role in signal transduction; indeed, individual NF-κB pathway constituents are frequently ubiquitinated when cells are stimulated (4, 14, 36). We reasoned that to maintain homeostasis, de novo-ubiquitinated proteins must eventually be deubiquitinated. To identify the cellular DUBs that might affect Tax-induced NF-κB activation, we tested 12 discrete DUBs and found that USP20 can suppress Tax-induced luciferase expression in a dose-dependent manner (Fig. 1A, lanes 1 to 9). By comparison, a related DUB, USP33 (and 10 other DUBs [data not shown]), failed to influence NF-κB activation by Tax (Fig. 1A, lanes 10 to 18). Next, we checked the activities of USP20 and USP33 in HTLV-1 transformed ATL2 leukemic cells which were directly derived from the leukemic cells of an ATL patient (46). We found that USP20, but not USP33, inhibited NF-κB activity in ATL2 cells (Fig. 1B). Collectively, the results suggest that the NF-κB activating pathways in HEK293T (Fig. 1A) and ATL2 (Fig. 1B) cells are both negatively regulated by USP20.
Fig. 1.
Expression of USP20 suppresses HTLV-1 Tax- and IL-1β-induced NF-κB activation. (A) HEK293T cells were transfected with a Tax-expressing plasmid, Hpx-Tax (0.05 μg in lanes 4 to 6 and 13 to 15; 0.15 μg in lanes 7 to 9 and 16 to 18), and κB-luc reporter (0.05 μg) with pcDNA3-USP20 (0.5 μg in lanes 2, 5, and 8; 1 μg in lanes 3, 6, and 9) or pCMV-USP33 (0.5 μg in lanes 11, 14, and 17; 1 μg in lanes 12, 15, and 18). In each case, a pCMV-β-Gal plasmid (0.01 μg) was included, and β-Gal values were used to normalize for transfection efficiency. Immunoblotting (lower panels) was done to confirm the expression of transfected and control (actin) protein using the indicated antibodies. (B) ATL2 cells were transfected with κB-luc reporter (1 μg) with pcDNA3-USP20 (2 μg in lane 2; 4 μg in lane 3) or pCMV-USP33 (2 μg in lane 5; 4 μg in lane 6). pCMV-β-Gal (0.2 μg) was included in each transfection as an internal normalization control. (C) HEK293T cells were transfected with κB-luc reporter (0.05 μg) with pcDNA3-USP20 (0.5 μg in lanes 2 and 5; 1 μg in lanes 3 and 6) or pCMV-USP33 (0.5 μg in lanes 8 and 11; 1 μg in lanes 9 and 12) and were treated with recombinant IL-1β (10 ng/ml) for 8 h (lanes 4 to 6 and 10 to 12). pCMV-β-Gal (0.01 μg) was included in each transfection as an internal control. Immunoblotting was performed to confirm the expression of transfected and control (actin) protein using the indicated antibodies. Total amounts of transfected DNA were equalized in each sample by the addition of vector DNA. Cell lysates were assayed for luciferase. The results from three independent experiments are shown as average values ± standard deviations (SD).
In order to characterize the roles of USP20 and USP33 in regard to other activators of NF-κB, we investigated their effects on IL-1β-mediated activation. As shown in Fig. 1C, NF-κB activation by IL-1β was suppressed when either USP20 (Fig. 1C, lanes 5 and 6) or USP33 (Fig. 1C, lanes 11 and 12) was expressed in HEK293T cells. These findings suggest that both USP20 and USP33 can negatively regulate IL-1β-mediated NF-κB signaling, while USP20, but not USP33, can modulate Tax-induced NF-κB activation (Fig. 1A).
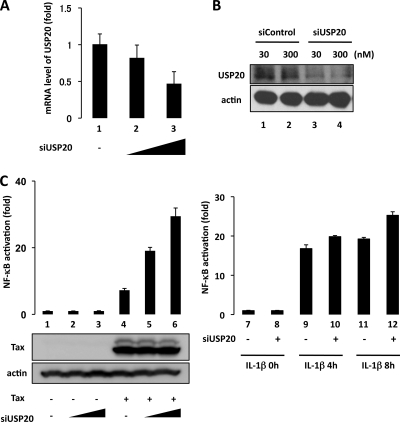
To check for physiological relevance and to rule out that the results were simply from nonphysiological overexpression, we knocked down cell-endogenous USP20 in HEK293T cells using siRNAs. Using real-time reverse transcription-PCR (RT-PCR), we documented an siRNA dose-dependent knockdown of USP20 mRNA in HEK293T (Fig. 2A, bars 2 and 3). To check the knockdown effect of the siRNA at the protein level, we transfected the siRNA into Jurkat cells and confirmed by immunoblotting that USP20 expression was reduced as expected (Fig. 2B). Next, at 48 h after USP20 siRNA transfection into HEK293T cells, we transfected a κB-luc reporter plasmid and checked for its induction by Tax or IL-1β. With either Tax (Fig. 2C, compare bar 4 to bars 5 and 6) or IL-1β (Fig. 2C, compare bar 9 to 10 and bar 11 to 12), the activity of the κB-luc reporter was higher in USP20-knocked-down cells than in control siRNA-treated cells. Collectively, the findings in Fig. 1 and 2 are consistent with USP20 serving to negatively modulate cellular NF-κB activity.
Fig. 2.
Knockdown of cell-endogenous USP20 enhances HTLV-1 Tax- and IL-1β-induced NF-κB activation. (A) Sequence-specific siRNA was used to knock down USP20. The knockdown of cell-endogenous USP20 by siRNA was confirmed with real-time RT-PCR. USP20-specific siRNAs at 20 nM (bar 2) or 40 nM (bar 3) were transfected into HEK293T cells, from which RNA was extracted. USP20 mRNA levels were quantified by real time RT-PCR. (B) The competent knockdown of cell-endogenous USP20 by siRNA was checked by immunoblotting. USP20-specific siRNAs at 30 nM (lane 3) or 300 nM (lane 4) were transfected into Jurkat cells, and cell-endogenous USP20 protein was assayed by immunoblotting. (C) HEK293T cells were transfected with Hpx-Tax (bars 4 to 6) or treated with IL-1β (50 ng/ml) at the indicated times (bars 9 to 12) with or without the cotransfection of siRNA for USP20 (20 nM in lanes 2 and 5; 40 nM in lanes 3, 6, 8, 10, and 12) (0 h of IL-1β means that no treatment occurred). Total amounts of transfected DNAs and siRNAs were equalized by the addition of either vector DNA or control siRNA, respectively. Cell lysates were assayed for luciferase. Immunoblotting was done to confirm the expression of transfected and control (actin) protein using the indicated antibodies. The results from three independent experiments are shown as average values ± SD.
TRAF6 interacts with USP20 and USP33.
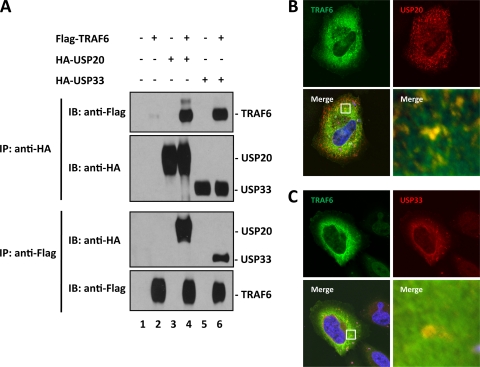
To our knowledge, the above observations on USP20 and USP33 represent the first demonstration of their effects on NF-κB signaling. We were thus interested in searching for potential USP20/33 cellular substrates among known NF-κB-signaling proteins. TRAF6 is an NF-κB-signaling protein that has been reported to be regulated by ubiquitination (8). We next asked if TRAF6 would interact with USP20 or USP33 in coimmunoprecipitations. We transfected Flag-tagged TRAF6 and either HA-tagged USP20 or HA-tagged USP33 into HEK293T cells. Cell lysates were immunoprecipitated using anti-Flag or anti-HA, and the presence of HA- or Flag-tagged proteins in the respective immunoprecipitates was assessed by immunoblotting. As shown in Fig. 3A, TRAF6 bound USP20 and USP33. As a control, we checked if another NF-κB-signaling protein also known to be ubiquitinated, IKKγ (47, 53), would interact with USP20 or USP33; however, the latter interaction was not observed (data not shown).
Fig. 3.
TRAF6 interacts with USP20 and USP33. (A) HEK293T cells were transfected with the indicated plasmids, and immunoprecipitation was done with either anti-Flag or anti-HA antibody as indicated. The immunoprecipitates and total cell extracts were resolved by SDS-PAGE and immunoblotted. The blots were probed with either anti-Flag or anti-HA. (B and C) Flag-TRAF6 plasmid with either HA-USP20 or HA-USP33 was cotransfected into HeLa cells. Subcellular localizations of TRAF6, USP20, or USP33 were visualized with anti-Flag (green) or anti-HA (red). The nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) (blue). The higher magnification of the “Merge” is shown in the lower right panels of panels B and C.
USP20 and USP33 have been reported to localize in perinuclear regions and in small vesicles throughout the cytoplasm (1, 7), and TRAF6 has been shown to locate diffusely in the cytoplasm (49). Next, we immunostained HeLa cells transfected with Flag-TRAF6 and either HA-USP20 or HA-USP33 (Fig. 3B and C). Although not all TRAF6 and USP20/USP33 colocalized together, we did observe partial colocalization of these proteins in the cytoplasm (see yellow costaining in magnified views of the “Merge” panels in Fig. 3B and C), consistent with the potential for intracellular protein-protein interaction.
TRAF6 is a substrate of USP20.
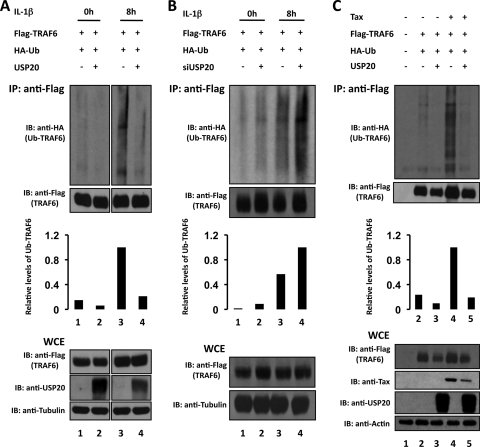
Previously, USP20 was reported to interact with HIF-1α (27), type2 iodothyronine deiodinase (7), and β2 adrenergic receptor (β2AR) (1). Our finding of an interaction between USP20 and TRAF6 prompted us to ask if TRAF6 might be a functional USP20 substrate. We therefore checked for the effect of USP20 on IL-1β-induced ubiquitination of TRAF6. We compared the levels of ubiquitinated TRAF6 in the absence or presence of transfected USP20 (Fig. 4A). IL-1β treatment of cells for 8 h increased the amount of ubiquitinated TRAF6 (Fig. 4A, compare lane 1 to 3), and this increase was diminished by the expression of USP20 (Fig. 4A, compare lane 3 to 4). In addition, siRNA knockdown of cell-endogenous USP20 enhanced the level of ubiquitinated TRAF6 in IL-1β-treated cells (Fig. 4B, compare lane 3 to 4). Altogether the results support an interpretation that USP20 promotes the deubiquitination of IL-1β-induced ubiquitinated TRAF6.
Fig. 4.
IL-1β- or Tax-induced ubiquitinated TRAF6 is deubiquitinated by USP20. (A) HEK293T cells were transfected as indicated with Flag-TRAF6, HA-Ub, and USP20. Cells without IL-1β treatment (lanes 1 and 2) and cells treated with IL-1β for 8 h (lanes 3 and 4) were subjected to immunoprecipitation (IP) followed by immunoblotting (IB) to detect ubiquitinated TRAF6 (anti-HA; top panel). WCE, whole-cell extract. (B) HEK293T cells were transfected as indicated with Flag-TRAF6, HA-Ub, and siRNA specific for USP20. Cells without treatment (0 h; lanes 1 and 2) and cells treated with IL-1β for 8 h (lanes 3 and 4) were immunoprecipitated and detected by immunoblotting for ubiquitinated TRAF6 (anti-HA; top panel). (C) HEK293T cells were transfected as indicated with Tax, Flag-TRAF6, HA-Ub, and USP20 (lanes 2 to 5). Cell lysates were immunoprecipitated and immunoblotted for detection of ubiquitinated TRAF6 (anti-HA; top panel). Total amounts of transfected DNA or transfected siRNA were equalized by the addition of empty vector or control siRNA, respectively. Immunoprecipitations were performed using anti-Flag antibody. Ubiquitinated proteins were detected by immunoblotting with anti-HA antibody. Anti-Flag (for TRAF6), anti-USP20, anti-Tax, anti-tubulin, and anti-actin antibodies were used to verify the respective proteins in immunoblottings. To normalize the amount of ubiquitinated TRAF6, signals of ubiquitinated TRAF6 and total TRAF6 in the immunoblots were quantified by densitometry, and relative levels of normalized ubiquitinated TRAF6 were calculated. The results are graphed in the middle of each panel.
In our experiments, Tax expression also increased intracellular ubiquitinated TRAF6, as reported previously (Fig. 4C, compare lane 2 to 4) (12). The Tax-induced ubiquitination of TRAF6 was also sensitive to deubiquitination by USP20 (Fig. 4C, compare lane 4 to 5). Thus, TRAF6 appears to be a bona fide USP20 substrate, since USP20 can efficiently deubiquitinate otherwise ubiquitinated TRAF6 that is induced by diverse stimuli such as Tax and IL-1β.
Ubiquitinated Tax is a substrate for USP20.
Ubiquitination, sumoylation, acetylation, and phosphorylation of Tax have been shown to influence its transcriptional function (5, 9, 22, 23, 25, 28, 34, 35, 40). Tax ubiquitination has been reported to be required for its activation of NF-κB, and it has been found that the Tax-polyubiquitin chains are predominantly K63 linked (40). Because it was observed that USP20 expression inhibited Tax-induced NF-κB activation (Fig. 1A), we wondered if this finding might also have resulted from USP20-mediated deubiquitination of ubiquitinated Tax.
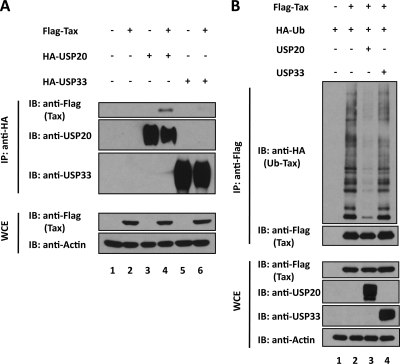
To check the potential interaction between Tax and either USP20 or USP33, we performed coimmunoprecipitation assays using cells transfected with Flag-tagged Tax and either HA-USP20 or HA-USP33. USP20 coimmunoprecipitated with Tax (Fig. 5A, top panel, lane 4), while USP33 did not distinctly coimmunoprecipitate with Tax (Fig. 5A, top panel, lane 6). Next, we asked how USP20 expression might affect the intracellular level of ubiquitinated Tax. Expression of transfected USP20 (Fig. 5B, compare lane 2 to 3), but not USP33 (Fig. 5B, compare lane 2 to 4), indeed reduced the abundance of ubiquitinated Tax in HEK293T cells. These results agree with the findings in Fig. 1A that USP20, but not USP33, inhibited Tax-induced NF-κB activation.
Fig. 5.
USP20, but not USP33, deubiquitinates Tax. (A) Tax coimmunoprecipitates with USP20 but not USP33. The indicated plasmids were transfected into HEK293T cells and immunoprecipitated using anti-HA for HA-USP20 (USP20; lanes 3 and 4) or HA-USP33 (USP33; lanes 5 and 6). The immunoprecipitates were blotted and probed with anti-USP20 or anti-USP33. The coimmunoprecipitated Flag-Tax was verified by immunoblotting with anti-Flag. WCE, whole-cell extract. (B) Flag-Tax and HA-Ub as indicated were cotransfected into HEK293T cells with USP20 (lane 3) or USP33 (lane 4). Immunoprecipitation was performed with anti-Flag (top panels). Ubiquitinated Tax (lanes 2, 3, and 4; top panel) was detected using anti-HA in immunoblotting of anti-Flag immunoprecipitates. The amount of ubiquitinated Tax was reduced by the cotransfection of USP20 (lane 3). Amounts of Tax, USP20, USP33, and actin in the whole-cell lysates (WCE) are verified in the bottom panels by immunoblotting.
USP20 levels are frequently low in HTLV-1-transformed cells, and overexpression of USP20 suppresses cellular proliferation.
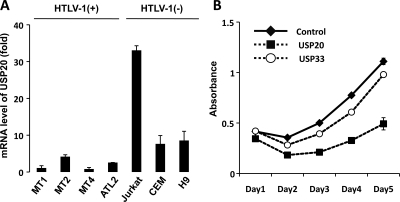
Many ATL cell lines that express Tax are activated for NF-κB (30). If ubiquitinated Tax is needed to activate NF-κB, then one prediction is that the level of USP20 in many ATL cells should be low. To examine this issue, the cell-endogenous levels of USP20 transcripts in several HTLV-1-positive or -negative T cell lines were compared by real-time RT-PCR. USP20 mRNA levels in HTLV-1-transformed Tax-expressing cell lines MT1, MT2, MT4, and ATL2 were indeed significantly lower than those in HTLV-1-negative cell lines Jurkat, CEM, and H9 (Fig. 6A). While we do not fully understand the mechanisms for why USP20 expression is reduced in HTLV-1 cells, our preliminary evidence suggests the involvement of epigenetic regulatory mechanisms, including DNA methylation and histone acetylation (data not shown).
Fig. 6.
USP20 is frequently expressed at a reduced level in HTLV-1-transformed cells. (A) USP20 transcripts in the indicated T cell lines were quantified by real-time RT-PCR. Compared to HTLV-1-negative cell lines (Jurkat, CEM, and H9), the HTLV-1 positive cell lines (MT1, MT2, MT4, and ATL2) have much reduced amounts of USP20 transcripts. (B) Ectopic expression of USP20 suppresses cell proliferation. ATL2 cells were transfected with USP20, USP33, or control plasmid, and cell proliferation was evaluated by MTT assay. Based on use of a GFP expression plasmid, the transfection efficiency of ATL2 cells was determined to be slightly greater than 60%.
Because NF-κB is a prosurvival and proproliferative factor in HTLV-1 cells, we next wondered if the overexpression of USP20, which negatively regulates NF-κB activity, would inhibit the proliferation of these cells. We transfected USP20, USP33, or a control GFP plasmid into ATL2 cells. Based on the visualization of GFP, our ATL2 transfection efficiency slightly exceeded 60% (data not shown). We then assessed the proliferation of USP20- or USP33-transfected ATL2 cells. As shown in Fig. 6B, ATL2 cells transfected with USP20 proliferated distinguishably slower than GFP- or USP33-transfected ATL2 cells. While there are many ways to interpret this result, one explanation consistent with our other current findings is that the “transfected” USP20 deubiquitinated TRAF6 and/or Tax in ATL2 cells, reducing their NF-κB activity and thus resulting in the slower cell growth (Fig. 6B).
DISCUSSION
NF-κB activation is an important host immune response triggered by pathogens. However, an excessive or a prolonged response can evoke autoimmune disorder, septic shock, neoplastic diseases, and death (36). For optimal homeostasis, positive and negative mechanisms serve to balance ambient NF-κB activity. Current evidence suggests that protein ubiquitination and deubiquitination may provide such a balancing process (43).
In T cells, TRAF6 is ubiquitinated upon receptor stimulation; the ubiquitinated TRAF6 positively regulates NF-κB signal transduction (8, 47, 53). Here we show that two ubiquitin-specific peptidases, USP20 and USP33, can negatively regulate TRAF–NF-κB signaling by targeting TRAF6 for deubiquitination. Other DUBs such as A20 and CYLD can also target TRAF6 (20, 24, 41, 48). Future studies are needed to fully understand the specificity and the breadth of redundancy of the various deubiquitinases for their substrates.
In our experiments, USP20 also deubiquitinated Tax and inhibited its activity. To our knowledge, USP20 is the first DUB shown to deubiquitinate Tax. Because the Tax–NF-κB pathway is important for cellular transformation by HTLV-1 and because ubiquitinated Tax has been shown to be necessary for NF-κB activation, our findings suggest that USP20 could be a key regulator of Tax that might influence ATL leukemogenesis. Elsewhere, it has been reported that A20 negatively regulates Epstein-Barr virus (EBV)-encoded LMP1 function and that the activity of LMP1 is important for EBV immortalization of B cells (33). Thus, HTLV-1 and EBV may be two viruses that similarly exploit the cellular ubiquitination-deubiquitination machinery for pathogenesis. Potentially, the ubiquitination-deubiquitination process could be a common focal point that could be targeted to interdict HTLV and EBV infections. Additional investigation will be needed to understand the general importance of ubiquitination and deubiquitination in other viral infections.
The reduced expression of several DUBs is associated with tumorigenesis (31). Thus, mutations in the CYLD gene are known to cause familial tumors of skin appendages called cylindromas (3, 24, 48). The inactivation of A20 by genetic changes has also been reported in malignant lymphomas (6, 16, 21, 38). Here, reduced USP20 activity is shown for HTLV-1-transformed MT1, MT2, MT4, and ATL2 cells (Fig. 6). Moreover it was recently reported that in 5% of adult T cell acute lymphoblastic leukemia (T-ALL) cases, an abnormal fusion transcript, TAF I-NUP214, is expressed from a chromosomal aberration. Intriguingly, in these chimeric fusion cases, the levels of USP20 transcript were significantly reduced (13). This clinical finding is additionally consistent with an association between USP20, NF-κB signaling, and T cell malignancies.
In summary, the salient findings from this study are the identification of USP20 as an inhibitor of NF-κB signaling and as a deubiquitinating enzyme for TRAF6 and Tax. Preliminary data suggest that USP20 overexpression may impede the proliferation of HTLV-I/ATL cells (Fig. 6B); however, this finding will need to be verified further through studying a large series of ATL clinical samples. If the notion can be demonstrated to be correct, then the screening for small-molecule compounds that enhance USP20 deubiquitinase activity may unveil new agents that are useful for treating ATL.
ACKNOWLEDGMENTS
We thank Alicia Buckler-White for assistance with DNA sequencing and Junko Tanabe for technical assistance. We thank laboratory members for critical readings of the manuscript.
Work in K.-T.J.'s laboratory is supported in part by intramural funds from NIAID, NIH. Xiongbin Lu is supported by grant R01CA136549 from NCI/NIH.
Footnotes
Published ahead of print on 27 April 2011.
REFERENCES
- 1. Berthouze M., Venkataramanan V., Li Y., Shenoy S. K. 2009. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 28:1684–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boxus M., et al. 2008. The HTLV-1 Tax interactome. Retrovirology 5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brummelkamp T. R., Nijman S. M., Dirac A. M., Bernards R. 2003. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424:797–801 [DOI] [PubMed] [Google Scholar]
- 4. Chen Z. J. 2005. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 7:758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiari E., et al. 2004. Stable ubiquitination of human T-cell leukemia virus type 1 tax is required for proteasome binding. J. Virol. 78:11823–11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Compagno M., et al. 2009. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459:717–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curcio-Morelli C., et al. 2003. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J. Clin. Invest. 112:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng L., et al. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351–361 [DOI] [PubMed] [Google Scholar]
- 9. Durkin S. S., Ward M. D., Fryrear K. A., Semmes O. J. 2006. Site-specific phosphorylation differentiates active from inactive forms of the human T-cell leukemia virus type 1 Tax oncoprotein. J. Biol. Chem. 281:31705–31712 [DOI] [PubMed] [Google Scholar]
- 10. Feuer G. 2009. “Tax-ing” the cancer stem cell. Blood 114:2568–2569 [DOI] [PubMed] [Google Scholar]
- 11. Feuer G., Green P. L. 2005. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 24:5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gohda J., et al. 2007. HTLV-1 Tax-induced NFkappaB activation is independent of Lys-63-linked-type polyubiquitination. Biochem. Biophys. Res. Commun. 357:225–230 [DOI] [PubMed] [Google Scholar]
- 13. Gorello P., et al. 2010. Combined interphase fluorescence in situ hybridization elucidates the genetic heterogeneity of T-cell acute lymphoblastic leukemia in adults. Haematologica 95:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haglund K., Dikic I. 2005. Ubiquitylation and cell signaling. EMBO J. 24:3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higuchi M., Fujii M. 2009. Distinct functions of HTLV-1 Tax1 from HTLV-2 Tax2 contribute key roles to viral pathogenesis. Retrovirology. 6:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honma K., et al. 2009. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 114:2467–2475 [DOI] [PubMed] [Google Scholar]
- 17. Hymowitz S. G., Wertz I. E. 2010. A20: from ubiquitin editing to tumour suppression. Nat. Rev. Cancer 10:332–341 [DOI] [PubMed] [Google Scholar]
- 18. Iha H., et al. 2000. Pleiotropic effects of HTLV type 1 Tax protein on cellular metabolism: mitotic checkpoint abrogation and NF-kappaB activation. AIDS Res. Hum. Retroviruses 16:1633–1638 [DOI] [PubMed] [Google Scholar]
- 19. Iha H., et al. 2003. Segregation of NF-kappaB activation through NEMO/IKKgamma by Tax and TNFalpha: implications for stimulus-specific interruption of oncogenic signaling. Oncogene 22:8912–8923 [DOI] [PubMed] [Google Scholar]
- 20. Iha H., et al. 2008. Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-kappaB activation. EMBO J. 27:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato M., et al. 2009. Frequent inactivation of A20 in B-cell lymphomas. Nature 459:712–716 [DOI] [PubMed] [Google Scholar]
- 22. Kfoury Y., et al. 2008. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene 27:1665–1676 [DOI] [PubMed] [Google Scholar]
- 23. Kfoury Y., et al. 2011. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 117:190–199 [DOI] [PubMed] [Google Scholar]
- 24. Kovalenko A., et al. 2003. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424:801–805 [DOI] [PubMed] [Google Scholar]
- 25. Lamsoul I., et al. 2005. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell. Biol. 25:10391–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li M., Kesic M., Yin H., Yu L., Green P. L. 2009. Kinetic analysis of human T-cell leukemia virus type 1 gene expression in cell culture and infected animals. J. Virol. 83:3788–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z., Wang D., Messing E. M., Wu G. 2005. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 6:373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lodewick J., et al. 2009. Acetylation of the human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-kappaB pathway. Virology 386:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuoka M., Jeang K. T. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7:270–280 [DOI] [PubMed] [Google Scholar]
- 30. Mori N., et al. 1999. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood 93:2360–2368 [PubMed] [Google Scholar]
- 31. Nicholson B., Marblestone J. G., Butt T. R., Mattern M. R. 2007. Deubiquitinating enzymes as novel anticancer targets. Future Oncol. 3:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nijman S. M., et al. 2005. A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786 [DOI] [PubMed] [Google Scholar]
- 33. Ning S., Pagano J. S. 2010. The A20 deubiquitinase activity negatively regulates LMP1 activation of IRF7. J. Virol. 84:6130–6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peloponese J. M., Jr., et al. 2004. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J. Virol. 78:11686–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peloponese J. M., Jr., Yasunaga J., Kinjo T., Watashi K., Jeang K. T. 2009. Peptidylproline cis-trans-isomerase Pin1 interacts with human T-cell leukemia virus type 1 Tax and modulates its activation of NF-kappaB. J. Virol. 83:3238–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perkins N. D. 2007. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 8:49–62 [DOI] [PubMed] [Google Scholar]
- 37. Rauch D., et al. 2009. T-cell activation promotes tumorigenesis in inflammation-associated cancer. Retrovirology 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitz R., et al. 2009. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J. Exp. Med. 206:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shembade N., et al. 2008. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat. Immunol. 9:254–262 [DOI] [PubMed] [Google Scholar]
- 40. Shembade N., Harhaj N. S., Yamamoto M., Akira S., Harhaj E. W. 2007. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J. Virol. 81:13735–13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shembade N., Ma A., Harhaj E. W. 2010. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327:1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shoji T., et al. 2009. Identification of a novel motif responsible for the distinctive transforming activity of human T-cell leukemia virus (HTLV) type 1 Tax1 protein from HTLV-2 Tax2. Retrovirology 6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skaug B., Jiang X., Chen Z. J. 2009. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 78:769–796 [DOI] [PubMed] [Google Scholar]
- 44. Sun S. C. 2008. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 8:501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun S. C., Yamaoka S. 2005. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene 24:5952–5964 [DOI] [PubMed] [Google Scholar]
- 46. Takeda S., et al. 2004. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int. J. Cancer 109:559–567 [DOI] [PubMed] [Google Scholar]
- 47. Tokunaga F., et al. 2009. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat. Cell Biol. 11:123–132 [DOI] [PubMed] [Google Scholar]
- 48. Trompouki E., et al. 2003. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424:793–796 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y., et al. 2006. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 7:139–147 [DOI] [PubMed] [Google Scholar]
- 50. Watashi K., et al. 2008. Human immunodeficiency virus type 1 replication and regulation of APOBEC3G by peptidyl prolyl isomerase Pin1. J. Virol. 82:9928–9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wertz I. E., et al. 2004. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430:694–699 [DOI] [PubMed] [Google Scholar]
- 52. Wilkinson K. D. 2000. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol. 11:141–148 [DOI] [PubMed] [Google Scholar]
- 53. Zhou H., et al. 2004. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature 427:167–171 [DOI] [PubMed] [Google Scholar]