Abstract
Rift Valley fever virus (RVFV) is a zoonotic pathogen that primarily affects ruminants but can also be lethal in humans. A negative-stranded RNA virus of the family Bunyaviridae, this pathogen is transmitted mainly via mosquito vectors. RVFV has shown the ability to inflict significant damage to livestock and is also a threat to public health. While outbreaks have traditionally occurred in sub-Saharan Africa, recent outbreaks in the Middle East have raised awareness of the potential of this virus to spread to Europe, Asia, and the Americas. Although the virus was initially characterized almost 80 years ago, the only vaccine approved for widespread veterinary use is an attenuated strain that has been associated with significant pathogenic side effects. However, increased understanding of the molecular biology of the virus over the last few years has led to recent advances in vaccine design and has enabled the development of more-potent prophylactic measures to combat infection. In this review, we discuss several aspects of RVFV, with particular emphasis on the molecular components of the virus and their respective roles in pathogenesis and an overview of current vaccine candidates. Progress in understanding the epidemiology of Rift Valley fever has also enabled prediction of potential outbreaks well in advance, thus providing another tool to combat the physical and economic impact of this disease.
INTRODUCTION
Rift Valley fever virus (RVFV) is an arthropod-borne pathogen that primarily affects ruminants in eastern and sub-Saharan Africa (7, 16). While periodic outbreaks of Rift Valley fever have resulted in significant losses to the African livestock industry, it has only been during the past decade that the international community has appreciated the potential dangers of the disease. In particular, recent outbreaks in the Middle East have demonstrated the potential of the disease to spread beyond the African continent (4) (Fig. 1). Moreover, other considerations, such as RVFV's recent classification as a potential bioterrorist agent (55), have renewed interest in the study of the virus as well as in the development of prophylactic measures to contain future outbreaks. Another important factor in recent Rift Valley fever outbreaks is the increasing number of human fatalities. Human symptoms of this disease range from photophobia and headaches to retinitis and encephalitis (18, 19). While Rift Valley fever was originally associated with livestock, recent outbreaks in Kenya have resulted in increased fatality rates among humans, thereby presenting an increased threat to public health (2, 11).
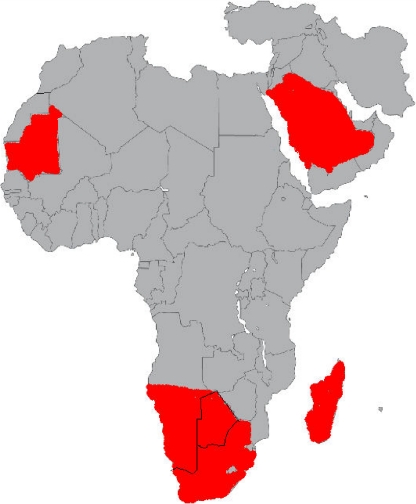
Fig. 1.
Countries with confirmed cases of Rift Valley fever from July 2009 to November 2010 (indicated in red). Data were obtained from ProMed-mail (International Society For Infectious Diseases [http://www.promedmail.org]).
Furthermore, the potential dangers of the disease recently came to the international community's attention earlier this year, when, based on a suspected case of Rift Valley fever in a German tourist following a trip to a South African game reserve, the World Health Organization issued travel advisories to tourists attending the 2010 FIFA World Cup. Ultimately, further testing revealed that the tourist suffered from rickettsia (67), thereby keeping Europe RVFV free for the time being.
Nevertheless, due to increased trade with and traffic to the African continent, as well as to climate change affecting northern Africa and southern Europe, there is an increasing consensus that it is only a matter of time before Rift Valley fever outbreaks will start to affect the agricultural industry in Europe and Asia. The combination of all these factors has resulted in the increased study of RVFV pathogenesis and to more comprehensive research on protection from and prevention of Rift Valley fever. This review summarizes recent advances in the structural and functional characterization of the virus, as well as recent progress on antiviral and epidemiological measures being proposed to limit future epidemics.
STRUCTURAL AND GENETIC ORGANIZATION OF RVFV
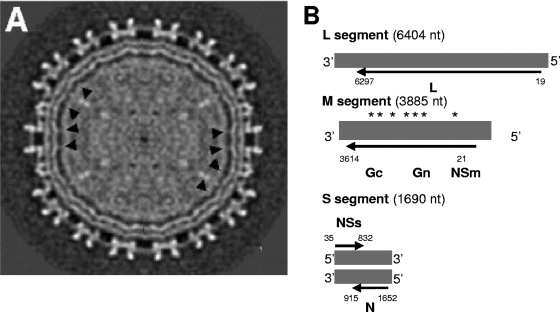
RVFV is a Phlebovirus within the family Bunyaviridae. As such, the virus has a trisegmented, single-stranded RNA genome, in which two segments (L and M) have a negative polarity, while the third (S segment) is ambisense (23) (Fig. 2). It has been proposed that similar to other bunyaviruses, RVFV has three segments with a circular, “panhandled” secondary structure (27, 52) formed by cRNA sequences on the ends of each segment (58). While the L segment encodes the viral RNA polymerase used for replication and mRNA transcription, the M segment encodes two glycoproteins (Gn and Gc) and a nonstructural protein that can be expressed by itself (Nsm1) or in fusion with Gn (NSm2) (22). In its antisense orientation, the S segment expresses the nucleoprotein (N protein), while its complementary orientation encodes the nonstructural protein NSs. When assembled, the RVFV particles possess a lipid-bilayered, enveloped virion, with the surface of each particle measuring 90 to 110 nm in diameter (15) and being composed of subunits of Gn and Gc heterodimers, forming an ordered icosahedral shell of 122 capsomers (29). Within the inner envelope of the virion, ribonucleoprotein complexes are layered proximal to the inner envelope, suggesting a possible interaction between the glycoproteins and the ribonucleoproteins (RNPs).
Fig. 2.
Structural and genomic organization of RVFV. (A) A single-particle cryo-electron microscopy reconstruction of RVFV MP-12 is shown. The black arrowheads point to connecting densities protruding through the lipid envelope, most likely representing glycoprotein cytoplasmic tails. (B) Genomic organization of RVFV. Asterisks over the M segment indicate possible hydrophobic domains of Gn and Gc. Reprinted from reference 59 with permission of the publisher.
FUNCTIONAL CHARACTERIZATION OF RVFV COMPONENTS AND THEIR ROLES IN DISEASE PATHOLOGY
Viral RNA polymerase.
Expressed on the L segment of the RVFV genome, L protein is the largest protein expressed by the virus, yet despite its large size, L protein has been expressed recombinantly, thereby enabling its functional characterization (Table 1) (41, 68). Experiments using a vaccinia virus expression vector found that L protein is capable of mRNA transcription of purified, transfected RVFV RNP complexes. Results from these experiments also led to the hypothesis that L protein may also play a role in the replication of the viral genome (41). It has also been suggested that the polymerase activity is mediated through an SDD motif, which was also demonstrated to mediate polymerization of recombinant L protein, suggesting that functionality is correlated with oligomerization of the protein (68).
Table 1.
RVFV components and their role in infection and pathology
| Protein | Mol wt | Genome segment | Role in pathogenesis | Reference(s) |
|---|---|---|---|---|
| Viral RNA polymerase | 244 | L | RNA replication and viral RNA transcription | 40, 68 |
| Gn/Gc | 59/55 | M | Mediates virus entry into cells through receptors; contributes to the assembly process and likely interacts with N protein | 16, 39 |
| NSm1/NSm2 | 78/14 | M | Suppresses virus-induced apoptosis | 67 |
| N | 27 | S | Induces humoral and T-cell immunity response | 36, 41, 42 |
| NSs | 31 | S | Interferon antagonist: limits IFN-mediated host antiviral responses, inhibiting cellular transcription and degrading protein kinase PKR; interacts with specific DNA regions of the host genome, inducing chromosome cohesion and segregation defects | 10, 24, 30, 38, 45 |
Bunyaviral RNA polymerases have been previously shown to utilize a “cap-snatching” mechanism for mRNA transcription. Specifically, bunyaviruses utilize the endonuclease activity associated with L protein to obtain 5′-capped RNA, which can then be used as primers for viral mRNA transcription (57). Recently, the crystal structure of another member of Bunyaviridae was deduced. La Crosse virus L protein was found to possess an endonuclease domain, and when aligned with the secondary structures of other bunyaviral L proteins (including that of RVFV), this domain was found to be conserved (57).
Nucleoprotein.
As previously mentioned, RVFV nucleoproteins, when associated with genomic viral RNA, result in the formation of RNP complexes. Interestingly, recent structural work on RVFV RNPs revealed a nonhelical complex unique among negative-sense RNA viruses (56). These RNPs, in turn, associate with glycoproteins to localize to the interior of the virion (Fig. 2A). While nucleoproteins are not known to be directly involved in pathogenesis, experiments using recombinant N have shown it to elicit a partial degree of immune protection (37, 42). Although strongly immunogenic, N cannot elicit neutralizing antibodies. Furthermore, it has been shown that RVFV-immunized sheep and mice exhibit lymphocytic proliferation in vitro in the presence of recombinant N protein (37, 42, 43). This suggests that this level of immunity is mediated through the interactions of antigen-presenting cells (APCs) with T helper cells.
Glycoproteins.
As the names suggest, Gn is expressed near the N terminus of the M segment and Gc is located at the C-terminal end of the open reading frame (ORF) of the M segment. Both proteins in conjunction make up the heterodimeric unit of the viral surface. It has been shown that recombinant Gc (40) and Gn (13) can form virus-like particles (VLPs) and induce complete immune protection from RVFV infection in mice. Furthermore, recombinant Gn protein has been shown to localize to the Golgi complex via its Golgi localization signal, as opposed to Gc, which when expressed by itself is retained in the endoplasmic reticulum (ER) (21). This suggests that Gn may ultimately be required for viral budding. DNA vaccination studies using Gn have demonstrated that a neutralizing antibody response can be generated without coexpression of Gc (6). When recombinantly expressed with RVFV nucleoprotein in a baculoviral system, Gc can form virus-like particles. However these VLPs were found to be highly pleiomorphic, suggesting that heterodimeric expression is required for homogenous expression of virion particle surfaces (40). Recently, virulence has been associated with a single amino acid substitution in the ORF of this glycoprotein. It has been demonstrated using reverse genetics that a single nucleotide substitution at nucleotide 847 of the M segment can determine the virulence phenotype of the RVFV ZH501 strain in mice (48).
Further experiments using RVFV reverse genetics have also indicated a role for cis-acting RNA signals in viral packaging (63). Work by Teraski et al. demonstrated that M segments lacking a portion of the 5′ UTR prevented efficient copackaging with both L and S segments.
It should also be noted that when expressed recombinantly in eukaryotic cell lines using an alphavirus replicon vector, glycoproteins can be exported to the cell surface (17). Interestingly, brief treatment with mildly acidic media was shown to induce glycoprotein-mediated cell-to-cell fusion. These results were reproduced in a variety of cell types, and it has been proposed that viral entry, while mediated through glycoproteins, may either (i) interact with a host receptor that is expressed in a wide variety of mammalian species, or (ii) not require a host-specific cellular receptor at all. However, it should be noted that these results contrast with work performed using another Phlebovirus, Uukuniemi virus (44). In this case, while virus-membrane fusion was found to occur at low pH, only certain cell lines could be infected. Therefore, the exact mechanism of RVFV entry is not as yet clearly defined.
Nonstructural protein: M segment.
The first detailed description of NSm was described by Kakach et al. (33). When recombinant RVFV M segments were expressed in vaccinia virus, radioimmunoprecipitation using anti-RVFV serum indicated the presence of four protein products: Gn, Gc, and two uncharacterized polypeptides, one 14 kDa and the other 78 kDa, both of which initiate from ATG codons upstream from the Gn ORF. In the case of the 78-kDa product, the translated product appeared to originate from the first coding ATG of the M segment and continue through the entire Gn-coding sequence. Current nomenclature designates the 78-kDa product as Nsm1, while the 14-kDa polypeptide has been designated NSm2 (22).
While the function of NSm has yet to be confirmed, experiments using RVFV mutants have suggested that the coding region upstream from Gn (known as pre-Gn) plays a role in modulating virus-induced apoptosis (66). It should also be noted that while these results were found with an attenuated strain of RVFV (MP-12), reverse genetics using an NSm-deficient virulent RVFV strain (ZH-501) does not seem to affect virulence or lethality in experimental rat infections (20). Therefore, the exact role of NSm in RVFV pathogenesis is still inconclusive.
Nonstructural protein: S segment.
Due to its antiviral-sense orientation (Fig. 2), NSs should theoretically be expressed later than other viral components. However, transcription inhibition assays of RVFV-infected cells have demonstrated that NSs is expressed early during viral infection (32). This discrepancy may be due to the small proportion of antiviral-sense S RNA that is packaged in the viral particles and may be responsible for early NSs expression, thus providing some initial inhibition of alpha/beta interferon (INF-α/β)-mediated responses. Unlike NSm, NSs has been extensively characterized and has been found to have antagonistic activity against the IFN antiviral response (10). This inhibitory activity appears to be mediated through multiple mechanisms. One such mechanism involves the competitive inhibition of formation of the transcription factor TFIIH (38). Protein interaction/colocalization experiments by Le May et al. have shown that NSs interacts with p44, a subunit of the transcription factor TFIIH (38). This interaction presents a mode of competitive inhibition with XPD, another component of TFIIH. This mode of transcriptional inhibition may play an important role in the suppression of the host antiviral response. Another mechanism involves transcriptional suppression through its interaction with SAP30 (39), thereby interfering with IFN-β expression. NSs has also been shown to disrupt double-stranded RNA-dependent protein kinase R (PKR) activity associated with cellular antiviral responses (25, 31). These mechanisms enable RVFV to infect the host cell while limiting IFN-mediated host antiviral responses. NSs forms a filamentous structure in the nucleus and interacts with some specific DNA regions of the host genome correlated with the induction of chromosome cohesion and segregation defects, which could be related to RVFV pathology (46). Conversely, as will be discussed later, RVFV strains deficient in functional NSs have been shown to be considerably less virulent than wild-type strains and could serve as potential attenuated vaccine candidates.
VACCINATION/ANTIVIRAL STRATEGIES
Within two decades of the characterization of RVFV, a neurotropic strain was developed by Smithburn (in the late 1940s) and was shown to be partially attenuated (60). This strain was subsequently used as a livestock vaccine, and it is still approved for veterinary use in African countries. While this attenuated strain (also known as the Smithburn strain) has shown to be potent in inducing protection from viral infection, its ability to induce abortions (34) and exhibit pathogenicity in European cattle (8) has limited its use to areas threatened by an imminent outbreak (5). Since then, other attenuated strains as well as other vaccine candidates have been developed to provide protection against Rift Valley fever outbreaks while exhibiting less adverse side effects than the Smithburn strain (Table 2). These candidates can be classified into four groups: live attenuated, inactivated, viral-recombinant, and DNA.
Table 2.
Summary of current vaccine strategies against RVFV
| Vaccine type | Description | Target | Example(s) | Reference(s) |
|---|---|---|---|---|
| Live attenuated | Viral mutants displaying decreased virulence | Whole virus | Smithburn strain, MP-12, clone 13 | 59, 48, 13 |
| Inactivated | Formalin-killed virus | Whole virus | TSI-GSD-200 | 53 |
| Virus-like particles | Recombinantly expressed glycoproteins that still possess neutralizing epitopes; resemble exterior of RVFV capsid | RVFV glycoproteins (Gn/Gc), sometimes in combination with RVFV nucleoprotein | VLPs generated from mammalian cell lines, Drosophila cell lines, baculovirus expression systems | 44, 50, 12, 52, 39 |
| Recombinant viral vectors | Induce viral infection and expression without possibility of viral revertant/recombination | RVFV glycoproteins (Gn/Gc) | Lumpy skin disease virus, alphavirus (i.e., VEEV), Newcastle disease virus, capripoxvirus, adenovirus | 64, 23, 34, 60, 27 |
| DNA | DNA plasmids that express recombinant RVFV proteins upon cellular transfection | RVFV glycoproteins and nucleoproteins | Mammalian expression DNA plasmids | 61, 41, 36 |
Live attenuated vaccines.
While the Smithburn strain continues to be the only strain approved for vaccine use in livestock, at least two other candidates that appear to exhibit a high level of protection while offering less virulence in the host, MP-12 and clone 13, have been developed.
MP-12 is an attenuated strain of RVFV that originated from the virulent Egyptian strain ZH548, which was isolated from a human patient. Created following 12 passages in the presence of the mutagen 5-fluorouracil, MP-12 was found to have mutations in all three segments (12). In a series of experiments performed by Morrill et al. (48, 49), inoculations of this strain were found to be effective in sheep and cattle. Furthermore, this vaccine has an important advantage for areas where RVFV is endemic: newborn lambs from immunized ewes acquire neutralizing antibodies via colostrums. However, experiments by Hunter et al. have shown that MP-12 can still be teratogenic in sheep (30), thereby raising some of the same safety concerns as those associated with the Smithburn strain.
The most recent attenuated strain of RVFV was obtained from an isolate from a mild human case of Rift Valley fever in the Central African Republic. This strain (then known as 74HB59) was shown to yield heterogeneous plaques when cultured in vitro. Upon further analysis of this strain, an individual clone was found to be highly immunogenic yet avirulent in mice and hamsters (the latter are particularly susceptible to RVFV infection) (50). Designated clone 13, this attenuated strain was found to have an in-frame deletion of most of the ORF of NSs. When assayed as a vaccine, this strain was shown to be effective in inducing protection in sheep without any noticeable pathogenic side effects. Furthermore, unlike the Smithburn and MP-12 strains, clone 13 does not appear to induce abortions in pregnant ewes (14), making it the most promising live attenuated vaccine candidate thus far.
Another potential attenuated vaccine was recently described. This new strain, designated R566, was derived from clone 13 and MP-12 by reassortment; it contains the S segment of clone 13 and the L and M segments of MP-12 and has a combination of attenuation markers from both strains (9). Initial challenge experiments using mice found that complete protection can be obtained following RVFV infection.
Inactivated vaccines.
Inactivated vaccines have the advantage of inducing a protective immune response without the concern of developing viral recombinant/revertant mutants associated with live vaccines. However, their use in RVFV vaccination strategies has been limited, mainly due to the expense associated with their preparation but also to the multiple inoculations required to elicit an effective level of immunity. Formalin-inactivated vaccines based on the formalin-killed Entebbe strain are available for use in livestock, although regular inoculation and boosting are required to maintain protective immunity (47, 54). This inconvenience, along with the overall cost of the vaccine, makes widespread vaccine regimens rather impractical for many African farmers/governments threatened with Rift Valley fever outbreaks.
Currently, formalin-inactivated RVFV TSI-GSD-200 is being used for vaccination of military and laboratory personnel who are or may be in contact with the virus (54). Originally isolated from an RVFV strain derived from African green monkey kidney cultures, this inactivated vaccine also requires multiple inoculations, thereby making its administration prohibitive for widespread human/animal use.
Virus-like particles.
Generated by reverse genetics or single eukaryotic expression vectors, VLPs offer the advantage of expressing the immunogenic components of a virus of interest (in this case, the neutralizing epitopes of RVFV glycoproteins) without the risks of using a functional replicating virus. In the case of RVFV, several reports have demonstrated that VLPs can be generated in mammalian cell lines and can induce protective immunity in mice (51, 53). It should also be noted that RVFV VLPs have been generated using baculovirus systems (40), although lysates from baculovirus-infected insect cells were used in the experiments and the use of Freund's complete adjuvant was required. However, it has recently been shown that baculovirus-generated VLPs purified from pooled supernatants can also confer protection in rats (45) when combined with an adjuvant. While the results are promising, current limitations in the large-scale preparation of VLPs must be addressed in order to make this vaccine candidate more economically viable for widespread animal inoculation.
Recombinant viral vectors.
Recombinant viruses offer two distinct advantages over conventional vaccine vectors: (i) they enable expression of the gene of interest through viral infection, thus enabling the host cell to process recombinant proteins in a manner similar to that with natural viral gene delivery, and (ii) the recombinant antigen of interest is not expressed through its natural viral host, thereby eliminating the risks associated with attenuated viruses (i.e., reversion to a pathogenic phenotype). To date, several different recombinant RVFV vaccine candidates have been described.
The first successful example of such a vaccine was demonstrated using lumpy skin disease virus (LSDV) (65). When RVFV Gn and Gc cDNA was expressed through this vector and inoculated into mice, neutralizing antibodies against RVFV were observed and complete protection from RVFV challenge was achieved. RVFV glycoproteins were also expressed in other established viral vector systems. A nonreplicating, complex adenovirus platform expressing RVFV Gn and Gc conferred complete protection in mice (28). Gn/Gc was also used as a vaccine target for an alphavirus vector, specifically, Venezuelan equine encephalitis virus (VEEV) (24). As is the case with LSDV and adenovirus vectors, the alphavirus vector conferred complete immunity from RVFV infection in mice. Finally, a modified vaccinia Ankara (MVA) vector encoding Gn/Gc has also been shown to elicit protection in mice upon lethal RVFV challenge (E. Lopez-Gil, unpublished observations).
Over the past year, two other recombinant vaccines have been described. The first is based on the avian paramyxovirus Newcastle disease virus (35). Similar to the other recombinant viral vectors, NDV expressing RVFV Gn and Gc provided complete protection in mice, and furthermore, a single intramuscular inoculation in lambs was sufficient to induce neutralizing antibodies (36), thereby making this vaccine a promising candidate for future investigation. Also, neutralizing antibodies against RVFV Gn were generated in calves, using a NDV expressing only the RVFV Gn protein (NDV-Gn) (13).
The second, and most recent, example uses poxvirus, in this case, an attenuated capripoxvirus KS1 strain encoding the viral glycoproteins. This system was also shown to exhibit protective capabilities upon challenge of mice and sheep with RVFV (61).
DNA vaccines.
DNA vaccines also hold promise as a prophylactic strategy against Rift Valley fever outbreaks. There are several advantages to genetic vaccines, such as (i) the ability to mass produce them at significantly less cost than that for attenuated or heat-killed vaccines; (ii) the ability to store/transport them under ambient conditions (i.e., no need for continuous refrigeration), a particular concern, considering the areas that are currently endemic for outbreaks of the disease; and (iii) their ability to express recombinant proteins that are highly similar to viral proteins expressed during infection (including conformational folding and posttranslational modifications), thereby increasing the chances of inducing potent immune responses against the antigen of interest. To date, two aspects of DNA immunization against RVFV have been studied. The first involves recombinant DNA expression of one or both RVFV glycoproteins (37, 42, 62, 65), while the second involves evaluation of the immunogenic properties of recombinantly expressed nucleoprotein (37, 42).
DNA vaccines encoding RVFV glycoproteins were originally described by Spik et al. (62) after previous studies in which vaccinia virus-induced expression of the glycoprotein-encoding M segment induced neutralizing antibodies in mice. In this study, two DNA vaccine constructs were generated, one beginning from the second AUG initiation site that encodes the nonstructural protein NSm (denoted NSm+) and both glycoproteins and the second initiating translation from the fourth AUG site, thereby omitting NSm (denoted NSm−). When administered in mice using a gene gun, the NSm construct was found to be the more potent of the two plasmids, resulting in the induction of neutralizing antibodies and conferring 100% protection from RVFV challenge, whereas mice inoculated with NSm+ constructs demonstrated significantly less protection, even when the constructs were used in conjunction with chemical adjuvants or in combination with other viral DNA vaccines.
Further studies using DNA vaccines were conducted in sheep, where pCMV plasmids encoding either the nucleoprotein (pCMV-N) or both glycoproteins (pCMV-M1) were evaluated for their ability to induce cell-mediated and/or humoral responses (43). In this case, 6-month-old sheep were inoculated three times every 3 weeks, and their sera were obtained. While no detectable antibodies were generated against the glycoproteins, transient cellular responses were found following a booster inoculation with the attenuated RVFV strain MP-12. This result demonstrated the use of plasmid DNA as a component in a prime-boost vaccination strategy.
Another advance in DNA vaccination involves the use of self-adjuvants fused to the cDNA of a protein of interest in order to augment the host immune response. Recently, this has been demonstrated using a plasmid vector encoding the C3d trimer fused to RVFV Gn (C3d-trimer-Gn). Compared to unfused Gn, C3d-trimer-Gn significantly decreased the level of morbidity in RVFV-challenged mice (6).
DISEASE TRANSMISSION/EPIDEMIOLOGY
While the current vaccine candidates described hold promise for preventing epidemics of Rift Valley fever in areas prone to outbreaks, even the most potent vaccines can take weeks to establish immunity in the individual. Therefore, it is imperative that techniques to predict future outbreaks be researched and refined in order to provide public health/veterinary officials advanced warning of pending RVFV transmissions. It is only with this vital information that meaningful vaccination strategies can be developed.
The first step in understanding RVFV epidemiology involves the study of how the virus is spread. As previously mentioned, RVFV is transmitted primarily through a variety of mosquito genera, in particular, Aedes, Culex, and Anopheles (12). While the virus is currently limited to Africa and the Middle East, recent studies have reported that regions in Europe (12) and North America (64) have mosquito genera capable of acting as RVFV transmission vectors. Furthermore, since RVFV can survive in dormant mosquito eggs for prolonged periods of time in arid conditions during periods referred to as interepizootic dry periods, RVF outbreaks tend to be associated with periods of prolonged rainfall (12, 26). It has been proposed that factors such as rainfall, ocean temperature, and climate change all play roles in determining the likelihood of an epidemic. Recently, an RVFV early warning system was developed using satellite measurements of sea surface temperatures, rainfall anomalies, and vegetation. This system was successful in predicting human/livestock outbreaks of Rift Valley fever in the horn of Kenya, Sudan, and southern Africa from 2006 to 2008 (3). Based on these results, it was further proposed that an early warning system, in conjunction with mosquito vector control programs in “preoutbreak” periods, could be effective in preventing large-scale fatalities associated with Rift Valley fever outbreaks.
Another risk assessment was recently made by Abdo-Salem et al. (1), this time looking at the probability of another introduction of RVFV to the Arabian Peninsula (following the first outbreak in 2000), with particular emphasis on Yemen. As in the previous study by Anyamba et al. (3), rainfall and mosquito vectors were considered, but this time, seasonal importation of livestock (due to religious festivals) was also evaluated. Ultimately, it was concluded that the probability of an outbreak was highest during the festival season, when RVFV transmission can occur via vector transmission (i.e., mosquitoes) and direct contact with infected livestock.
CONCLUDING REMARKS
As shown in this review, major advances in the study of RVFV and an extensive assortment of prophylactic strategies have been developed over the last several years. There is increasing consensus that the spread of Rift Valley fever beyond Africa is no longer a question of if, but when. While the scientific community has started to address the possibility of large-scale epidemics and preventive measures that can be used to stop them, there are still no low-cost, broadly effective vaccines approved for use by the general public. Furthermore, as previously discussed, the safety and cost limitations associated with the current approved veterinary vaccines leave significant room for improvement. Hopefully, some of the vaccine candidates discussed will be approved for wide-scale use in the near future. There is hope that in conjunction with recent epidemiological advances, the health and economic costs associated with Rift Valley fever virus can be contained.
ACKNOWLEDGMENTS
We thank S. M. de Boer (CVI, Lelystad, Netherlands) and D. Wallace (OVI, Onderstepoort, South Africa) for editorial assistance with the manuscript. We also thank S. Watowich (University of Texas Medical Branch, Galveston, TX) for contributing the electron microscopy image.
This work was supported by EPIZONE (EU Network of Excellence for Epizootic Disease Diagnosis and Control; contract CT2006-016236) and ARBO-ZOONET (EU Coordination and Support Action; grant KBBE-211757).
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Abdo-Salem S., et al. 2011. Risk assessment of the introduction of Rift Valley fever from the Horn of Africa to Yemen via legal trade of small ruminants. Trop. Anim. Health Prod. 43:471–480 [DOI] [PubMed] [Google Scholar]
- 2. Adam A. A., Karsany M. S., Adam I. 2010. Manifestations of severe Rift Valley fever in Sudan. Int. J. Infect. Dis. 14:e179–e180 [DOI] [PubMed] [Google Scholar]
- 3. Anyamba A., et al. 2009. Prediction of a Rift Valley fever outbreak. Proc. Natl. Acad. Sci. U. S. A. 106:955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balkhy H. H., Memish Z. A. 2003. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents 21:153–157 [DOI] [PubMed] [Google Scholar]
- 5. Bengis R. G., Swanepoel R., De Klerk M., Pienaar N. J., Prinsloo G. 2010. Rift Valley fever: current concepts and recent findings, p. 12–14 In Proceedings of the 9th Annual Congress of the South African Society for Veterinary Epidemiology and Preventive Medicine South African Society for Veterinary Epidemiology and Preventive Medicine, Pretoria, Republic of South Africa [Google Scholar]
- 6. Bhardwaj N., Heise M. T., Ross T. M. 2010. Vaccination with DNA plasmids expressing Gn coupled to C3d or alphavirus replicons expressing Gn protects mice against Rift Valley fever virus. PLoS Negl.Trop. Dis. 4:e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bird B. H., Ksiazek T. G., Nichol S. T., Maclachlan N. J. 2009. Rift Valley fever virus. J. Am. Vet. Med. Assoc. 234:883–893 [DOI] [PubMed] [Google Scholar]
- 8. Botros B., et al. 2006. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J. Med. Virol. 78:787–791 [DOI] [PubMed] [Google Scholar]
- 9. Bouloy M., Flick R. 2009. Reverse genetics technology for Rift Valley fever virus: current and future applications for the development of therapeutics and vaccines. Antiviral Res. 84:101–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouloy M., et al. 2001. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J. Virol. 75:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention 2007. Rift Valley fever outbreak—Kenya, November 2006-January 2007. MMWR Morb. Mortal. Wkly. Rep. 56:73–76 [PubMed] [Google Scholar]
- 12. Chevalier V., Pepin M., Plee L., Lancelot R. 2010. Rift Valley fever—a threat for Europe? Euro Surveill. 15:19506. [PubMed] [Google Scholar]
- 13. de Boer S. M., et al. 2010. Rift Valley fever virus subunit vaccines confer complete protection against a lethal virus challenge. Vaccine 28:2330–2339 [DOI] [PubMed] [Google Scholar]
- 14. Dungu B., et al. 2010. Evaluation of the efficacy and safety of the Rift Valley fever clone 13 vaccine in sheep. Vaccine 28:4581–4587 [DOI] [PubMed] [Google Scholar]
- 15. Ellis D. S., Simpson D. I., Stamford S., Abdel Wahab K. S. 1979. Rift Valley fever virus: some ultrastructural observations on material from the outbreak in Egypt 1977. J. Gen. Virol. 42:329–337 [DOI] [PubMed] [Google Scholar]
- 16. Faye O., et al. 2007. Rift Valley fever outbreak with East-Central African virus lineage in Mauritania, 2003. Emerg. Infect. Dis. 13:1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Filone C. M., Heise M., Doms R. W., Bertolotti-Ciarlet A. 2006. Development and characterization of a Rift Valley fever virus cell-cell fusion assay using alphavirus replicon vectors. Virology 356:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flick R., Bouloy M. 2005. Rift Valley fever virus. Curr. Mol. Med. 5:827–834 [DOI] [PubMed] [Google Scholar]
- 19. Gerdes G. H. 2004. Rift Valley fever. Rev. Sci. Tech. 23:613–623 [DOI] [PubMed] [Google Scholar]
- 20. Gerrard S. R., Bird B. H., Albarino C. G., Nichol S. T. 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359:459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerrard S. R., Nichol S. T. 2002. Characterization of the Golgi retention motif of Rift Valley fever virus GN glycoprotein. J. Virol. 76:12200–12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerrard S. R., Nichol S. T. 2007. Synthesis, proteolytic processing and complex formation of N-terminally nested precursor proteins of the Rift Valley fever virus glycoproteins. Virology 357:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giorgi C., et al. 1991. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian sandfly fever, and Uukuniemi viruses. Virology 180:738–753 [DOI] [PubMed] [Google Scholar]
- 24. Gorchakov R., et al. 2007. Comparative analysis of the alphavirus-based vectors expressing Rift Valley fever virus glycoproteins. Virology 366:212–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Habjan M., et al. 2009. NSs protein of Rift Valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 83:4365–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hendrickx G., Lancelot R. 2010. A perspective on emerging mosquito and phlebotomine-borne diseases in Europe. Euro Surveill. 15:19503. [PubMed] [Google Scholar]
- 27. Hewlett M. J., Pettersson R. F., Baltimore D. 1977. Circular forms of Uukuniemi virion RNA: an electron microscopic study. J. Virol. 21:1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holman D. H., et al. 2009. A complex adenovirus-vectored vaccine against Rift Valley fever virus protects mice against lethal infection in the presence of preexisting vector immunity. Clin. Vaccine Immunol. 16:1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huiskonen J. T., Overby A. K., Weber F., Grunewald K. 2009. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: evidence for GN-GC glycoprotein heterodimers. J. Virol. 83:3762–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hunter P., Erasmus B. J., Vorster J. H. 2002. Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J. Vet. Res. 69:95–98 [PubMed] [Google Scholar]
- 31. Ikegami T., et al. 2009. Dual functions of Rift Valley fever virus NSs protein: inhibition of host mRNA transcription and post-transcriptional downregulation of protein kinase PKR. Ann. N. Y. Acad. Sci. 1171(Suppl. 1):E75–S85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikegami T., Won S., Peters C. J., Makino S. 2005. Rift Valley fever virus NSs mRNA is transcribed from an incoming anti-viral-sense S RNA segment. J. Virol. 79:12106–12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kakach L. T., Wasmoen T. L., Collett M. S. 1988. Rift Valley fever virus M segment: use of recombinant vaccinia viruses to study Phlebovirus gene expression. J. Virol. 62:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamal S. A. 2009. Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol. J. 6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kortekaas J., et al. 2010. Rift Valley fever virus immunity provided by a paramyxovirus vaccine vector. Vaccine 28:4394–4401 [DOI] [PubMed] [Google Scholar]
- 36. Kortekaas J., et al. 2010. Intramuscular inoculation of calves with an experimental Newcastle disease virus-based vector vaccine elicits neutralizing antibodies against Rift Valley fever virus. Vaccine 28:2271–2276 [DOI] [PubMed] [Google Scholar]
- 37. Lagerqvist N., et al. 2009. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley fever virus cDNA constructs. Virol. J. 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le May N., et al. 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116:541–550 [DOI] [PubMed] [Google Scholar]
- 39. Le May N., et al. 2008. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu L., Celma C. C., Roy P. 2008. Rift Valley fever virus structural proteins: expression, characterization and assembly of recombinant proteins. Virol. J. 5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lopez N., Muller R., Prehaud C., Bouloy M. 1995. The L protein of Rift Valley fever virus can rescue viral ribonucleoproteins and transcribe synthetic genome-like RNA molecules. J. Virol. 69:3972–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lorenzo G., Martin-Folgar R., Hevia E., Boshra H., Brun A. 2010. Protection against lethal Rift Valley fever virus (RVFV) infection in transgenic IFNAR−/− mice induced by different DNA vaccination regimens. Vaccine 28:2937–2944 [DOI] [PubMed] [Google Scholar]
- 43. Lorenzo G., Martin-Folgar R., Rodriguez F., Brun A. 2008. Priming with DNA plasmids encoding the nucleocapsid protein and glycoprotein precursors from Rift Valley fever virus accelerates the immune responses induced by an attenuated vaccine in sheep. Vaccine 26:5255–5262 [DOI] [PubMed] [Google Scholar]
- 44. Lozach P. Y., et al. 2010. Entry of bunyaviruses into mammalian cells. Cell Host Microbe 7:488–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mandell R. B., et al. 2010. A replication-incompetent Rift Valley fever vaccine: chimeric virus-like particles protect mice and rats against lethal challenge. Virology 397:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mansuroglu Z., et al. 2010. Nonstructural NSs protein of Rift Valley fever virus interacts with pericentromeric DNA sequences of the host cell, inducing chromosome cohesion and segregation defects. J. Virol. 84:928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meadors G. F., III, Gibbs P. H., Peters C. J. 1986. Evaluation of a new Rift Valley fever vaccine: safety and immunogenicity trials. Vaccine 4:179–184 [DOI] [PubMed] [Google Scholar]
- 48. Morrill J. C., et al. 2010. Rapid accumulation of virulent Rift Valley fever virus in mice from an attenuated virus carrying a single nucleotide substitution in the mRNA. PLoS One 5:e9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morrill J. C., Mebus C. A., Peters C. J. 1997. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am. J. Vet. Res. 58:1104–1109 [PubMed] [Google Scholar]
- 50. Muller R., et al. 1995. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am. J. Trop. Med. Hyg. 53:405–411 [DOI] [PubMed] [Google Scholar]
- 51. Näslund J., et al. 2009. Vaccination with virus-like particles protects mice from lethal infection of Rift Valley fever virus. Virology 385:409–415 [DOI] [PubMed] [Google Scholar]
- 52. Pettersson R. F., von Bonsdorff C. H. 1975. Ribonucleoproteins of Uukuniemi virus are circular. J. Virol. 15:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pichlmair A., Habjan M., Unger H., Weber F. 2010. Virus-like particles expressing the nucleocapsid gene as an efficient vaccine against Rift Valley fever virus. Vector Borne Zoonotic Dis. 10:701–703 [DOI] [PubMed] [Google Scholar]
- 54. Pittman P. R., et al. 1999. Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine 18:181–189 [DOI] [PubMed] [Google Scholar]
- 55. Rabinowitz P., et al. 2006. Animals as sentinels of bioterrorism agents. Emerg. Infect. Dis. 12:647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raymond D. D., Piper M. E., Gerrard S. R., Smith J. L. 2010. Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation. Proc. Natl. Acad. Sci. U. S. A. 107:11769–11774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reguera J., Weber F., Cusack S. 2010. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 6:e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rönnholm R., Pettersson R. F. 1987. Complete nucleotide sequence of the M RNA segment of Uukuniemi virus encoding the membrane glycoproteins G1 and G2. Virology 160:191–202 [DOI] [PubMed] [Google Scholar]
- 59. Sherman M. B., Freiberg A. N., Holbrook M. R., Watowich S. J. 2009. Single-particle cryo-electron microscopy of Rift Valley fever virus. Virology 387:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smithburn K. C. 1949. Rift Valley fever: the neurotropic adaptation of the virus and the experimental use of this modified virus as a vaccine. Br. J. Exp. Pathol. 30:1–16 [PMC free article] [PubMed] [Google Scholar]
- 61. Soi R. K., et al. 2010. Protection of sheep against Rift Valley fever virus and sheep poxvirus with a recombinant capripoxvirus vaccine. Clin. Vaccine Immunol. 17:1842–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spik K., et al. 2006. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 24:4657–4666 [DOI] [PubMed] [Google Scholar]
- 63. Terasaki K., Murakami S., Lokugamage K. G., Makino S. 2011. Mechanism of tripartite RNA genome packaging in Rift Valley fever virus. Proc. Natl. Acad. Sci. U. S. A. 108:804–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Turell M. J., et al. 2008. Potential for North American mosquitoes to transmit Rift Valley fever virus. J. Am. Mosq. Control Assoc. 24:502–507 [DOI] [PubMed] [Google Scholar]
- 65. Wallace D. B., et al. 2006. Protective immune responses induced by different recombinant vaccine regimes to Rift Valley fever. Vaccine 24:7181–7189 [DOI] [PubMed] [Google Scholar]
- 66. Won S., Ikegami T., Peters C. J., Makino S. 2007. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J. Virol. 81:13335–13345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. World Health Organization 2010. Rift Valley fever, South Africa—update. Wkly. Epidemiol. Rec. 85:185–186 [PubMed] [Google Scholar]
- 68. Zamoto-Niikura A., Terasaki K., Ikegami T., Peters C. J., Makino S. 2009. Rift Valley fever virus L protein forms a biologically active oligomer. J. Virol. 83:12779–12789 [DOI] [PMC free article] [PubMed] [Google Scholar]