Abstract
Human herpesvirus 6 (HHV-6) is an important immunosuppressive and immunomodulatory virus that primarily infects immune cells and strongly suppresses the proliferation of infected cells. However, the mechanisms responsible for the regulation and suppression mediated by HHV-6 are still unknown. In this study, we examined the ability of HHV-6A to manipulate cell cycle progression in infected cells and explored the potential molecular mechanisms. We demonstrated that infection with HHV-6A imposed a growth-inhibitory effect on HSB-2 cells by inducing cell cycle arrest at the G2/M phase. We then showed that the activity of the Cdc2–cyclin B1 complex was significantly decreased in HHV-6A-infected HSB-2 cells. Furthermore, we found that inactivation of Cdc2–cyclin B1 in HHV-6A-infected cells occurred through the inhibitory Tyr15 phosphorylation resulting from elevated Wee1 expression and inactivated Cdc25C. The reduction of Cdc2–cyclin B1 activity in HHV-6-infected cells was also partly due to the increased expression of the cell cycle-regulatory molecule p21 in a p53-dependent manner. In addition, HHV-6A infection activated the DNA damage checkpoint kinases Chk2 and Chk1. Our data suggest that HHV-6A infection induces G2/M arrest in infected T cells via various molecular regulatory mechanisms. These results further demonstrate the potential mechanisms involved in immune suppression and modulation mediated by HHV-6 infection, and they provide new insights relevant to the development of novel vaccines and immunotherapeutic approaches.
INTRODUCTION
Human herpesvirus 6 (HHV-6) is a ubiquitous pathogen of the betaherpesvirus family, including cytomegalovirus and HHV-7, which primarily infects CD4+ T cells (49). Like other herpesviruses, HHV-6 establishes latency after the initial productive infection and thus is never cleared from its host (41). Two subtypes of HHV-6 have been identified: variants A and B (2, 42). Although these two variants are similar in sequence and genome organization, they are associated with different types of pathogenesis. HHV-6B causes exanthema subitum in young children (53). HHV-6A has been implicated in the etiology of several other pathologies, such as multiple sclerosis (47) and encephalitis (29). In addition, several lines of experimental and clinical evidence suggest that HHV-6A might accelerate AIDS progression (27). In particular, a recent study reported that HHV-6A can trigger faster progression of AIDS in simian immunodeficiency virus (SIV)-infected macaques (26).
Many viruses, including DNA viruses, retroviruses, and RNA viruses, can perturb the cell cycle during infection (5, 7). It has been speculated that HHV-6 infection might also disrupt a component of the cell cycle that links cytoplasmic growth with cell division (3). Recently, increasing evidence has demonstrated the different levels of cell cycle arrest in HHV-6-infected cells. It has been shown that HHV-6A infection induces cell cycle arrest at the G2/M phase in infected cord blood mononuclear cells (CBMCs) (9). Furthermore, recent studies have suggested that HHV-6A and HHV-6B infection can also alter E2F1/Rb pathways and cause cell cycle arrest in the G2/M phase in infected SupT1 T cells (30) and that HHV-6B infection of MOLT 3 cells causes cell cycle arrest at the G1 phase concomitant with p53 phosphorylation and accumulation (36). In addition, G1/S arrest induced by HHV-6 infection has been observed in other types of cells, such as epithelial cells and neural cells (11, 37). Although HHV-6 has been implicated in halting cell cycle progression, the precise mechanisms behind this phenomenon are not yet fully understood.
Cell cycle progression is critically regulated by sequential activation of cyclins and cyclin-dependent kinases (Cdks). In mammalian cells, the transition from G2 into mitosis is controlled by the activation of the Cdc2–cyclin B1 complex (4). The Cdc2 (also known as Cdk1) catalytic subunit is regulated by a series of coordinated phosphorylation and dephosphorylation events. Activation of Cdc2 is prevented by its phosphorylation at Thr14/Tyr15 by the protein kinases Wee1 and Myt1 (32, 39). Dephosphorylation of Thr14/Tyr15 by the protein phosphatase Cdc25 eventually activates the Cdc2–cyclin B1 complex, allowing progression to mitosis (48). The activity of Cdc2 is also regulated by the availability of the cyclin subunits. During S phase, cyclin B1 mRNA and protein begin to accumulate, and their levels become maximal at G2/M. As the cells pass through mitosis, cyclin B1 is ubiquitinated and degraded by the anaphase-promoting complex (APC) (34).
In further exploration of the mechanisms of immunosuppression and molecular regulation in infected cells mediated by HHV-6, we observed that HHV-6A infection of human T-lymphoblastoid HSB-2 cells promoted cell cycle arrest in the G2/M phase in infected cells. This cell cycle arrest was accompanied by inhibition of Cdc2–cyclin B1 kinase activity and a significant increase of phosphorylated Cdc2 at the Tyr15 inhibitory site. Furthermore, we show here for the first time that the G2/M arrest and reduced Cdc2–cyclin B1 activity induced by HHV-6A infection involve activation of the p53/p21 and Chk1/Chk2/Cdc25C pathways, as well as elevated Wee1 expression.
MATERIALS AND METHODS
Cells and viruses.
The human T-lymphoblastoid cell line HSB-2 was cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS). The GS strain of HHV-6 variant A was propagated in cord blood mononuclear cells (CBMCs), as described previously (51). HHV-6A in infected cell culture supernatants was concentrated by ultracentrifugation at 80,000 × g for 2 h and was then resuspended in a minimal volume of complete RPMI 1640 medium for experimental use. Supernatants from uninfected CBMCs cultured similarly to HHV-6A-infected cells were used as a mock infection control. Viral titers were determined as viral DNA equivalents by quantitative PCR and were confirmed by virus infection in inoculated cells. A multiplicity of infection (MOI) of 10 virus DNA copies per cell was used for all the experiments.
Immunofluorescence.
Mock-infected or HHV-6A-infected HSB-2 cells were fixed in 4% paraformaldehyde (in phosphate-buffered saline [PBS]), permeabilized in 0.5% Triton X-100 (in PBS), and stained with primary antibodies followed by a secondary antibody labeled with fluorescein isothiocyanate (FITC). The primary antibodies include anti-gp60/110 monoclonal antibodies (Chemicon International), anti-cyclin B1 (Santa Cruz Biotechnology), anti-phospho-Tyr15 Cdc2, anti-phospho-Ser216 Cdc25C, and anti-phospho-Ser15 p53 (Cell Signaling Technology).
Measurement of cell proliferation and viability.
HSB-2 cells were either mock infected or infected with HHV-6A. Cells were counted, and the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed, as described previously (23).
Cell cycle analysis by flow cytometry.
The cell cycle status and nuclear DNA contents were determined using propidium iodide (PI) staining and flow cytometry. Briefly, cells were collected and fixed with 70% ethanol. The cell pellets were first resuspended in 0.5 ml of PBS containing 50 μg/ml PI and 100 μg/ml RNase, then incubated at 4°C for 30 min, and finally analyzed by a flow cytometry.
Annexin V–PI staining.
Cells were collected and resuspended in the binding buffer, followed by the addition of annexin V–FITC (Bender) and PI solutions. The apoptotic cells were analyzed by flow cytometry.
Western blotting.
HHV-6A-infected and mock-infected cells were collected at 0, 24, 48, and 72 h postinfection. Cell lysates were prepared and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore) and were detected with the corresponding primary and secondary antibodies. The immune complexes were detected using an enhanced chemiluminescence kit (Applygen). The antibodies used in Western blotting included anti-Cdc2, anti-phospho-Tyr15 Cdc2, anti-p53, anti-phospho-Ser15 p53, anti-p21, anti-Wee1, anti-Cdc25C, anti-phospho-Ser216 Cdc25C, anti-Myt1, anti-Chk1, anti-phospho-Ser345 Chk1, anti-Chk2, anti-phospho-Thr68 Chk2, anti-β-actin (Cell Signaling Technology), and anti-cyclin B1 (Santa Cruz Biotechnology).
Immunoprecipitation and Cdc2–cyclin B1 kinase assay.
Cell lysates obtained from HHV-6A-infected, mock-infected, or nocodazole-treated cells were prepared as described previously (31). Lysate supernatants were incubated with an anti-cyclin B1 antibody (Santa Cruz Biotechnology) for 1 h at 4°C using a Catch and Release (version 2.0) reversible immunoprecipitation system (Millipore). The Cdc2–cyclin B1 kinase activity in the cells was measured using a Cyclex (Nagano, Japan) Cdc2–cyclin B kinase assay kit according to the manufacturer's protocol.
Statistical analysis.
Unless otherwise indicated, data were expressed as means ± standard deviations (SD). The significance of differences between groups was determined by a Student t test. A P value of <0.05 was considered significant.
RESULTS
Inhibition of cell proliferation mediated by HHV-6A infection in HSB-2 cells.
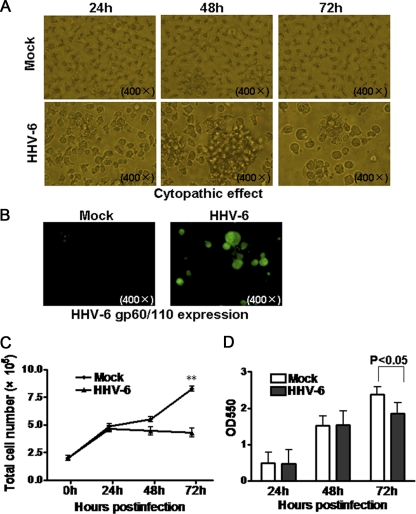
HSB-2, a T-lymphoblastoid cell line permissive for HHV-6A infection, was used in the current study. After infection with HHV-6A, HSB-2 cells showed typical cytopathic effects (CPE), rounding and forming giant multinucleated syncytia from the fusion of infected cells (Fig. 1A). To further confirm HHV-6A infection in HSB-2 cells, the expression of the late protein gp60/110, essential for HHV-6A propagation, in infected HSB-2 cells was analyzed using an immunofluorescence assay. Prominent expression of HHV-6 gp60/110 was detected in HHV-6A-infected HSB-2 cells compared with that in control mock-infected cells (Fig. 1B). These results indicate that HHV-6A could efficiently infect HSB-2 cells.
Fig. 1.
Inhibition of HSB-2 cell proliferation mediated by HHV-6A infection. (A) HHV-6A infection exhibited typical cytopathic effects in infected HSB-2 cells. The morphological characteristics of mock-infected or HHV-6A-infected HSB-2 cells were observed under a light microscope at various time points postinfection. (B) HHV-6 gp60/110 expression on mock-infected or HHV-6A-infected HSB-2 cells at 72 h postinfection. gp60/110 protein expression was determined by immunofluorescence analysis with an anti-gp60/110 monoclonal antibody. (C) HHV-6A significantly decreased HSB-2 cell numbers during virus infection. Cell numbers were counted and plotted at various time points postinfection. Data shown are means ± SD from five repeated experiments. Asterisks indicate a significant difference (**, P < 0.01) from the control group. (D) HHV-6A infection strongly inhibited the proliferation of infected HSB-2 cells at 72 h postinfection. Cell proliferation was determined by an MTT assay. Data shown are means ± SD from five repeated experiments.
We then determined whether HHV-6A infection affects the growth of HSB-2 cells. Viable HSB-2 cells, either infected with HHV-6A or mock infected, were counted at 24-h intervals. The numbers of HHV-6A-infected HSB-2 cells were significantly lower than the numbers of mock-infected cells at 72 h postinfection (Fig. 1C). Further analysis of cell proliferation using an MTT assay also showed that HHV-6A infection efficiently inhibited the growth of HSB-2 cells at 72 h postinfection (Fig. 1D).
HHV-6A infection results in G2/M arrest in HSB-2 cells.
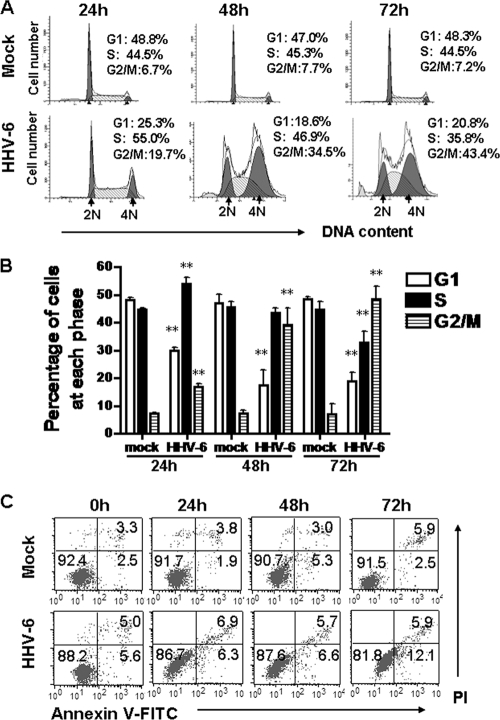
The inhibition of cell growth in HHV-6A-infected cells prompted us to determine whether viral infection is associated with an arrest of cell division during a specific phase in the cell cycle. To test this possibility, cell cycle analyses of mock- and HHV-6A-infected HSB-2 cells were performed at 24, 48, and 72 h postinfection by measuring DNA content with PI staining. Representative cell cycle profiles and histograms of mock- and HHV-6A-infected cells are presented in Fig. 2A and B, respectively. Cell cycle analyses showed that mock-infected HSB-2 cells maintained the normal cell cycle profile during 3 days of culture. However, HHV-6A infection remarkably decreased the proportion of cells in G1 phase and significantly increased the number of cells in G2/M phase with increasing infection time. The percentage of infected cells in the G2/M phase was almost 8-fold higher than the corresponding percentage of control cells (48.33% ± 4.82% versus 6.89% ± 1.05%) at 72 h postinfection. At the same time point, the percentage of HHV-6A-infected cells in the G1 phase was 2.5-fold lower than the corresponding percentage of mock-infected cells (18.92% ± 3.14% versus 48.05% ± 0.94%). In addition, the percentage of HHV-6A-infected HSB-2 cells in S phase was slightly higher than the corresponding percentage of mock-infected cells at 24 h postinfection (53.84% ± 1.62% versus 44.76% ± 0.50%) and then decreased by 48 h (43.60% ± 2.92% versus 45.59% ± 2.35%) and 72 h (32.75% ± 4.23% versus 44.81% ± 2.05%) postinfection (Fig. 2B). These results demonstrate that HHV-6A infection of HSB-2 cells promotes cell cycle progression from G1 and S into G2/M phase and then induces arrest in G2/M phase.
Fig. 2.
HHV-6A infection leads to growth arrest at the G2/M phase in infected HSB-2 cells. (A and B) HHV-6A infection remarkably decreased the proportions of HSB-2 cells in G1 phase and increased HSB-2 cell populations in G2/M phase with increasing infection time. (A) The relative DNA contents of HSB-2 cells at different time points after HHV-6A infection were determined by PI staining and were analyzed by flow cytometry. The 2N (diploid) and 4N (tetraploid) DNA contents represent the G1 and G2/M phases of the cell cycle, respectively. The cell populations in the sub-G1 (<2N) phase represent apoptotic cell fragments. (B) Histograms represent the percentages of mock- and HHV-6A-infected HSB-2 cells in the G1, S, and G2/M phases of the cell cycle at 24, 48, and 72 h postinfection. Data are means ± SD from three independent experiments. Asterisks indicate a significant difference (**, P < 0.01) from results for the control. (C) Mock- and HHV-6A-infected HSB-2 cells were stained with annexin V–PI and were analyzed by flow cytometry.
It has been demonstrated that HHV-6A infection can induce apoptosis in different types of cells (14, 19, 54). To investigate the apoptotic effect on infected cells mediated by HHV-6A, mock- and HHV-6A-infected HSB-2 cells were stained with annexin V–FITC and PI and were then analyzed by fluorescence-activated cell sorting (FACS) during the first 3 days after infection. As shown in Fig. 2C, more than 90% of mock-infected cells were viable and negative for annexin V staining during the 3 days of infection. Furthermore, only slightly increased apoptotic cell populations were observed in HHV-6A-infected cells at 72 h postinfection (the percentages of apoptotic cells at this time point were about 18% for virus-infected cells and 8.4% for mock-infected cells) (Fig. 2C), suggesting that only a minority of HHV-6A-infected cells underwent apoptosis during the first 3 days after infection. Taken together, these results clearly demonstrate that inhibition of cell proliferation by HHV-6A infection is due mainly to the induction of cell cycle arrest in infected cells rather than to induction of apoptosis during the first 3 days after infection.
The level of cyclin B1 is significantly increased in HHV-6-infected cells.
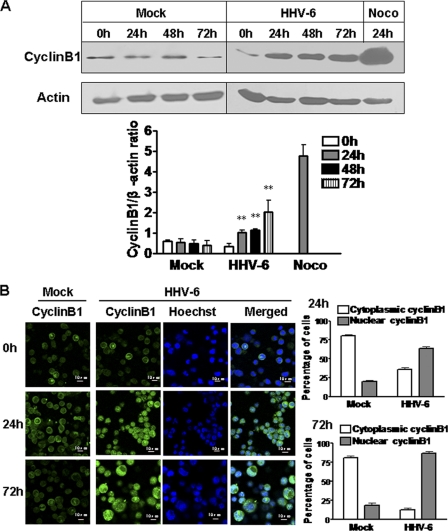
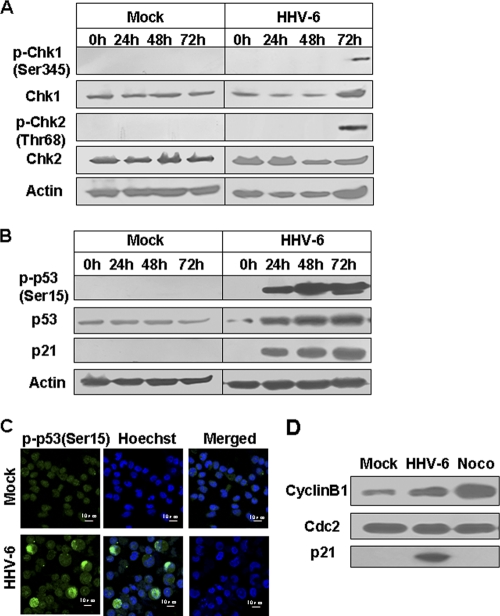
Cyclin B1 is an important regulator during normal cell cycle progression. It accumulates in the S and G2 phases to form a mitosis-promoting factor (MPF) with Cdc2 and is then ubiquitinated and degraded by the anaphase-promoting complex (APC) after the cells pass through mitosis (34). We thus determined whether HHV-6A infection altered the cyclin B1 expression level in HHV-6A-infected HSB-2 cells. At various intervals after infection, HSB-2 cell lysates were collected, and cyclin B1 protein expression was determined by Western blot analyses. As expected, HHV-6A infection significantly increased cyclin B1 expression in HSB-2 cells at 24 h postinfection (a 3-fold increase over the level in mock-infected cells), and the expression level was further progressively increased at 48 and 72 h postinfection (4-fold and 8-fold increases, respectively, over levels in mock-infected cells). However, only minimal cyclin B1 expression was detected in mock-infected cells at various times. In addition, we detected a significant increase in cyclin B1 levels in HSB-2 cells treated with the mitotic inhibitor nocodazole, which is known to block cell cycle progression in M phase through disruption of mitotic spindles, and which served as a positive control (Fig. 3A).
Fig. 3.
HHV-6A infection induces increased cyclin B1 expression. (A) HHV-6A infection significantly increased cyclin B1 expression in HSB-2 cells. Lysates from mock-infected or HHV-6A-infected HSB-2 cells were prepared at the indicated time points and were processed for Western blotting with a specific antibody against cyclin B1. HSB-2 cells treated with 0.5 μg/ml nocodazole (Noco) for 24 h served as a positive control. Cyclin B1 protein expression levels (shown in the histogram below the blot) were analyzed quantitatively and were compared with β-actin expression by use of a densitometer. Results are means ± SD from three independent experiments. Asterisks indicate significant differences (**, P < 0.01) from the results for the mock-infected control. (B) Cyclin B1 was highly expressed and prominently located in the nucleus in HHV-6A-infected HSB-2 cells. Cyclin B1 expression was determined by an indirect immunofluorescence assay. Mock- and HHV-6A-infected HSB-2 cells were stained for cyclin B1 (green) and DNA (blue) with an anti-cyclin B1 antibody and Hoechst stain, respectively, at 0, 24, and 72 h postinfection. The percentages of cells positive for cytoplasmic or nuclear cyclin B1 are shown in the histograms on the right. Results are means ± SD from three independent experiments.
Cyclin B1 shuttles between the nucleus and cytoplasm during interphase, but it is targeted to the nucleus during M phase (50). Several reports have shown that virus infection-induced cell cycle arrest in G2 is due to the prevention of nuclear localization of cyclin B1 (8, 31). We thus reasoned that HHV-6A infection may also alter cyclin B1 translocation in infected cells. We determined the subcellular localization of cyclin B1 in HHV-6A-infected HSB-2 cells at 24 and 72 h postinfection using immunofluorescence analysis with confocal microscopy. As shown in Fig. 3B, we observed that cyclin B1 existed mainly in the cytoplasm at a hardly detectable level in mock-infected cells, whereas cyclin B1 was highly expressed and prominently located in the nucleus in HHV-6A-infected cells (Fig. 3B). These results clearly indicate that HHV-6A-induced G2/M phase arrest in infected cells does not result from loss of cyclin B1 or from interference with its nuclear translocation.
HHV-6A infection inhibits Cdc2 activity and increases Cdc2 phosphorylation.
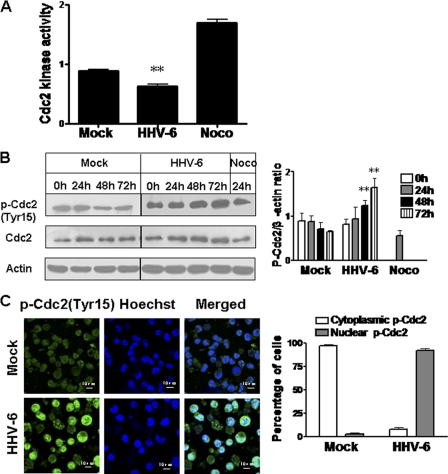
Cdc2 kinase activity plays an important regulatory role in the G2-to-M transition of the cell cycle (4). We therefore proceeded to examine Cdc2 activity in HSB-2 cells following HHV-6A infection. Cdc2–cyclin B1 complexes were isolated from HSB-2 cell lysates at 72 h postinfection by immunoprecipitation with an anti-cyclin B1 antibody, and Cdc2 kinase activity was determined. We found that HHV-6A infection significantly reduced Cdc2 kinase activity in infected cells from that in mock-infected cells (Fig. 4A). Furthermore, we observed that nocodazole-treated cells had markedly higher Cdc2 kinase activity than HHV-6-infected cells, although the percentages of cells arrested in the G2/M phase (approximately 43.4%) among HHV-6A-infected and nocodazole-treated cells were similar (data not shown). This finding suggested that HHV-6A infection downregulated the kinase activity of the Cdc2–cyclin B1 complex.
Fig. 4.
HHV-6A infection inhibits Cdc2 kinase activity and increases phosphorylated Cdc2 expression in HSB-2 cells. (A) HHV-6A infection significantly reduced Cdc2 kinase activity in infected HSB-2 cells. HSB-2 cells were either infected with HHV-6A or mock infected as a control for 72 h. HSB-2 cells treated with 0.5 μg/ml nocodazole (Noco) for 24 h served as a positive control. Cdc2 kinase activity was determined using a Cdc2–cyclin B kinase assay kit. Data are means ± SD from three independent experiments. Asterisks indicate significant differences (**, P < 0.01) from results for the two controls. (B) HHV-6A infection induced phosphorylation of Cdc2 in infected HSB-2 cells. (Left) Lysates from mock-infected or HHV-6A-infected HSB-2 cells were prepared at the indicated time points and were processed for Western blotting with specific antibodies against Cdc2 and phosphorylated Cdc2 (Tyr15). HSB-2 cells treated with 0.5 μg/ml Noco for 24 h served as a positive control. (Right) Phosphorylated Cdc2 protein levels were quantitatively analyzed and were compared with β-actin expression by use of a densitometer. Results are means ± SD from three independent experiments. Asterisks indicate significant differences (**, P < 0.01) from results for the mock-infected control. (C) Phosphorylated Cdc2 was highly expressed in HHV-6A-infected HSB-2 cells. (Left) Phospho-Cdc2 (Tyr15) expression was determined using an indirect immunofluorescence assay. Mock- and HHV-6A-infected HSB-2 cells were stained for Cdc2-Tyr15 (green) and DNA (blue) with an anti-phospho-Cdc2 (Tyr15) antibody and Hoechst stain at 72 h postinfection. (Right) Histogram showing percentages of cells positive for cytoplasmic phospho-Cdc2 (Tyr15) or nuclear phospho-Cdc2 (Tyr15). Results are means ± SD from three independent experiments.
Given that HHV-6A infection significantly upregulated cyclin B1 expression and decreased Cdc2 kinase activity in infected cells, we reasoned that the arrest of the G2-to-M transition induced by HHV-6A infection might be mediated by a specific inactivation of Cdc2. Since the Cdc2–cyclin B1 complex is maintained in an inactive form by phosphorylation of the residues Thr14 and Tyr15 in Cdc2, we examined Cdc2 expression and phosphorylation using Western blot analyses. The level of Cdc2-Tyr15 phosphorylation in HHV-6A-infected cells was obviously higher than that in mock-infected cells, by as much as 3-fold at 72 h postinfection. In addition, the level of Cdc2-Tyr15 phosphorylation in nocodazole-treated cells was markedly lower than that in HHV-6A-infected cells (Fig. 4B). The increase in Cdc2-Tyr15 phosphorylation and nuclear distribution in HHV-6A-infected cells was further confirmed by immunofluorescence analysis (Fig. 4C).
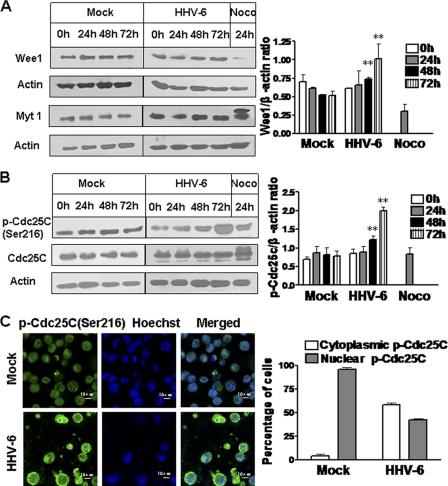
Effects of HHV-6A infection on G2/M cell cycle-regulatory proteins Wee1, Myt1, and Cdc25C.
Cdc2 kinase activity is negatively regulated by kinases Wee1 and Myt1 and is positively regulated by phosphatase Cdc25C (39, 48). We thus proceeded to determine whether these G2/M cell cycle-regulatory proteins were involved in the decrease in Cdc2 activity during HHV-6A infection. As shown in Fig. 5A, Wee1 expression levels were higher in HHV-6A-infected cells than in mock-infected cells at 48 h (1.4-fold) and 72 h (2-fold) postinfection, even through the majority of HHV-6-infected cells were still in G2/M phase at these time points. These data suggest that Wee1 is involved in the regulation of G2/M arrest induced by HHV-6A infection. As expected, Wee1 expression in nocodazole-treated cells was hardly detected, as a result of protein degradation during M phase. Furthermore, the effect of HHV-6A infection on Myt1 expression was also determined. Western blot analyses showed no obvious alteration of Myt1 expression in HHV-6A-infected cells from that in mock-infected cells (Fig. 5A). In addition, we found that nocodazole-treated cells revealed the presence of an additional, slower-migrating form of Myt1 (inactive form). Given that Myt1 has been shown to be inactivated by phosphorylation, our result suggested that HHV-6A infection may block Myt1 inactivation by its upstream kinase during the G2/M phase transition. Our results also suggested that enhancement of Wee1 expression and maintenance of steady Myt1 activity may be directly responsible for the increased Cdc2 phosphorylation in HHV-6A-infected cells.
Fig. 5.
HHV-6A infection enhances Wee1 expression, maintains Myt1 activity, and increases Cdc25C phosphorylation. (A) Wee1 and Myt1 expression in mock-infected or HHV-6A-infected HSB-2 cells. (Left) Cell lysates were collected at the indicated time points postinfection, and expression of Wee1 and Myt1 was determined by Western blotting. HSB-2 cells treated with 0.5 μg/ml nocodazole (Noco) for 24 h served as a positive control. (Right) Wee1 expression levels were quantitatively analyzed and were compared with β-actin expression by use of a densitometer. Results are means ± SD from three independent experiments. Asterisks indicate significant differences (**, P < 0.01) from results for the mock-infected control. (B) HHV-6A infection induced phosphorylation of Cdc25C in infected HSB-2 cells. (Left) Lysates from mock-infected or HHV-6A-infected HSB-2 cells were prepared at the indicated time points and were processed for Western blotting with specific antibodies against Cdc25C and phospho-Cdc25C (Ser216). HSB-2 cells treated with 0.5 μg/ml Noco for 24 h served as a positive control. (Right) Phosphorylated Cdc25C protein levels were quantitatively analyzed and were compared with β-actin expression levels by use of a densitometer. Results are means ± SD from three independent experiments. Asterisks indicate significant differences (**, P < 0.01) from results for the mock-infected control. (C) Cytoplasmic accumulation of phospho-Cdc25C (Ser216) in HHV-6A-infected HSB-2 cells. (Left) Mock- and HHV-6A-infected HSB-2 cells were stained for phospho-Cdc25C (Ser216) (green) and DNA (blue) with an anti-phospho-Cdc25C (Ser216) antibody and Hoechst stain at 72 h postinfection. (Right) Histogram showing percentages of cells positive for cytoplasmic phospho-Cdc25C (Ser216) or nuclear phospho-Cdc25C (Ser216). Results are means ± SD from three independent experiments.
To determine whether the increased inhibitory phosphorylation of Cdc2 following HHV-6A infection also resulted from inactivation of Cdc25C, a Cdc2-specific phosphatase, we analyzed the phosphorylation status of Cdc25C. As shown in Fig. 5B, HHV-6A-infected cells showed increases in the levels of the Ser216-phosphorylated form of Cdc25C compared with those in mock-infected controls at 48 and 72 h postinfection. Recent studies have suggested that phosphorylation of Cdc25C on Ser216 by Chk1 or Chk2 leads to 14-3-3 protein binding, resulting in the sequestration of Cdc25C in the cytoplasm. Cytoplasmic accumulation of phospho-Cdc25C (Ser216) denies access to its substrate Cdc2 subunit and prevents cells from going into mitosis by keeping the MPF inactive, resulting in the arrest of cells at G2/M (16). We next examined the distribution of phospho-Cdc25C (Ser216) in HHV-6-infected cells by immunofluorescence analysis. As shown in Fig. 5C, phospho-Cdc25C (Ser216) expression was obviously detectable and occurred mainly in the cytoplasm of HHV-6A-infected cells, in contrast with that in mock-infected cells. These results collectively suggest that HHV-6A infection not only can increase the level of Cdc2 phosphorylation in HHV-6A-infected cells by enhancing Wee1 expression but also can prevent Cdc2 dephosphorylation by inhibiting the Cdc2-dependent phosphatase Cdc25C, resulting in the inhibition of Cdc2 activity in infected cells.
HHV-6A infection results in changes in the expression and/or phosphorylation status of several cell cycle checkpoint proteins.
To further explore the molecular mechanisms responsible for HHV-6A-induced cell cycle arrest at the G2/M phase, we examined the expression and phosphorylation status of the key cell cycle checkpoint proteins, including Chk1, Chk2, p21, and p53, in HSB-2 cells. The DNA damage checkpoint kinases, Chk1 and Chk2, play important roles in regulating the G2/M checkpoint. They can phosphorylate Cdc25C on Ser216 by an ATM/ATR-dependent pathway (28, 55). Given that several studies have reported that Chk1 and Chk2 regulate cell cycle arrest in response to virus-induced stress (22, 24, 35) and that we also found increased phospho-Cdc25C (Ser216) levels in HHV-6A-infected cells, we asked whether DNA damage responses and these two kinases were also involved in the regulation of Cdc25C activation during HHV-6A infection. To test this possibility, Chk1 and Chk2 activation in HHV-6A-infected HSB-2 cells was assessed by Western blot analyses with phospho-specific anti-Chk1 (Ser317) and anti-Chk2 (Thr68) antibodies. As expected, HHV-6A infection significantly induced Chk1 and Chk2 activation in infected HSB-2 cells at 72 h postinfection, resulting in increased phosphorylation of Chk1 at Ser345 and of Chk2 at Thr68 (Fig. 6A).
Fig. 6.
Expression and/or phosphorylation of several cell cycle checkpoint proteins in HHV-6A-infected HSB-2 cells. (A) HHV-6A infection significantly induced phosphorylation of Chk1 and Chk2 at 72 h postinfection. Lysates from mock- or HHV-6A-infected HSB-2 cells were prepared at the indicated time points and were processed for Western blotting with specific antibodies against Chk1, phospho-Chk1 (Ser345), Chk2, and phospho-Chk2 (Thr68). (B) HHV-6A infection markedly induced the expression of p53, phosphorylated p53, and p21 in HSB-2 cells. Cell lysates were prepared as for panel A, and expression of p53, phosphorylated p53, and p21 was determined by Western blotting. (C) Phospho-p53 (Ser15) expression in HSB-2 cells was visualized using an indirect immunofluorescence assay. HHV-6A- and mock-infected cells were stained for phospho-p53 (Ser15) (green) and DNA (blue) with an anti-phospho-p53 (Ser15) antibody and Hoechst stain at 72 h postinfection. (D) Physical interactions between p21 and Cdc2–cyclin B1 in HSB-2 cells induced by HHV-6A infection. The Cdc2–cyclin B1 complex was precipitated with an anti-cyclin B1 antibody from lysates of HHV-6A-infected HSB-2 cells at 72 h postinfection. HSB-2 cells treated with 0.5 μg/ml nocodazole (Noco) for 24 h served as a positive control. Immunocomplexes were denatured and separated on denaturing polyacrylamide gels for Western blotting with anti-Cdc2, anti-cyclin B1, and anti-p21 antibodies.
The tumor suppressor protein p53 also plays a critical role in regulating cell cycle progression. We thus investigated whether HHV-6A infection could induce p53 expression. Indeed, we detected increased levels of total p53 in HHV-6A-infected cells in a time-dependent manner (Fig. 6B). Phosphorylation at Ser15 in p53 is a common event in response to DNA damage and other stresses, leading to enhanced p53 stability (43). To examine the consequences of HHV-6A infection for p53 phosphorylation, we next measured phospho-p53 (Ser15) levels in HHV-6A-infected HSB-2 cells using Western blot and immunofluorescence analyses with an anti-p53 (Ser15) antibody. As shown in Fig. 6B and C, higher phospho-p53 (Ser15) expression was observed in HHV-6A-infected cells than in control cells, consistent with the recently reported observation that HHV-6B induced phosphorylation of p53 at Ser15 in a manner dependent on ATM kinase in infected cells (36).
Phosphorylation of p53 usually correlates both with the accumulation of total p53 protein and with the ability of p53 to transactivate downstream target genes, including p21, a cyclin-dependent kinase inhibitor (CKI) that inhibits the activity of Cdc2–cyclin B1 complexes. We next determined the involvement of p53 and p21 in the reduction of Cdc2 activity following HHV-6A infection. As shown in Fig. 6B, we found remarkably elevated p21 expression in HHV-6A-infected cells at different time points postinfection. In addition, coimmunoprecipitation experiments further confirmed the interactions between p21 and Cdc2–cyclin B1 in HSB-2 cells induced by HHV-6A infection (Fig. 6D). These results suggest that activation of the p53/p21 signaling pathway in infected cells mediated by HHV-6A infection might account for part of the reduction in Cdc2 activity, leading to the accumulation of cells in the G2/M phase.
DISCUSSION
HHV-6 was initially isolated from peripheral blood mononuclear cells of immunologically deprived AIDS patients and patients with lymphoproliferative disorders (25, 40). Like other herpesviruses, HHV-6 is able to induce latent infection and to persist indefinitely in the host. However, in immunocompromised patients, such as organ transplant recipients and those with AIDS, HHV-6 reactivation or reinfection may cause severe opportunistic diseases, such as encephalitis, pneumonitis, hepatitis, and infectious mononucleosis-like disease, as well as bone marrow graft failure (1, 10, 52). Both HHV-6A and HHV-6B can infect several types of immune cells, either productively or nonproductively. The primary target for HHV-6 replication is CD4+ T lymphocytes, a pivotal cell type in the generation of humoral and cell-mediated adaptive immune responses. By modulating the specific antiviral immune response, HHV-6 can facilitate its own spread and persistence in vivo, as well as enhancing the pathogenic effects mediated by other infectious agents, such as human immunodeficiency virus (HIV). To date, the immune evasion and regulation mechanisms deployed by HHV-6 are poorly characterized. In this study, we investigated the molecular process by which HHV-6A infection induces cell cycle perturbation in infected T cells, which may be one of the key mechanisms responsible for HHV-6-induced immunosuppression in infected hosts.
Manipulation of the cell cycle in infected cells is a common strategy used by many viruses to regulate their infection. In this study, we demonstrated that HHV-6A infection promoted a dramatic level of cell cycle arrest in the G2/M phase in infected cells, resulting from the inhibition of Cdc2–cyclin B1 kinase activity. To explore the mechanisms responsible for the promotion of cell cycle arrest by HHV-6A, we further identified three potential mechanisms utilized by HHV-6A to cause the reduction of Cdc2–cyclin B1 kinase activity. First, HHV-6A infection increases Cdc2 phosphorylation at the inhibitory site in infected cells, which involves elevated Wee1 expression and the inactivation of Cdc25C. In support of our findings, recent studies with other viruses have shown that reovirus infection also induces G2/M-phase cell cycle arrest, which is associated with an inhibition of Cdc25C activity (38). In addition, HIV Vpr protein can inhibit Cdc2 activity via activation of Wee1 or inactivation of Cdc25C (15, 21). Second, HHV-6A infection activates the DNA damage responses. DNA damage checkpoint kinases Chk2 and Chk1 are under the control of the phosphatidylinositol 3-kinase-like proteins ATR and ATM. Several studies have reported that virus infection could elicit DNA damage responses in infected cells. It has been reported that herpes simplex virus (HSV) infection elicits a cellular DNA damage response dependent on ATM (44). Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment for viral lytic replication (22). Large T antigen promotes JC virus replication in G2-arrested cells by inducing ATM- and ATR-mediated G2 checkpoint signaling (35). In this study, we demonstrated that infection of HSB-2 cells with HHV-6A significantly induced Chk1 and Chk2 activation, resulting in increased phosphorylation of Chk2 at Thr68 and of Chk1 at Ser345. However, our studies showed that phosphorylation of Chk2 and Chk1 was seen only at 72 h postinfection (Fig. 6A). These data suggested that HHV-6A infection at a late stage might elicit the ATM/ATR DNA damage checkpoint signaling pathway, which responds to viral replication stress. We postulate that activation of DNA damage-associated protein checkpoint kinases, such as Chk, is not a direct cause for the G2/M arrest. However, the induced DNA damage responses might be a later event of virus infection that helps to maintain the inactivation of the Cdc2–cyclin B1 complex and the G2/M arrest in the late stage of HHV-6 infection.
Finally, HHV-6A infection induces the activation of p53/p21 signaling in infected cells, resulting in increased expression and phosphorylation of p21 and p53. The activation of p53 results in p21 expression and p21 protein subsequently binding to the Cdc2–cyclin B1 complex, which inhibits the activity of the complex and blocks the G2/M transition. Hollsberg and coworkers have shown that HHV-6B infection of MOLT 3 cells caused cell cycle arrest at the G1 phase concomitant with p53 phosphorylation and accumulation but that p21 was not upregulated in HHV-6B-infected T cells (36). Furthermore, studies with HHV-6A-infected cord blood mononuclear cells also showed accumulation of infected cells in the G2/M phase, but here, too, p21 accumulation was absent (9). In contrast, in our current study, we demonstrated significant induction of p21 expression in HHV-6-infected HSB-2 cells in a time-dependent manner, suggesting that unique regulatory pathways and mechanisms induced by HHV-6 infection may exist for individual types of infected cells. In support of our notion, we also determined whether HHV-6A infection can induce the same cell cycle arrest phenomena in other cell lines that occurred in HSB-2 cells. JJHAN, another T cell line for HHV-6 propagation, was used for this study. Cell cycle analyses of mock- and HHV-6A-infected JJHAN cells were performed at 24, 48, and 72 h postinfection. We found that HHV-6A infection decreased the proportion of JJHAN cells in the G1 phase and increased the number of cells in the S phase with increasing infection time (data not shown). In addition, studies from other groups suggested that G1/S arrest induced by HHV-6 infection has been observed in other types of cells, such as epithelial cells and neural cells (11, 37). These results clearly suggested that the regulatory pathways and mechanisms induced by HHV-6 infection might be different for different types of infected cells.
The present study has suggested that HHV-6A is able to regulate several key cellular regulatory proteins, resulting in G2/M cell cycle arrest. Many viruses interact with the host cell division cycle to create an optimal environment for their own growth. A recent study suggested that influenza A virus may produce favorable conditions for viral protein accumulation and virus production in infected cells by inducing G0/G1-phase cell cycle arrest (18). It was also discovered that viral protein expression and progeny virus production were greater in G2/M-phase-arrested cells. For example, it was reported that avian reovirus (ARV) p17 protein facilitates virus replication through initiation of G2/M arrest and host cellular translation shutoff (6). Cell cycle arrest at the G2/M phase was also observed to boost both early and late steps of HIV infection (17). In addition, the avian coronavirus infectious bronchitis virus (IBV) induces G2/M-phase arrest in infected cells to promote viral replication (12). Our studies suggested that HHV-6, like other viruses, may also have evolved mechanisms to alter the physiology of the host cells during viral infection in a manner beneficial to viral replication and pathogenesis. The benefits of HHV-6A-induced cell cycle arrest may be explained by several hypotheses, such as increasing the efficiency of transcription, translation, and virus assembly. In addition, it is possible that cell cycle arrest in HHV-6 infection prevents early death of infected cells, therefore gaining sufficient time and resources for the viral life cycle. Further studies should determine whether the G2/M phase in HHV-6-infected cells is a favorable condition for HHV-6 viral protein expression and progeny virus production.
HHV-6 is an important immunosuppressive and immunomodulatory virus, which has the ability to avoid, misguide, or impair immune system recognition, allowing the virus to persist within the host it infects. Previous studies have shown that HHV-6 infection negatively affects T-cell proliferation and interleukin-2 synthesis (13), interleukin-12 production (45), and dendritic cell maturation (46), as well as inducing phenotypic alterations in monocytes (20). Furthermore, in the current study, we discovered that HHV-6A replication has an effect on cell cycle progression in infected T cells. Our results showed that HHV-6A infection induced cell cycle arrest in the G2/M phase in infected T cells. We further demonstrated that the expression patterns of some molecules that are key in regulating the cell cycle were altered during HHV-6 infection, a finding consistent with the G2/M arrest profile in the cell cycle. HHV-6A may utilize these mechanisms to block the clonal expansion and proliferation of HHV-6-specific T cells that maintain immune suppression and to damage the antiviral immune responses. In addition, it has been reported that noncycling cells are refractory to killing by cytolytic T cells (33). Thus, HHV-6 may also use this cell-cycle-arrest strategy to avoid being killed by cytolytic T cells. Clarification of the molecular mechanisms by which HHV-6A disrupts the cell cycle machinery will not only be important for the study of cell cycle changes in HHV-6A replication but will also be crucial for better understanding of the potential mechanisms utilized by HHV-6 to induce immune suppression in hosts, and for the development of novel vaccines and/or therapeutic agents to inhibit HHV-6A infection.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grants 30771961 and 30901344), the Science Development Foundation of Nanjing Medical University (grant 08NMUZ003), and the Jiangsu Province Laboratory of Pathogen Biology (grant 08bykf01).
Footnotes
Published ahead of print on 27 April 2011.
REFERENCES
- 1. Akashi K., et al. 1993. Severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. N. Engl. J. Med. 329:168–171 [DOI] [PubMed] [Google Scholar]
- 2. Archives of Virology 1993. Human herpesvirus-6 strain groups: a nomenclature. Arch. Virol. 129:363–366 [DOI] [PubMed] [Google Scholar]
- 3. Braun D. K., Dominguez G., Pellett P. E. 1997. Human herpesvirus 6. Clin. Microbiol. Rev. 10:521–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castedo M., Perfettini J. L., Roumier T., Kroemer G. 2002. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 9:1287–1293 [DOI] [PubMed] [Google Scholar]
- 5. Chaurushiya M. S., Weitzman M. D. 2009. Viral manipulation of DNA repair and cell cycle checkpoints. DNA Repair (Amst.) 8:1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chulu J. L., Huang W. R., Wang L., Shih W. L., Liu H. J. 2010. Avian reovirus nonstructural protein p17-induced G2/M cell cycle arrest and host cellular protein translation shutoff involve activation of p53-dependent pathways. J. Virol. 84:7683–7694 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Davy C., Doorbar J. 2007. G2/M cell cycle arrest in the life cycle of viruses. Virology 368:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davy C. E., et al. 2005. Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes. J. Virol. 79:3998–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Bolle L., Hatse S., Verbeken E., De Clercq E., Naesens L. 2004. Human herpesvirus 6 infection arrests cord blood mononuclear cells in G2 phase of the cell cycle. FEBS Lett. 560:25–29 [DOI] [PubMed] [Google Scholar]
- 10. De Bolle L., Naesens L., De Clercq E. 2005. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin. Microbiol. Rev. 18:217–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dietrich J., et al. 2004. Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J. Neurosci. 24:4875–4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dove B., Brooks G., Bicknell K., Wurm T., Hiscox J. A. 2006. Cell cycle perturbations induced by infection with the coronavirus infectious bronchitis virus and their effect on virus replication. J. Virol. 80:4147–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flamand L., Gosselin J., Stefanescu I., Ablashi D., Menezes J. 1995. Immunosuppressive effect of human herpesvirus 6 on T-cell functions: suppression of interleukin-2 synthesis and cell proliferation. Blood 85:1263–1271 [PubMed] [Google Scholar]
- 14. Gardell J. L., et al. 2006. Apoptotic effects of human herpesvirus-6A on glia and neurons as potential triggers for central nervous system autoimmunity. J. Clin. Virol. 37(Suppl. 1):S11–S16 [DOI] [PubMed] [Google Scholar]
- 15. Goh W. C., Manel N., Emerman M. 2004. The human immunodeficiency virus Vpr protein binds Cdc25C: implications for G2 arrest. Virology 318:337–349 [DOI] [PubMed] [Google Scholar]
- 16. Graves P. R., Lovly C. M., Uy G. L., Piwnica-Worms H. 2001. Localization of human Cdc25C is regulated both by nuclear export and 14-3-3 protein binding. Oncogene 20:1839–1851 [DOI] [PubMed] [Google Scholar]
- 17. Groschel B., Bushman F. 2005. Cell cycle arrest in G2/M promotes early steps of infection by human immunodeficiency virus. J. Virol. 79:5695–5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He Y., et al. 2010. Influenza A virus replication induces cell cycle arrest in G0/G1 phase. J. Virol. 84:12832–12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inoue Y., Yasukawa M., Fujita S. 1997. Induction of T-cell apoptosis by human herpesvirus 6. J. Virol. 71:3751–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janelle M. E., Flamand L. 2006. Phenotypic alterations and survival of monocytes following infection by human herpesvirus-6. Arch. Virol. 151:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamata M., Watanabe N., Nagaoka Y., Chen I. S. 2008. Human immunodeficiency virus type 1 Vpr binds to the N lobe of the Wee1 kinase domain and enhances kinase activity for CDC2. J. Virol. 82:5672–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kudoh A., et al. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 280:8156–8163 [DOI] [PubMed] [Google Scholar]
- 23. Li F. Q., Tam J. P., Liu D. X. 2007. Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53. Virology 365:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li H., et al. 2008. Chk2 is required for HSV-1 ICP0-mediated G2/M arrest and enhancement of virus growth. Virology 375:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez C., et al. 1988. Characteristics of human herpesvirus-6. J. Infect. Dis. 157:1271–1273 [DOI] [PubMed] [Google Scholar]
- 26. Lusso P., et al. 2007. Human herpesvirus 6A accelerates AIDS progression in macaques. Proc. Natl. Acad. Sci. U. S. A. 104:5067–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lusso P., Gallo R. C. 1995. Human herpesvirus 6 in AIDS. Immunol. Today 16:67–71 [DOI] [PubMed] [Google Scholar]
- 28. Masrouha N., Yang L., Hijal S., Larochelle S., Suter B. 2003. The Drosophila chk2 gene loki is essential for embryonic DNA double-strand-break checkpoints induced in S phase or G2. Genetics 163:973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCullers J. A., Lakeman F. D., Whitley R. J. 1995. Human herpesvirus 6 is associated with focal encephalitis. Clin. Infect. Dis. 21:571–576 [DOI] [PubMed] [Google Scholar]
- 30. Mlechkovich G., Frenkel N. 2007. Human herpesvirus 6A (HHV-6A) and HHV-6B alter E2F1/Rb pathways and E2F1 localization and cause cell cycle arrest in infected T cells. J. Virol. 81:13499–13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morita E., et al. 2001. Human parvovirus B19 induces cell cycle arrest at G2 phase with accumulation of mitotic cyclins. J. Virol. 75:7555–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller P. R., Coleman T. R., Kumagai A., Dunphy W. G. 1995. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270:86–90 [DOI] [PubMed] [Google Scholar]
- 33. Nishioka W. K., Welsh R. M. 1994. Susceptibility to cytotoxic T lymphocyte-induced apoptosis is a function of the proliferative status of the target. J. Exp. Med. 179:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nurse P. 1990. Universal control mechanism regulating onset of M-phase. Nature 344:503–508 [DOI] [PubMed] [Google Scholar]
- 35. Orba Y., et al. 2010. Large T antigen promotes JC virus replication in G2-arrested cells by inducing ATM- and ATR-mediated G2 checkpoint signaling. J. Biol. Chem. 285:1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Øster B., Bundgaard B., Hollsberg P. 2005. Human herpesvirus 6B induces cell cycle arrest concomitant with p53 phosphorylation and accumulation in T cells. J. Virol. 79:1961–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Øster B., Kaspersen M. D., Kofod-Olsen E., Bundgaard B., Hollsberg P. 2006. Human herpesvirus 6B inhibits cell proliferation by a p53-independent pathway. J. Clin. Virol. 37(Suppl. 1):S63–S68 [DOI] [PubMed] [Google Scholar]
- 38. Poggioli G. J., Dermody T. S., Tyler K. L. 2001. Reovirus-induced σ1s-dependent G2/M phase cell cycle arrest is associated with inhibition of p34cdc2. J. Virol. 75:7429–7434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell P., Nurse P. 1987. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49:559–567 [DOI] [PubMed] [Google Scholar]
- 40. Salahuddin S. Z., et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596–601 [DOI] [PubMed] [Google Scholar]
- 41. Sandhoff T., Kleim J. P., Schneweis K. E. 1991. Latent human herpesvirus-6 DNA is sparsely distributed in peripheral blood lymphocytes of healthy adults and patients with lymphocytic disorders. Med. Microbiol. Immunol. 180:127–134 [DOI] [PubMed] [Google Scholar]
- 42. Schirmer E. C., Wyatt L. S., Yamanishi K., Rodriguez W. J., Frenkel N. 1991. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc. Natl. Acad. Sci. U. S. A. 88:5922–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shieh S. Y., Ikeda M., Taya Y., Prives C. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325–334 [DOI] [PubMed] [Google Scholar]
- 44. Shirata N., et al. 2005. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J. Biol. Chem. 280:30336–30341 [DOI] [PubMed] [Google Scholar]
- 45. Smith A., et al. 2003. Selective suppression of IL-12 production by human herpesvirus 6. Blood 102:2877–2884 [DOI] [PubMed] [Google Scholar]
- 46. Smith A. P., et al. 2005. Viral replication-independent blockade of dendritic cell maturation and interleukin-12 production by human herpesvirus 6. J. Virol. 79:2807–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soldan S. S., et al. 1997. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 3:1394–1397 [DOI] [PubMed] [Google Scholar]
- 48. Strausfeld U., et al. 1991. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature 351:242–245 [DOI] [PubMed] [Google Scholar]
- 49. Takahashi K., et al. 1989. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 63:3161–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Toyoshima-Morimoto F., Taniguchi E., Shinya N., Iwamatsu A., Nishida E. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215–220 [DOI] [PubMed] [Google Scholar]
- 51. Wang F., et al. 2006. Human herpesvirus-6-specific interleukin 10-producing CD4+ T cells suppress the CD4+ T-cell response in infected individuals. Microbiol. Immunol. 50:787–803 [DOI] [PubMed] [Google Scholar]
- 52. Yamane A., et al. 2007. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol. Blood Marrow Transplant. 13:100–106 [DOI] [PubMed] [Google Scholar]
- 53. Yamanishi K., et al. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065–1067 [DOI] [PubMed] [Google Scholar]
- 54. Yasukawa M., Inoue Y., Ohminami H., Terada K., Fujita S. 1998. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection. J. Gen. Virol. 79(Pt 1):143–147 [DOI] [PubMed] [Google Scholar]
- 55. Zhao H., Piwnica-Worms H. 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21:4129–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]