Abstract
Primary varicella-zoster virus (VZV) infection in humans produces varicella (chickenpox), after which the virus becomes latent in ganglionic neurons. Analysis of the physical state of viral nucleic acid and virus gene expression during latency requires postmortem acquisition of fresh human ganglia. To provide an additional way to study the VZV-host relationship in neurons, we developed an in vitro model of infected differentiated human neural stem cells (NSCs). NSCs were induced to differentiate in culture dishes coated with poly-l-lysine and mouse laminin in the presence of fibroblast growth factor 2 (FGF-2), nerve growth factor (NGF), brain-derived neurotropic factor (BDNF), dibutyryl cyclic AMP, and retinoic acid. Immunostaining with neuronal (MAP2a and β-tubulin), astrocyte (GFAP), and oligodendrocyte (CNPase) markers revealed that differentiated neurons constituted approximately 90% of the cell population. These neurons were maintained in culture for up to 8 weeks. No cytopathic effect (CPE) developed in neurons infected with cell-free VZV (Zostavax vaccine) compared to human fetal lung fibroblasts infected with VZV. Weeks later, VZV DNA virus-specific transcripts (open reading frames [ORFs] 21, 29, 62, and 63) were detected in infected neurons, and dual immunofluorescence staining revealed the presence of VZV IE63 and gE exclusively in healthy-appearing neurons, but not in astrocytes. Neither the tissue culture medium nor a homogenate prepared from VZV-infected neurons produced a CPE in fibroblasts. VZV induced apoptosis in fibroblasts, as shown by activation of caspase 3 and by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, but not in neurons. This model provides a unique in vitro system to study the VZV-neuronal relationship.
INTRODUCTION
Varicella zoster virus (VZV) is a ubiquitous, exclusively human, and highly neurotropic herpesvirus. Primary infection causes varicella (chickenpox), during which VZV replicates in the cells of every visceral organ and the skin (12). VZV establishes lifelong latency in neurons of cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis (9, 11, 17). VZV reactivates in elderly and immunocompromised individuals to produce shingles (herpes zoster), frequently complicated by chronic pain (postherpetic neuralgia) (10) and other serious neurological and ocular disorders, such as VZV vasculopathy, myelopathy, and retinal necrosis.
Analyses of latently infected human ganglia obtained postmortem within 24 h after death have provided useful information on the configuration and abundance of the VZV genome and the limited extent of viral gene expression (reviewed in reference 6). However, such studies are hampered by lack of sufficient tissue, RNA degradation postmortem, and possible virus reactivation after death. Because no satisfactory animal model exists to study the interaction of VZV with neurons, alternate models have been developed. For example, immune-deficient SCID mice have had human neural stem cells or human ganglia transplanted, followed by infection with VZV and examination of infected neurons (2, 21). VZV has been also shown to infect cultured neurons from guinea pig enteric ganglia (8). An in vitro model of cultured human neurons infected with VZV provides an opportunity to study the molecular events leading to the establishment of VZV latency and reactivation. Here, we developed a system in which human neural stem cells (NSCs) were induced to differentiate into neurons, infected with VZV, observed carefully, and analyzed for viral gene expression and for markers of apoptosis.
MATERIALS AND METHODS
Materials.
Cell culture media and supplies were purchased from Gemini Bio Products, Inc. (Woodland, CA), and Invitrogen-Life Technologies (Rockville, MD). NSCs derived from human fetal brain at 9 weeks gestational age were obtained as cryopreserved neurospheres from Lonza, Inc. (Walkersville, MD). Neurobasal medium and supplements for proliferation and differentiation of NSCs, i.e., nerve growth factor (NGF) and fibroblast growth factor (FGF), were purchased from Stemcell Technologies (Vancouver, BC, Canada). Poly-l-lysine, mouse laminin, DAPI (4′,6-diamidino-2-phenylindole), and dibutyryl cyclic AMP were purchased from Sigma Chemical Co. (St. Louis, MO). Brain-derived neurotropic factor (BDNF) and antibodies directed against the active cleaved form of caspase 3, MAP2a, β-tubulin, GFAP, and β-actin were obtained from Cell Signaling (Beverly, MA). Antibody to VZV glycoprotein E (gE) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-VZV IE63 antibody was used as described previously (18). Anti-rabbit IgG and anti-mouse IgG conjugated to fluorescent probes (Cy3 or fluorescein isothiocyanate [FITC]) were obtained from Jackson Immuno Research Laboratories (West Grove, PA). NGF and the Click-iT TUNEL Alexa Fluor 647 imaging assay kit were purchased from Invitrogen. Zostavax was purchased from the University of Colorado Hospital pharmacy.
Culture of NSCs.
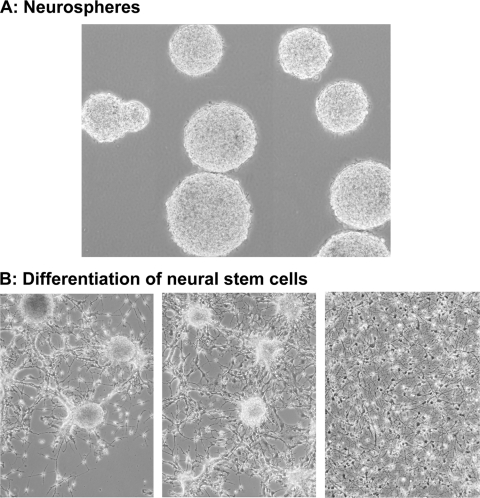
NSCs were cultured in suspension as neurospheres (Fig. 1A) in neurobasal medium, along with the proliferation supplements epidermal growth factor (EGF) (20 ng/ml) and FGF-2 (25 ng/ml). The neurospheres were transferred to 50-ml tubes and centrifuged at 1,000 rpm for 5 min. The supernatant was removed by aspiration, and the pellet was resuspended in 0.5 ml of NSC proliferation medium. The neurospheres were triturated 75 times to yield a single-cell suspension; within 1 week of culture, the cells proliferated and formed new neurospheres.
Fig. 1.
Differentiation of human NSCs. (A) NSCs derived from human fetal brain were propagated as neurospheres in suspension in neurobasal medium containing the proliferation supplements EGF and FGF-2. (B) Neurospheres were broken by trituration, seeded in dishes coated with poly-l-lysine and mouse laminin, and cultured in the presence of FGF-2, NGF, BDNF, dibutryl cyclic AMP, and retinoic acid. The images show neurons after 2, 4, and 10 days of differentiation (left to right).
Differentiation of human NSCs.
When cultured in vitro, NSCs can differentiate into neurons, astrocytes, or oligodendrocytes. To obtain a predominantly neuronal population, 300- to 500-μm neurospheres were centrifuged at 1,000 rpm for 5 min, and the pellet was resuspended in neurobasal medium with differentiation supplements and triturated. The resulting single-cell suspension was seeded in 6-well dishes (coated with 100 μg/ml poly-l-lysine, recoated with 5 μg/ml mouse laminin, and washed in phosphate-buffered saline [PBS]) and cultured in the presence of 25 ng/ml FGF-2, 20 ng/ml NGF, 10 ng/ml BDNF, 100 μM dibutryl cyclic AMP, and 1 μM retinoic acid. Figure 1B (left to right) shows the progressive differentiation of neurons at 2, 4, and 10 days. Fully differentiated neurons were maintained for up to 8 weeks.
Immunofluorescence staining of neurons.
Differentiated NSCs were fixed in 4% paraformaldehyde for 30 min, washed in PBS, treated with permeabilization buffer (5% bovine serum albumin [BSA] and 0.2% Triton X-PBS) for 90 min, and incubated with primary antibodies to MAP2a (1:200), β-tubulin (1:500), GFAP (1:1000), active caspase 3 (1:500), IE63 (1:1,000), and VZV gE (1:500) overnight at 4°C in a humidified chamber. After being washed in PBS, the cells were further incubated with anti-rabbit-Cy3, anti-mouse-FITC, and DAPI (2 μg/ml; nuclear stain) at room temperature for 90 min. The dishes were washed in PBS, mounted in mounting medium, and examined by fluorescence microscopy.
VZV DNA and VZV-specific transcripts in VZV-infected cells.
Cultured human fetal lung fibroblasts and differentiated neurons were infected with 2,500 PFU of Zostavax in a dish containing ∼1 million cells. After vaccine was added to the cells, the culture dish was gently mixed once every 2 h for 6 h, after which the medium was replaced. DNA isolated using a DNeasy kit (Qiagen, Valencia, CA) was examined for open reading frame (ORF) 63 levels by PCR. RNA was isolated using the Versagene RNA isolation kit (Fisher Scientific, Pittsburgh, PA) and examined for mRNA levels of VZV ORFs 21, 29, 62, and 63 by real-time quantitative RT-PCR using TaqMan probes. Primers and probes were designed with Primer Express (PE) (ABI, Foster City, CA) (20). The PCRs were monitored in real-time in an ABI Prism 7700 sequence detector (Perkin Elmer Corp/Applied Biosystems, Foster City, CA).
Western blot analysis.
Uninfected and VZV-infected fibroblasts and differentiated neurons were lysed using mammalian protein extraction reagent (M-PER) (Pierce, Rockford, IL) supplemented with phosphatase inhibitors (20 mM sodium fluoride, 1 mM sodium orthovanadate, and 500 nM okadaic acid) and protease inhibitor cocktail (Sigma). After centrifugation of cell lysates at 20,800 × g for 15 min, the protein content of the supernatant was measured (4). Diluted samples containing equal amounts of protein were mixed with 2× Laemmli sample buffer, resolved on 12% SDS-polyacrylamide gels, and transferred to polyvinylidene difluoride (PVDF) membranes. Blots were blocked with TBST (20 mM Tris-HCl, pH 7.9, 8.5% NaCl, and 0.1% Tween 20) containing 5% nonfat dry milk at room temperature for 1 h and exposed overnight at 4°C to a 1:1,000 dilution of antibody directed against the active cleaved form of caspase 3 in TBST containing 5% BSA. After the blots were washed with TBST, alkaline phosphatase-conjugated anti-rabbit IgG was added, followed by incubation for 1 h at room temperature. The blots were rinsed with wash buffer (10 mM Tris-HCl, pH 9.5, 10 mM NaCl, and 1 mM MgCl2), developed with CDP-Star reagent (New England BioLabs, Beverly, MA), and exposed to X-ray film. The membranes were then stripped in buffer containing 62.5 mM Tris-HCl, pH 6.7, 2% SDS, and 100 mM β-mercaptoethanol at 55°C for 45 min and reprobed with β-actin antibody. Band intensity was quantitated using Fluor-S MultiImager and Quantity One software from Bio-Rad (Hercules, CA).
Statistical analysis.
All statistical analyses were performed by one-way analysis of variance (ANOVA) with Dunnett's multiple-comparison tests.
RESULTS
Characterization of differentiated neurons.
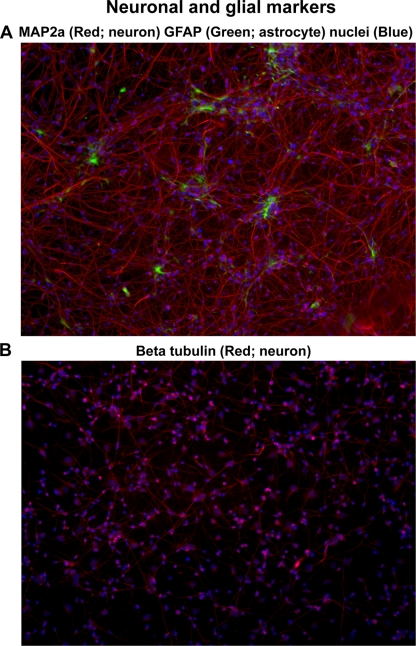
To confirm the neuronal nature of differentiated cells, immunofluorescence staining for multiple markers was performed. After attachment to the dishes, the neural stem cells differentiate from a glial to a neuronal phenotype with the addition of growth factors (NGF and BDNF), dibutyryl cyclic AMP, and retinoic acid. At 1 week, 70 to 80% of the cells were neurons. With continued culture for another 2 weeks, MAP2a-positive neurons (red) and GFAP-positive astrocytes (green) constituted ∼90% and ∼5% of the cell population, respectively (Fig. 2A). Neurons also stained positive for β-tubulin (Fig. 2B). CNPase staining for oligodendrocytes was seen in less than 5% of the cells (not shown).
Fig. 2.
Neurons and astrocytes in differentiated human NSCs. Differentiated NSCs cultured for 4 weeks were fixed, immunostained for MAP2a (a neuronal marker) and GFAP (astrocytes), mounted in mounting medium, and examined by fluorescence microscopy. (A) Neurons (stained red for MAP2a) constituted ∼90% of the cell population, and astrocytes (stained green for GFAP) formed ∼5% of the cells. At least 1,000 cells were counted to determine cell type percentages. (B) Neurons also stained positive (red) with the neuronal marker β-tubulin. The images are representative of 4 independent experiments.
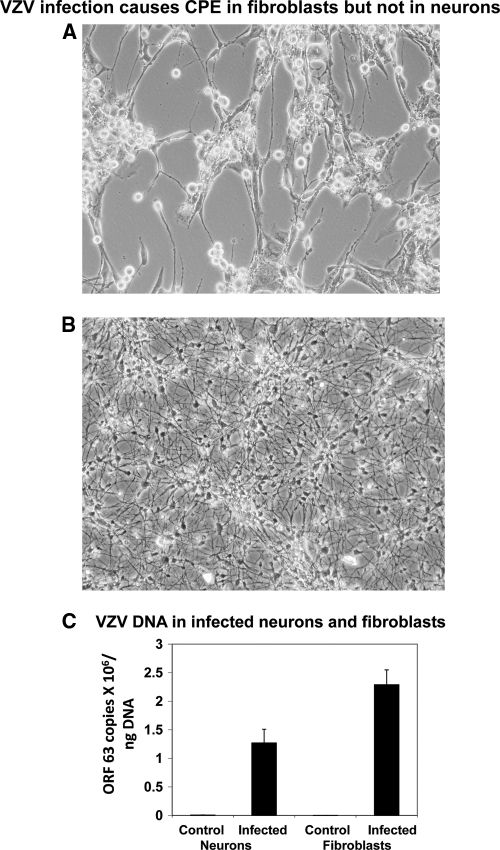
Infection of neurons with VZV did not produce a CPE.
One week after differentiation, cells in 35-mm dishes were infected with 2,500 PFU of cell-free VZV (Zostavax). Human lung fibroblasts were infected in parallel. A cytopathic effect (CPE) developed in infected fibroblasts at 5 to 7 days postinfection (p.i.) (Fig. 3A). No CPE developed in infected neurons even 3 weeks after infection (Fig. 3B). VZV DNA was found in both neurons and fibroblasts (Fig. 3C).
Fig. 3.
VZV does not produce a CPE in differentiated neurons. (A) A CPE developed in human fetal lung fibroblasts 6 days after infection with cell-free VZV (Zostavax) at 2,400 PFU/well. (B) No CPE developed in NSCs induced to differentiate in the presence of growth factors, cultured in dishes infected with cell-free VZV (Zostavax) at 2,400 PFU/well, and examined up to 3 weeks after infection. The images are representative of 4 independent experiments. (C) DNA was extracted from fibroblasts and differentiated neurons infected with Zostavax for 1 week, and VZV DNA was quantitated by PCR. The values are means (plus standard errors [SE]) of results from 4 independent experiments.
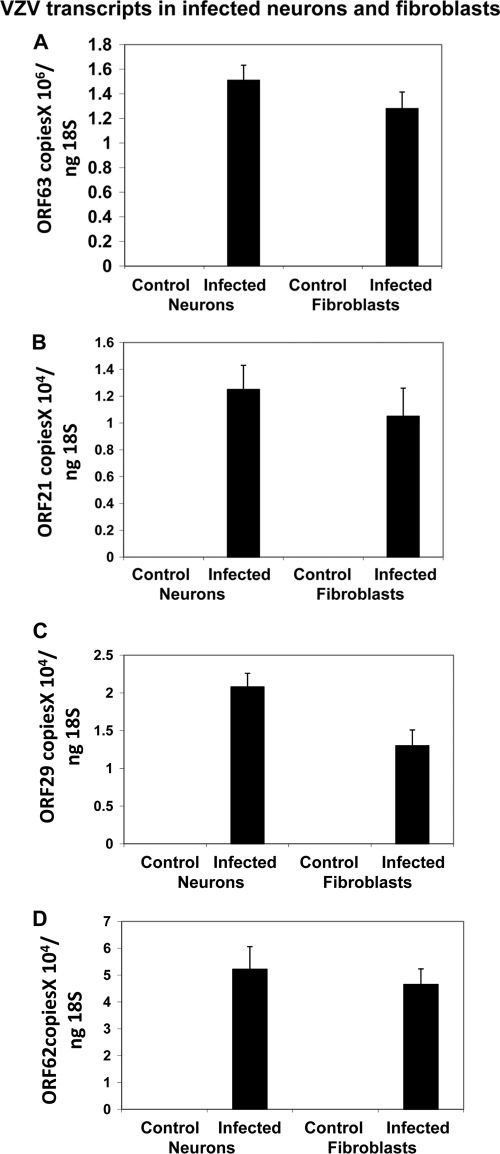
Detection of VZV transcripts in infected neurons.
One week after infection, we searched for multiple VZV transcripts corresponding to VZV ORFs 21, 29, 62, and 63, which have been found in latently infected human ganglia. VZV ORF 21, 29, 62, and 63 mRNA was detected in infected fibroblasts and neurons at comparable levels (Fig. 4). VZV 63 was the most abundant transcript in both cell types.
Fig. 4.
VZV-specific transcripts in infected cells. Human fetal lung fibroblasts and differentiated neurons were infected with Zostavax. One week later, RNA was isolated for quantitative RT-PCR analysis of the VZV-specific transcripts ORF 63 (A), ORF 21 (B), ORF 29 (C), and ORF 62 (D). The values are means (plus SE) of results from 4 independent experiments.
Detection of VZV-specific proteins in infected neurons.
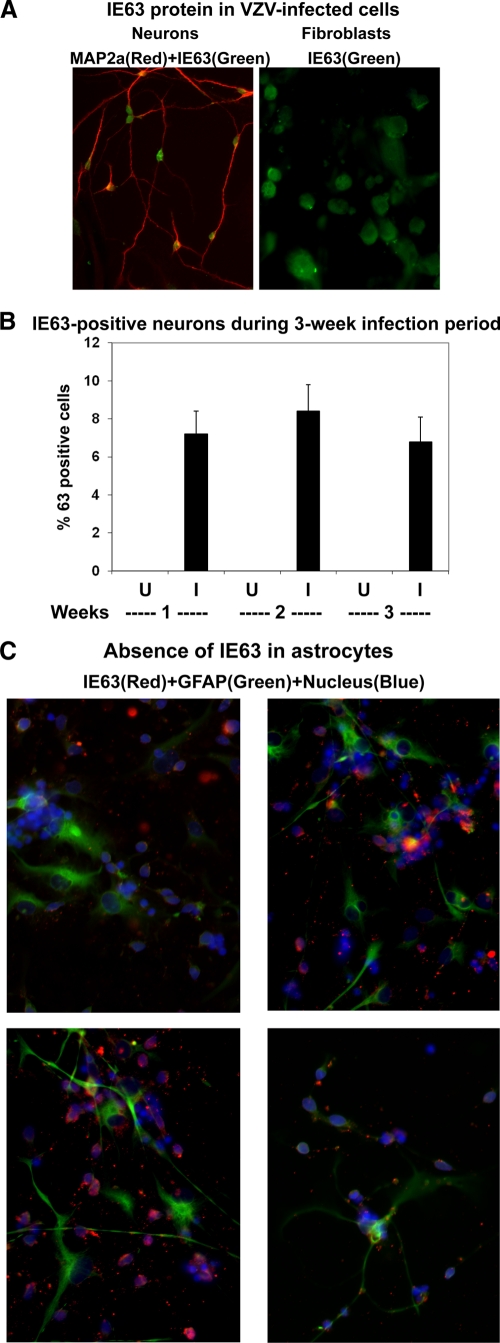
One week after infection, VZV IE63 protein was seen in most infected fibroblasts, and dual immunofluorescence revealed the presence of VZV IE63 in MAP2a-positive neurons (Fig. 5A). VZV IE63 was present in both the nuclei and cytoplasm of neurons. Additional experiments were conducted to detect MAP2a-positive neurons that contained VZV IE63 at multiple times after infection. One thousand neurons were counted in each independent experiment. At 1, 2, and 3 weeks after infection, VZV IE63 was seen in 5 to 10% of neurons, and the percentage of infected neurons did not increase during the 1- to 3-week postinfection observation period (Fig. 5B), indicating the absence of cell-to-cell spread. Importantly, to rule out the possibility that VZV persisted in astrocytes, three different cultures of infected cells were immunostained for VZV IE63 and GFAP. Examination of 200 GFAP-positive astrocytes in each experiment did not reveal VZV IE63 (Fig. 5C).
Fig. 5.
VZV IE63 protein in uninfected (U) and VZV-infected (I) cells. (A) Human fetal lung fibroblasts and differentiated neurons were infected with Zostavax and 1 week later were fixed and immunostained for VZV IE63 (green) and MAP2a (red). (B) One to 3 weeks after infection, neurons were immunostained for IE63, and the percentage of 63-positive neurons was determined. The percentage of 63-positive neurons did not change over a 3-week period after infection. The images are representative of 4 independent experiments. (C) To determine if any astrocytes contained VZV IE63, infected NSCs were also immunostained for VZV IE63 and GFAP. Nuclei stained blue with DAPI. The dishes were mounted in mounting medium and examined by fluorescence microscopy. The four random images show the complete absence of VZV IE63 protein in GFAP+ astrocytes; at least 200 astrocytes were examined for IE63 in each experiment.
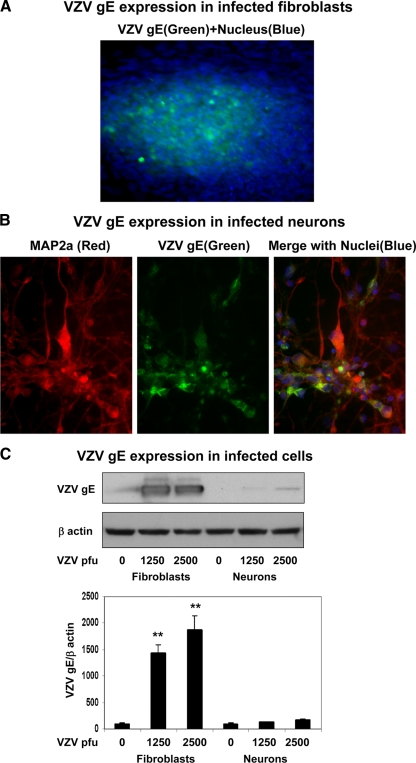
Immunofluorescence staining readily detected expression of VZV gE, a major envelope glycoprotein, in infected fibroblasts (Fig. 6A). VZV gE expression was also seen in 2 to 3% of MAP2a-positive neurons (Fig. 6B) and remained constant over the 3-week period. To compare the abundances of VZV gE protein in these two cell types, Western blot analysis was performed. An intense band of VZV gE protein was seen in productively infected fibroblasts, whereas only a faint band of gE was detected in infected neurons (Fig. 6C, top). Quantitation of the VZV gE bands revealed a 15- to 20-fold signal increase in VZV-infected fibroblasts (Fig. 6C, bottom).
Fig. 6.
VZV gE expression levels in infected human fetal lung fibroblasts and neurons. Fibroblasts and differentiated neurons were infected with Zostavax for 1 week. (A) Infected fibroblasts were fixed and immunostained for VZV gE, and nuclei stained blue with DAPI. Note the abundant expression of VZV gE. (B) Neurons were similarly fixed and also immunostained for MAP2a. The high-power image shows VZV gE expression in MAP2a-stained neurons. (C) VZV-infected cells were processed by Western blot analysis for VZV gE (top). The intensities of the bands were quantitated by scanning and corrected for β-actin levels (bottom). Abundant VZV gE was seen in infected fibroblasts in contrast to trace levels in infected neuronal cultures. The images are representative of 3 independent experiments. **, P < 0.001 versus uninfected cells. The error bars indicate SE of the means.
Examination of infected neurons for cell-free and cell-associated virus.
To determine if infectious virus is present in neurons infected with VZV, both the tissue culture medium and cell homogenates from VZV-infected neurons were used to infect fibroblasts. In three replicate experiments, neither the tissue culture medium nor homogenates prepared from VZV-infected neurons produced a CPE in uninfected fibroblasts. In contrast, both the tissue culture medium and homogenates from VZV-infected fibroblasts readily produced a CPE when added to uninfected fibroblasts. Because VZV is cell associated and the tissue culture medium from VZV-infected fibroblasts usually contains dead cells or cell debris with small amounts of virus, the tissue culture medium from VZV-infected fibroblasts, after removal of cell debris by centrifugation, did not produce a CPE when added to uninfected fibroblasts.
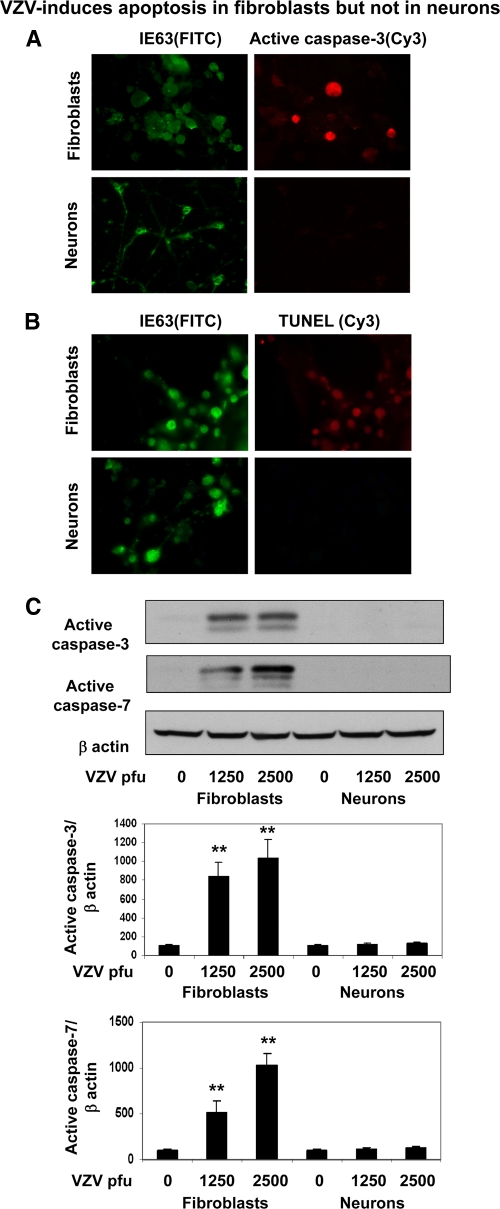
VZV does not induce apoptosis in neurons.
VZV induces apoptosis in MeWo cells (5) and in fibroblasts, but not in ganglionic neurons (14). To determine if neurons were resistant to apoptosis, VZV-infected neurons and fibroblasts were examined for markers of apoptosis. VZV IE63-positive fibroblasts stained for the active form of caspase 3 and in a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, but not infected neurons (Fig. 7A and B). Western blot analysis for the active forms of caspase 3 and caspase 7 further confirmed the induction of apoptosis by VZV in fibroblasts; levels of active caspase 3 and 7 increased by 8- to 10-fold and 5- to 10-fold, respectively, compared to uninfected fibroblasts or VZV-infected neurons (Fig. 7C).
Fig. 7.
VZV does not induce apoptosis in neurons. Human fetal lung fibroblasts and differentiated neurons were infected with Zostavax and, after 1 week, were fixed and immunostained for VZV IE63 (green) and for the active form of caspase 3 (red) (A) and by TUNEL assay (red) (B). Note active caspase 3 and TUNEL staining in VZV-infected fibroblasts, but not in VZV-infected neurons. (C) Western blot analysis of VZV-infected cells for the active cleaved forms of caspase 3 and caspase 7. The blots were reprobed for β-actin levels. The intensities of bands of activated caspases were quantitated and corrected for β-actin, and background signals in uninfected cells were taken as 100%. The images are representative of 4 independent experiments. **, P < 0.001 versus uninfected cells. The error bars indicate SE of the means.
DISCUSSION
Here, we developed an in vitro system in which human NSCs were induced to differentiate into cells that were more than 90% neuronal and viable in tissue culture for up to 8 weeks, providing sufficient time to infect them with VZV and to study virus-cell interactions. NSCs, which originate in the subgranular layer of the dentate gyrus of the hippocampus, are multipotent cells that can be induced to differentiate into neurons, astrocytes, and oligodendrocytes after adhesion to specific substrata, including laminin (3, 7). NSCs can potentially be transplanted into patients with various neurodegenerative disorders, including Parkinson's disease, amyotrophic lateral sclerosis, stroke, and Alzheimer's disease (16). An abundant supply of human NSCs was obtained by propagation of neurospheres in culture in the presence of EGF and FGF-2 for up to 10 passages. To enrich for neurons, neurotropic growth factors (NGF and BDNF) were added to the tissue culture medium, along with retinoic acid to induce cell cycle exit and with dibutyryl cAMP to activate CREB, a transcription factor that enhances neurogenesis. This is the first report of such a combination of agents that favors neuronal differentiation in vitro.
Cultures enriched for differentiated neurons were verified immunohistochemically and infected with cell-free VZV. Zostavax was a useful source of cell-free virus and did not produce a CPE in neurons in culture. A significant outcome was the complete lack of CPE in VZV-infected neurons even after 3 weeks, while the same input virus produced a CPE in fibroblasts in 6 to 7 days (Fig. 3). Despite the absence of a CPE in neurons, they contained VZV DNA, virus-specific transcripts, and protein weeks later. Both the tissue culture medium and cell homogenate from VZV-infected neurons were unable to infect fibroblasts, indicating that weeks after experimental infection, VZV in neurons is not infectious. Importantly, infected neurons did not become apoptotic.
There have been four other studies of VZV interaction with human neurons in vitro (2, 13, 14, 21). All were different than ours in that neuronal infection was established by cocultivation rather than with cell-free virus, and only one of the studies analyzed neurons for VZV gene expression weeks after infection (21). The first study used cocultivation to infect explants of human fetal dorsal root ganglia. Neurons were shown to be productively infected 2 days later. Although neurons were not examined weeks later, they were found to be resistant to apoptosis (14), as we found in our differentiated NSC cultures. The second study by the same laboratory also infected explants of human fetal dorsal root ganglia and showed that productive virus infection peaked at day 4 but dropped dramatically by day 5 (13); infected neurons were not analyzed weeks later, which would have allowed a comparison of viral gene expression with our neuronal cultures. The third study used human neural stem cells isolated from fetal brain that were transplanted into nonobese diabetic SCID mouse brains, after which they were allowed to differentiate in vivo, followed by infection with VZV (2). In this chimeric model, VZV was found in both neurons and glial cells. In a fourth study, human dorsal root ganglion xenografts were engrafted under the kidney capsule of SCID mice followed by infection with VZV. At 14 days postinfection, VZ virions were detected by electron microscopy (EM) in neuronal cell nuclei and cytoplasm, but not in satellite cells (21). The VZV genome copy number was 7.1 × 107 to 8.0 × 108 copies per 105 cells, and infectious virus was recovered. Four to 8 weeks later, no infectious virus was released from cells, there was no virion assembly, and there was a marked reduction in VZV genome copies to 3.7 × 105 to 4.7 × 106 per 105 cells. VZV ORF 63, but not ORF 62, continued to be transcribed; studies of the expression of other VZV ORFs at this time were not conducted.
The studies discussed above and ours indicate that VZV infection can persist in neurons and that those neurons are resistant to apoptosis. Viruses induce or inhibit apoptosis in a cell type- and context-dependent manner. During lytic infection, the decision to commit to apoptosis may come from the host cell with the objective of limiting cellular damage caused by the virus. We have previously shown that simian varicella virus-induced apoptosis in monkey kidney cells (20) and VZV-induced apoptosis in human MeWo cells (5) involved downregulation of the antiapoptotic bcl-2 gene. The absence of induction of apoptosis in neurons may enhance the chance of establishment of latency. Interestingly, VZV 63 protein inhibits apoptosis in human neurons (15). It will be important to determine how VZV 63 affects bcl-2 expression, a study eminently possible in our model.
Finally, our examination of neurons in culture weeks after infection revealed the presence of both early and late VZV-specific transcripts (ORFs 21, 29, 62, and 63) and proteins (63 and gE). Both VZV 63 and VZV gE proteins were found exclusively in neurons, but not in nonneuronal cells. Importantly, transcripts corresponding to VZV ORFs 21, 29, 62, 63, and 68 (gE) are present in latently infected human ganglia (1, 19). VZV gE is also present in vivo months after experimental infection of human dorsal root ganglia (22).
Overall, despite having established a nonproductive infection of neurons in culture with expression of many of the same VZV transcripts and proteins that have been found in latently infected human ganglia, we are reluctant to categorically state that the cultured neurons are latently infected. This is because increasing numbers of VZV transcripts are now being detected with more advanced technologies (19), and the exact extent of viral gene expression is still unknown in latently infected human ganglia. In parallel with our continuing studies of VZV latency in human ganglia, this promising neuronal model will be used to analyze the total degree of VZV transcription and translation and will also be used to attempt to reactivate virus from nonproductively infected neurons.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AG006127 (D.G.), AG032958 (D.G., S.P., R.M., and R.J.C.), and NS067070 (M.A.N.) from the National Institutes of Health and Merit Review grant NEUD-004-07F from the Veterans Administration (S.P.).
We thank Ron Bouchard at the Denver VAMC-Digital Deconvolution Microscopy Core facility and Umarani Pugazhenthi at the University of Colorado Cancer Center RT-PCR Core Facility for excellent technical support. We also thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
Footnotes
Published ahead of print on 27 April 2011.
REFERENCES
- 1. Azarkh Y., Gilden D., Cohrs R. J. 2010. Molecular characterization of varicella zoster virus (VZV) in latently infected human ganglia: physical state and abundance of VZV DNA, quantitation of viral transcripts and detection of VZV-specific proteins. Curr. Top. Microbiol. Immunol. 342:229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baiker A., et al. 2004. Varicella-zoster virus infection of human neural cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 101:10792–10797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bottai D., et al. 2003. Neural stem cells in the adult nervous system. J. Hematother. Stem Cell Res. 12:655–670 [DOI] [PubMed] [Google Scholar]
- 4. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 5. Brazeau E., et al. 2010. Varicella-zoster virus-induced apoptosis in MeWo cells is accompanied by down-regulation of Bcl-2 expression. J. Neurovirol. 16:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohrs R. J., Gilden D. H., Mahalingam R. 2004. Varicella zoster virus latency, neurological disease and experimental models: an update. Front. Biosci. 9:751–762 [DOI] [PubMed] [Google Scholar]
- 7. Galli R., Gritti A., Bonfanti L., Vescovi A. L. 2003. Neural stem cells: an overview. Circ. Res. 92:598–608 [DOI] [PubMed] [Google Scholar]
- 8. Gershon A. A., Chen J., Gershon M. D. 2008. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J. Infect. Dis. 197:S61–S65 [DOI] [PubMed] [Google Scholar]
- 9. Gilden D. H., et al. 1983. Varicella-zoster virus DNA in human sensory ganglia. Nature 306:478–480 [DOI] [PubMed] [Google Scholar]
- 10. Gilden D. H., Kleinschmidt-DeMasters B. K., LaGuardia J. J., Mahalingam R., Cohrs R. J. 2000. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 342:635–645 [DOI] [PubMed] [Google Scholar]
- 11. Gilden D. H., et al. 2001. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 23:145–147 [DOI] [PubMed] [Google Scholar]
- 12. Gilden D. H., Cohrs R. J., Mahalingam R. 2003. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 16:243–258 [DOI] [PubMed] [Google Scholar]
- 13. Gowrishankar K., et al. 2007. Productive varicella-zoster virus infection of cultured intact human ganglia. J. Virol. 81:6752–6756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hood C., Cunningham A. L., Slobedman B., Boadle R. A., Abendroth A. 2003. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J. Virol. 77:12852–12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hood C., et al. 2006. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J. Virol. 80:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindvall O., Kokaia Z. 2010. Stem cells in human neurodegenerative disorders—time for clinical translation? J. Clin. Invest. 120:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahalingam R., et al. 1990. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N. Engl. J. Med. 323:627–631 [DOI] [PubMed] [Google Scholar]
- 18. Mahalingam R., et al. 1996. Expression of protein encoded by varicella-zoster zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. U. S. A. 93:2122–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagel M. A., et al. 2011. Varicella-zoster virus transcriptome in latently infected human ganglia. J. Virol. 85:2276–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pugazhenthi S., et al. 2009. Simian varicella virus induces apoptosis in monkey kidney cells by the intrinsic pathway and involves downregulation of bcl-2 expression. J. Virol. 83:9273–9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zerboni L., Ku C.-C., Jones C. D., Zehnder J. L., Arvin A. M. 2005. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:6490–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zerboni L., et al. 2007. Aberrant infection and persistence of varicella-zoster virus in human dorsal root ganglia in vivo in the absence of glycoprotein I. Proc. Natl. Acad. Sci. U. S. A. 104:14086–14091 [DOI] [PMC free article] [PubMed] [Google Scholar]