Abstract
During lytic infection with Epstein-Barr virus (EBV), several viral lytic proteins function to evade immune recognition or to actively suppress immune cells. An EBV lytic transactivator, Zta, induces an immunosuppressive cytokine interleukin 10 (IL-10) in B cells, but whether it regulates IL-10 in the context of epithelial cells is unclear. In this study, we tested nasopharyngeal carcinoma (NPC) cell lines and found that Zta did not induce IL-10 in these epithelial cells. Interestingly, the conditioned medium of Zta-expressing NPC cells enhanced IL-10 production from monocytes. We further revealed that the IL-10-inducing effect involved at least two immunomodulators that were upregulated by Zta and secreted from NPC cells: granulocyte-macrophage colony-stimulating factor (GM-CSF) and prostaglandin E2 (PGE2). Zta was recruited to and activated the GM-CSF promoter, thus upregulating GM-CSF expression. Zta also activated the promoter of cyclooxygenase-2 (COX-2), and Zta-induced COX-2 increased downstream PGE2 production. Cotreatment with GM-CSF and PGE2 synergistically induced IL-10 production from monocytes. The IL-10-inducing effect of the Zta-conditioned medium was reduced when GM-CSF or the COX-2/PGE2 pathway was blocked. The conditioned medium of NPC cells with EBV lytic infection showed a similar increase of GM-CSF and PGE2 levels as well as the IL-10-inducing effect on monocytes, and knockdown of Zta abolished all the effects. Therefore, through Zta-induced immunomodulators, EBV lytic infection in NPC cells can direct bystander monocytes to produce IL-10, which may be a novel way of EBV to promote local immunosuppression.
INTRODUCTION
Epstein-Barr virus (EBV) establishes lifelong persistence in more than 90% of the adult population worldwide, showing its successful dealings with the human immune system (51). Compared with EBV latent infection, in which only few viral antigens are expressed, the lytic infection expresses abundant viral proteins with high antigenicity, serving as a more attractive target recognized and attacked by the host immune system. To survive under the immune surveillance, EBV is equipped with several lytic proteins that evade immune recognition. For example, major histocompatibility complex (MHC) class I-restricted antigen presentation is inhibited by EBV BNLF2a, which blocks peptide transport (25), and by BILF1, which promotes degradation of MHC class I molecules (62). MHC class II-restricted antigen presentation is hampered by the interaction between EBV BZLF2 and MHC class II molecules (50). Moreover, expression of MHC class I and II genes can be downregulated by other EBV lytic proteins: Zta acting at the transcriptional level and BGLF5 acting at the posttranscriptional level (32, 38, 52).
In addition to the strategies that prevent EBV from being recognized by immune cells, EBV may actively cause suppressive effects on immune cells during the lytic cycle, through several secreted factors that are encoded or induced by EBV. For example, a soluble form of EBV BARF1 functions as a decoy receptor of colony-stimulating factor and inhibits macrophage activation (58). An EBV-encoded cytokine BCRF1 reduces expression of MHC molecules, costimulatory molecules, and inflammatory cytokines from monocytes or macrophages, thus impeding T cell activation (53, 60). The EBV lytic transactivator Zta not only initiates expression cascade of viral lytic genes but also induces some cellular genes involved in immune regulation (7, 16, 29). Zta can turn on gene expression through binding to and activation of the target promoters (11, 34). Notably, a previous study shows that Zta induces transcription of human interleukin 10 (IL-10) in B cells (42). IL-10 is an anti-inflammatory cytokine and also a master regulator suppressing the activity of antivirus immune cells, such as Th1 cells, NK cells, and macrophages (14). Thus, IL-10 hinders virus clearance and facilitates chronic infection with several viruses (6, 14).
EBV also infects epithelial cells, but whether Zta regulates IL-10 expression in this cell type is unknown. In this study, we tested cell lines of nasopharyngeal carcinoma (NPC), an epithelial cancer associated with EBV infection. NPC represents a unique tumor microenvironment, where the virus-infected epithelial tumor cells flourish among abundant infiltrating immune cells (51). EBV-specific T lymphocytes are present in the tumor tissues, but their cytotoxic function is generally impaired (39). The functional inactivation of immune cells in NPC tumors may be attributed to several suppressive mechanisms in the microenvironment, including IL-10, gelactin-9, and regulatory T cells (33, 36, 61). Notably, IL-10 has been associated with poor prognosis of NPC, suggesting that it may blunt not only antivirus but also anticancer immune responses in the tumors (19).
In our previous study, Zta induces some chemokines from NPC cells (26). Hence, we wondered if Zta may regulate IL-10 production in these cells. Unexpectedly, different from what has been observed in B cells, Zta did not trigger IL-10 expression in NPC cells. Interestingly, monocytes secreted more IL-10 when they were cultured with the conditioned medium of Zta-expressing NPC cells. We further found that IL-10 production from monocytes was increased by granulocyte-macrophage colony-stimulating factor (GM-CSF) and prostaglandin E2 (PGE2), two immunomodulators that were augmented by Zta and secreted from NPC cells. Therefore, we propose that Zta-induced secreted factors can direct bystander monocytes to produce IL-10, which may serve as a novel way of EBV to promote local immunosuppression.
MATERIALS AND METHODS
Cell culture.
EBV-negative NPC cell lines (NPC-TW01 and HONE-1), EBV-positive NPC cell lines (NA, TW01-GV, and HOTZ-GV), monocytic cell lines (THP-1 and U937), and primary monocytes were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone) at 37°C with 5% CO2. EBV-infected NA cells were established previously (10). By following the same infection protocol, we established two other EBV-converted NPC cell lines by using a recombinant EBV expressing a neomycin-resistant gene and green fluorescent protein (43). TW01-GV was derived from NPC-TW01, and HOTZ-GV was derived from HONE-tetonZ, an NPC cell line carrying a doxycycline-inducible, Zta-expressing cassette (26). To induce the EBV lytic cycle, NA and TW01-GV cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA; 40 ng/ml; Sigma) and sodium n-butyrate (3 mM; Sigma) for 24 h, while HOTZ-GV cells were treated with doxycycline (1 μg/ml; Sigma) for 24 h (26). Human whole blood from healthy donors was obtained from Tainan Blood Center, with the approval of Research Ethics Committee of National Health Research Institutes (EC0971201-E). Mononuclear cells in the whole blood were collected after Ficoll centrifugation, and primary CD14-positive monocytes were further purified through positive selection by using magnetic microbeads (Miltenyi Biotec). Purity of the monocytes was confirmed in that more than 95% of purified cells were positive for CD14 in flow cytometry.
Treatment of monocytes with conditioned medium.
The conditioned medium of NPC cells was the serum-free medium that had been used to culture NPC cells for 48 h; cells and cell debris in the culture supernatants were removed by centrifugation and filtration. Monocytes were cultured with the NPC cell-conditioned medium for 72 h, and their culture supernatants were collected for further examination. In the indicated experiments (see Fig. 7), at 1 h before monocytes were incubated with the conditioned medium, a goat anti-GM-CSF antibody (R&D Systems) or a control goat IgG (MP Biomedicals) was added to the conditioned medium at a final concentration of 6.4 μg/ml, or monocytes were pretreated with 10 μM AH 6809 and AH 23848 (antagonists against EP2 and EP4; Cayman). In some other experiments, monocytes were treated with recombinant GM-CSF protein (PeproTech), sulprostone (an agonist for PGE2 receptors EP1 and EP3; Cayman), or misoprostol (an agonist for PGE2 receptors EP2 and EP4; Cayman) at indicated concentrations.
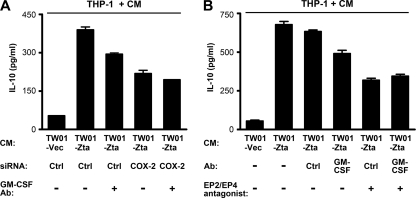
Fig. 7.
Zta-induced immunomodulators from NPC cells contribute to upregulation of IL-10 from monocytes. (A) NPC-TW01 cells were transfected with a vector plasmid (TW01-Vec) or a Zta-expressing plasmid (TW01-Zta), in combination with a control siRNA (Ctrl) or a COX-2-targeted siRNA for 48 h. The conditioned medium (CM) in the absence or presence of a GM-CSF-neutralizing antibody (GM-CSF Ab) was used to culture THP-1 cells. IL-10 concentrations in the final cell culture supernatants were measured by using ELISA. (B) NPC-TW01 cells were transfected with a vector plasmid or a Zta-expressing plasmid. The conditioned medium of the cells was used to culture THP-1 cells. As indicated, a control antibody (Ctrl) or a GM-CSF-neutralizing antibody (Ab) was added to the conditioned medium, or THP-1 cells were pretreated with antagonists against EP2 and EP4. IL-10 concentrations in the final cell culture supernatants were measured by using ELISA.
Plasmids and siRNAs.
Plasmids expressing wild-type Zta, transactivation-impaired Zta (with deletion of the amino acid residuals 52 to 78), or DNA-binding-defective Zta (with mutation of the amino acid residuals 187RKC189 into 187EES189) have been constructed in previous studies (17, 18, 41). The GM-CSF promoter fragment (−588 to −5) was PCR amplified by using the primers 5′-GGGGTACCATGGTGGTCATTCCCTCT-3′ (the KpnI site is underlined) and 5′-CCCTCGAGCTCACTGGCAAAAGA-3′ (the XhoI site is underlined). The cyclooxygenase (COX)-2 promoter fragment (−918 to +49) was PCR amplified by using the primers 5′-GGGGTACCGGACATTTAGCGTCCCTGCA-3′ (the KpnI site is underlined) and 5′-GGCTCGAGGAGTTCCTGGACGTGCTCCT-3′ (the XhoI site is underlined). The amplified DNA fragments were digested with KpnI and XhoI and then ligated to the KpnI/XhoI-digested pGL3-basic reporter plasmid (Promega) containing a firefly luciferase gene. The pSUPER-based plasmids expressing a control short-hairpin RNA (shRNA) or the shRNA targeted against Zta have been used previously (8). The chemical-modified, double-stranded small interfering RNAs (siRNAs) targeted against COX-2 (5′-AAAGACUGGUAUUUCAUCUGCCUGC-3′) and a control siRNA with comparable GC content were purchased from Invitrogen.
Cell transfection and reporter gene assay.
NPC-TW01 and HONE-1 cells were transfected with plasmid DNA and/or siRNA by using Lipofectamine 2000 reagent (Invitrogen) as described in our previous study (26). After 4 h of incubation with the DNA/RNA-liposome mixture, the cells were washed and cultured in serum-free medium. For an indicated experiment, the cells were treated with COX-2 inhibitor II (Calbiochem) at this time point. At 48 h posttransfection, cell lysates and the conditioned medium were collected for further examination or for monocyte culture. TW01-GV cells were transfected with shRNA-expressing plasmids as described previously (8). At 24 h posttransfection, the cells were treated with TPA and n-butyrate for 24 h and then washed to remove the lytic inducers. The washed cells were further cultured in serum-free medium for 48 h, and their conditioned medium was used for the indicated experiments. For reporter gene assays, cotransfection with reporter and effector plasmids was carried out according to our previous protocol (26). At 36 h posttransfection, the cells were subjected to the luciferase assay by using a Bright-Glo assay kit (Promega).
Cytokine antibody array and ELISA.
Expression profiles of cytokines in the conditioned medium were analyzed by using the human cytokine antibody array C series 1000 (RayBiotech) according to the manufacturer's instruction. Secreted IL-10 and GM-CSF in the conditioned medium were quantified by using the DuoSet enzyme-linked immunosorbent assay (ELISA) development system (R&D Systems) based on the forward sandwich binding technique, while PGE2 was quantified by using a Parameter PGE2 immunoassay kit (R&D Systems) based on the forward competitive binding technique. Each ELISA experiment was done in duplicate by following the protocol provided by the manufacturer.
Reverse transcription and quantitative real-time PCR.
Cellular RNAs were extracted by using a QIAamp RNA kit (Qiagen). Reverse transcription (RT) reaction was carried out by using the oligo(dT)15 primer and Superscript III reverse transcriptase (Invitrogen). The cDNAs of IL-10, GM-CSF, and COX-2 were quantified by real-time PCR by using LightCycler reagents and the detection system (Roche Applied Science). PCR primers for detecting IL-10 were 5′-TGGGGGAGAACCTGAAGAC-3′ and 5′-CAGGGAAGAAATCGATGACAG-3′, and the locked nucleic acid-based probe was 5′-GGCTGAGG-3′. Primers for detecting GM-CSF were 5′-TCTCAGAAATGTTTGACCTCCA-3′ and 5′-GCCCTTGAGCTTGGTGAG-3′, and the probe was 5′-CCTGGAGC-3′. Primers for detecting COX-2 were 5′-TGGGAAGCCTTCTCTAACCTC-3′ and 5′-TCAGGAAGCTGCTTTTTACCTT-3′, and the probe was 5′-CTTCCTCC-3′. The cDNA of TATA box-binding protein was quantified and used as an internal reference for calibration (primers, 5′-GCTGGCCCATAGTGATCTTT-3′ and 5′-TCCTTGGGTTATCTTCACACG-3′; probe, 5′-CCCAGCAG-3′). Relative mRNA levels were calculated by using the LightCycler software (Roche Applied Science). Each experiment was done twice independently.
Immunoblotting assay.
Protein extraction, electrophoresis, and an immunoblotting assay were performed as described previously (26). The primary antibodies used in the assay included mouse monoclonal antibodies recognizing Zta, BMRF1, and β-actin (Chemicon), rabbit antibodies recognizing COX-2 and COX-1 (Santa Cruz Biotechnology), and a human serum recognizing EBV nuclear antigen 1 (EBNA1). Corresponding secondary antibodies conjugated with horseradish peroxidase were purchased from Jackson Immunoresearch.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed according to our previous study with some modifications (26). Briefly, cells were fixed with formaldehyde, lysed, and sonicated, followed by incubation with an anti-Zta antibody (AZ-69; Argene) or a control mouse immunoglobulin at 4°C overnight. The immunocomplex was then precipitated with magnetic protein A-Dynabeads (Invitrogen), and DNA fragments in the precipitants were eluted and subjected to quantitative real-time PCR by using LightCycler reagents and the detection system (Roche Applied Science). PCR primers for quantifying the GM-CSF promoter were 5′-ACCTAGGGAAAAGGCTCACC-3′ and 5′-GGGGAACTACCTGAACTGTGG-3′, and the probe was 5′-TGTGGCTG-3′. PCR primers for quantifying the COX-2 promoter were 5′-CCGGGCTTACGCAATTTT-3′ and 5′-TTTCTTTCCGCCTTTTCGTA-3′, and the probe was 5′-GGAGAGGA-3′. PCR primers for control DNA were 5′-CTTATCCGAGCCGAGCTG-3′ and 5′-CGTTGTAGATGTGGTCGTTACC-3′, and the probe was 5′-CCAGCCAG-3′. The amounts of target DNA in the precipitants were compared with those before precipitation and expressed as the “percentage of input.” Each experiment was done twice independently.
RESULTS
The conditioned medium of Zta-expressing NPC cells enhances IL-10 production from monocytes.
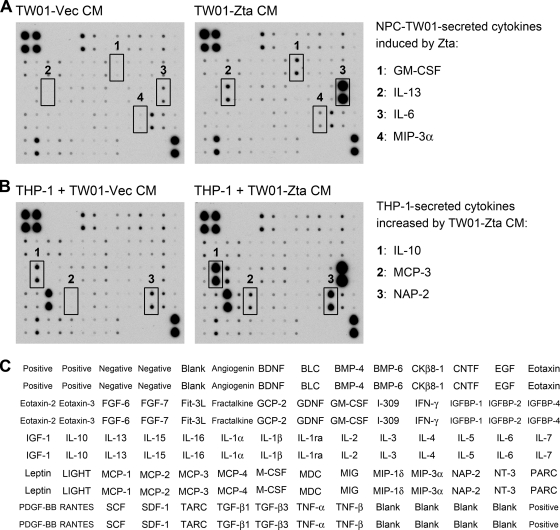
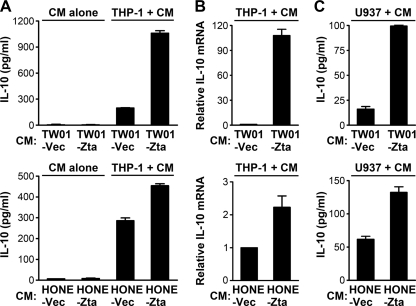
To identify Zta-induced cytokines secreted from NPC cells, an antibody array was used to analyze the conditioned medium of EBV-negative NPC-TW01 cells transfected with a vector plasmid or with a Zta-expressing plasmid. Figure 1A shows that Zta increased secretion of GM-CSF, IL-13, IL-6, and macrophage inflammatory protein 3α (MIP-3α). Zta-mediated upregulation of IL-13 and IL-6 in other cell types has been reported previously (29, 59). Notably, different from a previous study indicating that Zta upregulates IL-10 in B cells (42), Zta did not increase IL-10 secretion from NPC cells in the cytokine profile analysis (Fig. 1A). The negative result was confirmed by ELISA in that IL-10 was almost undetectable in the conditioned medium of NPC cells with or without Zta expression (Fig. 2A). Furthermore, in quantitative RT-PCR analysis, we did not detect a Zta-induced increase of IL-10 mRNA in NPC cells (data not shown).
Fig. 1.
Zta upregulates cytokines from NPC cells, and the conditioned medium of Zta-expressing NPC cells further induces cytokines from monocytes. (A) An antibody array was used to analyze cytokine profiles in the conditioned medium (CM) of NPC-TW01 cells transfected with a vector plasmid (TW01-Vec) or a Zta-expressing plasmid (TW01-Zta). Four NPC-TW01-secreted cytokines induced by Zta are indicated. (B) An antibody array was used to analyze cytokine profiles in the final culture supernatants of THP-1 cells that were cultured in the NPC cell-conditioned medium mentioned for panel A. Three additional THP-1-secreted cytokines whose levels were increased by the conditioned medium of Zta-expressing NPC-TW01 cells (TW01-Zta CM) are indicated. (C) Target cytokines and their positions in the antibody array used in panels A and B are provided.
Fig. 2.
The conditioned medium of Zta-expressing NPC cells enhances IL-10 production from monocytes. (A) NPC-TW01 cells and HONE-1 cells were transfected with a vector plasmid (TW01-Vec and HONE-Vec) or a Zta-expressing plasmid (TW01-Zta and HONE-Zta). An ELISA was used to measure IL-10 concentrations in their conditioned medium (CM alone) or in the final supernatants of THP-1 cells that were cultured with the indicated NPC cell-conditioned medium (THP-1 + CM). (B) Quantitative RT-PCR was used to measure the relative levels of IL-10 mRNA in THP-1 cells that were cultured with the indicated NPC cell-conditioned medium. (C) An ELISA was used to measure IL-10 concentrations in the final supernatants of U937 cells that were cultured with the indicated NPC cell-conditioned medium (U937 + CM).
Considering that monocytes and monocyte-derived cells are potent producers of cytokines, we wondered if cytokine production from monocytes can be affected by certain immunomodulators secreted from Zta-expressing NPC cells. Therefore, the conditioned medium of NPC-TW01 cells with or without Zta expression was used to culture a monocytic cell line THP-1, and cytokine profiles in the final culture supernatants were further analyzed by using the antibody array. Compared with the monocytes cultured in the vector control medium, those cultured in the conditioned medium of Zta-expressing NPC cells secreted some cytokines in higher levels. Apart from IL-13 and IL-6, which had increased in the conditioned medium of Zta-expressing NPC cells (Fig. 1A), THP-1 cells secreted more IL-10, macrophage chemoattractant protein 3 (MCP-3), and neutrophil-activating protein 2 (NAP-2) in the Zta-conditioned medium than in the vector control medium (Fig. 1B). We focused on the indirect upregulation of IL-10 in the following studies. An ELISA confirmed that IL-10 secretion from THP-1 cells was augmented by the conditioned medium of two NPC cell lines with ectopic Zta expression (Fig. 2A). Meanwhile, quantitative RT-PCR showed that THP-1 cells expressed more IL-10 mRNA when they were cultured with the Zta-conditioned medium (Fig. 2B). The enhancement of IL-10 production by the Zta-conditioned medium was also observed when another monocytic cell line, U937, was tested (Fig. 2C). The Zta-triggered, IL-10-inducing effect of the conditioned medium from HONE-1 cells was less prominent than that from NPC-TW01 cells, which may be attributed to higher basal levels of secretion of certain IL-10-inducing factors from HONE-1 cells in the absence of Zta expression.
GM-CSF and PGE2 synergistically induce IL-10 production from monocytes.
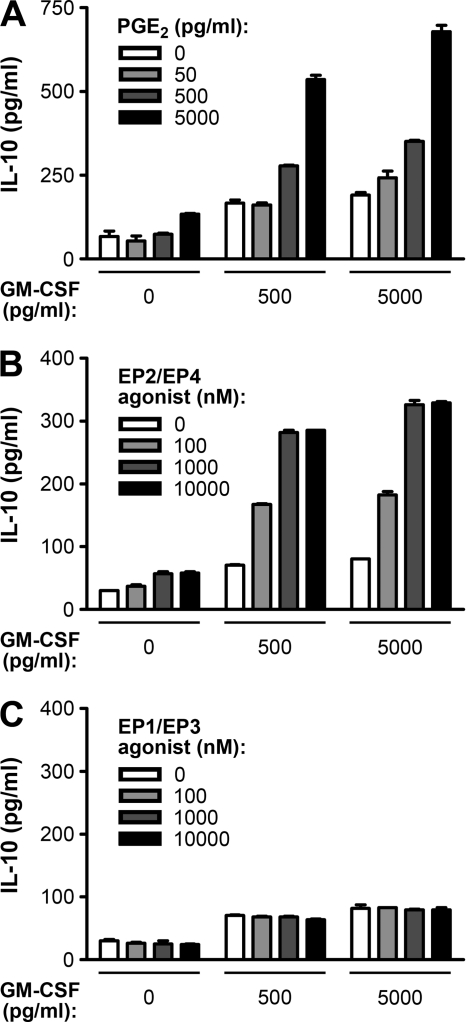
Previous studies have indicated that two immunomodulators, GM-CSF and PGE2, can upregulate IL-10 expression in monocytes or macrophages (20, 37, 49). Interestingly, our cytokine antibody array showed that Zta increased GM-CSF secretion from NPC-TW01 cells (Fig. 1A). To verify the effects of GM-CSF and PGE2 on IL-10 production from THP-1 cells, we treated the cells with GM-CSF protein and PGE2 separately or in combination. IL-10 secretion was slightly increased by treatment with GM-CSF alone or PGE2 alone but was remarkably induced by cotreatment with increasing dosages of GM-CSF and PGE2 (Fig. 3A), which is similar to the observation in a previous study using U937 cells (20). GM-CSF also significantly enhanced IL-10 secretion from THP-1 cells in cooperation with an agonist of the PGE2 receptors EP2 and EP4 (Fig. 3B) but not with an agonist of the PGE2 receptors EP1 and EP3 (Fig. 3C), indicating that the effect of PGE2 was through EP2/EP4. These results corroborate the synergistic effect of GM-CSF and PGE2 to induce IL-10 production from monocytes.
Fig. 3.
GM-CSF and PGE2 synergistically induce IL-10 production from monocytes. (A) GM-CSF and PGE2 at the indicated concentrations were used to treat THP-1 cells for 72 h, and then IL-10 concentrations in the cell culture supernatants were quantified by using ELISA. (B) GM-CSF and misoprostol (an agonist for PGE2 receptors EP2 and EP4) at the indicated concentrations were used to treat THP-1 cells for 72 h, and then IL-10 concentrations in the cell culture supernatants were quantified by using ELISA. (C) GM-CSF and sulprostone (an agonist for PGE2 receptors EP1 and EP3) at the indicated concentrations were used to treat THP-1 cells for 72 h, and then IL-10 concentrations in the cell culture supernatants were quantified by using ELISA.
Zta induces GM-CSF expression in NPC cells.
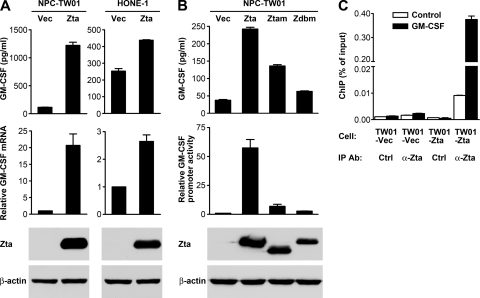
To confirm Zta-mediated induction of GM-CSF, a Zta-expressing plasmid was transfected into two NPC cell lines, and GM-CSF expression was examined by using ELISA and quantitative RT-PCR. While HONE-1 cells showed higher basal levels of production of GM-CSF than NPC-TW01 cells, ectopic Zta expression increased levels of mRNA and secretory protein of GM-CSF in both cell lines (Fig. 4 A). Zta is a DNA-binding transcription factor that activates many viral and cellular promoters, and we found that Zta also transactivated the GM-CSF promoter in a reporter gene assay (Fig. 4B). Zta mutants defective in either transactivation function or DNA-binding ability showed reduced activity to activate the GM-CSF promoter and to induce GM-CSF secretion (Fig. 4B). Furthermore, recruitment of Zta to the GM-CSF promoter in vivo was demonstrated by using a ChIP assay, in which DNA of the target promoter was preferentially detected in the Zta-immunoprecipitant from Zta-expressing NPC cells (Fig. 4C). These results support the assertion that Zta can bind to and activate the GM-CSF promoter, driving GM-CSF expression.
Fig. 4.
Zta upregulates GM-CSF expression in NPC cells. (A) NPC-TW01 and HONE-1 cells were transfected with a vector plasmid (Vec) or a Zta-expressing plasmid (Zta). GM-CSF concentrations in the cell culture supernatants were measured by using ELISA. GM-CSF mRNA was measured by quantitative RT-PCR. Intracellular Zta and β-actin proteins were detected by an immunoblotting assay. (B) NPC-TW01 cells were transfected with a vector plasmid or a plasmid expressing wild-type Zta, a transactivation-impaired Zta mutant (Ztam), or a DNA-binding-defective Zta mutant (Zdbm). GM-CSF concentrations in the cell culture supernatants were measured by using ELISA. Intracellular Zta and β-actin proteins were detected by an immunoblotting assay. These effector plasmids were also cotransfected with a GM-CSF reporter plasmid into NPC-TW01 cells, and their effects on GM-CSF promoter activity were examined by using a luciferase reporter gene assay. (C) NPC-TW01 cells were transfected with a vector plasmid (TW01-Vec) or a Zta-expressing plasmid (TW01-Zta) and then subjected to a ChIP assay. An anti-Zta antibody (α-Zta) and a control antibody (Ctrl) were used as the immunoprecipitating antibodies (IP Ab). The amounts of GM-CSF promoter DNA (black bars) and control DNA (white bars) in the immunoprecipitants were quantified by using real-time PCR and expressed as “percentage of input.”
Zta induces PGE2 production through upregulation of COX-2 in NPC cells.
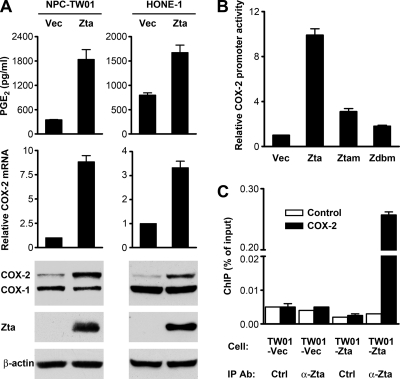
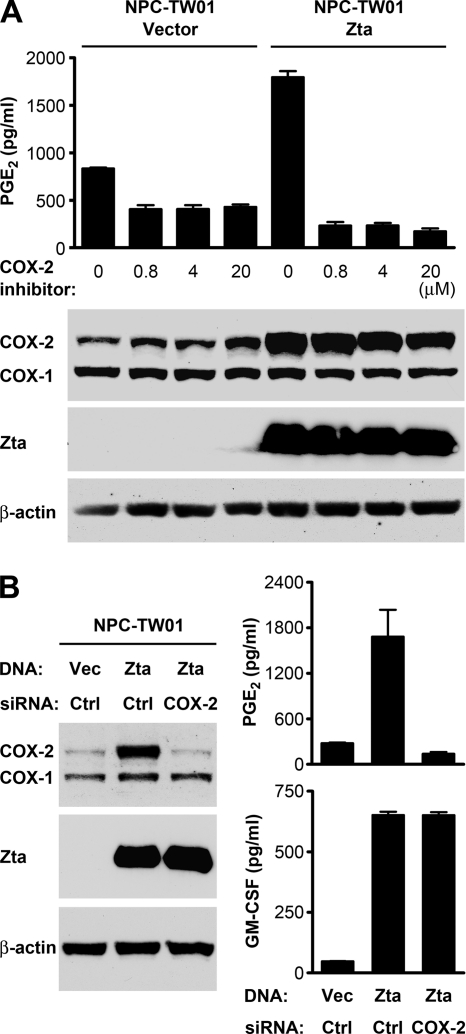
Next, we studied whether and how Zta can induce PGE2 production. While HONE-1 cells showed higher basal levels of PGE2 production than NPC-TW01 cells, ectopic Zta expression increased PGE2 levels in the conditioned medium of both cell lines (Fig. 5A). Since COXs are the key upstream enzymes for metabolic production of PGE2 (21), we wondered if Zta can induce COX expression. An immunoblotting assay showed that Zta upregulated the protein level of COX-2 but not COX-1, and quantitative RT-PCR indicated that Zta increased COX-2 mRNA in NPC cells (Fig. 5A). Zta also transactivated the COX-2 promoter in a reporter gene assay, and Zta mutants defective in gene transactivation or DNA binding exhibited a diminished ability to activate the promoter (Fig. 5B). In addition, Zta recruitment to the COX-2 promoter in vivo was demonstrated by using a ChIP assay (Fig. 5C). To confirm that COX-2 mediated Zta-induced PGE2 production, we treated cells with a COX-2 inhibitor. While the inhibitor did not affect the protein levels of Zta and Zta-induced COX-2, it blocked the PGE2 production induced by Zta (Fig. 6 A). Knockdown of COX-2 by using a COX-2-specific siRNA also abolished Zta-induced secretion of PGE2 but did not influence Zta-induced secretion of GM-CSF (Fig. 6B). Therefore, Zta can promote PGE2 production through induction of COX-2 but upregulates GM-CSF in a COX-2-independent manner.
Fig. 5.
Zta upregulates PGE2 production and COX-2 expression in NPC cells. (A) NPC-TW01 and HONE-1 cells were transfected with a vector plasmid (Vec) or a Zta-expressing plasmid (Zta). PGE2 concentrations in the cell culture supernatants were measured by using ELISA. COX-2 mRNA levels were measured by quantitative RT-PCR. Intracellular proteins of Zta, COX-1, COX-2, and β-actin were detected by an immunoblotting assay. (B) NPC-TW01 cells were cotransfected with a COX-2 reporter plasmid and an effector plasmid expressing wild-type Zta (Zta), a transactivation-impaired Zta mutant (Ztam), or a DNA-binding-defective Zta mutant (Zdbm). The effects of Zta and its mutants on COX-2 promoter activity were examined by using a luciferase reporter gene assay. (C) NPC-TW01 (TW01) cells were transfected with a vector plasmid or a Zta-expressing plasmid and then subjected to a ChIP assay that was performed and presented as described in the legend to Fig. 4C.
Fig. 6.
COX-2 mediates Zta-induced PGE2 production from NPC cells. (A) NPC-TW01 cells were transfected with a vector plasmid or a Zta-expressing plasmid and treated with COX-2 inhibitor II at the indicated concentrations for 48 h. Intracellular proteins of Zta, COX-1, COX-2, and β-actin were detected by an immunoblotting assay. PGE2 concentrations in the cell culture supernatants were measured by using ELISA. (B) NPC-TW01 cells were transfected with a vector plasmid (Vec) or a Zta-expressing plasmid (Zta), in combination with a control siRNA (Ctrl) or a COX-2-targeted siRNA for 48 h. Intracellular proteins of Zta, COX-1, COX-2, and β-actin were detected by an immunoblotting assay. Concentrations of PGE2 and GM-CSF in the cell culture supernatants were measured by using ELISA.
Zta-induced immunomodulators from NPC cells contribute to upregulation of IL-10 from monocytes.
We further evaluated the roles of the Zta-induced, NPC cell-produced immunomodulators in promotion of IL-10 production from monocytes. Treatment of the Zta-conditioned medium with a GM-CSF-neutralizing antibody partially reduced the IL-10 induction from THP-1 cells (Fig. 7A and B). The IL-10-inducing effect was also diminished when the COX-2/PGE2 pathway was blocked by either COX-2-targeted siRNA (Fig. 7A) or antagonists against the PGE2 receptors EP2 and EP4 (Fig. 7B). When the COX-2/PGE2 pathway was blocked, neutralization of GM-CSF did not further reduce the IL-10-inducing effect (Fig. 7A and B), suggesting that GM-CSF may have little effect in the absence of PGE2. These results indicate that GM-CSF and PGE2 in the conditioned medium of Zta-expressing NPC cells contribute to upregulation of IL-10 from monocytes.
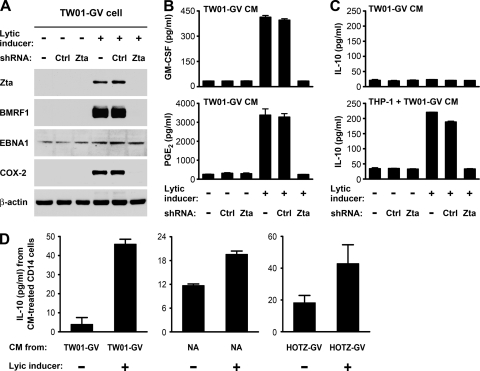
EBV lytic infection in NPC cells increases production of GM-CSF and PGE2 and enhances IL-10 production from monocytes.
We used the EBV-positive NPC cells, TW01-GV, to test whether EBV lytic infection in NPC cells can also induce the immunomodulators and enhance IL-10 production from monocytes. EBV infection in TW01-GV cells was predominantly latent, and treatment with TPA and n-butyrate activated the cells into a state expressing viral lytic proteins such as Zta and BMRF1 (Fig. 8A). The EBV lytic infection was accompanied by induction of COX-2 and increased secretion of GM-CSF and PGE2 (Fig. 8A and B). Since Zta serves as an essential immediate-early protein that initiates an EBV lytic expression cascade, a Zta-targeted shRNA efficiently blocked expression of Zta and its downstream lytic genes but did not affect expression of an EBV latent protein, EBNA1 (Fig. 8A). Notably, induction of COX-2, GM-CSF, and PGE2 in the chemically activated TW01-GV cells was abolished by the Zta shRNA but not by a control shRNA (Fig. 8A and B), suggesting that Zta (or other downstream viral gene products) contributed to the induction effects. The EBV lytic inducers did not promote IL-10 secretion from TW01-GV cells, but the conditioned medium of the activated TW01-GV cells enhanced IL-10 secretion from THP-1 cells (Fig. 8C). The IL-10-inducing effect on monocytes was blocked when the EBV lytic cycle in TW01-GV cells was inhibited by Zta shRNA (Fig. 8C), recapitulating that EBV lytic infection in NPC cells is essentially involved in the induction of IL-10 from monocytes.
Fig. 8.
EBV lytic infection in NPC cells induces production of GM-CSF and PGE2 and enhances IL-10 production from monocytes. TW01-GV cells were mock transfected or transfected with the indicated shRNA-expressing plasmids, followed by treatment with or without lytic inducers. The lytic inducers were removed 24 h later, and then the cells were cultured in serum-free medium for 48 h before collection of their conditioned medium for further study. (A) Intracellular proteins of Zta, BMRF1, EBNA1, COX-2, and β-actin were detected by an immunoblotting assay. (B) Concentrations of PGE2 and GM-CSF in the conditioned medium (CM) of the TW01-GV cells with the indicated treatments were measured by using ELISA. (C) An ELISA was used to measure IL-10 concentrations in the conditioned medium of the TW01-GV cells with indicated treatments (TW01-GV CM) and IL-10 concentrations in the final supernatants of THP-1 cells that were cultured with the indicated TW01-GV cell-conditioned medium (THP-1 + TW01-GV CM). (D) The conditioned medium (CM) of three EBV-infected NPC cell lines (TW01-GV, NA, and HOTZ-GV) with or without lytic induction was used to treat primary CD14-positive monocytes. Secretion of IL-10 from monocytes was quantified by using ELISA.
We also tested whether the IL-10-inducing effect of EBV lytic infection can apply to primary monocytes. CD14-positive monocytes were purified from peripheral blood mononuclear cells of healthy donors and treated with the conditioned medium of TW01-GV cells with or without lytic induction. The conditioned medium of the lytically infected cells enhanced IL-10 production from primary monocytes, compared with the medium of latently infected cells (Fig. 8D). We further tested two other EBV-infected NPC cell lines, NA and HOTZ-GV. The EBV lytic cycle can be reactivated by TPA and n-butyrate in NA cells (10) and by doxycycline, which triggers Zta expression, in HOTZ-GV cells (26). The condition medium of these two cell lines under lytic infection also increased IL-10 production from primary monocytes (Fig. 8D).
DISCUSSION
During pathogen infection, the suppressive cytokine IL-10 prevents overactivated immune responses and alleviates immunopathogenesis, but inappropriately prolonged IL-10 production causes failure of pathogen clearance and facilitates chronic infection (14). To achieve persistent infection, viruses may enhance IL-10 production from infected cells or other uninfected immune cells (4, 6, 28). In addition, several viruses encode homologs of cellular IL-10 to modulate host immune responses (57). Being a virus that successfully establishes persistence in human population, EBV also utilizes IL-10 and IL-10-like proteins to deal with the host immune system. BCRF1 is the EBV homolog of IL-10 and exerts immunosuppressive functions at the late stage of lytic infection (53, 60). Furthermore, in B cells, expression of human IL-10 can be induced by some EBV gene products, including the immediate-early lytic protein Zta and two latent products, latent membrane protein 1 (LMP1) and EBV-encoded small RNA (EBER) (35, 42, 54). On the other hand, how EBV regulates IL-10 expression in the context of epithelial cells is unclear. In the present study, we tested NPC cells and found that Zta did not induced IL-10 expression in the cells. Our unpublished data indicated that IL-10 expression in NPC cells was also not affected by LMP1 and EBER. Therefore, we speculated an alternative mechanism whereby EBV may indirectly promote IL-10 production from bystander immune cells. Our study using the conditioned medium of NPC cells with Zta expression or EBV lytic infection supported this hypothesis (Fig. 2 and 8), which prompted us to propose the model illustrated in Fig. 9. In NPC cells, Zta not only induces GM-CSF expression but also upregulates COX-2, which increases PGE2 production, so the cells with EBV lytic infection and Zta expression secrete more GM-CSF and PGE2. The secreted GM-CSF and PGE2 may cooperatively promote IL-10 production from monocytes. Thus, through the Zta-induced immunomodulators, EBV lytic infection in NPC cells may drive nearby monocytes into IL-10-producing cells, facilitating local immunosuppression.
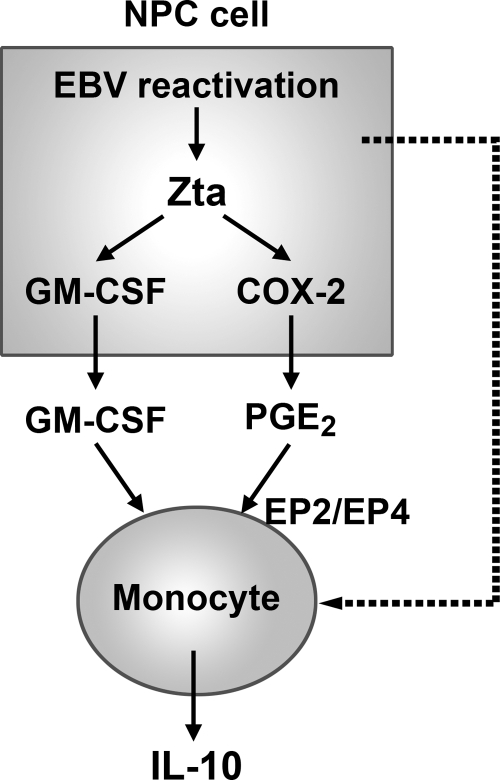
Fig. 9.
A model illustrates how EBV lytic infection in NPC cells may upregulate IL-10 production from monocytes. During EBV reactivation in NPC cells, the lytic protein Zta not only induces GM-CSF expression but also upregulates COX-2 that increases production of PGE2. The secreted GM-CSF and PGE2 may cooperatively promote IL-10 production from monocytes. Through the Zta-induced immunomodulators, EBV lytic infection in NPC cells can direct bystander monocytes to produce IL-10. Meanwhile, we do not rule out that other immunomodulators or other EBV proteins expressed during lytic infection also contribute to induction of IL-10 from monocytes (the dotted line).
Serving as a transcription factor, Zta can activate promoters containing the Zta-binding DNA sequences, which partly resemble the DNA elements targeted by cellular AP-1 or C/EBP proteins (11, 34). Notably, the Zta-responsive elements are found in promoters of many cellular genes involved in immune regulation. For example, cytokines, such as IL-6, IL-8, IL-10, IL-13, and transforming growth factor β1 (TGF-β1), have been reported as Zta-targeted genes (7, 26, 29, 59). Therefore, Zta may function as a potent immune regulator upon EBV reactivation into the lytic cycle. Nevertheless, Zta does not induce these cytokines in all cell types. In our cytokine profile analysis, Zta increased production of IL-6 and IL-13 from NPC cells but did not induce IL-10 and TGF-β1 (Fig. 1). Even though multiple Zta-binding elements have been identified in the IL-10 promoter (42), whether the promoter is activated by Zta seems to depend on the cellular context. The differential Zta-mediated regulation of IL-10 may be attributed to epigenetic modification of the promoter region (3, 30) or to the presence of other cofactors that regulate Zta activity (1). In addition, as has been observed previously, the effects of Zta on cytokine production may depend on coexpression of other EBV proteins (29). Further studies are required for identification of critical factors affecting Zta-mediated cytokine regulation. On the other hand, our present study puts GM-CSF and COX-2 into the list of Zta-targeted genes associated with immune regulation. Zta activates promoters of these two genes, which requires the DNA binding and transactivation activity of Zta (Fig. 4 and 5). Our preliminary analysis predicted a cluster of potential Zta-binding sequences in the proximal region (−161 to −5) of the GM-CSF promoter and more than 10 potential Zta-binding elements scattering in the 1-kb COX-2 promoter (−918 to + 49) (data not shown). The genuine elements responsible for Zta binding and transactivation in the two promoters remain to be determined.
Zta-mediated induction of GM-CSF and COX-2/PGE2 may trigger, directly or indirectly, multiple suppressive events that inactivate local immune responses. Although GM-CSF is recognized as an immune stimulator and used as an adjuvant for some vaccines (47), it may exert immunosuppressive effects under certain circumstances, especially in some tumor microenvironments. For example, tumor-produced GM-CSF dysregulates maturation of antigen-presenting cells and thus inhibits CD8 T cell responses (5); it also impairs local immunity through recruitment of myeloid-derived suppressor cells (55). On the other hand, increase of COX-2 expression and its downstream product PGE2 is frequently detected in tumors and linked to immunosuppressive events (21). PGE2 exerts its inhibitory effects on many immune cells, including lymphocytes and antigen-presenting cells (12, 22). Both GM-CSF and PGE2 have been shown to induce development of immunosuppressive cells, such as regulatory T cells and myeloid-derived suppressor cells, which produce more suppressive factors, including IL-10 (2, 15, 31, 56). In addition, as has been reported previously (20) and confirmed in our study, GM-CSF and PGE2 synergistically promote IL-10 production from monocytes (Fig. 3). Therefore, Zta may induce these two immunomodulators to bias a microenvironment in favor of immune suppression.
Whether the findings of this study apply to NPC in vivo is intriguing. It is unknown if GM-CSF is expressed in NPC tissues or even by the tumor cells undergoing EBV lytic infection. It is also unclear if NPC-infiltrating monocytes produce IL-10 in vivo. Nevertheless, there are several indirect clues. First, since NPC resembles an inflamed tissue in histological features and cytokine profiles (27, 51), it will not be surprising that the inflammatory cytokine GM-CSF can be expressed in the tumor tissues. In a previous study of immunoglobulin A-mediated EBV infection, GM-CSF expression in NPC cells is increased at the initial infection stage, where the viral lytic cycle may occur (40). On the other hand, in a previous study examining IL-10 expression in NPC biopsy specimens, IL-10 is not always detectable in tumor cells but is generally detectable among tumor-infiltrating mononuclear cells, which may include lymphocytes and monocytes (19). Even though the clues are very limited, our findings draw attention to the potential contribution of EBV reactivation to local immunosuppression of NPC. While only a small subset of NPC tumor cells shows EBV lytic infection and Zta expression (13), these cells may secrete critical factors that benefit the whole tumor. In a mouse model of EBV-associated lymphoproliferative disease, lytically infected B cells produce more cytokines and angiogenic factors, which contribute to early outgrowth of EBV-infected B cells in vivo (23, 24). Our present study suggests that EBV lytic infection in NPC cells may upregulate immunomodulators that affect infiltrating immune cells, shielding tumor cells from immune attack. Notably, strong immunosuppression in the tumors could be a major obstacle to the development of immunotherapy for NPC (39), so alleviation of EBV-induced suppressive effects may improve the cancer treatment.
Meanwhile, we do not exclude the possibility that other immunomodulators or other EBV proteins also contribute to the induction of IL-10 from monocytes (Fig. 9). When we simultaneously blocked GM-CSF and COX-2/PGE2 pathways, the IL-10-inducing effect of the Zta-conditioned medium was reduced but not completely abolished (Fig. 7). It suggests that other factor(s) in the conditioned medium may be involved. We are currently testing other Zta-induced cytokines or chemokines for their ability to enhance IL-10 production from monocytes. Whether NPC cell-produced exosomes in the conditioned medium also play a role therein is another interesting direction for further study (33, 44). On the other hand, when we used Zta-targeted shRNA to inhibit EBV lytic infection in TW01-GV cells, the induction of GM-CSF, COX-2, and PGE2, as well as the IL-10-inducing effect on monocytes, was almost entirely abolished (Fig. 8). Considering that the Zta shRNA blocks expression of not only Zta but also its downstream lytic genes (8), we do not rule out that, in addition to Zta, other EBV proteins(s) may cause similar effects. EBV LMP1 is a potential candidate because its expression is upregulated during lytic infection and it can induce COX-2 and PGE2 in NPC cells (9, 48). A recent study using microarray analysis shows that LMP1 also augments transcription of GM-CSF in keratinocytes (46). In addition, multiple roles of LMP1 in immune evasion have been reported (45). It would be of interest to test whether LMP1 expressed in NPC cells also induces similar immunomodulators to upregulate IL-10 production from monocytes.
ACKNOWLEDGMENTS
We thank Pin Ling for providing THP-1 cells, Ping-Kun Chen for providing the plasmid containing COX-2 promoter, and Ko-Jiunn Liu for inspiring discussion.
This study was supported by the National Health Research Institutes (ID-099-PP-16, ID-099-SP-06, and ID-100-PP-15) and the National Science Council (NSC99-2628-B-400-001-MY3).
This study has no potential conflicts of interest.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Bailey S. G., et al. 2009. Functional interaction between Epstein-Barr virus replication protein Zta and host DNA damage response protein 53BP1. J. Virol. 83:11116–11122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baratelli F., et al. 2005. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175:1483–1490 [DOI] [PubMed] [Google Scholar]
- 3. Bhende P. M., Seaman W. T., Delecluse H. J., Kenney S. C. 2004. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat. Genet. 36:1099–1104 [DOI] [PubMed] [Google Scholar]
- 4. Brady M. T., MacDonald A. J., Rowan A. G., Mills K. H. 2003. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur. J. Immunol. 33:3448–3457 [DOI] [PubMed] [Google Scholar]
- 5. Bronte V., et al. 1999. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J. Immunol. 162:5728–5737 [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks D. G., et al. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cayrol C., Flemington E. K. 1995. Identification of cellular target genes of the Epstein-Barr virus transactivator Zta: activation of transforming growth factor βigh3 (TGF-βigh3) and TGF-β1. J. Virol. 69:4206–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang Y., et al. 2004. Inhibition of the Epstein-Barr virus lytic cycle by Zta-targeted RNA interference. J. Gen. Virol. 85:1371–1379 [DOI] [PubMed] [Google Scholar]
- 9. Chang Y., et al. 2004. Induction of Epstein-Barr virus latent membrane protein 1 by a lytic transactivator Rta. J. Virol. 78:13028–13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang Y., et al. 1999. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J. Virol. 73:8857–8866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang Y. N., Dong D. L., Hayward G. S., Hayward S. D. 1990. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J. Virol. 64:3358–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chemnitz J. M., et al. 2006. Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck: implications in Hodgkin's lymphoma. Cancer Res. 66:1114–1122 [DOI] [PubMed] [Google Scholar]
- 13. Cochet C., et al. 1993. Expression of the Epstein-Barr virus immediate early gene, BZLF1, in nasopharyngeal carcinoma tumor cells. Virology 197:358–365 [DOI] [PubMed] [Google Scholar]
- 14. Couper K. N., Blount D. G., Riley E. M. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771–5777 [DOI] [PubMed] [Google Scholar]
- 15. Dolcetti L., et al. 2010. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 40:22–35 [DOI] [PubMed] [Google Scholar]
- 16. Feederle R., et al. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flemington E. K., Borras A. M., Lytle J. P., Speck S. H. 1992. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J. Virol. 66:922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flemington E. K., Lytle J. P., Cayrol C., Borras A. M., Speck S. H. 1994. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol. Cell. Biol. 14:3041–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujieda S., et al. 1999. Staining of interleukin-10 predicts clinical outcome in patients with nasopharyngeal carcinoma. Cancer 85:1439–1445 [PubMed] [Google Scholar]
- 20. Grant V., King A. E., Faccenda E., Kelly R. W. 2005. PGE/cAMP and GM-CSF synergise to induce a pro-tolerance cytokine profile in monocytic cell lines. Biochem. Biophys. Res. Commun. 331:187–193 [DOI] [PubMed] [Google Scholar]
- 21. Greenhough A., et al. 2009. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30:377–386 [DOI] [PubMed] [Google Scholar]
- 22. Harizi H., Juzan M., Grosset C., Rashedi M., Gualde N. 2001. Dendritic cells issued in vitro from bone marrow produce PGE2 that contributes to the immunomodulation induced by antigen-presenting cells. Cell. Immunol. 209:19–28 [DOI] [PubMed] [Google Scholar]
- 23. Hong G. K., et al. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 79:13993–14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong G. K., et al. 2005. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 79:13984–13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horst D., et al. 2009. Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J. Immunol. 182:2313–2324 [DOI] [PubMed] [Google Scholar]
- 26. Hsu M., et al. 2008. Epstein-Barr virus lytic transactivator Zta enhances chemotactic activity through induction of interleukin-8 in nasopharyngeal carcinoma cells. J. Virol. 82:3679–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang Y. T., et al. 1999. Profile of cytokine expression in nasopharyngeal carcinomas: a distinct expression of interleukin 1 in tumor and CD4+ T cells. Cancer Res. 59:1599–1605 [PubMed] [Google Scholar]
- 28. Ji J., Sahu G. K., Braciale V. L., Cloyd M. W. 2005. HIV-1 induces IL-10 production in human monocytes via a CD4-independent pathway. Int. Immunol. 17:729–736 [DOI] [PubMed] [Google Scholar]
- 29. Jones R. J., et al. 2007. Roles of lytic viral infection and IL-6 in early versus late passage lymphoblastoid cell lines and EBV-associated lymphoproliferative disease. Int. J. Cancer 121:1274–1281 [DOI] [PubMed] [Google Scholar]
- 30. Kalla M., Schmeinck A., Bergbauer M., Pich D., Hammerschmidt W. 2010. AP-1 homolog BZLF1 of Epstein-Barr virus has two essential functions dependent on the epigenetic state of the viral genome. Proc. Natl. Acad. Sci. U. S. A. 107:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kared H., et al. 2008. Role of GM-CSF in tolerance induction by mobilized hematopoietic progenitors. Blood 112:2575–2578 [DOI] [PubMed] [Google Scholar]
- 32. Keating S., Prince S., Jones M., Rowe M. 2002. The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules. J. Virol. 76:8179–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klibi J., et al. 2009. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 113:1957–1966 [DOI] [PubMed] [Google Scholar]
- 34. Kouzarides T., Packham G., Cook A., Farrell P. J. 1991. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene 6:195–204 [PubMed] [Google Scholar]
- 35. Lambert S. L., Martinez O. M. 2007. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J. Immunol. 179:8225–8234 [DOI] [PubMed] [Google Scholar]
- 36. Lau K. M., et al. 2007. Increase in circulating Foxp3+CD4+CD25high regulatory T cells in nasopharyngeal carcinoma patients. Br. J. Cancer 96:617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lehmann M. H. 1998. Recombinant human granulocyte-macrophage colony-stimulating factor triggers interleukin-10 expression in the monocytic cell line U937. Mol. Immunol. 35:479–485 [DOI] [PubMed] [Google Scholar]
- 38. Li D., et al. 2009. Down-regulation of MHC class II expression through inhibition of CIITA transcription by lytic transactivator Zta during Epstein-Barr virus reactivation. J. Immunol. 182:1799–1809 [DOI] [PubMed] [Google Scholar]
- 39. Li J., et al. 2007. Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS One 2:e1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin C. T., et al. 2000. Response of nasopharyngeal carcinoma cells to Epstein-Barr virus infection in vitro. Lab. Invest. 80:1149–1160 [DOI] [PubMed] [Google Scholar]
- 41. Lu J., et al. 2000. Upregulation of tyrosine kinase TKT by the Epstein-Barr virus transactivator Zta. J. Virol. 74:7391–7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahot S., Sergeant A., Drouet E., Gruffat H. 2003. A novel function for the Epstein-Barr virus transcription factor EB1/Zta: induction of transcription of the hIL-10 gene. J. Gen. Virol. 84:965–974 [DOI] [PubMed] [Google Scholar]
- 43. Maruo S., Yang L., Takada K. 2001. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J. Gen. Virol. 82:2373–2383 [DOI] [PubMed] [Google Scholar]
- 44. Meckes D. G., Jr., et al. 2010. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. U. S. A. 107:20370–20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Middeldorp J. M., Pegtel D. M. 2008. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin. Cancer Biol. 18:388–396 [DOI] [PubMed] [Google Scholar]
- 46. Morris M. A., et al. 2008. Epstein-Barr virus-encoded LMP1 induces a hyperproliferative and inflammatory gene expression programme in cultured keratinocytes. J. Gen. Virol. 89:2806–2820 [DOI] [PubMed] [Google Scholar]
- 47. Morrissey P. J., Bressler L., Park L. S., Alpert A., Gillis S. 1987. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J. Immunol. 139:1113–1119 [PubMed] [Google Scholar]
- 48. Murono S., et al. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 98:6905–6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nemeth K., et al. 2009. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ressing M. E., et al. 2003. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. Proc. Natl. Acad. Sci. U. S. A. 100:11583–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rickinson A. B., Kieff E. 2007. Epstein-Barr virus, p. 2655–2700In Knipe D. M., Howley P. M.(ed.), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 52. Rowe M., et al. 2007. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc. Natl. Acad. Sci. U. S. A. 104:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salek-Ardakani S., Arrand J. R., Mackett M. 2002. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology 304:342–351 [DOI] [PubMed] [Google Scholar]
- 54. Samanta M., Iwakiri D., Takada K. 2008. Epstein-Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene 27:4150–4160 [DOI] [PubMed] [Google Scholar]
- 55. Serafini P., et al. 2004. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 64:6337–6343 [DOI] [PubMed] [Google Scholar]
- 56. Sinha P., Clements V. K., Fulton A. M., Ostrand-Rosenberg S. 2007. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67:4507–4513 [DOI] [PubMed] [Google Scholar]
- 57. Slobedman B., Barry P. A., Spencer J. V., Avdic S., Abendroth A. 2009. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J. Virol. 83:9618–9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strockbine L. D., et al. 1998. The Epstein-Barr virus BARF1 gene encodes a novel, soluble colony-stimulating factor-1 receptor. J. Virol. 72:4015–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsai S. C., et al. 2009. EBV Zta protein induces the expression of interleukin-13, promoting the proliferation of EBV-infected B cells and lymphoblastoid cell lines. Blood 114:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vieira P., et al. 1991. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc. Natl. Acad. Sci. U. S. A. 88:1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yao M., et al. 1997. Interleukin-10 expression and cytotoxic-T-cell response in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int. J. Cancer 72:398–402 [DOI] [PubMed] [Google Scholar]
- 62. Zuo J., et al. 2009. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 5:e1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]