Abstract
Human T cell leukemia virus type 1 (HTLV-1) inhibits host antiviral signaling pathways although the underlying mechanisms are unclear. Here we found that the HTLV-1 Tax oncoprotein induced the expression of SOCS1, an inhibitor of interferon signaling. Tax required NF-κB, but not CREB, to induce the expression of SOCS1 in T cells. Furthermore, Tax interacted with SOCS1 in both transfected cells and in HTLV-1-transformed cell lines. Although SOCS1 is normally a short-lived protein, in the presence of Tax, the stability of SOCS1 was greatly increased. Accordingly, Tax enhanced the replication of a heterologous virus, vesicular stomatitis virus (VSV), in a SOCS1-dependent manner. Surprisingly, Tax required SOCS1 to inhibit RIG-I-dependent antiviral signaling, but not the interferon-induced JAK/STAT pathway. Inhibition of SOCS1 by RNA-mediated interference in the HTLV-1-transformed cell line MT-2 resulted in increased IFN-β expression accompanied by reduced HTLV-1 replication and p19Gag levels. Taken together, our results reveal that Tax inhibits antiviral signaling, in part, by hijacking an interferon regulatory protein.
INTRODUCTION
Human T cell leukemia virus type 1 (HTLV-1) is etiologically linked to the development of adult T-cell leukemia/lymphoma (ATLL) and the demyelinating disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (45). The HTLV-1-encoded viral protein Tax plays an essential role in HTLV-1-mediated pathogenesis. Tax is a trans-activating protein that dysregulates cellular gene expression by modulating the activity of signaling pathways, including NF-κB (44). Tax is thought to be required for HTLV-1-mediated transformation of T lymphocytes, but is clearly not required for the maintenance of the transformed phenotype in ATLL (25). Tax is also the main viral target of host cytotoxic T cells (CTLs) and is highly expressed in HAM/TSP patients with high proviral loads (49).
Viruses are first detected by the innate immune system via cytoplasmic pattern recognition receptors (PRRs), including RIG-I and MDA-5 which recognize specific features of viral nucleic acids (20, 51). RIG-I, upon detection of viral RNAs bearing 5′-triphosphate, initiates a signaling cascade that ultimately leads to the activation of the interferon regulatory factor 3 (IRF-3) transcription factor (16, 30). RIG-I interacts with the mitochondrial protein IPS-1 (also known as mAVs and Cardif) which leads to the formation of a protein complex anchored to the mitochondrial membrane consisting of TRAF3, IKKγ (also known as NEMO), TBK1, and IKKi (8, 21, 27, 36, 54). TBK1 phosphorylates several C-terminal serine residues in IRF-3, triggering its dimerization and nuclear translocation where it activates expression of the beta interferon (IFN-β) gene (37). IFN-β is a member of the type I interferon family of genes that binds to the IFN alpha/beta receptor 1 (IFNAR1) triggering the JAK/STAT pathway which in turn activates the expression of hundreds of interferon stimulated genes (ISGs) that together coordinate the host antiviral response (31).
Suppressor of cytokine signaling (SOCS) proteins are comprised of a family of eight cytokine inhibitors (CIS and SOCS 1 to 7) (10). The SOCS proteins are induced in a negative-feedback loop and inhibit activated JAK kinases or cytokine receptors to ensure transient activation of cytokine signaling pathways (52). SOCS proteins all contain an amino-terminal kinase inhibitory domain of variable length, a central Src homology 2 (SH2) domain that binds to phosphorylated tyrosines and a carboxy-terminal 40-amino-acid domain known as the SOCS box which promotes the degradation of protein substrates (5, 43). The SOCS box recruits several regulatory proteins, including elongin B, elongin C, cullin-5 and RING-box-2 (RBX2) to couple SOCS proteins to the ubiquitin-proteasome pathway (19). Gene targeting studies in mice have confirmed an essential role for SOCS1 as a negative regulator of IFN-γ and IL-2 pathways (2) (4). SOCS1 also inhibits IFN-α/β signaling and the induction of antiviral genes suggesting that SOCS1 may mediate IFN resistance during antiviral treatment (47).
Many viruses have devised multiple mechanisms to inhibit host antiviral pathways, a necessary step to avoid immune detection and promote viral replication. With regard to HTLV-1, little is known regarding viral mechanisms of counteracting host antiviral signaling. T cell clones derived from HAM/TSP patients are resistant to the anti-proliferative effects of IFN-β through an undefined mechanism (41). A subsequent study demonstrated that HTLV-1, when expressed from an infectious molecular clone, inhibited an interferon-stimulated response element (ISRE) reporter (7). HTLV-1 blocked IFN-α-mediated induction of ISGs by antagonizing the phosphorylation of TYK2 and STAT2 (7); however, the viral protein responsible for the IFN inhibitory effect was not identified. Another study implicated HTLV-1 Tax as an inhibitor of IFN signaling via competition of Tax with STAT2 for the coactivators CBP/p300 (53). Taken together, it appears that HTLV-1, and possibly Tax, inhibits IFN signaling.
In this report, we demonstrate that HTLV-1-transformed cell lines all exhibit elevated levels of SOCS1 mRNA. Tax upregulates the expression of SOCS1 via the NF-κB pathway. Furthermore, Tax interacts with SOCS1 and promotes its stabilization. Tax enhances the replication of the heterologous rhabdovirus vesicular stomatitis virus (VSV) in a SOCS1-dependent manner. Tax does not require SOCS1 to inhibit IFN-α/β signaling, but rather uses SOCS1 to inhibit innate antiviral signaling pathways.
MATERIALS AND METHODS
Cell lines, plasmids, and reagents.
293-T cells and Jurkat cells were purchased from the American Type Culture Collection (ATCC). C8166, MT-2, SLB-1, Jurkat-SVT and SVT-2C cells were a gift from S. C. Sun (12, 48). Jurkat Tax Tet-On cells were provided by W. Greene (23). SOCS1 cDNA was amplified by PCR from peripheral blood mononuclear cell (PBMC) cDNA and cloned into the pCMV-HA vector (BD Biosciences) using EcoRI and XhoI sites. pCMV-Flag SOCS3 was from C. R. Kahn and obtained from Addgene (plasmid 11486) (46). The Tax expression vector (pCMV4-Tax) and Tax mutants M22 and M47 were provided by S. C. Sun. The pCLXSN retroviral vector plasmids (pCL-Ampho, pCLXSN-Tax and VSV-G) were provided by S. C. Sun. ISRE-Luc, IFN-β Luc and NF-κB Luc reporter plasmids were previously described (29). Control scrambled and SOCS1 siGENOME SMARTpool small interfering RNAs (siRNAs) were purchased from Thermo Fisher Scientific/Dharmacon. The following antibodies were used in this study: anti-SOCS1 (Imgenex and Invitrogen), anti-β-actin (Abcam), anti-Flag (M2; Sigma), anti-hemagglutinin (anti-HA; Roche), anti-HTLV-1 p19Gag (ZeptoMetrix), anti-control Ig (Santa Cruz), and anti-Tax (AIDS Research and Reference Program). IFN-α, IFN-β and IFN-γ were purchased from Sigma. Poly(I:C) was purchased from Invivogen. Doxycycline (Dox) was purchased from Clontech. IKK-2 inhibitor IV was purchased from EMD Biosciences.
Transfections.
293-T cells were transfected with FuGENE 6 (Roche). Primary CD4+ T cells were transfected with FuGENE HD (Roche). siRNA (60 pmol) transfections were carried out using Lipofectamine 2000 (Invitrogen). MT-2 cells (5 × 106) were transfected with siRNA either by Lipofectamine LTX (Invitrogen) or by electroporation with a Gene Pulser XCell (Bio-Rad Laboratories).
Purification of primary CD4+ T cells.
PBMCs were isolated by density gradient centrifugation from whole blood using Ficoll-Paque Plus (GE Healthcare Biosciences Corp.). CD4+ T cells were purified from PBMCs using negative selection and stimulated with 1 μg/ml phytohemagglutinin (PHA) for 24 h prior to transfection. Purified cells were 96% CD4 positive as determined by flow cytometry.
Luciferase assays.
Cells were transfected with either IFN-β Luc, NF-κB Luc or ISRE-Luc together with the Renilla reporter pRL-tk as an internal control. Cells were lysed after 2 days and subjected to dual-luciferase assays as recommended by the manufacturer (Promega). Results are reported as the relative firefly luciferase activity over the Renilla luciferase activity.
qRT-PCR and RT-PCR.
Reverse transcription-PCR (RT-PCR) and quantitative RT-PCR (qRT-PCR) were performed as described previously (15). The Tax forward primer sequence was 5′-CGG ATA CCC AGT CTA CGT G. The Tax reverse primer sequence was 5′-GAG GTA CAT GCA GAC AAC GG. The GAPDH forward primer sequence was 5′-CCA CAG TCC ATG CCA TCA C. The GAPDH reverse primer sequence was 5′-GCT TCA CCA CCT TCT TGA TG. TaqMan probes specific for SOCS1, IFN-β, and β-actin were purchased from Applied Biosystems. SOCS1 mRNA levels were normalized to the expression of β-actin mRNA.
Retroviral infections.
293-T cells were transfected with pCLXSN, pCLXSN-Tax, pCLXSN-Tax M22 or pCLXSN-Tax M47 together with pCL-Ampho and VSV-G as described previously (13). After 36 h, supernatants were filtered and used to infect Jurkat, Jurkat SVT WT or Jurkat SVT 2C cells.
ELISA.
MT-2 cells were transfected with either control scrambled or SOCS-1 siRNA and after 48 h were treated with TNF-α (20 ng/ml) for 2 h. Supernatants were collected for an enzyme-linked immunosorbent assay (ELISA). The HTLV-1 p19 Gag ELISA was performed using a kit from ZeptoMetrix according to the manufacturer's instructions.
VSV infections.
293-T cells were infected with VSV expressing GFP (VSV-GFP) (17) at an MOI of 0.1 for 24 h.
Co-IPs and immunoblotting.
Coimmunoprecipitations (co-IPs) and immunoblotting were done essentially as described previously (38). Briefly, whole-cell lysates were generated by lysing cells in radioimmunoprecipitation assay (RIPA) buffer. For co-IPs, lysates were diluted 1:1 in RIPA buffer and incubated with the indicated antibodies at 4°C overnight. Protein A-agarose beads (25 μl) were added and incubated for 2 h at 4°C. Three washes were performed and 2× Laemmli sample buffer (LSB) was added to disrupt the protein-agarose bead interactions.
Yeast two-hybrid binding assays.
SOCS1 cDNA was cloned into pGBKT7, which contains a tryptophan (Trp) selection marker, to generate a SOCS1-GAL4 DNA binding domain fusion protein. Tax, Tax M22 and Tax M47 were cloned into pGADT7, which contains a leucine (Leu) selection marker, to generate a Tax-GAL4 activation domain fusion protein. Histidine (His) and adenine (Ade) are downstream reporter genes that are transcribed when the bait and prey proteins interact. SOCS1 and Tax plasmids were cotransformed in yeast strain AH109 and selected on minimal medium lacking either leucine (Leu), tryptophan (Trp), histidine (His), or adenine (Ade). Colony growth under stringent conditions in minimal medium lacking Leu, Trp, His and Ade indicates a positive interaction in the yeast two-hybrid assay.
Cycloheximide chase assays.
293-T cells were transfected with HA-SOCS1 or Flag-SOCS3 and/or Tax expression plasmids and after 36 h were treated with cycloheximide (100 μg/ml) for various times prior to lysing the cells and subjecting the lysates to Western blotting.
Statistical analysis.
All error bars represent the standard deviation of triplicate samples. Statistical significance was determined by Student's t test. * indicates a P value of <0.05. ** indicates a P value of <0.005.
RESULTS
Tax induces the expression of SOCS1.
Since it was previously demonstrated that HTLV-1 antagonizes type I IFN signaling (7), we examined the expression of SOCS1, a potent inhibitor of IFN signaling, in a panel of HTLV-1-transformed cell lines by qRT-PCR. SOCS1 mRNA was significantly upregulated in the HTLV-1-transformed cell lines MT-2, MT-4, SLB-1 and C8166 compared to HTLV-1-negative T cell lines Jurkat and SUP-T1 (Fig. 1 A). As expected, Tax mRNA was detected selectively in the HTLV-1-transformed cell lines (Fig. 1A, right panel). Because Tax is a trans-activating viral protein and has been implicated in the inhibition of IFN signaling previously (7), we next determined if Tax was responsible for the increased expression of SOCS1. For this purpose, we used a Jurkat Tax Tet-On cell line that expresses Tax upon doxycycline (Dox) treatment (23, 39). As shown in Fig. 1B, treatment of Jurkat Tax Tet-On cells with Dox resulted in an enhancement of SOCS1 expression as detected by qRT-PCR. Dox treatment was confirmed to activate Tax expression (Fig. 1B, right panel). Jurkat Tax Tet-On cells treated with Dox also exhibited an increase in SOCS1 protein levels (Fig. 1C). Therefore, Tax clearly upregulates the expression of SOCS1, and HTLV-1-transformed cell lines all overexpress SOCS1. We next transfected a Tax plasmid into primary CD4+ T cells to determine if Tax upregulates SOCS1 in untransformed cells. Tax expression was confirmed by Western blotting, which correlated with upregulated SOCS1 protein (Fig. 1D). Thus, Tax induces SOCS1 expression in primary CD4+ T cells.
Fig. 1.
Tax induces the expression of SOCS1. (A) SOCS1 mRNA is overexpressed in HTLV-1-transformed cell lines. qRT-PCR measuring SOCS1 and β-actin was performed using mRNA from control Jurkat and SUP-T1 cell lines and HTLV-1-transformed cell lines MT-2, MT-4, SLB-1 and C8166. The expression of Tax and GAPDH was examined by RT-PCR (right panel). **, P < 0.005 for Jurkat versus HTLV-1-transformed cell lines. (B, C) Tax induces the expression of SOCS1 mRNA and protein. Jurkat Tax Tet-On cells were treated with Dox (1 μg/ml) for 24 h to turn on Tax expression. qRT-PCR measuring SOCS1 and β-actin was performed using mRNA from untreated and Dox-treated cells (B). RT-PCR was also performed for Tax and GAPDH expression (B-left panel). Immunoblotting was conducted with whole-cell lysates from untreated and Dox-treated cells (C). Immunoblotting was performed with anti-SOCS1 and anti-β-actin. **, P < 0.005 for untreated versus Dox-treated samples in panel B. (D) Tax upregulates SOCS1 protein in primary CD4+ T cells. Empty vector or Tax plasmid were transfected into primary CD4+ T cells. Immunoblotting was performed with anti-Tax, anti-SOCS1 and anti-β-actin.
Tax requires NF-κB, but not CREB, to upregulate SOCS1.
Tax modulates the expression of host genes by dysregulating cellular signaling pathways-two of the best characterized targets of Tax are the NF-κB and CREB pathways. Tax point mutants selectively defective in either NF-κB or CREB are useful to delineate the requirement of each of these pathways in a particular function of Tax (42). The Tax mutant M22 is defective for NF-κB, but is wild-type for CREB activation. Conversely, Tax mutant M47 is defective for CREB, but is competent for NF-κB activation. Tax was expressed in Jurkat T cells using murine retroviral vectors expressing wild-type Tax, Tax M22 or Tax M47 (13). Although wild-type Tax and M47 induced SOCS1 expression, M22 was largely defective in the induction of SOCS1, despite similar expression levels of Tax and the two mutants (Fig. 2A).
Fig. 2.
Tax requires NF-κB to induce SOCS1 expression. (A) Tax requires NF-κB to upregulate SOCS1. qRT-PCR measuring SOCS1 and β-actin was performed using mRNA from Jurkat cells infected with retroviral vectors expressing Tax, Tax M22 or Tax M47. The expression of Tax and GAPDH was examined by RT-PCR (right panel). **, P < 0.005 for mock versus Tax and Tax M47 samples. (B) Tax induction of SOCS1 is impaired in IKKγ-deficient Jurkat cells. qRT-PCR measuring SOCS1 and β-actin was performed using mRNA from parental Jurkat SVT cells or SVT 2C IKKγ-deficient cells infected with a Tax retroviral vector. The expression of Tax and GAPDH was examined by RT-PCR (right panel). **, P < 0.005 for Tax samples in Jurkat SVT WT versus those in Jurkat SVT 2C. (C) Inhibition of IKKβ reduces SOCS1 expression in HTLV-1-transformed cells. (Left) A luciferase assay was performed with lysates from 293-T cells transfected with an NF-κB-Luc reporter and treated with TNF for 8 h. Where indicated, cells were also pretreated with inhibitor IV (40 nm) for 30 min prior to TNF stimulation. (Right) qRT-PCR measuring SOCS1 and β-actin was performed using mRNA from the HTLV-1-transformed cell line MT-2 treated with vehicle or inhibitor IV (40 nm) for 2 h. **, P < 0.005 for vehicle versus inhibitor IV treatment.
Tax interacts with the IKKγ subunit of the IKK complex as an essential step in activation of NF-κB (3, 14, 18). Accordingly, Tax is unable to activate NF-κB in a Jurkat cell line variant lacking IKKγ expression (SVT 2C) (12). Therefore, wild-type Tax was expressed using a retroviral vector in the SVT 2C-IKKγ-deficient cells and the SVT parental cell line. As expected, Tax upregulated the expression of SOCS1 in the parental SVT cells, but was significantly attenuated in the IKKγ-deficient Jurkat cells, despite similar levels of Tax expression in both cell types (Fig. 2B).
To further demonstrate the role of NF-κB in Tax-mediated induction of SOCS1, we used a cell permeable small molecule inhibitor with selectivity for IKKβ (IKK2 inhibitor IV) (11). We first validated the inhibitor by demonstrating a potent block in TNF-induced activation of an NF-κB luciferase reporter gene (Fig. 2C, left panel). Treatment of the HTLV-1-transformed cell line MT-2 with inhibitor IV significantly reduced SOCS1 mRNA levels as detected by qRT-PCR (Fig. 2C, right panel). These data provide further support that NF-κB plays an important role in the regulation of SOCS1 expression in the context of HTLV-1-transformed cell lines.
Tax interacts with and stabilizes SOCS1.
Since Tax exerts many of its effects on cellular signaling pathways by directly interacting with proteins (24), we next examined if Tax interacts with SOCS1. Indeed, transfected Tax interacted with ectopic HA-tagged SOCS1, but not Flag-SOCS3, in 293-T cells (Fig. 3A). Furthermore, endogenous Tax and SOCS1 formed a stable complex in the HTLV-1-transformed cell lines C8166 and MT-4 that was detectable by coimmunoprecipitation (Fig. 3B). Next, we examined the binding of Tax and Tax mutants with SOCS1 via a yeast two-hybrid assay. pGBKT7-SOCS1 and pGADT7-Tax were cotransformed in yeast and selected on minimal medium lacking either leucine (Leu−), tryptophan (Trp−), histidine (His−) or adenine (Ade−). As shown in Fig. 3C, only when yeast were cotransformed with SOCS1 and Tax (or Tax mutants), colonies formed under highly stringent conditions on plates lacking histidine, leucine, tryptophan and adenine (His− Leu− Trp− Ade−). These results indicate interactions between wild-type Tax, Tax M22 and Tax M47 with SOCS1 in the yeast two-hybrid assay. Thus, although Tax M22 is unable to induce the expression of SOCS1, it is able to interact with SOCS1. Collectively, these data indicate that SOCS1 is a novel Tax interacting protein.
Fig. 3.
Tax interacts with SOCS1 and promotes SOCS1 stability. (A) Ectopic SOCS1, but not SOCS3, interacts with Tax. A co-IP assay was performed with lysates from 293-T cells expressing Tax, HA-SOCS1 and Flag-SOCS3 as indicated. Immunoprecipitations were conducted with anti-Tax and immunoblotting was performed with anti-HA, anti-Flag, and anti-Tax as a control for the IPs. Immunoblotting was also performed with lysates using anti-Tax, anti-HA and anti-Flag. (B) Endogenous SOCS1 interacts with Tax in HTLV-1-transformed cell lines. A co-IP assay was performed with lysates from C8166 and MT-4 cells as indicated. Immunoprecipitations were conducted with an isotype control immunoglobulin (αIgG) or anti-SOCS1 followed by immunoblotting with anti-Tax. Immunoblotting was also performed with lysates using anti-Tax and anti-SOCS1. (C) Tax and SOCS1 interact in a yeast two-hybrid assay. Various combinations of pGBKT7-SOCS1 and pGADT7-Tax (also Tax M22 or Tax M47) or empty vectors were cotransformed in the yeast strain AH109 and plated on minimal medium lacking Leu, Trp, His or Ade as indicated. (D) Tax enhances the stability of SOCS1, but not SOCS3. A cycloheximide chase assay was performed to examine the stability of SOCS1 and SOCS3. 293-T cells expressing HA-SOCS1 (left panel) or Flag-SOCS3 (right panel), in the absence or presence of Tax, were treated with CHX for the indicated times and lysates were subjected to immunoblotting. Immunoblotting was performed with anti-HA, anti-Flag, and anti-Tax.
SOCS1 is a relatively unstable protein with a half-life of approximately 1 to 2 h (40). We next examined if Tax had any effect on the stability of the SOCS1 protein. Therefore, we conducted cycloheximide (CHX) chase assays to examine SOCS1 stability in the absence or presence of Tax. As expected, SOCS1 was a labile protein which was mostly lost by 6 h of the CHX chase (Fig. 3D, left panel). However, Tax enhanced the stability of SOCS1, and no loss of SOCS1 protein was observed during the CHX chase (Fig. 3D, left panel). In contrast, Tax had no effect on the stability of another SOCS family member SOCS3 (Fig. 3D, right panel). Therefore, it appears that Tax specifically interacts with SOCS1 as a mechanism to antagonize its degradation and promote its stability.
Tax requires SOCS1 to inhibit antiviral signaling.
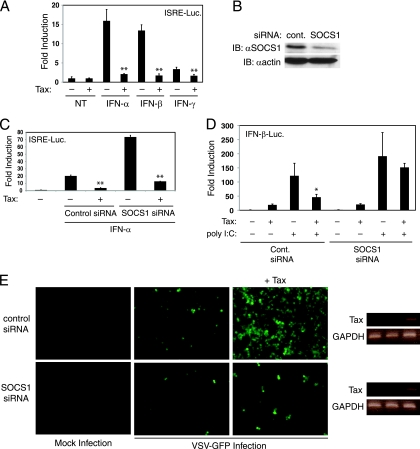
Tax has previously been shown to inhibit type I interferon signaling by competition with STAT2 for the coactivators CBP/p300. Since other viruses such as herpes simplex virus type 1 (HSV-1) (9) and influenza A (32) virus enhance SOCS1 expression to inhibit IFN signaling, we next investigated if Tax was dependent on SOCS1 to inhibit IFN signaling. Consistent with previous studies (7), Tax expression significantly reduced the activation of an ISRE reporter by IFN-α or IFN-β stimulation (Fig. 4 A). To determine the contribution of SOCS1 to the inhibition of IFN signaling by Tax, we used RNA-mediated interference (RNAi) to inhibit SOCS1 expression. A pool of four different SOCS1 siRNAs effectively downregulated SOCS1 protein levels in 293-T cells (Fig. 4B). Knockdown of SOCS1 by RNAi enhanced IFN signaling, although Tax was still able to inhibit IFN signaling (Fig. 4C) suggesting that Tax inhibits IFN signaling independently of SOCS1. We have found that Tax also functions as a potent inhibitor of the RIG-I/TLR3 antiviral pathways (Hyun et al., submitted). Thus, we next examined if Tax inhibition of the RIG-I/MDA5 antiviral pathway was dependent on SOCS1. Indeed, Tax blocked the activation of an IFN-β reporter in response to the double-stranded RNA mimetic poly(I:C) that was largely abolished by siRNA knockdown of SOCS1 (Fig. 4D). Therefore, Tax requires SOCS1 to effectively block poly(I:C)-mediated activation of the IFN-β promoter.
Fig. 4.
Tax requires SOCS1 to inhibit antiviral signaling. (A) Tax blocks type I interferon signaling. A luciferase assay was performed with lysates from 293-T cells transfected with an ISRE-Luc reporter and Tax where indicated. Cells were also stimulated with IFN-α, IFN-β, or IFN-γ. **, P < 0.005 for empty vector versus Tax samples for IFN-α, IFN-β, and IFN-γ treatments. (B) SOCS1 siRNA inhibits SOCS1 expression. Immunoblotting was performed with lysates of 293-T cells transfected with control siRNA or SOCS1 siRNA. Immunoblotting was performed with anti-SOCS1 and anti-β-actin. (C) Tax does not require SOCS1 to inhibit IFN-α signaling. A luciferase assay was performed with lysates from 293-T cells transfected with an ISRE-Luc reporter, Tax, control siRNA or SOCS1 siRNA as indicated. Cells were also treated with IFN-α. **, P < 0.005 for empty vector versus Tax samples (for both cont. siRNA and SOCS1 siRNA). (D) Tax requires SOCS1 to inhibit antiviral signaling. A luciferase assay was performed with lysates from 293-T cells transfected with an IFN-β-Luc reporter, poly(I:C), Tax, control siRNA or SOCS1 siRNA as indicated. *, P < 0.05 for empty vector versus Tax samples [+cont. siRNA and poly(I:C)]. (E) Tax requires SOCS1 to enhance the replication of VSV-GFP. Micrographs of 293-T cells transfected with Tax, control siRNA or SOCS1 siRNA as indicated. VSV-GFP, at an MOI of 0.1, was used to infect cells 24 h after transfections. Pictures were taken 24 h postinfection. The expression of Tax and GAPDH was examined by RT-PCR (right panel).
Next, the effect of Tax and SOCS1 on the replication of VSV expressing the green fluorescent protein (GFP) was observed, using fluorescence microscopy. Remarkably, Tax significantly enhanced the replication of VSV-GFP in the presence of a control scrambled siRNA. However, RNAi-mediated inhibition of SOCS1 resulted in only low levels of VSV-GFP replication in the presence of Tax (Fig. 4E). Tax expression was similar in the presence of either control or SOCS1 siRNA (Fig. 4E). Thus, Tax clearly requires SOCS1 as part of its mechanism to enhance viral replication.
SOCS1 enhances replication of HTLV-1 in MT-2 cells.
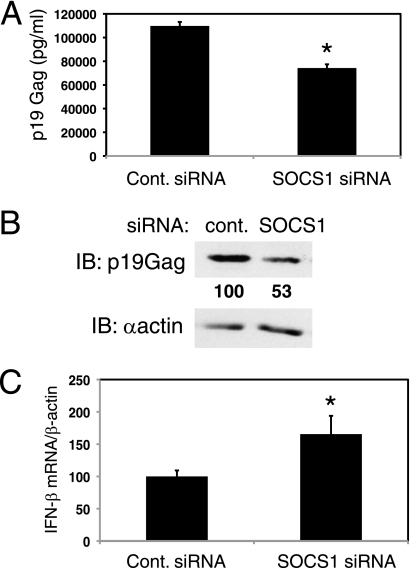
Since SOCS1 is overexpressed in HTLV-1-transformed cell lines (Fig. 1A), we next asked if SOCS1 modulated HTLV-1 replication. We conducted our studies with MT-2 cells, a T-cell line chronically infected with HTLV-1 (22). MT-2 cells produce low levels of infectious virus that can be increased by 1 to 2 logs with TNF-α stimulation (1). MT-2 cells were transfected with control or SOCS1 siRNA, and treated with TNF-α for 2 h. Viral replication was measured by the detection of the viral structural protein p19Gag in both the supernatants and cell lysates using immunoblotting and ELISA. Transfection efficiency was typically between 15 to 30% as measured by flow cytometry of MT-2 cells transfected with FITC-labeled control siRNA (data not shown). An ELISA for p19Gag was conducted with supernatants from transfected MT-2 cells, and p19Gag was significantly reduced in the presence of SOCS1 siRNA (Fig. 5A). Knockdown of SOCS1 also resulted in a decrease in p19Gag in cell lysates suggesting that SOCS1 regulates viral replication (Fig. 5B). Furthermore, the decrease in p19Gag levels was accompanied by an increase in IFN-β mRNA as measured by qRT-PCR (Fig. 5C). Taken together, these results reveal that SOCS1 is an important host factor induced by HTLV-1 that enhances viral replication by inhibiting antiviral signaling and IFN-β production.
Fig. 5.
SOCS1 is a positive regulator of HTLV-1 replication. (A) Loss of SOCS1 decreases extracellular HTLV-1 levels. p19Gag ELISA was conducted using supernatants from MT-2 cells transfected with control siRNA or SOCS1 siRNA. *, P < 0.05 for control siRNA versus SOCS1 siRNA samples. (B) Loss of SOCS1 decreases cellular HTLV-1 p19Gag. Immunoblotting was performed with lysates from MT-2 cells transfected with control siRNA or SOCS1 siRNA. Immunoblotting was performed with anti-p19Gag and anti-β-actin. p19Gag proteins were quantified with ImageJ software and relative levels are represented as a percentile compared to control siRNA (arbitrarily set at 100). (C) SOCS1 negatively regulates IFN-β in MT-2 cells. qRT-PCR measuring IFN-β and β-actin was performed using mRNA from the HTLV-1-transformed cell line MT-2 transfected with control scrambled siRNA or SOCS1 siRNA. *, P < 0.05 for control siRNA versus SOCS1 siRNA samples.
DISCUSSION
SOCS1 is induced by cytokines and functions in a classical negative-feedback loop to restrain activation of cytokine signaling pathways (43). SOCS1 inhibits JAK/STAT signaling by directly binding to JAK proteins and inhibiting their kinase activity (52). Due to the importance of SOCS1 in limiting IFN signaling, viruses, including HSV-1 and influenza A induce the expression of SOCS1 as a mechanism to evade innate immune detection (9, 32). Our study indicates that SOCS1 gene expression is upregulated in HTLV-1 infected cell lines. The HTLV-1-encoded Tax protein induces SOCS1 expression in an NF-κB dependent manner. Tax interacts with SOCS1, but not SOCS3, to mitigate its degradation and inhibit antiviral signaling, ultimately leading to the enhancement of viral replication.
Prior studies examining the regulatory elements in the SOCS1 promoter have identified IRF1 and -2 and Egr-1 binding sites (26). To our knowledge, NF-κB has not been previously implicated in the regulation of SOCS1 expression. TNF-α induces the expression of SOCS1 in mouse hepatocytes, although it is uncertain if NF-κB is important for SOCS1 expression in this regard (35). A search of the SABiosciences (Qiagen) regulatory transcription factor search portal (http://www.sabiosciences.com/chipqpcrsearch.php?app=TFBS) reveals several putative NF-κB binding sites in the SOCS1 promoter. Whether Tax promotes direct binding of NF-κB to any of these sites or NF-κB indirectly activates SOCS1 via other transcription factors will be important to address in future studies.
It is surprising that SOCS1 is dispensable for Tax to inhibit IFN signaling, but is required for Tax to inhibit antiviral signaling that regulates IFN-β transcription. Although SOCS1 is well known as an inhibitor of JAK/STAT signaling, its role in antiviral signaling is poorly understood. Overexpression of SOCS1, but not SOCS3, inhibits influenza A virus-mediated activation of an IRF-3 reporter suggesting that SOCS1 may directly inhibit host antiviral signaling (32). Indeed, a recent study demonstrated that HTLV-1 infection results in the induction of SOCS1 which promotes the degradation of IRF-3 (28). Our data confirm and extend the results of Oliere et al. since we have shown that Tax induces SOCS1 in an NF-κB-dependent manner and Tax subsequently interacts with and stabilizes SOCS1 protein. Tax relies on SOCS1 to effectively thwart RIG-I/MDA5-mediated antiviral signaling. Finally, our preliminary results indicate that SOCS1 is a potent inhibitor of the RIG-I antiviral pathway, although it appears to function upstream of IRF-3 (data not shown).
Human immunodeficiency virus type 1 (HIV-1) also induces the expression of SOCS1 which regulates the intracellular trafficking and maturation of HIV-1 Gag (34). With regard to HTLV-1, IFN-α inhibits viral assembly by preventing Gag interaction with lipid rafts, although the mechanism is not clear (6). Knockdown of SOCS1 with siRNA reduced the levels of p19Gag in the supernatant and lysates likely due to enhanced expression of IFN-β (Fig. 5). Thus, the expression of viral proteins and viral replication is likely reduced when SOCS1 is silenced because of a more robust antiviral response that is typically restrained by SOCS1. The specific antiviral pathways elicited by HTLV-1, and retroviruses in general, are poorly understood. A recent study has indicated that HIV-1 DNA is recognized by an unknown cellular DNA sensor that signals to the adaptor molecule STING, TBK-1 and IRF-3 which activates IFN-β expression (50). HIV-1 inhibits the host innate immune response via the exonuclease TREX1 which digests HIV-1 nucleic acid (50). Additional studies are necessary to determine if TREX1 is also used by HTLV-1 to counteract innate immune responses. Furthermore, it will be necessary to determine if SOCS1 negatively regulates the host DNA anti-retroviral pathway.
In summary, we have found that SOCS1 is induced by HTLV-1 as a mechanism to evade innate immunity and promote virus replication. Tax not only induces SOCS1 expression, but also interacts with SOCS1 to enhance its stability and prevent its proteasomal degradation. These findings have implications for HTLV-1-associated diseases, particularly HAM/TSP which is associated with high Tax expression and a high proviral load. Small molecule antagonists of SOCS1 have been described (9) and may potentially be useful to reduce the proviral load in asymptomatic individuals who are at high risk for HAM/TSP. Since IFN-α is used in the clinic as a therapeutic for ATL and HAM/TSP patients (33), elevated SOCS1 may also confer resistance to antiviral drugs.
ACKNOWLEDGMENTS
We thank S. C. Sun for plasmids and cells, W. Greene for Jurkat Tax Tet-On cells, J. Heiber and G. Barber for providing VSV-GFP, M. Roach and the University of Miami DCFAR Laboratory Core for primary CD4+ T cells, and T. Venkataraman for assistance with yeast two-hybrid assays.
This study was supported, in whole or in part, by NIH grants PO1CA128115 and RO1CA135362, awarded to E.W.H.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Agbottah E., et al. 2008. Two specific drugs, BMS-345541 and purvalanol A induce apoptosis of HTLV-1 infected cells through inhibition of the NF-κB and cell cycle pathways. AIDS Res. Ther. 5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander W. S., et al. 1999. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98:597–608 [DOI] [PubMed] [Google Scholar]
- 3. Chu Z. L., Shin Y. A., Yang J. M., DiDonato J. A., Ballard D. W. 1999. IKKγ mediates the interaction of cellular IκB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 274:15297–15300 [DOI] [PubMed] [Google Scholar]
- 4. Cornish A. L., et al. 2003. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J. Biol. Chem. 278:22755–22761 [DOI] [PubMed] [Google Scholar]
- 5. Endo T. A., et al. 1997. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387:921–924 [DOI] [PubMed] [Google Scholar]
- 6. Feng X., Heyden N. V., Ratner L. 2003. Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts. J. Virol. 77:13389–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng X., Ratner L. 2008. Human T-cell leukemia virus type 1 blunts signaling by interferon alpha. Virology 374:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitzgerald K. A., et al. 2003. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491–496 [DOI] [PubMed] [Google Scholar]
- 9. Frey K. G., et al. 2009. HSV-1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J. Immunol. 183:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujimoto M., Naka T. 2003. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 24:659–666 [DOI] [PubMed] [Google Scholar]
- 11. Gavriil M., et al. 2009. Specific IKKβ inhibitor IV blocks Streptonigrin-induced NF-κB activity and potentiates its cytotoxic effect on cancer cells. Mol. Carcinog. 48:678–684 [DOI] [PubMed] [Google Scholar]
- 12. Harhaj E. W., et al. 2000. Somatic mutagenesis studies of NF-κB signaling in human T cells: evidence for an essential role of IKKγ in NF-κB activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene 19:1448–1456 [DOI] [PubMed] [Google Scholar]
- 13. Harhaj E. W., et al. 2005. Human T cell leukemia virus type I Tax activates CD40 gene expression via the NF-κB pathway. Virology 333:145–158 [DOI] [PubMed] [Google Scholar]
- 14. Harhaj E. W., Sun S. C. 1999. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911–22914 [DOI] [PubMed] [Google Scholar]
- 15. Harhaj N. S., Janic B., Ramos J. C., Harrington W. J., Jr., Harhaj E. W. 2007. Deregulated expression of CD40 ligand in HTLV-I infection: distinct mechanisms of downregulation in HTLV-I-transformed cell lines and ATL patients. Virology 362:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hornung V., et al. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997 [DOI] [PubMed] [Google Scholar]
- 17. Ishikawa H., Barber G. N. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jin D. Y., Giordano V., Kibler K. V., Nakano H., Jeang K. T. 1999. Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I Tax interacts directly with IKKγ. J. Biol. Chem. 274:17402–17405 [DOI] [PubMed] [Google Scholar]
- 19. Kamura T., et al. 2004. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 18:3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kato H., et al. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105 [DOI] [PubMed] [Google Scholar]
- 21. Kawai T., et al. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981–988 [DOI] [PubMed] [Google Scholar]
- 22. Koyanagi Y., et al. 1984. Expression of HTLV-specific polypeptides in various human T-cell lines. Med. Microbiol. Immunol. 173:127–140 [DOI] [PubMed] [Google Scholar]
- 23. Kwon H., et al. 2005. Lethal cutaneous disease in transgenic mice conditionally expressing type I human T cell leukemia virus Tax. J. Biol. Chem. 280:35713–35722 [DOI] [PubMed] [Google Scholar]
- 24. Legros S., et al. 2009. Protein-protein interactions and gene expression regulation in HTLV-1 infected cells. Front. Biosci. 14:4138–4148 [DOI] [PubMed] [Google Scholar]
- 25. Matsuoka M., Jeang K. T. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7:270–280 [DOI] [PubMed] [Google Scholar]
- 26. Mostecki J., Showalter B. M., Rothman P. B. 2005. Early growth response-1 regulates lipopolysaccharide-induced suppressor of cytokine signaling-1 transcription. J. Biol. Chem. 280:2596–2605 [DOI] [PubMed] [Google Scholar]
- 27. Oganesyan G., et al. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208–211 [DOI] [PubMed] [Google Scholar]
- 28. Oliere S., et al. 2010. HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1). PLoS Pathog. 6:e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parvatiyar K., Barber G. N., Harhaj E. W. 2010. TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1-IKKi kinases. J. Biol. Chem. 285:14999–15009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pichlmair A., et al. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001 [DOI] [PubMed] [Google Scholar]
- 31. Platanias L. C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375–386 [DOI] [PubMed] [Google Scholar]
- 32. Pothlichet J., Chignard M., Si-Tahar M. 2008. Cutting edge: innate immune response triggered by influenza A virus is negatively regulated by SOCS1 and SOCS3 through a RIG-I/IFNAR1-dependent pathway. J. Immunol. 180:2034–2038 [DOI] [PubMed] [Google Scholar]
- 33. Ramos J. C., et al. 2007. IRF-4 and c-Rel expression in antiviral-resistant adult T-cell leukemia/lymphoma. Blood 109:3060–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryo A., et al. 2008. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc. Natl. Acad. Sci. U. S. A. 105:294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sass G., Shembade N. D., Tiegs G. 2005. Tumour necrosis factor alpha (TNF)-TNF receptor 1-inducible cytoprotective proteins in the mouse liver: relevance of suppressors of cytokine signalling. Biochem. J. 385:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seth R. B., Sun L., Ea C. K., Chen Z. J. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122:669–682 [DOI] [PubMed] [Google Scholar]
- 37. Sharma S., et al. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148–1151 [DOI] [PubMed] [Google Scholar]
- 38. Shembade N., Harhaj N. S., Liebl D. J., Harhaj E. W. 2007. Essential role for TAX1BP1 in the termination of TNF-α-, IL-1- and LPS-mediated NF-κB and JNK signaling. EMBO J. 26:3910–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shembade N., et al. 2008. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat. Immunol. 9:254–262 [DOI] [PubMed] [Google Scholar]
- 40. Siewert E., Muller-Esterl W., Starr R., Heinrich P. C., Schaper F. 1999. Different protein turnover of interleukin-6-type cytokine signalling components. Eur. J. Biochem. 265:251–257 [DOI] [PubMed] [Google Scholar]
- 41. Smith D., Buckle G. J., Hafler D. A., Frank D. A., Hollsberg P. 1999. HTLV-I-infected T cells evade the antiproliferative action of IFN-beta. Virology 257:314–321 [DOI] [PubMed] [Google Scholar]
- 42. Smith M. R., Greene W. C. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875–1885 [DOI] [PubMed] [Google Scholar]
- 43. Starr R., et al. 1997. A family of cytokine-inducible inhibitors of signalling. Nature 387:917–921 [DOI] [PubMed] [Google Scholar]
- 44. Sun S. C., Yamaoka S. 2005. Activation of NF-κB by HTLV-I and implications for cell transformation. Oncogene 24:5952–5964 [DOI] [PubMed] [Google Scholar]
- 45. Uchiyama T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15–37 [DOI] [PubMed] [Google Scholar]
- 46. Ueki K., Kondo T., Kahn C. R. 2004. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell Biol. 24:5434–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vlotides G., et al. 2004. SOCS-1 and SOCS-3 inhibit IFN-α-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem. Biophys. Res. Commun. 320:1007–1014 [DOI] [PubMed] [Google Scholar]
- 48. Xiao G., et al. 2001. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKα. EMBO J. 20:6805–6815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamano Y., et al. 2002. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 99:88–94 [DOI] [PubMed] [Google Scholar]
- 50. Yan N., Regalado-Magdos A. D., Stiggelbout B., Lee-Kirsch M. A., Lieberman J. 2010. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoneyama M., et al. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737 [DOI] [PubMed] [Google Scholar]
- 52. Yoshimura A., Naka T., Kubo M. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7:454–465 [DOI] [PubMed] [Google Scholar]
- 53. Zhang J., et al. 2008. Human T-cell leukemia virus type 1 Tax modulates interferon-alpha signal transduction through competitive usage of the coactivator CBP/p300. Virology 379:306–313 [DOI] [PubMed] [Google Scholar]
- 54. Zhao T., et al. 2007. The NEMO adaptor bridges the nuclear factor-κB and interferon regulatory factor signaling pathways. Nat. Immunol. 8:592–600 [DOI] [PubMed] [Google Scholar]