Abstract
Cells that can participate in an innate immune response within the central nervous system (CNS) include infiltrating cells (polymorphonuclear leukocytes [PMNs], macrophages, and natural killer [NK] cells) and resident cells (microglia and sometimes astrocytes). The proinflammatory cytokine interleukin-6 (IL-6) is produced by all of these cells and has been implicated in the development of behavioral seizures in the Theiler's murine encephalomyelitis virus (TMEV)-induced seizure model. The assessment, via PCR arrays, of the mRNA expression levels of a large number of chemokines (ligands and receptors) in TMEV-infected and mock-infected C57BL/6 mice both with and without seizures did not clearly demonstrate the involvement of PMNs, monocytes/macrophages, or NK cells in the development of seizures, possibly due to overlapping function of the chemokines. Additionally, C57BL/6 mice unable to recruit or depleted of infiltrating PMNs and NK cells had seizure rates comparable to those of controls following TMEV infection, and therefore PMNs and NK cells do not significantly contribute to seizure development. In contrast, C57BL/6 mice treated with minocycline, which affects monocytes/macrophages, microglial cells, and PMNs, had significantly fewer seizures than controls following TMEV infection, indicating monocytes/macrophages and resident microglial cells are important in seizure development. Irradiated bone marrow chimeric mice that were either IL-6-deficient mice reconstituted with wild-type bone marrow cells or wild-type mice reconstituted with IL-6-deficient bone marrow cells developed significantly fewer behavioral seizures following TMEV infection. Therefore, both resident CNS cells and infiltrating cells are necessary for seizure development.
INTRODUCTION
Viral encephalitis (inflammation within the brain) has recently been calculated to affect ∼7.5 persons per 100,000 in the general population (reviewed in reference 16). Many of these patients will develop seizures during the acute infection. The risk of seizures in viral encephalitis patients is enhanced by more than 20% over that in the general population. Additionally, 4 to 20% of viral encephalitis survivors develop epilepsy. Epilepsy has been estimated to affect 8 persons per 1,000 in the general population (reviewed in reference 16).
Behavioral seizures can be induced in C57BL/6 mice through infection with the neurotropic virus Theiler's murine encephalomyelitis virus (TMEV) (22). Infection with either the Daniels (DA) or GDVII strain of TMEV results in acute seizures occurring in more than 50% of both male and female C57BL/6 mice between days 3 and 10 postinfection (p.i.). Both the seizure score, based on the Racine scale, for any given day and the pattern of days on which the mice were observed to have seizures varied from mouse to mouse. Typically, the majority of seizures reached a score of 3 and above. Day 3 p.i. was the first day mice were observed to have seizures, day 6 p.i. was the peak of seizure activity, and the acute seizures usually resolved by day 10 p.i. The afebrile seizures appeared limbic in nature, and the mice displayed forelimb clonus with rearing and falling (22). Mice experiencing seizures were impaired in both motor function and coordination (22). Damage, in the form of neuronal loss early after viral infection, was largely restricted to the CA1-CA2 pyramidal layer of the hippocampus (3, 19, 22), and this damage is likely due to apoptosis triggered by a combination of the disruption in hippocampal circuits, the innate immune response, and direct viral infection (3). Observation of the mice that had acute seizures for several months demonstrated that an asymptomatic or latent period was followed by the development of spontaneous seizures/epilepsy (40, 41). Thus, this novel virus infection-induced seizure model, the TMEV-induced seizure model, has an advantage over previously described virus infection-induced seizure models in that the animals survive the acute seizures and are available for studies investigating the mechanisms of epileptogenesis (41).
Activation of the innate immune system and associated inflammatory changes within the central nervous system (CNS) have previously been linked to the development of seizures (reviewed in references 53 and 55). Examination of the role played by the innate immune system in the TMEV-induced seizure model implicated two proinflammatory cytokines (tumor necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]) and concomitant inflammatory changes (perivascular cuffing comprised of infiltrating mononuclear cells, infiltration of macrophages, and/or activation of microglial cells and gliosis) in the brain as contributors to acute seizure development (19). In contrast, viral persistence, the proinflammatory cytokine IL-1, and TMEV-specific CD8+ T cells did not play a major role in seizures (19). Thus, the innate immune response to viral infection of the CNS is a critical factor in the development of acute seizures in this model.
Cells of the innate immune system include polymorphonuclear leukocytes (PMNs; neutrophils, basophils, and eosinophils), macrophages, and natural killer (NK) cells from outside the CNS (infiltrating cells), and microglia and astrocytes within the CNS (resident cells) can also participate (14). Microglia, the resident macrophages of the CNS, are myeloid lineage cells, whereas astrocytes, the most abundant glial cell population, are of neuroectodermal origin (14). TMEV infection of neurons within the CNS causes activation of microglia and astrocytes which in turn produce an array of cytokines and chemokines (29, 32, 45).
Chemokines (chemotactic cytokines) function to recruit PMNs, monocytes (which give rise to macrophages), and NK cells into the CNS (6, 27, 36). The chemokine system is comprised of a large number of ligands, divided into subgroups to include CC, CXC, CX3C, and C chemokines, and a smaller number of promiscuous receptors, which are G-protein-coupled receptors (6, 27, 36). The chemokine ligand environment is modulated by both the G-protein-coupled chemokine signaling receptors and by nonsignaling chemokine receptor-like molecules as a means of preventing harmful, indiscriminate inflammation (36). The importance of infiltrating PMNs in the development of seizures was recently demonstrated by using the pilocarpine model of status epilepticus in C57BL/6 mice (13). It was found that genetic interference, or antibody blockade, of the recruitment of leukocytes, particularly neutrophils, into the CNS resulted in reduced seizure activity (13). In addition to the expression of chemokine receptors by infiltrating cells, chemokine ligands and receptors have been found to be expressed by resident CNS cells, including microglia, astrocytes, oligodendrocytes, and neurons, where they are thought to function in development, physiology, and repair (6, 27, 36).
Proinflammatory cytokines (TNF-α and IL-6) are produced in the brain early after viral infection by both cells infiltrating into the CNS from the periphery and/or resident cells in the CNS (20, 35, 38). IL-6 production is induced by other cytokines, including TNF-α (11, 51). Outside of the brain, IL-6 production by fibroblast-like synoviocytes isolated from rheumatoid arthritis patients was enhanced by TNF-α and the chemokines CCL2, CCL5, and CXCL12 (31). Conversely, interaction of IL-6 with the soluble form of its receptor, sIL-6R, has been shown to induce endothelial cells, which express the signal transduction component gp130 that interacts with the IL-6-IL-6R complex, to produce the chemokines CCL2 and CCL7 (37).
The goal of the present study was to examine the contribution of the infiltrating cells (PMNs, macrophages, and NK cells) compared to the resident cells (microglia and astrocytes) to the production of IL-6 in the brain and in the development of seizures in the TMEV-induced seizure model. Various antibody treatments were used to deplete or block entry of PMNs and to deplete NK cells. Minocycline treatment was used to block PMN recruitment and microglial/macrophage activation. We found that mice treated with minocycline, which blocked PMN recruitment into the CNS and microglial/macrophage activation, had significantly fewer seizures than wild-type (WT) control mice, thereby implicating both infiltrating cells and the resident CNS cells in the development of seizures. Using irradiated bone marrow chimeric mice generated from IL-6-deficient mice, we confirmed the role of IL-6 produced within the CNS by both the resident CNS cells and the infiltrating cells in the development of seizures.
MATERIALS AND METHODS
Animals.
C57BL/6 male mice and mice deficient in IL-6 on a C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). Transgenic mice that express the green fluorescent protein (GFP) [C57BL/6CrSlc-Tg(ACTb-EGFP)OsbC14-Y01-FM131] were kindly provided by Gerald Spangrude (University of Utah).
To generate irradiated bone marrow chimeric mice, bone marrow donor mice were euthanized using an overdose of isoflurane. Bone marrow cells were isolated from the femurs and tibias of donor mice that were at least 8 weeks old by flushing with sterile phosphate-buffered saline (PBS) containing 5% calf serum. Red blood cells were lysed, and bone marrow cells were washed and resuspended in sterile PBS for injection. For chimeric generation, bone marrow from one mouse donor was used to transplant up to 5 individual irradiated animals to reconstitute the bone marrow. Recipient mice were placed on autoclaved, antibiotic-supplemented (neomycin sulfate; 2 mg/ml) acidified water for at least 4 days prior to irradiation and continuing until 3 weeks following bone marrow transplant. On the day of chimera generation, recipient mice received a lethal whole-body irradiation dose of 1,200 rads total (600 rads given 3 h apart) by a cesium irradiator. Recipient mice were anesthetized with isoflurane, and at least 2 × 106 donor bone marrow cells were injected by the retro-orbital route in 100 μl PBS. Mice were weighed daily for the 6 weeks required for engraftment before infection with TMEV. The success of engraftment was determined by measuring the level of CD45+ cells in the peripheral blood. Blood was collected from mice by puncture of the submandibular vein. Cells were isolated using a dextran solution, and red blood cells were lysed using ACK solution (0.15 M ammonium chloride, 10 mM potassium bicarbonate [pH 7.2 to 7.4]). The remaining cells were stained with an anti-CD45 antibody conjugated to phycoerythrin (clone 30-F11; BD Biosciences, San Jose, CA), which stains all hematopoietic cells, except erythrocytes and platelets, and propidium iodide to determine cell viability. Cells were then analyzed by flow cytometry (FACScan and CELL Quest software; BD Biosciences). The percentage of cells expressing GFP was determined from live cells that were CD45+.
The care and use of the mice described above were in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council.
Infection.
On day 0, 5- to 6-week-old mice or 11- to 12-week-old chimeric mice were anesthetized with isoflurane (inhalation) and infected intracerebrally with 2 × 104 or 2 × 105 PFU of the Daniels (DA) strain of TMEV or mock infected with 20 μl of PBS (19). The DA strain of TMEV was propagated as previously described (59).
Observations.
To monitor for seizure activity, mice were observed continuously for 2 h, randomly between the hours of 9:00 a.m. and 5:00 p.m., each day p.i. Cages were gently removed from the containment racks and placed on a clean benchtop surface in the same negative-pressure animal room in which the animals are housed. The microisolator top and wire lid were removed from the cage, the mice were lightly aroused and weighed, and a righting reflex test was performed as described previously (22). Mice were then returned to the cage, with the microisolator top and wire lid off, and observed for seizure activity. Seizure activity was graded using the Racine scale as follows: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; and stage 5, rearing and falling (2, 34). After the 2-h observation period, the wire lids and microisolator tops were replaced, and the cages were returned to the containment racks.
PCR arrays.
Five- to 6-week-old C57BL/6 mice (three to four per group) infected with 2 × 104 PFU DA virus or injected with PBS were euthanized with isoflurane on days 2 and 6 p.i., and brains were harvested and frozen. Brains from naïve mice were used as a normal control. RNA was isolated by homogenizing the brains in Trizol reagent (Invitrogen, San Diego, CA), performing a chloroform extraction, and then further purifying the RNA by means of the RNeasy maxikit (Qiagen, Chatsworth, CA). From the RNA, cDNA was made with Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) according to the manufacturer's recommendations and using random primers. cDNAs from three to four brains were pooled from the following groups: PBS, day 2; infected, day 2; PBS, day 6; no seizures (infected), day 6; and seizures (infected), day 6. cDNA was assayed on a LC480 Light Cycler (Roche, Indianapolis, IN) 96-well block, via a PCR array specific for mouse inflammatory cytokines and receptors (SABiosciences, Frederick, MD) as per the manufacturer's instructions.
Antibody treatment.
C57BL/6 mice were injected intraperitoneally (i.p.) with 150 μg (150 μl) of a rat anti-mouse Gr-1 monoclonal antibody (clone RB6-8C5; BD Biosciences) 5 h prior to infection with 2 × 105 PFU of the DA strain of TMEV (9).
C57BL/6 mice were injected i.p. with 500 μl of anti-CXCR2 antibody (a generous gift from Tom Lane, University of California, Irvine) on the day of infection with 2 × 105 PFU of the DA strain of TMEV and 2 days p.i. (7). As a control, C57BL/6 mice were injected i.p. with 500 μl of PBS.
C57BL/6 mice were injected i.p. with 25 μg of mouse anti-NK1.1 antibody (clone PK-136; BioXCell, West Lebanon, NH) in 200 μl of PBS on the day before infection with 2 × 104 PFU of the DA strain of TMEV and 4 days p.i. (44; R. Welsh, personal communication). As an isotype control, C57BL/6 mice were injected i.p. with 25 μg of mouse IgG2a (clone Cl.18.4; BioXCell) in 200 μl of PBS. As another control, C57BL/6 mice were injected i.p. with 200 μl of PBS.
Minocycline treatment.
Minocycline (50 mg/kg; Sigma-Aldrich, St. Louis, MO) was administered i.p. twice a day (4) to 5- to 6-week-old C57BL/6 mice infected with 2 × 104 PFU of the DA strain of TMEV or injected with PBS from day 0 through day 8 p.i. Mice were monitored daily for seizures through day 21 p.i.
Immunohistochemistry.
Additional infected or mock-infected mice were euthanized with isoflurane on days 7 and 14 p.i. in order to enumerate adoptively transferred infiltrating cells in the brains of the chimeric mice. Animals were perfused with PBS, followed by a buffered 4% paraformaldehyde solution. Brains were harvested and fixed in 4% paraformaldehyde, divided into five coronal slabs per brain, and embedded in paraffin. Multiple 4-μm-thick tissue sections, containing sections from all five coronal slabs per brain, were cut and mounted on slides. The tissue section of only one of the five coronal slabs represented per slide contained the hippocampal/dentate gyral regions of the brain.
GFP-positive cells were detected on paraffin sections with GFP antibody (Abcam, Cambridge, MA) as previously described (48, 49). The slides were labeled by the avidin-biotin peroxidase complex technique with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) in 0.01% hydrogen peroxide (Sigma) in PBS. Specificity of antibody binding was confirmed by parallel staining minus the GFP antibody.
Enumeration of GFP-positive cells was performed in a blinded fashion with a light microscope, using one slide per brain and evaluating tissue sections from all five coronal slabs represented per slide (n = 1 to 19 brains per experimental group for the chimeric mice). GFP-positive cells were enumerated and summed in the following brain regions: frontal lobe, septum, caudoputamen, hippocampus, thalamus, midbrain, cortex, hypothalamus, cerebellum, and brain stem.
Statistical analysis.
The StatView program (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. Analysis of variance (ANOVA), followed where indicated by Fisher's protected least significant difference (PLSD) post hoc test, was performed to determine group differences for continuous data (GFP-positive cells). The chi-square test was utilized for nominal data (“yes” or “no” for the occurrence of seizures).
RESULTS
PCR arrays.
mRNA expression levels for chemokines involved in cell trafficking into the CNS were assayed via PCR arrays as described in Materials and Methods. mRNA expression was compared between PBS-injected and TMEV-infected mice on day 2 p.i., prior to the development of seizures, and day 6 p.i., when seizures were clearly evident. PBS-injected day-2 and day-6 mouse brains were not different from normal control brains. Chemokines can affect recruitment of PMNs and monocytes/macrophages and can affect NK cells either individually or in combination. The relative mRNA expression levels of chemokines that affect a single cell type are summarized in Table 1, and the levels of those that affect multiple cell types are summarized in Table 2.
Table 1.
Relative mRNA expression levels of chemokines (ligands and receptors) that affect a single cell typea
| Ligand or receptor | Other name | Relative mRNA expression in groupb: |
|||
|---|---|---|---|---|---|
| TMEV infected/PBS (day 2 p.i.) | Day 6 p.i. |
||||
| Seizures/PBS | No seizures/PBS | Seizures/no seizures | |||
| PMN recruitment | |||||
| CXCL1 | NAP-3 | 1.6 | 15.7 | 3.4 | 4.7 |
| CCL11 | Eotaxin | 3.5 | 1.1 | −1.0 | 1.2 |
| CCL24 | Eotaxin-2 | 1.6 | −1.2 | −2.3 | 1.9 |
| CCR3 | 2.3 | 12.9 | −1.5 | 19.8 | |
| CXCL5 | ENA-78 | 1.3 | −1.5 | 1.0 | −1.5 |
| CXCL15 | LGK | 1.6 | −1.1 | −1.1 | −1.1 |
| Monocyte/macrophage recruitment | |||||
| CCL12 | MCP-5 | 46.7 | 54.0 | 91.8 | −1.7 |
| CCL25 | TECK | 1.5 | −1.2 | −1.7 | 1.5 |
| CCR9 | −1.2 | 2.1 | −3.0 | 6.3 | |
| Affects NK cells | |||||
| CXCL9 | MIG | 51.4 | 233.3 | 168.9 | 1.4 |
| CXCL11 | IP-9 | 34.8 | 39.3 | 12.9 | 3.0 |
| XCR1 | 1.6 | 1.1 | −3.6 | 4.0 | |
CCL, chemokine (C-C motif) ligand; CCR, chemokine (C-C motif) receptor; CXCL, chemokine (C-X-C motif) ligand; ENA-78, epithelial derived neutrophil attractant 78; IP-9, IFN-γ-inducible protein 9; LGK, lungkine; MCP-5, monocyte chemoattractant protein 5; MIG, monocyte induced by IFN-γ; NAP-3, neutrophil activating protein 3; NK, natural killer; PBS, phosphate-buffered saline; p.i., postinfection; PMN, polymorphonuclear leukocyte; TECK, thymus-expressed chemokine; TMEV, Theiler's murine encephalomyelitis virus; XCR, chemokine (C motif) receptor.
mRNA expression normalized to normal-brain control group.
Table 2.
Relative mRNA expression levels of chemokines (ligands and receptors) that affect multiple cell typesa
| Ligand or receptor | Other name | Relative mRNA expression in groupb: |
|||
|---|---|---|---|---|---|
| TMEV infected/PBS (day 2 p.i.) | Day 6 p.i. |
||||
| Seizures/PBS | No seizures/PBS | Seizures/no seizures | |||
| PMN and monocyte/macrophage recruitment | |||||
| CCL1 | I-309 | 1.6 | 2.2 | 1.0 | 2.1 |
| CCR8 | 1.6 | 4.2 | 1.5 | 2.7 | |
| CXCL4 | PF-4 | −1.5 | −2.6 | −1.6 | −1.6 |
| PMN recruitment and affects NK cells | |||||
| CCL20 | MIP-3α | 1.6 | 1.0 | −2.6 | 2.7 |
| Monocyte/macrophage recruitment and affects NK cells | |||||
| CCL6 | MRP-1 | 1.5 | 2.4 | 1.6 | 1.5 |
| CXCL10 | IP-10 | 637.1 | 1,266.0 | 837.5 | 1.5 |
| CCL19 | MIP-3β | 2.1 | 3.5 | 5.2 | −1.5 |
| CXCL12 | SDF-1 | −1.1 | −2.1 | −1.1 | −1.9 |
| CX3CL1 | C3Xkine | −1.1 | −1.7 | 1.6 | −2.8 |
| CCR7 | 1.6 | 11.3 | 3.4 | 3.4 | |
| PMN and monocyte/macrophage recruitment and affects NK cells | |||||
| CCL2 | MCP-1 | 208.3 | 453.8 | 72.5 | 6.3 |
| CCL4 | MIP-1β | 10.8 | 105.9 | 85.0 | 1.2 |
| CCL3 | MIP-1α | 6.8 | 16.4 | 12.9 | 1.3 |
| CCL5 | RANTES | 19.2 | 122.5 | 122.8 | −1.0 |
| CCL7 | MCP-3 | 43.8 | 186.9 | 99.0 | 1.9 |
| CCL8 | MCP-2 | 5.9 | 31.0 | 19.0 | 1.6 |
| CCL22 | MDC | 1.2 | 1.7 | 2.2 | −1.3 |
| CCR1 | 2.5 | 19.1 | 4.9 | 3.9 | |
| CCR2 | 6.1 | 26.3 | 2.4 | 11.2 | |
| CCR5 | 2.4 | 12.8 | 1.6 | 7.8 | |
| CCR4 | 1.6 | 1.9 | −2.2 | 4.1 | |
| CXCR3 | −1.4 | 38.5 | 79.9 | −2.1 | |
CCL, chemokine (C-C motif) ligand; CCR, chemokine (C-C motif) receptor; CX3CL, chemokine (C-X3-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; CXCR, chemokine (C-X-C motif) receptor; IP-10, IFN-γ-inducible protein 10; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory protein; MRP-1, MIP-related protein 1; NK, natural killer; PBS, phosphate-buffered saline; PF-4, platelet factor 4; p.i., postinfection; PMN, polymorphonuclear leukocyte; RANTES, regulated on activation, normal T-cell expressed and secreted; SDF-1, stromal cell-derived factor 1; TMEV, Theiler's murine encephalomyelitis virus.
mRNA expression normalized to normal-brain control group.
The mRNA expression levels of two chemokines (CXCL1 and CCR3) involved in PMN recruitment were elevated as early as day 2 p.i. and remained elevated through day 6 p.i. (Table 1). At day 2 p.i., the mRNA expression of CXCL1 was only marginally elevated, with a 1.6-fold increase in the TMEV-infected mice; however, by day 6 p.i., the levels of mRNA expression of CXCL1 increased such that they were 3.4-fold and 15.7-fold greater in infected mice without seizures and infected mice with seizures, respectively, than in the PBS-injected mice. Therefore, on day 6 p.i., the mRNA expression of CXCL1 was 4.7-fold greater in the infected mice with seizures than that in the infected mice without seizures. The mRNA expression of CCR3, the receptor for both CCL11 and CCL24 (both somewhat increased at days 2 and 6 p.i.), was 2.3-fold increased at day 2 p.i. and 19.8-fold greater in the infected mice with seizures than the level in the infected mice without seizures at day 6 p.i. Two other chemokines, CXCL5 and CXCL15, involved in PMN recruitment (Table 1), had marginally increased mRNA expression at day 2 p.i.; however, by day 6 p.i., mRNA expression of these chemokines was greater in the infected mice without seizures than in the infected mice with seizures.
Chemokines involved in monocyte/macrophage recruitment include CCL25; its receptor, CCR9; and CCL12. The mRNA expression levels of CCL25 and CCR9 were in disagreement at day 2 p.i., with CCL25 increased and CCR9 decreased (Table 1). By day 6 p.i., the mRNA expression levels of both CCL25 and CCR9 were greater in the infected mice with seizures than the levels in the infected mice without seizures. CCL12 had greatly increased mRNA expression at day 2 p.i.; however, by day 6 p.i., mRNA expression was greater in the infected mice without seizures than in the infected mice with seizures (Table 1).
The chemokines CXCL9, CXCL11, and XCR1 all affect NK cells. The mRNA expression levels of CXCL9 and CXCL11 were greatly increased at day 2 p.i. (51.4-fold and 34.8-fold, respectively), but this great increase was not maintained when the infected mice with seizures were compared to the infected mice without seizures at day 6 p.i. (Table 1). The mRNA expression of XCR1, which binds XCL2 (not on the PCR array), which also affects NK cells, was only marginally increased at day 2 p.i. but was 4.0-fold greater in the infected mice with seizures than the level in the infected mice without seizures at day 6 p.i. (Table 1).
Of the three chemokines (CCL1, CCR8, and CXCL4) that affect PMN and monocyte/macrophage recruitment, the mRNA expression of two, CCL1 and its receptor, CCR8, was increased at days 2 and 6 p.i., and the mRNA expression of one, CXCL4, was decreased at both time points (Table 2). Only one chemokine ligand, CCL20, affects both PMN recruitment and NK cells, and its mRNA expression was increased at days 2 and 6 p.i. (Table 2). The mRNA expression levels of those chemokines (CCL6, CXCL10, CCL19, CXCL12, CX3CL1, and CCR7) that affect monocyte/macrophage recruitment and NK cells did not show any clear pattern of increase or decrease (Table 2). However, CXCL10, otherwise known as IP-10 (gamma interferon [IFN-γ]-inducible protein 10), had the greatest increase in mRNA expression of any of the samples included on the PCR array. At day 2 p.i., the mRNA expression of CXCL10 was 637.1-fold greater in the TMEV-infected mice, and by day 6 p.i., the mRNA expression of CXCL10 increased such that the levels were 837.5-fold and 1,266.0-fold greater in infected mice without seizures and infected mice with seizures, respectively, than those in the PBS-injected mice. The list of chemokines that affect all three cell types (PMNs, monocytes/macrophages, and NK cells) is much longer (Table 2). The majority of these showed an increase in mRNA expression at days 2 and 6 p.i., with the greatest increase occurring in CCL2: a 208.3-fold increase at day 2 p.i. and 72.5-fold and 453.8-fold increases in infected mice without seizures and infected mice with seizures, respectively, at day 6 p.i. The notable exception was CXCR3, which showed a decrease in mRNA expression at both time points.
Based on the PCR array data summarized in Tables 1 and 2, PMNs and NK cells appeared to be cell types that should be considered in greater detail by other means. PMNs were implicated by the increased mRNA expression levels at day 2 p.i. of the chemokines CCL11 and CCR3 and by the increased mRNA expression levels at day 6 p.i. of the chemokines CXCL1 and CCR3. NK cells were implicated by the increased mRNA expression levels at day 2 p.i. of the chemokines CXCL9 and CXCL11 and by the increased mRNA expression levels at day 6 p.i. of the chemokines CXCL11 and XCR1. Finally, the CXCL10 and CCL2 chemokines, which affect NK cells and/or PMN recruitment in addition to other cell types, were also shown to have increased mRNA expression levels at the day 2 and/or 6 p.i. time point.
Role of PMNs in acute seizures.
As a means of determining the contributions made by PMNs to the development of seizures in our TMEV-induced seizure model, infected mice were treated with various depleting or blocking antibodies, as described in Materials and Methods. In vivo treatment of mice with the anti-Gr-1 antibody, which recognizes a surface marker (Gr-1) on mature murine granulocytes, has been shown to deplete peripheral blood neutrophils (to <10% of pretreatment levels) for up to 5 days (18) and splenic neutrophils for up to 3 days (9). This antibody treatment was found not to affect the numbers of macrophages or CD4+ T cells in the spleen, and NK cell activity was unaffected at 1 and 3 days following antibody treatment (9). We found that neutrophil depletion through antibody treatment did not affect the number of TMEV-infected mice displaying seizures (Racine scale stages 3 to 5) through day 21 p.i. (50%; difference not significant, chi-square test) (Fig. 1).
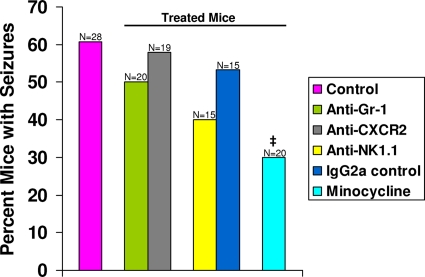
Fig. 1.
Seizure (Racine scale stages 3 to 5) frequency in antibody-treated and minocycline-treated mice. C57BL/6 mice were treated, as described in Materials and Methods, with various antibodies or minocycline in conjunction with a DA viral infection, and these mice were monitored for the development of seizures. No significant differences in seizure frequency were seen in any of the mice undergoing antibody treatments compared to wild-type control mice. Minocycline-treated mice had significantly fewer seizures (30%) than wild-type control mice (60.7%). ‡, P < 0.05 (chi-square test). The total number of mice infected (N) is shown over the individual bars of the graph. The percentages of mice with seizures (y axis) were calculated as follows: (no. of mice with seizures/total no. of mice infected) × 100.
To test the role of PMNs by a different approach, we used CXCR2 antibody. In vivo treatment of mice with the anti-CXCR2 antibody, which recognizes an ELR+ (containing a tripeptide motif glutamic acid-leucine-arginine) CXC chemokine receptor expressed predominantly by PMNs, has been shown to block PMN activation and/or migration into the CNS in an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (7). Although this antibody blocked PMN activation and/or migration, treatment with this antibody failed to deplete the experimental animal of PMNs in the periphery when it was administered every third day (7). We found that CXCR2 blockade through antibody treatment did not affect the number of TMEV-infected mice displaying seizures (Racine scale stages 3 to 5) through day 21 p.i. (57.9%; difference not significant, chi-square test) (Fig. 1). The PBS-treated mice (data not shown) were not significantly different from the wild-type control mice (60.7%).
Role of NK cells in acute seizures.
As a means of determining the contributions made by NK cells to the development of seizures in our TMEV-induced seizure model, infected mice were treated in vivo with the anti-NK1.1 antibody, which recognizes the NK1.1 surface antigen expressed on NK cells in the C57BL/6 strain of mice (15, 44). This treatment has been shown to deplete splenic NK cells (to <0.08% of untreated levels) for up to 4 days following infection with murine cytomegalovirus (44). We found that NK cell depletion somewhat decreased the number of TMEV-infected mice displaying seizures (Racine scale stages 3 to 5) through day 21 p.i. to 40% (difference not significant, chi-square test) (Fig. 1). The isotype control-treated mice also did not differ significantly (chi-square test) in the percentage of mice with seizures (53.3%) from wild-type control mice (60.7%) (Fig. 1). The PBS-treated mice (data not shown) were not significantly different from the wild-type control mice (60.7%).
Minocycline treatment.
Minocycline, a semisynthetic derivative of tetracycline, has been found to have antimicrobial, anti-inflammatory, and antiapoptotic properties (reviewed in reference 42). Due to the effects (discussed below) of minocycline on PMNs, monocytes/macrophages, and microglial cells, we decided to test whether minocycline could modulate seizures in C57BL/6 mice infected with DA virus. DA virus-infected mice were administered minocycline twice a day, as described in Materials and Methods, and mice were monitored for 21 days p.i. for seizures. Minocycline treatment significantly reduced the number of mice displaying seizures (Racine scale stages 3 to 5) to 30%, compared to 60.7% for wild-type control mice (P < 0.05, chi-square test) (Fig. 1). The PBS-treated mice (data not shown) were not significantly different from the wild-type control mice (60.7%).
Role of IL-6, produced by resident CNS cells and infiltrating cells, in acute seizures.
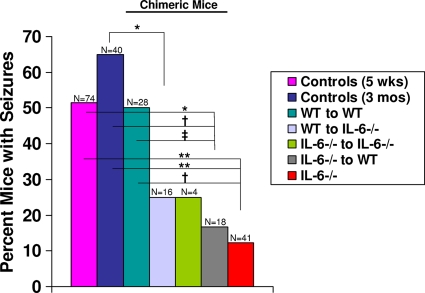
As a means of determining the contributions made by resident CNS cells versus infiltrating cells to the development of seizures in our TMEV-induced seizure model, chimeric mice were generated. Since our previous studies implicated IL-6 in the brain as contributing to acute seizure development (19), various chimeric mice were generated by using IL-6-deficient mice. Irradiated IL-6-deficient mice were reconstituted with either wild-type or IL-6-deficient donor cells, and irradiated wild-type mice were reconstituted with either wild-type or IL-6-deficient donor cells. Chimeric mice were infected with DA virus (2 × 105 PFU) and observed for the development of seizures (Racine scale stages 3 to 5) through day 21 p.i. Irradiated wild-type mice reconstituted with wild-type donor cells did not differ from irradiated wild-type mice reconstituted with GFP-expressing donor cells in the percentage of mice with seizures (50%), so these mice were combined into one group (WT to WT) (Fig. 2). Significantly fewer irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with wild-type donor cells (WT to IL-6−/−) developed seizures (25%) compared to 3-month-old control mice (65%; P < 0.01, chi-square test) (Fig. 2). Also, significantly fewer irradiated 3-month-old wild-type chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/− to WT) developed seizures (16.7%) compared to 5-week-old control mice (51.4%; P < 0.01, chi-square test), 3-month-old control mice (65%; P < 0.001, chi-square test), and irradiated 3-month-old wild-type chimeric mice reconstituted with wild-type donor cells (WT to WT) (50%; P < 0.05, chi-square test) (Fig. 2). Finally, significantly fewer 5-week-old IL-6-deficient mice (IL-6−/−) (12.2%) had seizures compared to 5-week-old control mice (51.4%; P < 0.0001, chi-square test), 3-month-old control mice (65%; P < 0.0001, chi-square test), and irradiated 3-month-old wild-type chimeric mice reconstituted with wild-type donor cells (WT to WT) (50%; P < 0.001, chi-square test) (Fig. 2). Twenty-five percent of irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/− to IL-6−/−) developed seizures; however, this group only numbered four mice. Therefore, there were no significant differences (chi-square test) between this group and any of the other groups (Fig. 2).
Fig. 2.
Seizure (Racine scale stages 3 to 5) frequency in chimeric mice. Chimeric mice, generated as described in Materials and Methods, were infected at 3 months of age with DA virus and monitored for the development of seizures. Significantly fewer irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with wild-type donor cells (WT to IL-6−/−; 25%) had seizures compared to 3-month-old control mice (65%). Significantly fewer 5-week-old IL-6-deficient mice (IL-6−/−; 12.2%) and irradiated 3-month-old wild-type chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/− to WT; 16.7%) had seizures compared to 5-week-old control mice (51.4%), 3-month-old control mice (65%), and irradiated 3-month-old wild-type chimeric mice reconstituted with wild-type donor cells (WT to WT; 50%). ‡, P < 0.05; *, P < 0.01; †, P < 0.001; **, P < 0.0001 (chi-square test). The total number of mice infected (N) is shown over the individual bars of the graph. The percentages of mice with seizures (y axis) are calculated as follows: (no. of mice with seizures/total no. of mice infected) × 100.
GFP expression in chimeric mice.
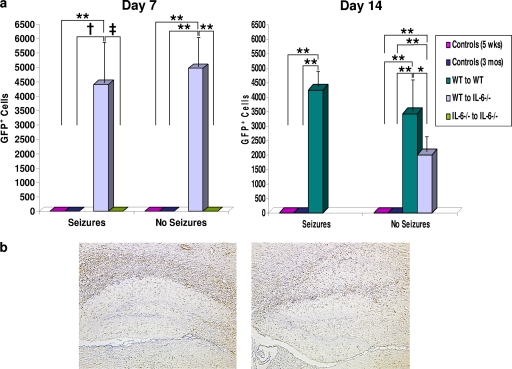
GFP-positive cells were examined in chimeric mice sacrificed on days 7 and 14 p.i. Unlike as described above, where the group of irradiated wild-type mice reconstituted with wild-type donor cells was combined with the irradiated wild-type mice reconstituted with GFP-expressing donor cells, here GFP-positive cells were enumerated only in the irradiated wild-type mice reconstituted with GFP-expressing donor cells (WT to WT) (Fig. 3a) as the irradiated wild-type mice reconstituted with wild-type donor cells were all negative for GFP immunostaining. Likewise, the 5-week-old control mice, the 3-month-old control mice, and the 3-month-old IL-6-deficient mice reconstituted with IL-6-deficient donor cells (IL-6−/− to IL-6−/−) were all negative for GFP immunostaining (Fig. 3a). The IL-6-deficient mice were not chimeras, and GFP immunostaining was not performed as they too would have been all negative for GFP immunostaining. GFP-positive cells could not be counted in the irradiated 3-month-old wild-type chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/− to WT) as all of the cells in the tissues examined were GFP positive due to the use of GFP-expressing mice as the recipients. Therefore, with the above caveats, the irradiated 3-month-old wild-type mice reconstituted with GFP-expressing donor cells (WT to WT) had more GFP-positive cells than the 5-week-old control mice and the 3-month-old control mice (P < 0.0001, ANOVA, Fisher's PLSD post hoc test) for both mice with seizures and those without seizures at day 14 p.i. and more GFP-positive cells than the irradiated 3-month-old IL-6-deficient mice reconstituted with wild-type donor cells (WT to IL-6−/−) for those mice without seizures at day 14 p.i. (P < 0.01, ANOVA, Fisher's PLSD post hoc test) (Fig. 3a). The irradiated 3-month-old IL-6-deficient mice reconstituted with wild-type donor cells (WT to IL-6−/−) had more GFP-positive cells than the 5-week-old control mice at day 7 p.i. with and without seizures and day 14 p.i. without seizures (P < 0.0001, ANOVA, Fisher's PLSD post hoc test), the 3-month-old control mice at day 7 p.i. with seizures (P < 0.001, ANOVA, Fisher's PLSD post hoc test) and without seizures and day 14 p.i. without seizures (P < 0.0001, ANOVA, Fisher's PLSD post hoc test), and the 3-month-old IL-6-deficient mice reconstituted with IL-6-deficient donor cells (IL-6−/− to IL-6−/−) at day 7 p.i. with seizures (P < 0.05, ANOVA, Fisher's PLSD post hoc test) and without seizures (P < 0.0001, ANOVA, Fisher's PLSD post hoc test) (Fig. 3a). Representative tissue sections in Fig. 3b show infiltrating GFP-positive cells in both mice with seizures (left panel) and without seizures (right panel). These results demonstrate that bone marrow-derived microglial progenitors and/or peripheral cells infiltrate the CNS, under pathological conditions, within the time frame of these studies.
Fig. 3.
GFP expression within the brains of chimeric mice. (a) GFP-positive cells, as determined by immunohistochemistry, demonstrate the presence of adoptively transferred infiltrating cells in the brains of infected mice sacrificed on days 7 and 14 p.i. For the mice on day 7 p.i. that had seizures and those that did not, the irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with wild-type donor cells (WT to IL-6−/−) had significantly more GFP-positive cells than the 5-week-old control mice, the 3-month-old control mice, and the irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/− to IL-6−/−). For the mice on day 14 p.i. that had seizures and those that did not, the irradiated 3-month-old wild-type chimeric mice reconstituted with wild-type donor cells (WT to WT) had significantly more GFP-positive cells than the 5-week-old control mice and the 3-month-old control mice. In addition, for those mice on day 14 p.i. that did not have seizures, the irradiated 3-month-old wild-type chimeric mice reconstituted with wild-type donor cells (WT to WT) had significantly more GFP-positive cells than the irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with wild-type donor cells (WT to IL-6−/−), while the irradiated 3-month-old IL-6-deficient chimeric mice reconstituted with wild-type donor cells (WT to IL-6−/−) had significantly more GFP-positive cells than the 5-week-old control mice and the 3-month-old control mice. ‡, P < 0.05; *, P < 0.01; †, P < 0.001; **, P < 0.0001 (ANOVA, Fisher's PLSD post hoc test). Results are means + standard errors from groups containing 1 to 19 mice. (b) Representative tissue sections showing infiltrating GFP cells in mice with seizures (left) and those without seizures (right).
DISCUSSION
It is difficult to clearly determine the contributions made by individual cell types to the development of seizures in our TMEV-induced seizure model based on the PCR array data. Many chemokine receptors are expressed by multiple cell types, thus allowing chemokine ligands to affect multiple cell types. The PCR array data can, however, provide a clue as to which cell types should be considered in greater detail by other means. For example, the mRNA expression levels of chemokines that affect (i) PMN recruitment (6 total) (Table 1), (ii) NK cells (3 total) (Table 1), and (iii) PMN recruitment and NK cells (1 total) (Table 2) were all elevated (10/10) at day 2 p.i. in the TMEV-infected mice compared to the PBS-injected mice. As seizures develop starting at day 3 p.i., PMNs and NK cells could be important in our TMEV-induced seizure model. However, the use of antibodies to either deplete PMNs (anti-Gr-1) or block PMN infiltration into the CNS (anti-CXCR2) or to deplete splenic NK cells (anti-NK1.1) did not significantly affect the number of TMEV-infected mice displaying seizures (Fig. 1).
It may be argued that in vivo administration of anti-Gr-1 antibody (RB6-8C5) depletes not only neutrophils but also Gr-1+ dendritic cells, lymphocytes, and monocytes/macrophages (10). In fact, Gr-1+ CCR2+ CX3CR1lo Ly-6Chi cells have been demonstrated to be a monocyte subpopulation that are the direct circulating precursors to microglia in peripheral blood (26). Therefore, anti-Gr-1 antibody treatment is not representative exclusively of neutropenia, although this antibody has been used extensively to examine neutrophil function in various murine disease models (10). In vivo treatment with the anti-Gr-1 antibody reduced the seizure activity in the pilocarpine model of status epilepticus in C57BL/6 mice, although the treatment regimen (200 μg intravenously 24 h before and days 3, 6, and 9 after status epilepticus onset) differed from that used in our study (150 μg i.p. 5 h before infection) (13). To circumvent the issue of the anti-Gr-1 antibody depleting other cell types in addition to neutrophils, we used the anti-CXCR2 antibody to block entry of PMNs into the CNS, since CXCR2 is expressed predominantly by PMNs (7).
The mRNA expression levels of chemokines that affect (i) monocyte/macrophage recruitment (3 total) (Table 1), (ii) monocyte/macrophage and PMN recruitment (3 total) (Table 2), and (iii) monocyte/macrophage recruitment and NK cells (6 total) (Table 2) varied as to whether they were elevated (8/12) or decreased (4/12) in the TMEV-infected mice compared to the PBS-injected mice at day 2 p.i. So monocytes/macrophages may be important in the development of seizures in our TMEV-induced seizure model. In vitro and in vivo studies have shown that minocycline can affect monocytes/macrophages, microglial cells, and PMNs. Peripherally administered minocycline has been shown to be able to penetrate into the brain (8; reviewed in reference 25). In vitro studies by several groups have demonstrated that minocycline significantly inhibits PMN chemotaxis, an effect not seen with other antibiotics/antimicrobials tested (43, 52). Minocycline also blocked PMN recruitment in vivo in response to intracerebral lipopolysaccharide, which, in the absence of minocycline, occurs through microglial activation and release of TNF-α (58). In vitro studies have demonstrated that minocycline prevents excitotoxin (N-methyl-d-aspartate, glutamate, and kainate)-induced microglial activation and proliferation in mixed spinal cord cultures and pure microglial cultures (46, 47). Minocycline has also been shown, through in vivo studies, to decrease activation of microglial cells in the CNS following traumatic brain injury (4), to decrease monocyte/macrophage activation and macrophage infiltration into the CNS following simian immunodeficiency virus infection of rhesus macaques (5), and to decrease microglial activation and monocyte/macrophage infiltration into the CNS following Japanese encephalitis virus (JEV) infection of mice (12, 28). Minocycline treatment significantly reduced the seizure frequency in TMEV-infected mice (Fig. 1). Interestingly, minocycline treatment following infection with JEV dramatically decreased the level of the chemokine CCL2 in the brain compared to untreated, JEV-infected mice (12, 28). Expression of CCL2 was also greatly increased in the brain following TMEV infection (Table 2), and examination of the expression of CCL2 following minocycline treatment of TMEV-infected mice could suggest a mechanism of action of minocycline during viral CNS infections. In general, minocycline may modulate the production of chemokines and/or chemokine receptors, thus modulating immune cell invasion and migration (reviewed in reference 42).
Finally, the mRNA expression levels of chemokines that affect all three cell types, monocyte/macrophage and PMN recruitment and NK cells (12 total) (Table 2) were mostly elevated (11/12) in the TMEV-infected mice compared to the PBS-injected mice at day 2 p.i. One interpretation of these many varied results is that both the resident cells within the CNS (microglia and astrocytes) and the infiltrating cells (monocytes/macrophages) contribute to the development of seizures.
Previously, we demonstrated that the proinflammatory cytokines TNF-α and IL-6 are induced in the brain in our TMEV-induced seizure model (19). Interestingly, minocycline treatment following infection with JEV significantly decreased the levels of TNF-α and IL-6 in the brain compared to those in untreated, JEV-infected mice (28). In the CNS, the production of TNF-α and IL-6 can be induced in microglia and astrocytes by CNS infection or injury (20, 35, 38, 51) and by seizures (reviewed in references 54 to 56). Resident CNS microglia and infiltrating macrophages have been separated to high purity based on their level of CD45 expression (CD45lo microglia and CD45hi macrophages), and both of these cell populations have been shown to express similar levels of IL-6 following TMEV infection (24). In addition, the production of IL-6 can be induced in neurons (17, 39).
The contributions that resident CNS cells, versus infiltrating cells, make to the production of IL-6 and the development of seizures were examined through the use of irradiated bone marrow chimeras. Of importance, the irradiation/transplantation protocol has not been found to compromise or alter the innate immune response in the brains of chimeric mice (50). Lethal irradiation of recipient mice destroys the bone marrow which can then be reconstituted through adoptive transfer of bone marrow from a suitable donor. Parenchymal microglial cells are radioresistant. Although it has been widely reported that resident CNS microglial cells, astrocytes, and neurons are not replaced in chimeric mice, particularly the microglial cells, in the time frame of these studies (21, 23, 30, 33, 57), newer studies demonstrate that, under pathological conditions, up to 20 to 22% of total parenchymal microglial cells can be traced to the donor following irradiation and transplantation (1). Therefore, the CNS phenotype of these chimeric mice, to a great extent (around 80% in the case of microglial cells), maintains the host phenotype following irradiation and transplantation, while the periphery acquires the distinct donor phenotype. GFP immunostaining of the brain sections of chimeric mice generated through the adoptive transfer of GFP-expressing donor cells demonstrates that bone marrow-derived microglial progenitors and/or peripheral cells infiltrate the CNS, under pathological conditions, within the time frame of these studies—7 and 14 days p.i. (Fig. 3).
DA virus infection of chimeric mice generated from irradiated IL-6-deficient mice (IL-6 deficient in the CNS) reconstituted with cells from wild-type donors (IL-6 normal in the periphery) resulted in fewer mice experiencing seizures (WT to IL-6−/−) (Fig. 2), thus implicating IL-6 production by CNS resident cells in the development of seizures in our TMEV-induced seizure model. Based on the work by Ajami et al. (1), we can estimate that 80% of the resident microglial cells in this chimera would be IL-6 deficient, while the remaining 20% would be IL-6 normal after having been replaced by IL-6-normal microglial precursors from the periphery. The decrease in seizure frequency in this chimera implies that the amount of IL-6 produced by the 20% wild-type microglia is below the threshold for seizure development. Alternately, DA virus infection of chimeric mice generated from irradiated wild-type mice (IL-6 normal in the CNS) reconstituted with cells from IL-6-deficient mice (IL-6 deficient in the periphery) also resulted in fewer mice experiencing seizures (IL-6−/− to WT) (Fig. 2). Again from Ajami et al. (1), we can estimate that 80% of the resident microglial cells in this chimera would be IL-6 normal, while the remaining 20% would be IL-6 deficient after having been replaced by IL-6-deficient microglial precursors from the periphery. So either the presence of ≥20% IL-6-deficient resident CNS microglial cells (20% in IL-6−/−-to-WT chimeras and 80% in WT-to-IL-6−/− chimeras) lowers the amount of IL-6 produced in the CNS to a level below the threshold required for seizure development or IL-6-producing infiltrating cells, such as macrophages, are important in the development of seizures, or both. Control chimeras generated by transfer of wild-type bone marrow into irradiated wild-type recipients were created to assess any nonspecific effects of irradiation on immune activation. There were no differences in the percentages of DA virus-infected mice experiencing seizures when the group made up of irradiated wild-type recipients of wild-type bone marrow (WT to WT) were compared to wild-type controls. Finally, chimeric mice were generated via transfer of IL-6-deficient bone marrow into irradiated IL-6-deficient recipients. These mice (IL-6−/− to IL-6−/−) were comparable in seizure frequency to both irradiated IL-6-deficient chimeric mouse recipients of wild-type bone marrow (WT to IL-6−/−) and irradiated wild-type chimeric mouse recipients of IL-6-deficient bone marrow (IL-6−/− to WT) (Fig. 2), thus implicating IL-6 production by both CNS resident cells and infiltrating cells in the development of seizures in our TMEV-induced seizure model; however, the number of mice in this group was too small for any statistical comparison.
When comparing the antibody-treated and minocycline-treated mouse experiment (Fig. 1) to the chimeric mouse experiment (Fig. 2), there was a discrepancy in the seizure frequency for 5-week-old, wild-type C57BL/6 control mice infected with the DA strain of TMEV. For the antibody-treated and minocycline-treated mouse experiment, the seizure frequency was 60.7% (n = 28), and for the chimeric mouse experiment, the seizure frequency was 51.4% (n = 74). When directly compared, there was no statistical difference between these seizure frequencies (P = 0.40, chi-square test). The seizure frequencies would be expected to converge with an increase in sample number (n).
In the present study, we have examined the contribution of infiltrating peripheral cells and resident CNS cells to the production of IL-6 in the brain and to the development of seizures in the TMEV-induced seizure model. Through the use of irradiated bone marrow chimeric mice generated from IL-6-deficient mice, we found that both resident CNS cells and infiltrating cells are important for the production of IL-6 in the brain and the development of seizures. Various antibody treatments to deplete or block entry of PMNs and to deplete NK cells demonstrated that infiltrating PMNs and NK cells contributed very little to the development of seizures. Minocycline treatment demonstrated that infiltrating monocytes/macrophages and resident microglial cells may play a role in the development of seizures. From this, we hypothesize that the infiltrating cells that produce IL-6 are most likely macrophages, and the resident CNS cells that produce IL-6 are most likely microglia and/or astrocytes. We are currently testing whether monocytes/macrophages could be the infiltrating cell-source of IL-6 and whether microglia could be the resident CNS cell source of IL-6.
ACKNOWLEDGMENTS
We thank Daniel J. Doty, Braden T. McElreath, and Jordan T. Sim for excellent technical assistance. We are grateful to Thomas E. Lane, University of California, Irvine, for the generous gift of the anti-CXCR2 antibody. We wish to acknowledge Kathleen Borick for the outstanding preparation of the manuscript.
This work was supported by funding from NIH 1R01NS065714, the Margolis Foundation, Citizens United for Research in Epilepsy (CURE), and the Emma Mary Deland Foundation.
We have read the Journal of Virology position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. We have no conflicts of interest to disclose.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Ajami B., Bennett J. L., Krieger C., Tetzlaff W., Rossi F. M. V. 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10:1538–1543 [DOI] [PubMed] [Google Scholar]
- 2. Benkovic S. A., O'Callaghan J. P., Miller D. B. 2004. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 1024:59–76 [DOI] [PubMed] [Google Scholar]
- 3. Buenz E. J., et al. 2009. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am. J. Pathol. 175:668–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bye N., et al. 2007. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 204:220–233 [DOI] [PubMed] [Google Scholar]
- 5. Campbell J. H., et al. 2009. The tetracycline antibiotic minocycline prevents the activation of CD14/CD16 monocytes in blood and accumulation in the brain, as well as the development of SIV encephalitis in rhesus macaques. J. Neurovirol. 15(Suppl. 1):1419085205 [Google Scholar]
- 6. Cardona A. E., Li M., Liu L., Savarin C., Ransohoff R. M. 2008. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J. Leukoc. Biol. 84:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlson T., Kroenke M., Rao P., Lane T. E., Segal B. 2008. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 205:811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colovic M., Caccia S. 2003. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 791:337–343 [DOI] [PubMed] [Google Scholar]
- 9. Czuprynski C. J., Brown J. F., Maroushek N., Wagner R. D., Steinberg H. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836–1846 [PubMed] [Google Scholar]
- 10. Daley J. M., Thomay A. A., Connolly M. D., Reichner J. S., Albina J. E. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70 [DOI] [PubMed] [Google Scholar]
- 11. de Araujo E. G., da Silva G. M., dos Santos A. A. 2009. Neuronal cell survival. The role of interleukins. Ann. N. Y. Acad. Sci. 1153:57–64 [DOI] [PubMed] [Google Scholar]
- 12. Dutta K., Kumawat K. L., Nazmi A., Mishra M. K., Basu A. 2010. Minocycline differentially modulates viral infection and persistence in an experimental model of Japanese encephalitis. J. Neuroimmune Pharmacol. 5:553–565 [DOI] [PubMed] [Google Scholar]
- 13. Fabene P. F., et al. 2008. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 14:1377–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farina C., Aloisi F., Meinl E. 2007. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28:138–145 [DOI] [PubMed] [Google Scholar]
- 15. Fritz R. B., Zhao M.-L. 2001. Regulation of experimental autoimmune encephalomyelitis in the C57BL/6J mouse by NK1.1+, DX5+, αβ+ T cells. J. Immunol. 166:4209–4215 [DOI] [PubMed] [Google Scholar]
- 16. Getts D. R., Balcar V. J., Matsumoto I., Müller M., King N. J. C. 2008. Viruses and the immune system: their roles in seizure cascade development. J. Neurochem. 104:1167–1176 [DOI] [PubMed] [Google Scholar]
- 17. Hopkins S. J., Rothwell N. J. 1995. Cytokines and the nervous system. I. Expression and recognition. Trends Neurosci. 18:83–88 [PubMed] [Google Scholar]
- 18. Jensen J., Warner T., Balish E. 1993. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J. Infect. Dis. 167:912–919 [DOI] [PubMed] [Google Scholar]
- 19. Kirkman N. J., Libbey J. E., Wilcox K. S., White H. S., Fujinami R. S. 2010. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 51:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konsman J. P., Drukarch B., Van Dam A.-M. 2007. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin. Sci. (Lond.) 112:1–25 [DOI] [PubMed] [Google Scholar]
- 21. Korngold R., Feldman A., Rorke L. B., Lublin F. D., Doherty P. C. 1986. Acute experimental allergic encephalomyelitis in radiation bone marrow chimeras between high and low susceptible strains of mice. Immunogenetics 24:309–315 [DOI] [PubMed] [Google Scholar]
- 22. Libbey J. E., et al. 2008. Seizures following picornavirus infection. Epilepsia 49:1066–1074 [DOI] [PubMed] [Google Scholar]
- 23. Lublin F. D., Knobler R. L., Doherty P. C., Korngold R. 1986. Relapsing experimental allergic encephalomyelitis in radiation bone marrow chimeras between high and low susceptible strains of mice. Clin. Exp. Immunol. 66:491–496 [PMC free article] [PubMed] [Google Scholar]
- 24. Mack C. L., Vanderlugt-Castaneda C. L., Neville K. L., Miller S. D. 2003. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J. Neuroimmunol. 144:68–79 [DOI] [PubMed] [Google Scholar]
- 25. Mika J. 2008. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol. Rep. 60:297–307 [PubMed] [Google Scholar]
- 26. Mildner A., et al. 2007. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10:1544–1553 [DOI] [PubMed] [Google Scholar]
- 27. Miller R. J., et al. 2008. Chemokine action in the nervous system. J. Neurosci. 28:11792–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mishra M. K., Basu A. 2008. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J. Neurochem. 105:1582–1595 [DOI] [PubMed] [Google Scholar]
- 29. Murray P. D., et al. 2000. Biphasic and regionally-restricted chemokine expression in the central nervous system in the Theiler's virus model of multiple sclerosis. J. Neurovirol. 6(Suppl. 1):S44–S52 [PubMed] [Google Scholar]
- 30. Myers K. J., Dougherty J. P., Ron Y. 1993. In vivo antigen presentation by both brain parenchymal cells and hematopoietically derived cells during the induction of experimental autoimmune encephalomyelitis. J. Immunol. 151:2252–2260 [PubMed] [Google Scholar]
- 31. Nanki T., Nagasaka K., Hayashida K., Saita Y., Miyasaka N. 2001. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Immunol. 167:5381–5385 [DOI] [PubMed] [Google Scholar]
- 32. Palma J. P., Kim B. S. 2001. Induction of selected chemokines in glial cells infected with Theiler's virus. J. Neuroimmunol. 117:166–170 [DOI] [PubMed] [Google Scholar]
- 33. Ponomarev E. D., Shriver L. P., Maresz K., Dittel B. N. 2005. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 81:374–389 [DOI] [PubMed] [Google Scholar]
- 34. Racine R. J. 1972. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32:281–294 [DOI] [PubMed] [Google Scholar]
- 35. Raivich G., et al. 1998. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J. Neurosci. 18:5804–5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ransohoff R. M. 2009. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romano M., et al. 1997. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6:315–325 [DOI] [PubMed] [Google Scholar]
- 38. Rubio N., Sierra A. 1993. Interleukin-6 production by brain tissue and cultured astrocytes infected with Theiler's murine encephalomyelitis virus. Glia 9:41–47 [DOI] [PubMed] [Google Scholar]
- 39. Sallmann S., et al. 2000. Induction of interleukin-6 by depolarization of neurons. J. Neurosci. 20:8637–8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart K.-A. A., Wilcox K. S., Fujinami R. S., White H. S. 2008. A novel model of infection-induced epilepsy: chronic seizures and neuronal cell loss in Theiler's virus infected C57BL/6 mice. Epilepsia 49(Suppl. 7):323–324 [Google Scholar]
- 41. Stewart K.-A. A., Wilcox K. S., Fujinami R. S., White H. S. 2010. Theiler's virus infection chronically alters seizure susceptibility. Epilepsia 51:1418–1428 [DOI] [PubMed] [Google Scholar]
- 42. Stirling D. P., Koochesfahani K. M., Steeves J. D., Tetzlaff W. 2005. Minocycline as a neuroprotective agent. Neuroscientist 11:308–322 [DOI] [PubMed] [Google Scholar]
- 43. Sugita K., Nishimura T. 1995. Effect of antimicrobial agents on chemotaxis of polymorphonuclear leukocytes. J. Chemother. 7:118–125 [DOI] [PubMed] [Google Scholar]
- 44. Szomolanyi-Tsuda E., Liang X., Welsh R. M., Kurt-Jones E. A., Finberg R. W. 2006. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J. Virol. 80:4286–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Theil D. J., Tsunoda I., Libbey J. E., Derfuss T. J., Fujinami R. S. 2000. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler's virus infections. J. Neuroimmunol. 104:22–30 [DOI] [PubMed] [Google Scholar]
- 46. Tikka T., Fiebich B. L., Goldsteins G., Keinänen R., Koistinaho J. 2001. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 21:2580–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tikka T. M., Koistinaho J. E. 2001. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 166:7527–7533 [DOI] [PubMed] [Google Scholar]
- 48. Tsunoda I., McCright I. J., Kuang L.-Q., Zurbriggen A., Fujinami R. S. 1997. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J. Neuropathol. Exp. Neurol. 56:1302–1313 [DOI] [PubMed] [Google Scholar]
- 49. Tsunoda I., et al. 2001. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J. Virol. 75:7494–7505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turrin N. P., Plante M.-M., Lessard M., Rivest S. 2007. Irradiation does not compromise or exacerbate the innate immune response in the brains of mice that were transplanted with bone marrow stem cells. Stem Cells 25:3165–3172 [DOI] [PubMed] [Google Scholar]
- 51. Tuttolomondo A., Di Raimondo D., di Sciacca R., Pinto A., Licata G. 2008. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 14:3574–3589 [DOI] [PubMed] [Google Scholar]
- 52. Ueyama Y., Misaki M., Ishihara Y., Matsumura T. 1994. Effects of antibiotics on human polymorphonuclear leukocyte chemotaxis in vitro. Br. J. Oral Maxillofac. Surg. 32:96–99 [DOI] [PubMed] [Google Scholar]
- 53. Vezzani A. 2005. Inflammation and epilepsy. Epilepsy Curr. 5:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vezzani A., Balosso S., Ravizza T. 2008. The role of cytokines in the pathophysiology of epilepsy. Brain Behav. Immun. 22:797–803 [DOI] [PubMed] [Google Scholar]
- 55. Vezzani A., Granata T. 2005. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743 [DOI] [PubMed] [Google Scholar]
- 56. Vezzani A., Ravizza T., Balosso S., Aronica E. 2008. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia 49(Suppl. 2):24–32 [DOI] [PubMed] [Google Scholar]
- 57. Willenborg D. O., Fordham S. A., Staykova M. A., Ramshaw I. A., Cowden W. B. 1999. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J. Immunol. 163:5278–5286 [PubMed] [Google Scholar]
- 58. Zhou H., Lapointe B. M., Clark S. R., Zbytnuik L., Kubes P. 2006. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J. Immunol. 177:8103–8110 [DOI] [PubMed] [Google Scholar]
- 59. Zurbriggen A., Fujinami R. S. 1989. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J. Virol. 63:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]