Abstract
Cellular apoptosis induced by viral genes can play a critical role in determining virulence as well as viral persistence. This form of cell death has been of interest with respect to Theiler's murine encephalomyelitis virus (TMEV) because the GDVII strain and members of the GDVII subgroup are highly neurovirulent, while the DA strain and members of the TO subgroup induce a chronic progressive inflammatory demyelination with persistence of the virus in the central nervous system. The TMEV L protein has been identified as important in the pathogenesis of Theiler's virus-induced demyelinating disease (TMEV-IDD). We now show that DA L is apoptotic following transfection of L expression constructs or following DA virus infection of HeLa cells; the apoptotic activity depends on the presence of the serine/threonine domain of L, especially a serine at amino acid 57. In contrast, GDVII L has little apoptotic activity following transfection of L expression constructs in HeLa cells and is antiapoptotic following GDVII infection of HeLa cells. Of note, both DA and GDVII L cleave caspase-3 in BHK-21 cells, although neither implements the full apoptotic machinery in this cell type as manifested by the induction of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. The differences in apoptotic activities of DA and GDVII L in varied cell types may play an important role in TMEV subgroup-specific disease phenotypes.
INTRODUCTION
Viruses frequently have genes with proapoptotic or antiapoptotic activity that may vary in different cell types. The apoptosis that is induced can trigger either a protective or destructive immune response, thereby facilitating virus clearance or persistence of the virus. Apoptotic and antiapoptotic genes have been identified in a number of picornaviruses (reviewed in reference 1). Genes regulating apoptosis in Theiler's murine encephalomyelitis virus (TMEV) have been of special interest because of their potential importance in the pathogenesis of TMEV-induced diseases (reviewed in reference 16).
TMEV is a member of the Theilovirus species of the Cardiovirus genus of the Picornaviridae; the Cardiovirus genus also includes the Encephalomyocarditis virus (EMCV) species, which comprises EMCV and mengovirus. TMEV strains can be divided into two subgroups on the basis of their differing biological properties. The GDVII strain and other members of the GDVII subgroup of TMEV are highly virulent and produce a fatal, acute polioencephalomyelitis in mice with no persistence of the virus. In contrast, DA, BeAn, and other members of the less-virulent TO subgroup induce an early transient subclinical neuronal disease followed by a chronic progressive inflammatory demyelination, TMEV-induced demyelinating disease (TMEV-IDD), with persistence of the virus in the central nervous system (CNS) for the life of the mouse. During TMEV-IDD, relatively large amounts of the TMEV genome persist in oligodendrocytes and microglia, with low levels of infectious virus and viral antigen, i.e., there is a restricted expression of DA viral proteins. TMEV-IDD serves as a model of multiple sclerosis because of the similarity in the demyelinating pathology and because the immune system appears to contribute to pathology in both disorders. The remarkable disease phenotype of TO subgroup strains has made TMEV a subject of continuing interest.
Apoptosis has been described during early infection of mice with strains from both subgroups of TMEV and during the late TMEV-IDD. During TMEV-IDD, apoptosis of T cells, microglia/macrophages, and oligodendrocytes has been described (2, 5, 20, 26). In vitro studies have implicated the cardiovirus L protein, which is encoded between the start of the polyprotein and the P1 capsid proteins (Fig. 1), in regulating apoptosis. In vitro studies of TMEV carried out by Fan et al. (9) showed that transfection of an expression construct of BeAn L into BHK-21 cells and a mouse macrophage cell line led to cell death and apoptosis, while Romanova et al. (18) found that L of other cardioviruses has antiapoptotic activity, since infection with a mengovirus with a mutation in the L zinc-binding domain led to apoptosis of HeLa cells that was not seen following wild-type (wt) mengovirus infection. In order to clarify the latter observations and further characterize the apoptotic activity of TMEV, we investigated apoptosis in different cell types following transfection of DA and GDVII L expression constructs and following infection with DA and GDVII wt and L mutant viruses. Our study demonstrated that DA and GDVII L have different apoptotic activities that vary in different cell types. These differences in apoptotic activity may play a role in the TMEV subgroup-specific disease phenotypes.
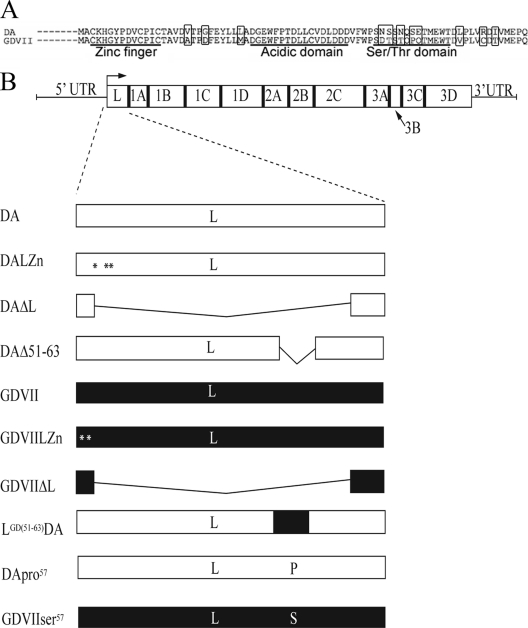
Fig. 1.
DA and GDVII wt and mutant L protein sequences. (A) Sequences of DA and GDVII L proteins. The zinc (Zn) finger, acidic domain, and serine/threonine (Ser/Thr) domain are noted, and the locations of amino acids that vary between these two TMEV strains are highlighted. (B) The TMEV genome with a long 5′-untranslated region (5′-UTR) and coding regions for L and other viral proteins are shown. The L coding region was mutated in different ways to generate the selected plasmids and viruses that are shown. The three amino acid mutations in L of DALZn are shown with asterisks: a change of the cysteine to an arginine at amino acid 11, from the proline to a threonine at amino acid 12, and from the cysteine to an arginine at amino acid 14. The two amino acid mutations in L of GDVIILZn are shown with asterisks: a change of the cysteine at amino acid 3 to alanine and from histidine to leucine at amino acid 5. The open part of the rectangle corresponds to the DA L sequence, while the shaded part corresponds to the GDVII L sequence.
MATERIALS AND METHODS
Cells.
BHK-21 cells were used for plaque assays and the growth of virus stocks, as previously described (6). Studies examining apoptosis were performed in HeLa cells, BHK-21 cells, and HCT116 colorectal cancer cells that constitutively express p53 or do not express p53 (a gift of Marcus Peter) (3).
Viruses and plasmids.
The sequences of DA and GDVII L and the positions of the zinc finger, acidic domain, and serine/threonine domain are shown in Fig. 1A. Mutations that were prepared in DA L in plasmids and viruses are shown in Fig. 1B.
pDAL (which expresses wt DA L) and pGDVIIL (which expresses wt GDVII L), which were previously described (23), were used for experiments involving transfection (see below).
The viruses listed below were prepared from in vitro-derived transcripts of full-length infectious clones. Cells were generally infected at a multiplicity of infection (MOI) of 10:1 and harvested 9 h following infection. DA and GDVII wt viruses were derived from pDAFL3 (19) and pGDVIIFL2 (10). The DAΔL virus, which has a deletion of amino acids 2 to 67 of L, was previously described (23). The GDVIIΔL virus has a deletion identical to that of the DAΔL virus and was prepared in a similar way. The DALZn virus was prepared from pDALZn as previously described (23), with 3 mutations in the zinc binding domain of DA L: a change from the cysteine to an arginine at amino acid 11, from the proline to a threonine at amino acid 12, and from the cysteine to an arginine at amino acid 14. The GDVIILZn virus was prepared from pGDVIIZn, with 2 mutations in the zinc binding domain of GDVII L: a change from the cysteine to an alanine at amino acid 3 and from the histidine to a leucine at amino acid 5. pGDVIILZn was prepared by PCR with the forward primer GTCAATATGGCTGCCAAACTCGGATACCCAGACG and reverse primer CGTCTGGGTATCCGAGTTTGGCAGCCATATTGAC. The LGDVIIDA virus (in which the 2nd through the 76th amino acids of DA L were replaced by the corresponding region of GDVII L in the DA backbone) was prepared from pLGDVIIDA, which was generated by first amplifying the GDVII L sequence from amino acid 2 to 76 as well as adding MluI sites with the forward primer ACGCGTGCTTGCAAACACGGATACCCAGAC and reverse primer GGTGCGCACTGGGGTTCCATGACAGTACGCATA. MluI sites were inserted into the L sequence of pDAFL3 at amino acid 2 by PCR with the forward primer CTATTGACACTATGACGCGTGCTTGCAAACATGGATAC and reverse primer GTATCCATGTTTGCAAGCACGCGTCATAGTGTCAATAG and at amino acid 76 with the forward primer GATATTGTCATGGAACCCCAGACGCGTGGAAACGCCTCTTC and reverse primer GAAGAGGCGTTTCCACGCGTCTGGGGTTCCATGACAATATC. The construct was then MluI digested and ligated into MluI-digested pDAFL3. The MluI sites were eliminated with PCR using the forward primer CTATTGACACTATGGCTTGCAAACACGGATACCCAGAC and reverse primer GTCTGGGTATCCGTGTTTGCAAGCCATAGTGTCAATAG and then the forward primer TATGCGTACTGTCATGGAACCCCAGGGAAACGCCTCTTC and reverse primer GAAGAGGCGTTTCCCTGGGGTTCCATGACAGTACGACATA. The LGD(51–63)/DA virus, which has a replacement of the serine/threonine domain of DA L (Fig. 1) with the corresponding domain of GDVIIL, was prepared from pLGDVII51-63/DA, which was generated by PCR with the forward primer GGCCTTCGGACACGAGCACTCAACCTCAAACAATGGAATGGACTG and reverse primer CAGTCCATTCCATTGTTTGAGGTTGAGTGCTCGTGTCCGAAGGCC. The DAΔ51-63 virus, which has a deletion between amino acids 51 and 63 in L, was prepared from pDALΔ51-63. MluI sites were introduced at amino acid 51 with PCR and the forward primer GACGTCTTCTGGCCTACGCGTCGAACTCGAGCAATC and reverse primer GATTGCTCGAGTTCGACGCGTAGGCCAGAAGACGTC and at amino acid 51 by the forward primer CAATGGAATGGACTACGCGTGACTACCGCTCGTACG and reverse primer CGTACGAGCGGTAGTCACGCGTAGTCCATTCCATTG. The construct was digested with MluI, and following religation, the resultant MluI site was deleted with PCR using the forward primer GACGTCTTCTGGCCTGACTACCGCTCGTACG and reverse primer CGTACGAGCGGTAGTCAGGCCAGAAGACGTC. The DApro57 virus (in which proline, the amino acid normally found at position 57 in GDVII L, replaced the serine that is found in DA L) and GDVIIser57 virus (in which serine, the amino acid normally found at position 57 of DA L, replaced the proline that is normally found in GDVII L) were provided by Yoshiro Ohara (24).
Transfection.
Subconfluent monolayers of BHK-21 and HeLa cells in 35-mm six-well plates were transfected with reaction mixtures consisting of 2 to 4 μg of DNA of an expression vector using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) at a 1:2 ratio of DNA to reagent. The mixtures were incubated for 20 min at room temperature (RT) and then placed on the cell monolayers at 37°C in 5% CO2. Unless specifically indicated, cells were harvested 20 h after transfection.
Reagents and antibodies.
The following antibodies were obtained from Cell Signaling Technology (Beverly, MA): rabbit anti-cleaved caspase-3 antibody, mouse anti-cleaved caspase-9, rabbit anti-poly(ADP-ribose) polymerase (PARP), rabbit anti-cytochrome c, rabbit anti-p53, rabbit antiactin, and rabbit monoclonal anti-Myc. Additional antibodies and reagents included goat anti-mouse and anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase (Chemicon, Bullerica, MA) and streptavidin Alexa Fluor 488 (Invitrogen, Carlsbad, CA).
Western blots.
Cell lysates were subjected to electrophoresis on 10% SDS polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Whatman, Stanford, ME). Membranes were blocked overnight at 4°C in 5% (wt/vol) skim milk in Tris-borate-EDTA (TBS)–0.1% Tween 20, followed by washing three times with TBS–0.1% Tween 20 and then incubation for 1 h at RT with antibody in TBS–0.1% Tween 20 with 5% milk. Membranes were then washed three times, followed by 1 h of incubation at RT with horseradish peroxidase anti-rabbit or anti-mouse IgG antibody in 5% (wt/vol) skim milk in TBS–0.1% Tween 20. The experiments involving Western blots were generally repeated at least three times.
TUNEL staining.
Cells were grown on coverslips for 24 h and then infected or mock infected or transfected. Nine hours after infection and 20 h after transfection, the cells were fixed at RT with 4% paraformaldehyde and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) stained according to the manufacturer's instructions (Promega, Madison, WI). Cells were also stained with Hoechst 33342 for 5 min at room temperature and after a 5-min wash were mounted in antifade mounting solution (Fisher, Pittsburgh, PA) prior to imaging. Images were captured using a Leica SP2 A OBS laser scanning confocal microscope (Leica, Bannockburn, IL). The ImageJ (National Institutes of Health) and Adobe Photoshop 7.0 (Adobe Systems, Inc.) software programs were used to process the images, add pseudocolors, and merge them. Quantitation of TUNEL staining was performed by counting the total number of cells that were TUNEL positive over the total number of cells in at least 8 microscopic fields. The Hoechst 33342 staining allowed one to calculate the total number of cells.
To examine the antiapoptotic activity of TMEV strains, BHK-21 cells were transfected with pDAL or pGDVIIL. Sixteen hours later, 1 μM staurosporine was added to the medium. Three hours later, the cells were TUNEL stained and the TUNEL-positive cells were counted.
Mitochondrial fractionation.
Virus-infected and mock-infected HeLa cells were fractionated using a mitochondrial isolation kit for cultured cells (Thermo, Rockford, IL) according to the manufacturer's recommendations.
RESULTS
DA L is proapoptotic in HeLa cells following DA virus infection, while GDVII L is antiapoptotic following GDVII virus infection.
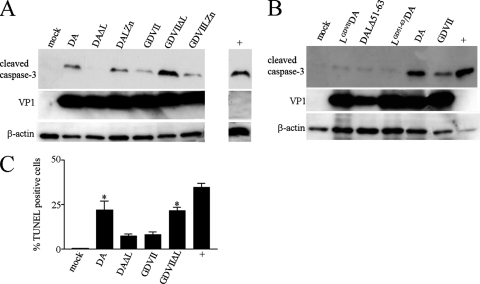
In order to clarify the apoptotic activity of TMEV, we subjected lysates from HeLa cells to Western blotting 9 h after infection with wt and L mutant TMEV (Fig. 1 and 2). Figure 2A shows that there was robust cleavage of caspase-3 following infection with DA wt virus but not DAΔL virus, suggesting that DA L is proapoptotic. The small amount of caspase-3 cleavage seen following DAΔL virus infection (compared to results with mock infection) suggests that there may be a minor apoptotic effect of DA virus infection unrelated to DA L. DALZn virus infection led to little if any diminution in the caspase-3 cleavage seen following infection with DA wt virus. Infection with GDVII wt virus induced cleavage of caspase-3 but at a lower level than that seen with DA wt virus infection. Surprisingly, infection with the GDVIIΔL virus induced a high level of cleaved caspase-3 that was significantly above that seen with GDVII virus infection. The latter results suggested that GDVII virus infection induces apoptosis, that GDVII L is not proapoptotic, and that GDVII L inhibits the apoptosis caused by GDVII virus infection. Similar but less robust results were seen following infection at 6 h (data not shown). At 15 h after infection, similar results were seen in the case of the DA virus; however, most of the monolayer infected with GDVII virus was destroyed (data not shown). The apoptosis caused by GDVIIΔL virus infection was not associated with a disturbance in virus growth since there were levels of VP1 capsid protein following GDVIIΔL infection comparable to those seen following GDVII wt virus infection.
Fig. 2.
DA L is apoptotic while GDVII L is antiapoptotic following infection of HeLa cells. Western blots of lysates of HeLa cells harvested 9 h after infection were immunostained with anti-cleaved caspase-3 antibody (A and B). The lysates were also probed with anti-TMEV VP1 and anti-β-actin antibody. TUNEL staining of cells (C) showed significantly more TUNEL-positive cells following infection with DA wt and GDVIIΔL viruses than following infection with DAΔL and GDVII wt viruses (P < 0.05) and significantly more TUNEL-positive cells with DAΔL and GDVII wt virus infection than with mock infection (P < 0.05). The positive control (“+”) in this Western blot and TUNEL assay and in all subsequent figures is a lysate from cells that were incubated for 3 h with 1 μM staurosporine following incubation overnight in medium without serum. The bars in this and subsequent figures correspond to means ± SEM.
In order to confirm these findings, we prepared a recombinant DA virus, LGDVIIDA virus, in which DA L was replaced by GDVII L (Fig. 2B). Infection of HeLa cells with LGDVIIDA virus led to slightly increased levels of cleaved caspase-3 compared to results with mock infection but substantially less caspase-3 cleavage than was seen following infection with DA wt virus, suggesting that DA L induces the apoptosis associated with DA virus infection and that GDVII L does not have apoptotic activity. There was significantly less caspase-3 cleavage following infection with a mutant DA virus with a deletion of L amino acids 51 to 63 (the serine/threonine domain) (Fig. 1A), DAΔ51-63 virus, than following DA wt virus infection (Fig. 2B). The importance of this site was supported by results of experiments with a recombinant DA virus, LGD(51–63)DA virus, in which L amino acids 51 to 63 were replaced by the corresponding residues from GDVII L: there was significantly less caspase-3 cleavage in HeLa cells infected with the LGD51-63DA virus than was seen following DA wt virus infection (Fig. 2B).
Further confirmation of these findings was made by TUNEL staining of HeLa cells infected with wt and L mutant TMEV (Fig. 2C). TUNEL staining was seen in 21.99% ± 4.89% (mean ± standard error of the mean [SEM]) of cells infected with DA wt virus, 7.41% ± 1.07% of cells infected with DAΔL virus, 8.29% ± 1.22% of cells infected with GDVII wt virus, and 21.76% ± 1.58% of cells infected with GDVIIΔL virus. There were significantly more TUNEL-positive cells (P < 0.05) associated with DA wt virus infection than with DAΔL virus infection and significantly fewer TUNEL-positive cells (P < 0.05) associated with GDVII wt virus infection than with GDVIIΔL virus infection, confirming that DA L has apoptotic activity associated with DA virus infection, while GDVII L has antiapoptotic activity in GDVII virus infections. There were significantly more TUNEL-positive cells following infection with DAΔL virus (and GDVII wt virus) than following mock infection (P < 0.002), suggesting that infection with DA virus can lead to cellular apoptosis because of an additional apoptotic activity unrelated to DA L.
Transfection of DA L induces apoptosis while GDVII L has little apoptotic effect in HeLa cells.
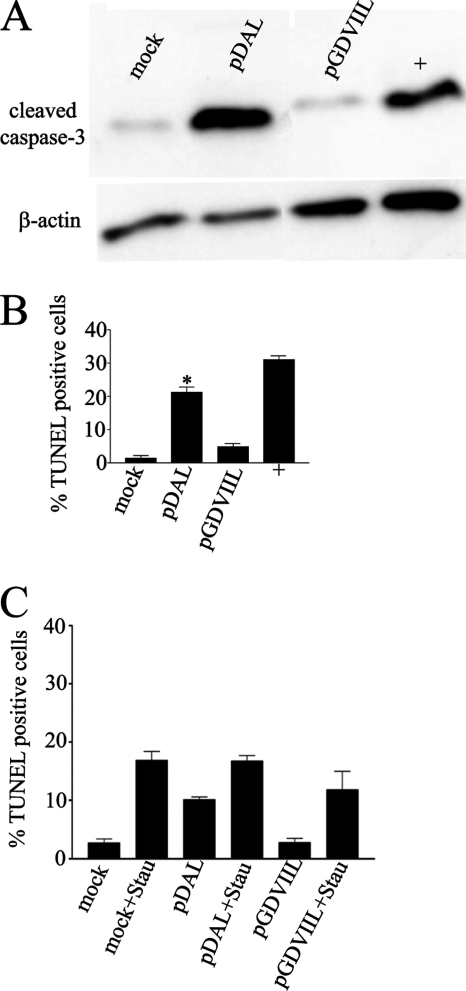
The above findings suggested that DA L induces apoptosis following DA wt virus infection of HeLa cells but that GDVII L is antiapoptotic following GDVII virus infection of these cells. In order to more directly test the apoptotic activities of DA L and GDVII L, we transfected plasmids expressing these genes into HeLa cells. Transfection of DA L induced cleavage of caspase-3, while transfection of GDVII L induced little if any caspase-3 cleavage above the level seen in the mock-transfected cells (Fig. 3 A). These results were confirmed by TUNEL staining (Fig. 3B).
Fig. 3.
DA L induces apoptosis, while GDVII L has little apoptotic effect following transfection of expression constructs into HeLa cells. Western blots of transfected lysates harvested 20 h after transfection were immunostained with anti-cleaved caspase-3 antibody (A). The lysates were also probed for β-actin. TUNEL staining of the cells (B) shows that transfection of pDAL leads to higher levels of apoptosis than transfection of pGDVIIL (*, P < 0.05). (C) GDVII L does not interfere with the apoptosis induced by staurosporine. There were no statistically significant differences (P > 0.05) in TUNEL staining when cells were exposed to staurosporine and transfected with empty vector or with pDAL or pGDVIIL.
We questioned whether GDVII L had antiapoptotic activity in HeLa cells against a nonviral inducer of apoptosis, as found in the case of EMCV and mengovirus L (18). In order to investigate this, we transfected HeLa cells with pGDVIIL and pDAL and then added staurosporine to the cultures 16 h later and finally, after an additional 3 h, fixed and TUNEL stained the cells. Figure 3C shows that DA L expression and staurosporine induce apoptosis in HeLa cells, while GDVII L expression fails to induce apoptosis above levels seen following transfection with vector alone. Of note, there were similar levels of TUNEL staining seen in cells exposed to staurosporine whether or not they had been transfected with pDAL or pGDVII L. These data indicate that GDVII L (and DA L) does not manifest antiapoptotic activity against staurosporine.
Serine at position 57 is important in the apoptotic activity of DA L in HeLa cells.
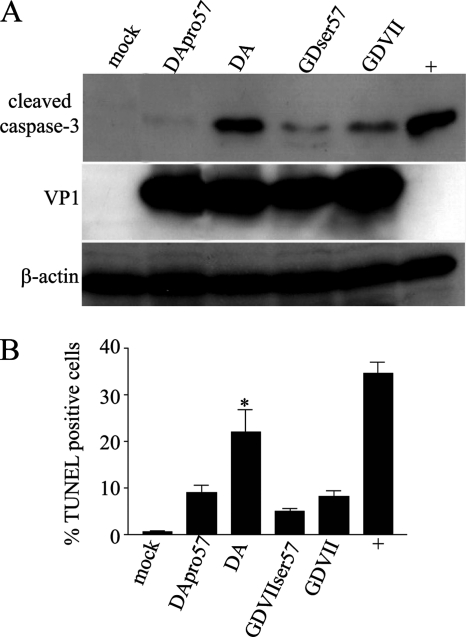
DA L and GDVII L differ by only 11 amino acids (Fig. 1A). The above studies (Fig. 2B) suggested that the serine/threonine domain of DA L is important in its apoptotic activity. We next focused on amino acid 57, which is within this domain, since this amino acid has been implicated in TMEV subgroup-specific growth in BHK-21 cells (24). Of note, this amino acid is serine in the case of all TO subgroup strains and proline in the case of GDVII subgroup strains. Infection with DApro57 virus (which has a proline, the amino acid normally found at position 57 of GDVII L, replacing a serine, which is the amino acid normally found at position 57 in DA L) failed to induce caspase-3 cleavage, as seen following DA wt virus infection (Fig. 4 A). On the other hand, infection with GDVIIser57 virus (which has a serine, the amino acid normally found at position 57 of DA L, replacing a proline, which is normally found in GDVII L) did not lead to an increase in apoptosis following infection of GDVII virus (Fig. 4A). The results suggested that the apoptotic activity of DA virus depends on serine at position 57 and cannot be substituted for proline and that replacement of proline at position 57 in GDVII L with serine does not enhance the apoptotic activity of GDVII virus, for example, either by directly inducing apoptosis or by decreasing the antiapoptotic activity of L. These findings were confirmed by TUNEL staining of cells infected with DApro57 and GDVIIser57 viruses (Fig. 4B).
Fig. 4.
A serine at amino acid 57 in DA is important for apoptosis following DA virus infection in HeLa cells. (A) Western blots of lysates from infected cells immunostained with anti-cleaved caspase-3 antibody show that infection of HeLa cells with DApro57 virus is associated with a substantial decrease in apoptosis compared to DA wt virus infection; however, infection with GDVIIser57 virus induces a similar amount of apoptosis to that seen following GDVII wt virus infection. The lysates were also probed for VP1 and β-actin. (B) TUNEL staining shows that infection with DApro57 virus leads to significantly fewer TUNEL-positive cells than are seen following infection with DA wt virus (*, P < 0.05); however, GDVIIser57 virus has a similar amount of apoptosis to that seen following GDVII wt virus infection.
DA and GDVII viruses induce apoptosis in HeLa cells via the intrinsic apoptotic pathway.
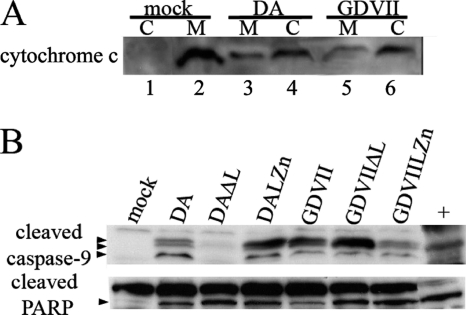
In order to clarify whether DA and GDVII virus use the intrinsic apoptotic pathway in HeLa cells, we examined markers of this pathway. In the intrinsic pathway, cytochrome c is released by the mitochondria, leading to activation of caspase-9, with a resultant cleavage of caspase-3. Infection with DA wt virus led to a release of cytochrome c from the mitochondria into the cytoplasmic fraction (Fig. 5 A), with activation of caspase-9 into cleavage products (Fig. 5B). Of interest, we found that GDVII wt virus infection led to levels of cytochrome c and levels of activated caspase-9 that were similar to those seen with DA wt virus infection (Fig. 5B), in contrast to the differences seen in the levels of caspase-3 and TUNEL (Fig. 2). This result suggests that the site of GDVII L antiapoptotic activity is downstream of activated caspase-9 and at the level of or upstream of caspase-3. Interestingly, we found that the level of cleaved PARP was also similar in the HeLa cells infected with either DA or GDVII wt viruses (Fig. 5B), perhaps because a number of caspases can cleave PARP, including caspase-9 (15).
Fig. 5.
Apoptosis of DA and GDVII virus-infected HeLa cells proceeds via the intrinsic pathway. Western blot of mitochondrial (M) (lanes 2, 3, and 5) and cytoplasmic (C) (lanes 1, 4, and 6) fractions of infected lysates that were immunostained with anti-cytochrome c antibody (A) show that infection of HeLa cells with DA or GDVII wt virus leads to release of cytochrome c from the mitochondrial fraction into the cytoplasm. Western blots of the same lysates used in Fig. 2 that were immunostained with anti-cleaved caspase-9 antibody and anti-PARP antibody (B) show that infection of HeLa cells with DA and GDVII viruses leads to cleavage of caspase-9 and PARP. The arrowheads show the locations of the cleavage products of caspase-9 and one cleavage product of PARP. Although there are three cleavage products of caspase-9, the upper and middle bands are not well separated on this gel.
DA and GDVII virus-induced apoptosis requires p53.
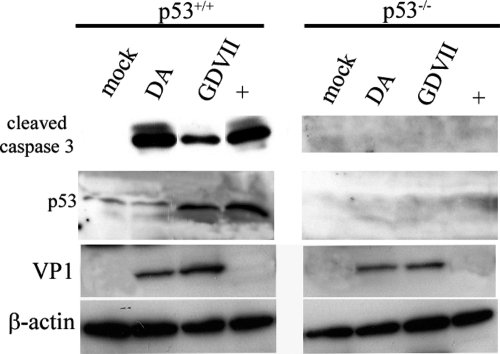
A previous study found that apoptosis triggered by transfection in BHK-21 cells of expression constructs of BeAn L required p53 (9). In order to further investigate this issue, we infected HCT116 colorectal cancer cells that constitutively express p53 or do not express p53. Figure 6 shows that cleavage of caspase-3 occurs following DA and GDVII wt virus infection of HCT116 cells that express p53 but not HCT116 cells that do not express p53.
Fig. 6.
Cleavage of caspase-3 following infection of HCT116 cells with DA and GDVII wt viruses requires p53. Cleavage of caspase-3 occurred following DA and GDVII wt virus infection of HCTT116 p53+/+ cells but not HCT116 p53 knockout cells. Western blots of infected lysates harvested 15 h following infection immunostained with anti-cleaved caspase-3 antibody (A) show that caspase-3 cleavage occurs in cells expressing p53 but not in cells lacking p53. The lysates were also probed for p53, VP1, and β-actin.
DA and GDVII virus infection and transfection of DA and GDVII L induce caspase-3 cleavage in BHK-21 cells but do not induce TUNEL staining.
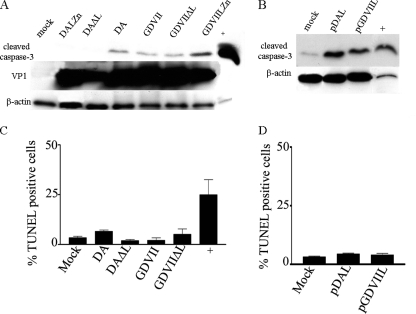
As noted above, Fan et al. (9) reported that apoptosis was induced in BHK-21 cells following transfection of constructs expressing BeAn L. In order to determine whether the apoptotic activity of TMEV was similar in BHK-21 cells to that seen in HeLa cells, we infected BHK-21 cells with DA and GDVII wt and mutant L viruses and also transfected these cells with DA and GDVII L (Fig. 7). As in the case with HeLa cells, infection of BHK-21 cells with DA wt virus led to caspase-3 cleavage, while DAΔL virus infection of BHK-21 cells showed no evidence of caspase-3 cleavage (Fig. 7A). However, GDVII wt virus infection had a different effect on BHK-21 cells than that seen following infection of HeLa cells. In contrast to the findings for infected HeLa cells, there was a similar amount of cleavage of caspase-3 in BHK-21 cells following infection with DA wt, GDVII wt, and GDVIIΔL viruses (Fig. 7A). In order to confirm that DA L and GDVII L have similar effects in BHK-21 cells, we transfected these cells with plasmids expressing DA L and GDVII L. A representative Western blot (Fig. 7B) shows that there are similar levels of caspase-3 cleavage in BHK-21 cells following transfection of DA L and GDVII L.
Fig. 7.
DA and GDVII virus infection and pDAL and pGDVIIL transfection in BHK-21 cells induce similar amounts of cleaved caspase-3 but do not induce TUNEL-positive staining. (A) Western blots of infected lysates harvested 9 h following infection were immunostained with anti-cleaved caspase-3 antibody; the lysates were also probed for VP1 and β-actin. (B) Western blots of lysates transfected with constructs expressing DA or GDVII L were immunostained with anti-cleaved caspase-3 antibody; the same lysates were also probed for β-actin. Cells infected with wt DA or GDVII virus (C) or transfected with pDAL or pGDVIIL (D) were TUNEL stained. There was no significant increase in TUNEL staining in TMEV-infected or TMEV L-transfected cells above the level with mock infection (P > 0.05).
In order to further investigate whether DA and GDVII L are similarly apoptotic in BHK-21 cells, we TUNEL stained infected and transfected cells. Surprisingly, DA and GDVII wt virus-infected (Fig. 7C) and pDAL- and pGDVII L-transfected (Fig. 7D) BHK-21 cells showed no increase in TUNEL-positive cells over mock infection.
DISCUSSION
The apoptosis induced by viral genes can play a critical role in determining virulence as well as viral persistence. This form of cell death has been of interest with respect to TMEV because of the marked difference in virulence and persistence manifested by strains of the two subgroups of TMEV: GDVII and other members of the GDVII subgroup are highly virulent, causing a fatal acute disease with no virus persistence, while DA, BeAn, and other members of the TO subgroup cause a chronic, progressive demyelinating disease in which virus routinely persists in the CNS for the life of the mouse. Apoptosis has been reported both in the acute disease caused by DA and GDVII virus and during chronic TMEV-IDD (2, 5, 20, 26). A number of different cell types have been found to undergo apoptosis, including activated macrophages/microglia, T cells, and oligodendrocytes.
In vitro studies of cardioviruses have sought to identify apoptotic and antiapoptotic viral genes. Fan et al. (9) recently reported that transfection of an expression construct of BeAn L into BHK-21 cells and a mouse macrophage cell line led to cell death and apoptosis. Romanova et al. (18) found evidence of antiapoptotic activity of EMCV and mengovirus L in infections by these cardioviruses in HeLa cells. The present study attempted to clarify these findings and elucidate the pathogenesis of TMEV subgroup-specific disease phenotypes by investigating DA and GDVII strains in more than one cell type.
We found that DA and GDVII L proteins differ in their apoptotic properties in HeLa cells. In HeLa cells, there was robust cleavage of caspase-3 following DA wt virus infection but not infection with DAΔL virus, suggesting that DA L led to apoptosis of HeLa cells. This was confirmed in studies involving transfection of DA L expression constructs and in TUNEL assays. GDVII virus infection induced a low level of apoptosis of HeLa cells; however, GDVIIΔL virus infection led to significantly higher levels of cleaved caspase-3, suggesting that L in GDVII virus has antiapoptotic activity, as was reported for L in the case of EMCV and mengovirus infection in these same cells (18). Transfection studies confirmed that DA L had apoptotic activity, while GDVII L had little apoptotic effect in HeLa cells. Further confirmation of the low level of proapoptotic activity of GDVII L was demonstrated following infection of HeLa cells with a recombinant virus in which GDVII L replaced DA L in DA virus. TUNEL staining provided additional support for these conclusions. In contrast to EMCV and mengovirus L (18), GDVII L had no antiapoptotic activity against a nonviral inducer of apoptosis; however, it may be that GDVII L has antiapoptotic activity against other nonviral inducers of apoptosis that were not tested.
It is of interest that DA and GDVII L have differing apoptotic activities in HeLa cells despite the fact that their sequences differ by only 11 amino acids. Our investigations found that the serine normally found at position 57 in DA L was important in the apoptotic activity in these cells since a change to proline, the corresponding amino acid in GDVII L, significantly attenuated the apoptosis. We suspect that there are a number of determinants in L that are also important in apoptotic activity. Mutation of the DA and GDVII L Zn finger had a somewhat unpredictable effect, perhaps because this mutation leads to major structural changes in L and because interactions of L with other proteins or nucleic acid are disrupted.
The studies with GDVIIΔL virus infection in HeLa cells indicated that GDVII virus infection induced apoptosis that was not related to expression of DA L. The apoptosis may have resulted because of cellular sensors of the viral RNA or because of an apoptotic activity of a GDVII viral gene distinct from L. There was also evidence from DAΔL virus infection that DA virus-induced apoptosis resulted from an activity unrelated to DA L. Varied picornaviral genes have been reported to be apoptotic (reviewed in reference 1), including poliovirus 2A and 3C (4), as well as capsid proteins (12). It will be of interest if the GDVII apoptotic protein is found to be a capsid protein, since this region of GDVII has been found to be critical for neurovirulence (11).
As found in the case of other viruses, the apoptotic activity of TMEV varied depending on the cell type that was infected. In contrast to results with HeLa cells, GDVII virus infection and GDVII L transfection of BHK-21 cells showed an amount of caspase-3 cleavage similar to that seen with DA virus and DA L transfection, respectively. Despite the presence of caspase-3 cleavage in BHK-21 cells infected with DA or GDVII virus or transfected with DA or GDVII L, there was no evidence of TUNEL staining above levels seen in the mock infection. The reason for this failure to implement the full machinery of apoptosis in BHK-21 cells (and not HeLa cells) is unclear. Of note, EMCV L has been found to interfere with the apoptotic pathway downstream of caspase activation in EMCV infections of HeLa cells (18). The degree of apoptosis in different cell types may be related to the following: the timing of nonapoptotic lysis vis-à-vis apoptosis in a TMEV-infection, the interference by L of nucleocytoplasmic transport (8, 17) in a particular cell type, and the absence of a component of an apoptotic pathway in a particular cell type following TMEV L expression. Fan et al. found evidence of apoptosis in BHK-21 cells induced by BeAn L (9); however, this study examined only markers for apoptosis that are upstream of TUNEL staining.
There is evidence to implicate both an intrinsic and extrinsic apoptosis pathway following infection with BeAn virus of different cell types. The intrinsic apoptotic pathway appears to be used in the case of BeAn infection of differentiated, nonactivated macrophage cell lines (e.g., M1-D cells) (21, 22), while the extrinsic pathway is used in the case of BeAn virus-infected gamma interferon (IFN-γ)-activated macrophages (14). In M1-D cells, BeAn L is reported to carry out Bax-mediated apoptosis by activation of p38 mitogen-activated protein kinase (MAPK), leading to p53 phosphorylation with transcriptional upregulation of Puma and Noxa (and loss of prosurvival Mcl-1 and A1), resulting in release of Bax with permeabilization of mitochondria and the subsequent release of cytochrome c; apoptosis was significantly decreased by inhibitors of phospho-p38 MAPK (22). Our studies indicated that DA wt virus and GDVIIΔL infection activate the intrinsic mitochondrial death pathway in HeLa cells. Support for the use of this pathway came from the presence of activated intermediates of the intrinsic apoptotic pathway in infected HeLa cells. Of note, we found similar levels of cytochrome c and activated caspase-9 (which leads to cleaved caspase-3) following DA and GDVII wt virus infection, despite our finding that DA wt virus infection leads to substantially more activated caspase-3 and TUNEL-positive cells than is seen following GDVII wt virus infection. These results suggest that GDVII L blocks the apoptotic activity of GDVII virus in HeLa cells at a site downstream of activated caspase-9 and at the level of or upstream of caspase-3. Our studies also suggested that TMEV-induced apoptosis of HCT116 cells requires p53.
Recent studies of cardioviruses have focused attention on the L protein because of its multiple functions and importance in disease pathogenesis. In addition to its effect on apoptosis, cardiovirus L has been found to inhibit type I IFN transcription (13, 23) and nucleocytoplasmic transport (which causes a block in mRNA egress the nucleus and therefore blocks host cell translation) (8, 17). We recently found that although DA L and GDVII L both block type IFN transcription, they block at different steps in the IFN-dependent IRF-3 pathway (23). One question that arises is whether the different sites at which DA L and GDVII L carry out the type I IFN block are associated with the induction of their different apoptotic activities. The association of the anti-type I IFN activity and apoptotic activity of L is suggested by the fact that the apoptotic activity of GDVII L differs from that of DA L in HeLa cells (which are type I IFN competent), but both GDVII L and DA L similarly cleave caspase-3 in BHK-21 cells (which have a defect in the type I IFN pathway) (7).
Do the differences in apoptotic activities of DA versus GDVII play a role in TMEV subgroup-specific CNS disease phenotypes? GDVII virus is thought to kill mice because of neurovirulent determinants in capsid proteins (11), allowing the virus to quickly replicate to high titers in the CNS; in addition, the block in type I IFN production by GDVII L will interfere with virus clearance (23). In the case of DA virus infection, apoptotic activity induced by this slower-growing virus may limit replication; in addition, the block in type I IFN production may foster virus persistence. Later during the infection, L-induced apoptosis of DA virus-infected cells may set the stage for the immune-mediated demyelinating disease, since innate recognition of infected apoptotic cells induces a T-helper-17 adaptive immune response that is associated with autoimmune diseases (25). In persistently infected oligodendrocytes, the continuing production of low levels of type I IFN may modulate the expression of L, leading to dysfunction of oligodendrocytes, thereby contributing to the subsequent demyelination (23). The differences in apoptotic activity between TMEV strains and in different cell types suggest investigations of apoptosis following TMEV infection of different neural cell types may help clarify the pathogenesis of TMEV-induced CNS diseases.
ACKNOWLEDGMENTS
We thank Marcus Peter (Feinberg School of Medicine) for advice and reagents and Yoshiro Ohara (Kanazawa Medical School) for viruses.
The work was partly funded by grants from the National Institutes of Health (to R.P.R.) (1R01 NS37958-07), the National Multiple Sclerosis Society (to R.P.R.), and the Multiple Sclerosis Foundation (to S.S.).
Footnotes
Published ahead of print on 11 May 2011.
REFERENCES
- 1. Buenz E. J., Howe C. L. 2006. Picornaviruses and cell death. Trends Microbiol. 14:28–36 [DOI] [PubMed] [Google Scholar]
- 2. Buenz E. J., et al. 2009. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am. J. Pathol. 175:668–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bunz F., et al. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497–1501 [DOI] [PubMed] [Google Scholar]
- 4. Calandria C., Irurzun A., Barco A., Carrasco L. 2004. Individual expression of poliovirus 2Apro and 3Cpro induces activation of caspase-3 and PARP cleavage in HeLa cells. Virus Res. 104:39–49 [DOI] [PubMed] [Google Scholar]
- 5. Carlson N. G., Hill K. E., Tsunoda I., Fujinami R. S., Rose J. W. 2006. The pathologic role for COX-2 in apoptotic oligodendrocytes in virus induced demyelinating disease: implications for multiple sclerosis. J. Neuroimmunol. 174:21–31 [DOI] [PubMed] [Google Scholar]
- 6. Chen H. H., Kong W. P., Zhang L., Ward P. L., Roos R. P. 1995. A picornaviral protein synthesized out of frame with the polyprotein plays a key role in a virus-induced immune-mediated demyelinating disease. Nat. Med. 1:927–931 [DOI] [PubMed] [Google Scholar]
- 7. Chinsangaram J., Piccone M. E., Grubman M. J. 1999. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 73:9891–9898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delhaye S., van Pesch V., Michiels T. 2004. The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins. J. Virol. 78:4357–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fan J., Son K. N., Arslan S. Y., Liang Z., Lipton H. L. 2009. Theiler's murine encephalomyelitis virus leader protein is the only nonstructural protein tested that induces apoptosis when transfected into mammalian cells. J. Virol. 83:6546–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu J., Rodriguez M., Roos R. P. 1990. Strains from both Theiler's virus subgroups encode a determinant for demyelination. J. Virol. 64:6345–6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu J. L., et al. 1990. Neurovirulence determinants of genetically engineered Theiler viruses. Proc. Natl. Acad. Sci. U. S. A. 87:4125–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gullberg M., et al. 2010. A single coxsackievirus B2 capsid residue controls cytolysis and apoptosis in rhabdomyosarcoma cells. J. Virol. 84:5868–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hato S. V., et al. 2007. The mengovirus leader protein blocks interferon-alpha/beta gene transcription and inhibits activation of interferon regulatory factor 3. Cell Microbiol. 9:2921–2930 [DOI] [PubMed] [Google Scholar]
- 14. Jelachich M. L., Lipton H. L. 2001. Theiler's murine encephalomyelitis virus induces apoptosis in gamma interferon-activated M1 differentiated myelomonocytic cells through a mechanism involving tumor necrosis factor alpha (TNF-alpha) and TNF-alpha-related apoptosis-inducing ligand. J. Virol. 75:5930–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margolin N., et al. 1997. Substrate and inhibitor specificity of interleukin-1 beta-converting enzyme and related caspases. J. Biol. Chem. 272:7223–7228 [DOI] [PubMed] [Google Scholar]
- 16. Michiels T., Roos R. P. 2010. Theiler's virus central nervous system infection, p. 411–428 In Ehrenfeld E., Domingo E., Roos R. P. (ed.), The picornaviruses. ASM Press, Washington, DC [Google Scholar]
- 17. Porter F. W., Bochkov Y. A., Albee A. J., Wiese C., Palmenberg A. C. 2006. A picornavirus protein interacts with Ran-GTPase and disrupts nucleocytoplasmic transport. Proc. Natl. Acad. Sci. U. S. A. 103:12417–12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romanova L. I., et al. 2009. Antiapoptotic activity of the cardiovirus leader protein, a viral “security” protein. J. Virol. 83:7273–7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roos R. P., Stein S., Ohara P., Fu J. L., Semler B. L. 1989. Infectious cDNA clones of Theiler's murine encephalomyelitis virus. J. Virol . 63:5492–5496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlitt B. P., Felrice M., Jelachich M. L., Lipton H. L. 2003. Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions. J. Virol. 77:4383–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Son K. N., Becker R. P., Kallio P., Lipton H. L. 2008. Theiler's virus-induced intrinsic apoptosis in M1-D macrophages is Bax mediated and restricts virus infectivity: a mechanism for persistence of a cytolytic virus. J. Virol. 82:4502–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Son K. N., Pugazhenthi S., Lipton H. L. 2009. Activation of tumor suppressor protein p53 is required for Theiler's murine encephalomyelitis virus-induced apoptosis in M1-D macrophages. J. Virol. 83:10770–10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stavrou S., Feng Z., Lemon S. M., Roos R. P. 2010. Different strains of Theiler's murine encephalomyelitis virus antagonize different sites in the type I interferon pathway. J. Virol. 84:9181–9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takano-Maruyama M., Ohara Y., Asakura K., Okuwa T. 2006. Theiler's murine encephalomyelitis virus leader protein amino acid residue 57 regulates subgroup-specific virus growth on BHK-21 cells. J. Virol. 80:12025–12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torchinsky M. B., Garaude J., Martin A. P., Blander J. M. 2009. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 458:78–82 [DOI] [PubMed] [Google Scholar]
- 26. Tsunoda I., Kurtz C. I., Fujinami R. S. 1997. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology 228:388–393 [DOI] [PubMed] [Google Scholar]