Abstract
The nonpathogenic human GB virus C (GBV-C), a member of the Flaviviridae, is highly prevalent in individuals with HIV-1 infections or with parenteral and sexual risk factors. Long-term GBV-C viremia has been associated with better survival or improved diagnosis in several epidemiological studies. In a previous study we reported that the E2 glycoprotein of GBV-C interferes with HIV-1 entry in vitro. To address the question what region of the E2 protein is involved in suppression of HIV-1 replication, we performed an E2-derived peptide scanning and determined the HIV-inhibitory activity of each peptide in HIV replication assays. We demonstrate here that peptides representing the N-terminal part of the E2 protein from amino acids (aa) 29 to 72 are able to inhibit efficiently HIV-1 replication in vitro. In particular, the peptides P6-2 (representing the E2-region from aa 45 to 64) and P4762 (aa 37 to 64) showed the highest potency in HIV replication assays performed on TZM-bl cells with 50% inhibitory concentrations between 0.1 and 2 μM. However, primary HIV-1 isolates representing clades A to H showed a high variability in their sensitivity to E2 peptides. Pseudovirus inhibition assays revealed that the sensitivity is determined by the gp120/gp41 envelope proteins. Using HIV-1 BlaM-Vpr-based fusion assays, we demonstrate that the E2-derived peptides prevent HIV-1 binding or fusion, presumably via interaction with the HIV-1 particle. Together, these findings reveal a new mechanism of viral interference, suggesting that the envelope protein E2 of GBV-C target directly HIV-1 particles to avoid entry of these virions.
INTRODUCTION
GB virus C (GBV-C), a positive-strand RNA virus of the Flaviviridae is thought to be a nonpathogenic virus that replicates primarily in CD4+, CD8+, and B lymphocytes (1, 9, 27). The main transmission routes are sexual and parenteral leading to a rather high prevalence of 1 to 3% viremic individuals among healthy blood donors and 15 to 40% among HIV-1 positive individuals (8, 17, 26, 28, 40). In immunocompetent individuals GBV-C viremia is mostly cleared within the first years concomitantly by the development of antibodies directed against the envelope glycoprotein E2. GBV-C became of interest because several epidemiological studies demonstrated that coinfection of HIV-1 and GBV-C is associated with a slower progression to AIDS and prolonged survival (18, 31, 33, 36, 39). In contrast, other groups could not confirm the positive effect of GBV-C infection for HIV-infected individuals (3, 4, 32). Van der Bij et al. (32) also referred that the loss of GBV-C viremia without production of anti-E2 antibodies is associated with the worst prognosis for HIV-1 patients. However, Williams et al. (33) could demonstrate that long-term GBV-C viremia for more than 5 to 6 years after HIV-1 seroconversion is essential to benefit from GBV-C coinfections. Several mechanisms have been postulated to be responsible for GBV-C-mediated HIV-1 suppression. In peripheral blood mononuclear cells (PBMC), GBV-C induces the release of HIV-inhibitory chemokines RANTES, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and SDF-1, the natural ligands for HIV-1 coreceptors. Thereby, CCR5 surface expression is downregulated due to internalization of chemokine receptors upon ligand binding (14, 22, 34). Two viral proteins have been shown to inhibit HIV-1 replication in vitro. Xiang et al. demonstrated that expression of NS5A induces the expression of SDF-1 and the downregulation of CXCR4 (35, 38). Previously, we demonstrated that the GBV-C envelope glycoprotein E2 is involved in HIV-1 inhibition (13). Incubation of PBMC with recombinant truncated E2 protein of GBV-C, where the hydrophobic transmembrane anchor was exchanged by the human IgG1 Fc fragment, led to significant replication suppression of clinical HIV-1 isolates and HIV-1 reporter particles pseudotyped with X4- and R5-tropic HIV-1 envelope proteins. In contrast, the replication efficiency of HIV reporter particles pseudotyped with heterologous envelope proteins such as vesicular stomatitis virus G protein (VSV-G) was not affected by recombinant E2 protein, implying that E2 specifically interferes with gp120/gp41-mediated HIV-1 entry. E2 is predicted to form heterodimers with E1 and seems to be involved in cell binding. However, no cellular receptor(s) for GBV-C E2 has been identified yet. The E2 region between the amino acids 279 to 298 is supposed to represent the fusogenic peptide and can adopt helical structure slightly inserted into membranes (16, 20). Haro and coworkers (10) could also demonstrate in biophysical assays that the peptide from amino acids 269 to 286 interacts with the fusion peptide of HIV-1 gp41. Latest results suggested that a broad range of E2-derived peptides interact with the gp41 fusion peptide and that the identified peptides were able to mediate HIV-1 inhibition in vitro (11). In the present study, we tested a panel of E2-derived peptides regarding their HIV-inhibitory capacity in various replication assays. We identified a distinct region in E2 that is crucial for HIV-1 inhibition. Peptides derived from that region presumably target the virus particle and mediate strain-specific HIV-1 inhibition.
MATERIALS AND METHODS
Peptide design and synthesis.
Based on the GBV-C glycoprotein E2 sequence from a genotype 2a isolate (cloned by Xiang et al. [37], GenBank accession no. AF121950 nucleotides 1164 to 2184), we designed 20-mer peptides overlapping in 10 amino acids. The peptides were synthesized from EMC Microcollections (Tuebingen, Germany). They are N acetylated and high-pressure liquid chromatography-purified to a range of ≥90%. For screenings, the peptides were purified by precipitation. Peptide stocks were dissolved in 75% dimethyl sulfoxide-H2O and diluted for experiments in respective buffers or medium.
Isolation of PBMC.
A portion (25 ml) of EDTA blood (1:2 diluted in phosphate-buffered saline) was overlaid on 20 ml of Ficoll separation solution and centrifuged at 3,200 rpm for 20 min and decelerated without braking. After centrifugation, the lymphocyte band was transferred to fresh 40 ml of RPMI 1640 and centrifuged for additional 5 min at 300 × g. The supernatant was discarded, and the cells were washed twice with RPMI 1640. PBMC were cultured in 50% Panserin-401 (Pan Biotech, Germany), 40% RPMI 1640, and 10% fetal calf serum (FCS)-glutamine-gentamicin and then stimulated for 2 days with 10 U of interleukin-2 and 10 μg of phytohemagglutinin/ml.
HIV variants and virus stocks.
Primary HIV-1 isolates, laboratory-adapted strains, and simian immunodeficiency virus (SIV)/HIV-2 variants were obtained through the NIH AIDS Reagent Program and the German National Reference Center for Retroviruses, Institute for Virology, Erlangen, Germany. Virus stocks were generated on CEMx174-M7-R5 or PMBC. Cells were infected with infectious supernatants in 25-cm2 cell culture flasks (Greiner Bio-One, Germany) in respective media. Syncytium-inducing (SI) strains were controlled for cytopathic effect (CPE) and expanded to 75-cm2 cell culture flasks when a moderate CPE could be observed and harvested when a robust CPE indicated a strong infection. Non-syncytium-inducing (NSI) strains were expanded approximately 5 days after the initial infection and harvested between 7 to 10 days postinoculation. Cells were centrifuged for 5 min at 300 × g, and the supernatants were filtrated through 0.2-μm-pore-size disposable filters to obtain cell-free virus stocks. Aliquots were frozen at −80°C until usage.
Virus stocks for the HIV-1 virion-based fusion assay were generated on 293T cells. Cells were seeded in 10-cm dishes prior transfection and cotransfected with pNL4-3 proviral DNA (13 μg), pCMV-BlaM-Vpr (5 μg), and pAdVAntage vectors (2 μg). At day 2 after transfection, the virus-containing supernatant was centrifuged for 10 min at 300 × g to remove cellular debris. The HIV-1 virions containing the supernatant were overlaid onto 20% sucrose cushion and ultracentrifuged at 35,000 × g at 4°C for 90 min. The resulting pellet was resuspended in RPMI medium, and aliquots were frozen at −80°C until usage.
Generation of pseudotyped HIV reporter particles.
293T cells were cultured in Dulbecco modified Eagle medium (DMEM), along with 10% FCS, glutamine, and gentamicin. Cells were seeded in 25-cm2 cell culture flasks 2 days prior transfection and cotransfected with pNL4-3.luc.R-E- and the expression plasmids pADAenv, pHxB2env, or pHEF-VSV-Genv, respectively, coding for various HIV-1 or heterologous envelope proteins. Pseudotyped HIV reporter particles were secreted into the supernatant and harvested 2 days after transfection. To obtain cell-free infectious supernatants, the medium was filtered through 0.2-μm-pore-size disposable filters. The supernatants were stored in aliquots at −80°C until use.
Single-round of infection HIV replication assay.
CEMx174-M7-R5 cells were propagated in Panserin-401, 40% RPMI 1640, and 10% FCS supplemented with glutamine, and gentamicin. A total of 5 × 104 cells were seeded in round-bottom 96-well plates (Greiner Bio-One). Cells were infected with pseudotyped HIV reporter particles after the addition of 10 μM concentrations of the respective peptides. Infection was performed in 200 μl of medium. The replication efficiency could be quantified through measurement of the luciferase activity in cell lysates. Cells were lysed at 3 days postinfection and transferred to white flat-bottom 96-well plates (Corning, Germany). After the addition of the substrate luciferine to cell lysates, the luciferase activity was quantified in an Orion II microplate luminometer (Berthold Detection Systems, Germany). The HIV inhibition mediated by E2 peptides was calculated in relation to mock-incubated cells as the percent reduction of the luciferase activity in cell lysates.
HIV wild-type infections.
TZM-bl cells were cultured in DMEM–10% FCS-glutamine-gentamicin. A total of 104 cells were seeded 1 day prior infection in flat-bottom 96-well plates (Greiner Bio-One). TZM-bl cells were infected with various clinical and laboratory-adapted HIV-1, SIV, and HIV-2 strains, respectively, after the addition of various concentrations of peptides in a volume of 200 μl of medium. The replication efficiency was quantified at day 3 postinfection by determination of the luciferase activity in cell lysates. Data were obtained from three independent experiments performed in triplicate.
CEMx174-M7-R5 HIV-reporter cells that express exogenous CCR5 and cassettes for enhanced green fluorescent protein and luciferase under the control of the viral long terminal repeat (LTR) were cultured in 50% Panserin-401, 40% RPMI 1640, and 10% FCS-glutamine-gentamicin (12, 19). A total of 5 × 104 cells were seeded in round-bottom 96-well plates (Greiner Bio-One) and infected with various clinical HIV-1 isolates after the addition of 20 μM concentrations of each peptide in a volume of 200 μl of medium. Replication efficiency was quantified at day 3 postinfection by determination of the luciferase activity in cell lysates. Data were obtained from two independent experiments performed in triplicate.
Stimulated PBMC were cultured in 50% Panserin-401, 40% RPMI 1640, and 10% FCS-glutamine-gentamicin. A total of 2 × 105 PBMC were seeded in round-bottom 96-well plates (Greiner Bio-One) and infected with various clinical HIV-1 strains isolates after the addition of 20 μM concentrations of the respective E2 peptide in a volume of 200 μl of medium. Supernatants were harvested at days 4, 7, and 10 postinfection. The efficiency of infection was monitored on HIV-1 reporter cells (CEMx174-M7-R5) by the quantification of infectious particles in supernatants from the PBMC. The inhibition of HIV-1 replication in peptide-treated cells was calculated in relation to mock-treated cells. Data were obtained from two independent experiments performed in triplicate.
HIV-1 virion-based fusion assay.
The virion-based fusion assay was performed essentially as described elsewhere (6). In brief, 3 × 105 CEMx174-M7-R5 cells or 1 × 106 PBMC were infected with sucrose-purified HIV-1 NL4-3BlaM-Vpr virions, (5 ng of p24-Gag per approach). Infection was performed in 100 μl of medium for 4 h at 37°C in 96-well plates. The cells were washed with Hanks balanced salt solution (HBSS) to remove unbound virus particles and incubated with the CCF2-AM substrate as described by the manufacturer (Invitrogen, Germany). To constitute the loading solution, 2 μl of CCF2-AM was mixed with 8 μl of 0.1% acetic acid containing 0.1 μg of Pluronic-F127R/ml and 990 μl of HBSS. The cells were incubated in 100 μl of loading solution at room temperature overnight, harvested, and washed twice with HBSS. Finally, the cells were fixed in 1.2% paraformaldehyde. After cleavage by BlaM-Vpr, the emission change of CCF2-AM from 520 nm (uncleaved dye) to 447 nm (cleaved dye) was measured by flow cytometry using a BD LSR II flow cytometer (Becton Dickinson Biosciences, Germany) after excitation with 409 nm. Flow cytometer data were collected with a BD FACSDiva and analyzed with FCS Express V3.
To determine the inhibitory capacity of E2-derived peptides, 3 × 105 CEMx174-M7-R5 cells were incubated with 50 μM concentrations of each peptide or their respective controls, T-20 (10 μM), AMD-3100 (4 μM), or TAK-779 (40 μM) for 1 h at 37°C in a total volume of 100 μl of medium. After incubation, the cells were infected with NL4-3BlaM-Vpr, and the experiment was continued as described above. To analyze the cell binding of E2-derived peptides, 3 × 105 CEMx174-M7-R5 cells were incubated with 50 μM concentrations of each peptide and their respective controls (b12, 2G12, B4, and AMD-3100, each at 0.1 μg/μl) for 1 h at 37°C in 100 μl of medium. After incubation, the cells were centrifuged for 10 min at 1,500 rpm, washed with 200 μl of medium, and centrifuged for a further 10 min to remove unbound peptides or antibodies. Cells were infected with NL4-3BlaM-Vpr virions, and the assay was continued as described above. For determination of the putative binding of peptides to virus particles, sucrose-cushion-purified NL4-3BlaM-Vpr was incubated with 50 μM concentrations of the peptides and respective controls (12G5, 4E10, b12, and 2G12, each at 0.1 μg/μl) for 1 h at 37°C in 100 μl of medium. After incubation, the virus was centrifuged at 15,000 rpm for 30 min, washed with 1 ml of medium, and centrifuged for another 30 min at 15,000 rpm. A total of 3 × 105 CEMx174-M7-R5 cells were infected with washed virus particles for 4 h at 37°C, and the experiment was carried out as described above.
Determination of IC50.
TZM-bl cells were treated and infected as described above. The 50% inhibitory concentrations (IC50s) were calculated with SigmaPlot 11 (Systat Software GmbH, Germany).
RESULTS
Screening for HIV-inhibitory regions within GBV-C E2.
In order to perform an E2 peptide scanning to identify the region(s) responsible for E2-mediated HIV-1 inhibition, 20mer peptides overlapping in 10 amino acids (aa) were synthesized, representing the truncated soluble version of the GBV-C E2 protein (according to GenBank accession number AF121950) from aa 1 to 340 used in earlier studies (13) (Fig. 1). These peptides were evaluated in a single-round replication assay using HIV-1 reporter viruses (pNL4-3.luc.R-E-) pseudotyped with X4-tropic envelope protein derived from the laboratory HIV-1 strain HxB2. The lymphoid cell line CEMx174-M7-R5 that expresses the HIV-relevant receptors (CD4, CXCR4, CCR5) and an HIV-1 LTR-driven luciferase reporter was infected with HIV-1 particles, directly after addition of respective peptides (10 μM). The results of this initial screening are shown in Fig. 2. The data reveal an inhibitory effect on HIV-1 replication mediated by peptides P4 and P6 that represent the N terminus of GBV-C E2 from aa 31 to 70, whereas no or only a weak and unspecific impact on HIV replication could be observed with the remaining peptides. The same results have been obtained, when HIV-1 particles were preincubated with peptides for 1 h at 4°C and at 37°C, respectively, before inoculation of the target cells (data not shown). Cytotoxic effects that might lead to unspecific HIV-1 inhibition caused by peptides or by respective solvents could be excluded by using the CellTiter 96 AQueous One Solution cell proliferation assay (Promega GmbH, Germany; data not shown).
Fig. 1.
Genome organization of GB virus C (GBV-C) and schematic localization of E2-derived peptides. GBV-C contains a 5′ untranslated region (UTR), including an internal ribosomal entry site directing translation of the polyprotein that is cleaved by cellular signal peptidases and viral proteases. Ψ, predicted glycosylation sites in E2.
Fig. 2.
Identification of HIV-inhibitory peptides derived from GBV-C glycoprotein E2. CEMx174-M7-R5 cells were infected with HIV reporter particles pseudotyped with HIVHxB2 envelope (X4) after the addition of 10 μM concentrations of the respective peptides. The replication efficiency was quantified at day 3 postinfection by detection of the luciferase activity in cell lysates. The bars show the average values from at least four independent experiments.
To define the E2 region responsible for HIV-1 suppression more precisely, we synthesized 20mer peptides with an 18-aa overlap representing aa 23 to 78 of the GBV-C E2 N terminus. CEMx174-M7-R5 cells were infected with HxB2-enveloped HIV-1 reporter particles, using defined concentrations of 5 μM for the respective peptides. This analysis revealed that the 20mer peptides P4-4 to P6-5, representing the aa 29 to 72 within the GBV-C E2 protein, were able to inhibit HxB2 pseudotyped human immunodeficency (HI) viruses, whereas the peptides flanking this region showed no relevant HIV-inhibitory activity (Fig. 3A).
Fig. 3.
Definition of the HIV-inhibitory E2 region. (A) A panel of 20mer peptides with 18-aa overlaps representing the E2 region of aa 23 to 78 was designed. CEMx174-M7-R5 cells were infected with HIV reporter particles pseudotyped with HIVHxB2 envelope (X4) after the addition of 5 μM concentrations of the respective peptides. The replication efficiency was measured as described above. Average values of HIV inhibition of three independent experiments are shown. (B) Titration of the best HIV-inhibitory peptides. TZM-bl cells were infected with strains 92UG024 and RU570 after the addition of increasing amounts of peptides. The replication efficiency was measured at day 3 postinfection as described above. The graphs show the mean values from two independent experiments. The table indicates the IC50s in μM of the respective peptides.
We chose the best peptide candidates from that screening and determined the inhibitory potency (i.e., the IC50) on the HIV-susceptible reporter cell line (TZM-bl) using two clinical HIV-1 isolates with either X4 or R5 tropism. The data revealed that both clinical HIV-1 isolates were potently suppressed by these E2-derived peptides. In particular, the peptide P4-7 ranging from aa 37 to 56 was ∼0.5 log more potent than the original peptide P4 (aa 31 to 50). Comparable to P4-7, an IC50 between 1 and 4 μM could be determined for peptides P6-1 (aa 43 to 62), P6-2 (aa 45 to 64), and P6-3 (aa 47 to 66), being slightly more potent than peptides P6-4 (aa 49 to 68) and P6 (aa 51 to 70) (Fig. 3B). In summary, these data imply that the N-terminal part of the GBV-C E2 protein ranging from aa 29 to 72 is involved in HIV-1 suppression and that peptides representing the core region from aa 37 to 66 appear to be most potent to mediate HIV-1 replication inhibition.
It is noteworthy that, within this core region, three cysteine residues are present, raising the question whether the formation of intra- or intermolecular disulfide bonds might play an essential role in HIV-1 suppression. Therefore, we exchanged in peptide P4-7 and P6-2 the existing cysteine into serine residues. To further prove the specificity of those peptides, we additionally synthesized scrambled peptide variants of P4-7 and P6-2, where the respective amino acid sequence was arranged randomly (Table 1). For each peptide the IC50s were determined with two laboratory HIV-1 strains representing both coreceptor tropisms. As shown in Table 2, the change of cysteine residues to serine reduced the potency of the respective peptides remarkably. Up to the highest concentration of 100 μM used in our analysis, no residual inhibitory activity was detectable. These findings argue on one hand for the relevance of the cysteine-derived sulfhydryl groups themselves within the respective E2 peptides. On the other hand, this might suggest that the formation of intermolecular dimers or oligomers or the formation of cyclic peptide structures is a prerequisite for the observed E2-peptide-mediated HIV-1 suppression mechanism. When we tested the HIV-inhibitory potency of peptides with randomly arranged amino acid sequence, we observed discrepant results for NL4-3 and YU-2. Whereas the IC50s for NL4-3 increased above 100 μM, the values for YU-2 increased just between 1 and 2 orders of magnitude in comparison to the parental peptides. Both peptides P4-7 and P6-2 have a high content of hydrophobic residues of 60%. Apparently, for the HIV-1 isolate YU-2 the general distribution of nonpolar molecules seems to be sufficient to retain a residual inhibitory activity. However, the molecular reason for this phenomenon remains to be determined.
Table 1.
Sequences of HIV-inhibitory E2-derived peptides
| Peptide | Residue positions | Sequence |
|---|---|---|
| P4 | 31–50 | Ac-PTGERVWDRGNVTLLCDCPN |
| P4-7 | 37–56 | Ac-WDRGNVTLLCDCPNGPWVWV |
| P4-7s | 37–56 | Ac-WDRGNVTLLSDSPNGPWVWV |
| P4-7scr | 37–56 | Ac-LNWGTPDWDVRNCVGVLWCP |
| P5 | 41–60 | Ac-NVTLLCDCPNGPWVWVPAFC |
| P6-2 | 45–64 | Ac-LCDCPNGPWVWVPAFCQAVG |
| P6-2s | 45–64 | Ac-LSDSPNGPWVWVPAFSQAVG |
| P6-2scr | 45–64 | Ac-PLCVNCWPQVCGDFPWGAVA |
| P6 | 51–70 | Ac-GPWVWVPAFCQAVGWGDPIT |
| P4762 | 37–64 | Ac-WDRGNVTLLCDCPNGPWVWVPAFCQAVG |
| P28 | 271–290 | Ac-TEVSEALGGAGLTGGFYEPL |
Table 2.
IC50s of modified E2-derived peptides
| Peptide | IC50 (μM) |
|
|---|---|---|
| NL4-3 | YU-2 | |
| P4-7 | 3.0 | 5.2 |
| P6-2 | 2.3 | 2.4 |
| P4-7s | >100 | >100 |
| P6-2s | >100 | >100 |
| P4-7scr | >100 | 86.5 |
| P6-2scr | >100 | 23.5 |
Breadth and potency of E2-derived peptides.
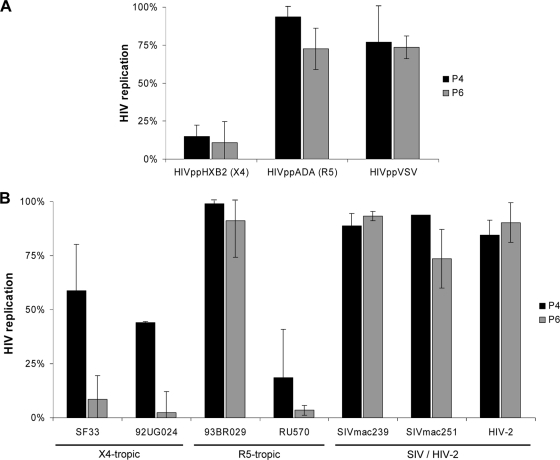
In the following, the antiretroviral activity of the E2-derived peptides P4 and P6 was tested with HIV particles carrying the X4-tropic HIV-1 envelope HxB2, the R5-tropic envelope ADA, and the heterologous envelope protein G derived from vesicular stomatitis virus (VSV-G). A defined concentration (10 μM) of peptides P4 and P6 was added shortly before infection of CEMx174-M7-R5 cells, and the replication efficiency was measured 3 days postinfection as described above. It should be noted that the respective E2-derived peptides were able to inhibit HxB2 viruses but failed to mediate substantial inhibition of ADA or VSV-G pseudotyped viruses (Fig. 4A). Since these HIV reporter viruses differ only in the envelope proteins, these data imply that the viral envelope determines the sensitivity to E2 peptides.
Fig. 4.
Activity spectrum of inhibitory peptides. (A) CEMx174-M7-R5 cells were infected with various pseudotyped HIV reporter particles (HxB2env [X4], ADAenv [R5], and VSV-Genv) after the addition of inhibitory peptides P4 and P6, respectively. The replication efficiency was quantified as described previously. HIV replication in peptide-treated cultures was calculated as the reduction in luciferase activity in cell lysates compared to mock-incubated cells. The bars show average values from three independent experiments. (B) TZM-bl cells were infected with different clinical HIV-1, SIV, and HIV-2 strains.
To further analyze the differences in sensitivity to retroviral isolates, clinical and laboratory HIV and SIV strains were used to infect TZM-bl cells in the presence or absence of P4 or P6, respectively. The results confirmed the selective inhibitory activity of the E2-derived peptides. Whereas most of the HIV-1 clinical isolates were sensitive toward P6 and to a lesser extent toward P4 peptides, one clinical R5-tropic isolate (93BR029) was not sensitive to both peptides. Furthermore, no replication suppression could be observed with both E2-derived peptides when HIV-2 and SIV isolates were used for infection (Fig. 4B).
To determine the breadth and potency of the E2-derived peptides more precisely, the IC50s were determined with a panel of fully replicative clinical and laboratory HIV-1 isolates ranging from clades A to H. Again, we included HIV-2 and SIV isolates in these titrations. We tested the more potent peptides P4-7 and P6-2; the peptide P5, which is located between P4-7 and P6-2; and a longer 28mer peptide, representing the region covered by P4-7 and P6-2 (P4762). For comparison, VIRIP-576 (VIR-576) was used as a positive control. Representative IC50 titrations are shown in Fig. 5, and the complete data set is summarized in Table 3. The results from this analysis revealed remarkable differences in E2-derived peptide sensitivity between the tested retroviral isolates. As expected, all tested E2-derived peptides and VIR-576 did not show any antiviral activity against SIVmac251 and HIV-2mir when concentrations of up to 100 μM of the respective peptide were applied. However, using a panel of different primary and laboratory-adapted HIV-1 isolates ranging from clades A to H, only 9 of 13 tested HIV-1 isolates were highly or partly sensitive to the E2-derived peptides. The nonsensitive HIV-1 strains (92TH026, 92BR025, 92UG035, and 93BR029) were not inhibited by peptide concentrations up to 100 μM. These HIV-1 isolates were all R5-tropic and belong to clades B, C, D, and F. Nevertheless, the clade origin does not seem to determine the sensitivity toward E2-derived peptides, since other representatives from clades B, D, and F appear to be sensitive to peptide-mediated HIV-1 suppression. With respect to coreceptor tropism and E2-peptide sensitivity, we also observed an inconsistent pattern. All X4-tropic HIV-1 strains could be suppressed by the respective peptides, with the restriction that one isolate (92BR020) was only partially sensitive. In the group of R5-tropic and X4/R5-dualtropic HIV-1 strains, four of the eight tested isolates were highly or at least moderately sensitive, whereas four isolates were not susceptible for E2-peptide-mediated suppression at all, when peptides at a concentration of up to 100 μM were used. Therefore, the coreceptor tropism does not clearly determine the sensitivity of HIV-1 strains to E2 peptides. The antiviral potency of the HIV-inhibitory E2-derived peptides varied between the peptides and the HIV-1 isolates that were tested, although the pattern of susceptibility of different HIV-1 isolates was remarkably similar. This complete concordance might be explained by the fact that all E2-derived peptides used in these experiments overlap with 12 aa with each other (LCDCPNGPWVWV), representing the aa 45 to 56 within the E2 protein. However, the most potent E2-derived peptides appear to be the 20mer P6-2 (aa 45 to 64) and the 28mer P4762 (aa 37 to 64). For these, the IC50s ranged from <0.1 to ∼2 μM for susceptible HIV-1 strains and were in some degree comparable to the activity of VIR-576 in our settings. In addition, we tested the GBV-C E2-derived peptide P28 (aa 271 to 290) for antiretroviral activity with selected primary HIV-1 isolates. P28 represents the E2 region, which was postulated by Herrera et al. to interact with the HIV-1 fusion peptide (10). However, we did not observe any anti-HIV-1 activity using 100 μM this GBV-C E2-derived peptide (Table 3).
Fig. 5.
Titration curves of different HIV subtypes. TZM-bl cells were infected with different clinical and laboratory-adapted HIV-1, SIV, and HIV-2 strains, respectively, after the addition of increasing amounts of respective peptides. The replication efficiency was quantified as described previously. The graphs show average values from at least three independent experiments, each performed in triplicate. Concentrations are given in μM.
Table 3.
IC50s of E2-derived peptides
| HIV strain | Tropism | Clade | IC50 (μM) of peptide: |
|||||
|---|---|---|---|---|---|---|---|---|
| VIR-576 | P4-7 | P5 | P6-2 | P4762 | P28 | |||
| 92UG029 | X4 | A | NDa | 0.76 | 5.05 | 1.44 | 0.85 | ND |
| NL4-3 | X4 | B | 0.11 | 3.01 | 5.72 | 2.30 | 1.03 | >100 |
| SF33 | X4 | B | ND | 10.21 | 3.84 | 0.96 | 1.92 | ND |
| YU-2 | R5 | B | 0.55 | 5.21 | 7.30 | 2.39 | 1.97 | >100 |
| JRCSF | R5 | B | ND | 98.68 | 85.08 | >100 | >100 | ND |
| 92TH026 | R5 | B | ND | >100 | >100 | >100 | >100 | ND |
| 92BR025 | R5 | C | ND | >100 | >100 | >100 | >100 | ND |
| 92UG035 | R5 | D2 | ND | >100 | >100 | >100 | >100 | ND |
| 92UG024 | X4 | D3 | 0.31 | 0.33 | 0.71 | 0.08 | 0.13 | >100 |
| 93BR029 | R5 | F1 | ND | >100 | >100 | >100 | >100 | ND |
| 93BR020 | X4 | F2 | ND | >100 | 94.35 | >100 | 20.78 | ND |
| RU570 | R5 | G | 21.82 | 0.74 | 1.96 | 0.58 | 0.09 | >100 |
| V1557 | X4/R5 | H | ND | 72.23 | 80.54 | >100 | >100 | ND |
| SIVmac251 | >100 | >100 | >100 | >100 | >100 | ND | ||
| HIV-2 | >100 | >100 | >100 | >100 | >100 | ND | ||
ND, not determined.
To further address the relevance of the observed variability in sensitivity of HIV-1 primary isolates to GBV-C E2-derived peptides and to exclude the possibility that the cell line used in our HIV replication assays may account for these variations, we also tested the anti-HIV-1 activity of P6-2 with a selection of primary HIV-1 strains on the lymphoid HIV reporter cell line CEMx174-M7-R5 and on PBMC. A defined concentration (20 μM) of peptide P6-2 was added shortly before infection, and the replication efficiency was compared to mock-treated cells measured 3 days postinfection on CEMx174-M7-R5 cells and 7 or 10 days postinfection on PBMC. The results are summarized in Table 4. We define here the reduction of replication efficiency (>70%) as “+” and, accordingly, as “−” when preincubation with P6-2 had no substantial impact on the HIV-1 replication. In addition, we compared the influence of GBV-C coinfection on a panel of HIV-1 isolates evaluated in a previous study (14). The data revealed almost the same pattern of variability in sensitivity for the tested HIV-1 strains toward P6-2-mediated HIV-1 replication suppression on the two reporter cell lines and on PBMC. In comparing the replication inhibition on TZM-bl and CEMx174-M7-R5 cells, we found that all HIV-1 isolates were congruently responsive or nonresponsive to E2-derived peptide-mediated HIV-1 suppression using 20 μM P6-2. However, on primary cells (PBMC) we observed that one HIV-1 isolate was suppressible that was not sensitive on TZM-bl or CEMx174-M7-R5 cells. In combination with these findings, we were able to show in three independent replication assays that GBV-C E2-derived peptides are able to inhibit the replication of primary HIV-1 isolates in a strain-specific manner. However, this observation is partly influenced by the cell type used for HIV-1 infection. In contrast, in GBV-C coinfections performed on PBMC in a previous study, all tested HIV-1 strains were suppressible. This implies that the HIV-1 inhibition mediated by the GBV-C E2-derived peptides does not reflect all interference mechanisms between the replication of GBV-C and HIV-1.
Table 4.
Reactivity of HIV-1 strains toward E2 peptide treatment compared to the reactivity in HIV-GBV-C coinfection assays
| HIV strain | Clade | Tropism | Reactivity of HIV strains with P6-2 on different cell typesa |
Reactivity of HIV strains in GBV-C coinfection assaysb | ||
|---|---|---|---|---|---|---|
| TZM-bl | CEMx174-M7-R5 | PBMC | ||||
| 92UG029 | A | X4 | + | + | + | + |
| 92UG024 | D3 | X4 | + | + | + | + |
| RU570 | G | R5 | + | + | + | + |
| 92TH026 | B | R5 | – | – | + | + |
| 93BR020 | F2 | X4 | – | – | – | + |
| JRCSF | B | R5 | – | – | – | ND |
+, Inhibition of HIV replication >70%; –, inhibition of HIV replication <30%.
As reported by Jung et al. (14). ND, not determined.
Elucidation of the HIV-inhibitory mechanism mediated by E2-derived peptides.
To identify the mode of action of GBV-C E2-derived peptides mediating HIV-1 inhibition, an HIV-1 virion-based fusion assay was performed. This assay allows exclusively the detection of early steps within the HIV-1 life cycle by measuring a shift in the fluorescence due to cleavage of a substrate (CCF2-AM) by the β-lactamase–Vpr chimeric protein present in HIV BlaM-Vpr particles (6). We used NL4-3BlaM-Vpr viruses to infect PBMC as well as CEMx174-M7-R5 cells. After virus fusion, the cells were stained with the substrate CCF2-AM, and enzymatic processing of the substrate was monitored via flow cytometry. Comparable to the known entry inhibitors T20 and AMD-3100, the peptides derived from the N-terminal part of E2 (P4, P4-7, P6, and P6-2) prevented virus entry efficiently, whereas P28 and P29 that represent the E2 region proximal the transmembrane anchor did not inhibit HIV-1 infection (Fig. 6). Identical results were obtained using R5-tropic NL4-3BlaM-Vpr virions in this fusion assay (data not shown). Therefore, HIV-inhibitory E2-derived peptides reflecting the N terminus of E2 interfere exclusively with HIV-1 binding or membrane fusion but not with postfusion events.
Fig. 6.
E2-derived peptides inhibit HIV entry. The binding and subsequent membrane fusion of HIV was determined by a virion-based fusion assay, and the inhibitory capacity of peptides on HIV entry was analyzed. (A and B) PBMC (A) and CEMx174-M7-R5 cells (B) were infected with NL4-3BlaM-Vpr virions after the addition of E2-derived peptides or the entry inhibitors T-20 and AMD3100. HIV-cell fusion was determined by flow cytometry; infected cells are depicted in blue. Fusion analyses with PBMC from two different donors were performed in duplicate in five independent experiments; fusion analyses with CEMx174-M7-R5 were performed in triplicate in five independent experiments. For each cell type, the results of one representative experiment are shown.
Next, we wanted to evaluate the binding target of the HIV-inhibitory peptides. To differentiate whether the peptides P4 and P6 mediate HIV-1 replication inhibition via binding to the HI virion or to a cellular structure, E2-derived peptides, respective control antibodies, or substances with defined cellular or viral targets were either preincubated with CEMx174-M7-R5 cells or with sucrose-cushion-purified HIV-1 particles (NL4-3BlaM-Vpr). Prior infection respective cells or viruses were washed intensively to remove unbound substances. As shown in Fig. 7, HIV-1 entry could not be prevented when cells were preincubated with E2-derived peptides. After the removal of unbound peptides no residual HIV-inhibitory activity could be detected (Fig. 7A), whereas E2-derived peptides still retain their anti-HIV-1 activity after incubation with HI viruses when unbound peptides were removed by two sequential washing and high-speed centrifugation procedures (30 min, 15,000 rpm). Under these conditions the HIV-1 neutralizing antibody 12G5 that binds to the cellular receptor CXCR4 had no residual HIV-inhibitory effect (Fig. 7B), whereas the HIV-1 particle-binding anti-gp41 antibody 4E10 and the anti-gp120 antibodies b12 and 2G12 still led to HIV-1 suppression. Therefore, we assume that the E2-derived peptides do not target HIV-1 host cells but supposedly interact with the HIV-1 particle itself.
Fig. 7.
E2-derived peptides prevent HIV entry by targeting the virus particle. (A) CEMx174-M7-R5 cells were incubated with the respective peptides or controls for 1 h, intensively washed, and infected with NL4-3BlaM-Vpr. The anti-gp120 antibodies b12 and 2G12 served as negative controls and the anti-CD4 antibody B4 and the CXCR4-agonist AMD-3100 served as positive controls for cell binding. (B) NL4-3BlaM-Vpr virus particles were incubated with inhibitory peptides or control antibodies for 1 h, intensively washed, and used to infect CEMx174-M7-R5 cells. The anti-CXCR4 antibody 12G5 was used as a negative control and the anti-gp120 antibodies b12 and 2G12 and the anti-gp41 antibody 4E10 served as positive controls for virus binding. Virus-cell fusion was determined by flow cytometry. Percentages were calculated in relation to mock-treated cells. Columns show average values from two independent experiments, each performed in triplicate.
DISCUSSION
In this study, we demonstrate that peptides representing a distinct region within the N terminus of the GBV-C E2 protein are able to inhibit efficiently HIV-1 replication in vitro. The responsible region is located between aa 29 and 72 of the E2 protein, whereas peptides representing the E2 region between aa 45 to 64 (P6-2) and aa 37 to 64 (P4762), respectively, showed the highest potency in an HIV replication assay on TZM-bl cells. Depending on the HIV-1 isolate, the potency (IC50) of these two E2-derived peptides varied between approximately 2 and 0.1 μM. Even though, the E2 peptides are less potent than the entry inhibitor T-20 or the derivative T-1249, their potency against primary HIV-1 isolates is comparable to that of VIR-576. This peptide is a 2 orders of magnitude more potent derivative of the HIV-1 virus-inhibitory peptide (VIRIP) that corresponds to the C-proximal region of the most abundant circulating serine protease inhibitor α1-antitrypsin and is reported to possibly be involved in early innate immune defense against HIV-1 infection (15, 21).
The antiviral activity of peptides derived from the N terminus of E2 seems to be restricted to HIV-1 isolates since HIV-2mir, SIVmac251, and SIVmac239 strains were not sensitive to high concentrations of the respective peptides (up to 100 μM). However, using a panel of different primary and laboratory-adapted HIV-1 isolates ranging from clades A to H, a highly reduced responsiveness against E2-mediated entry inhibition could be observed for some HIV-1 isolates as well. E2 responsiveness does not depend on the coreceptor usage or on the clade of the respective HIV-1 isolates, even though X4-tropic isolates are more likely to be suppressed by E2-derived peptides than R5-tropic HIV-1 strains. The variability in sensitivity of primary HIV-1 strains could be observed in three different cell types. However, on PBMC the E2 peptides seem to be able to inactivate HIV-1 isolates that are not suppressible on HIV-1 reporter cell lines. Therefore, these results imply that the sensitivity to E2 peptides is not only determined by the HIV-1 isolate itself but also by the interaction of the HIV-1 isolate with the host cell. Furthermore, when we compare the effect of GBV-C coinfection on HIV-1 replication with the E2 peptide effect, we observe that GBV-C coinfection led to the suppression of a broader range of HIV-1 isolates. This implies that the HIV-1 suppression mediated by the N-terminal part of the E2 does not reflect all interference mechanisms of GBV-C against HIV-1. One possible explanation could be the assumption that more E2 regions than only the N-terminal part between aa 29 and 72 are necessary to provide full HIV-1 interference. Not surprisingly, the effect mediated by E2 peptides is not fully identical in quantitative and qualitative concerns to the HIV-inhibitory effect induced by the recombinant truncated E2 protein (E2340Fc) used in earlier studies. Recombinant E2 protein is approximately 2 orders of magnitude more potent compared to P4-7 or P6-2, it is effective toward R5-tropic HIV-1 reporter viruses pseudotyped with the ADA envelope, and preincubation of cells is sufficient to induce the HIV-inhibitory effect, while with E2-derived peptides only preincubation with virus led to HIV-1 inhibition (13). Despite the fact that recombinant E2 protein and E2-derived peptides both interfere with early HIV-1 entry events, 20mer peptides derived from the N terminus of the E2 protein apparently do not mediate the full “E2 effect.” We cannot exclude the possibility that a three-dimensional protein structure within the native GBV-C E2 protein is important for the E2-mediated HIV-1 entry inhibition that might not be represented by a single peptide. Notably, Herrera et al. could identify several regions within the GBV-C E2 protein that are supposed to interact with the fusion peptide of HIV-1 and thereby mediate antiretroviral activity in HIV-1 replication assays (11). In our study we screened for antiretroviral activity of peptides that exclusively represent the GBV-C E2 protein without the transmembrane anchor (aa 1 to 340) in a therapeutically relevant concentration (10 μM). We can confirm the N-terminal part of E2 being involved in HIV-1 entry inhibition, but we did not observe any other E2-derived peptide sequences that confer potent inhibition in HIV replication assays. Another explanation of why infection with GBV-C isolates leads to suppression of a broader range of HIV-1 isolates might be due to the fact that clinical GBV-C isolates mediate HIV-1 interference not only via the E2 protein. This is supported by earlier studies, where it has been reported that GBV-C infection stimulates the induction of HIV-inhibitory chemokines in primary lymphocytes and that the expression of GBV-C NS5A in lymphoid cell lines can efficiently reduce HIV-1 susceptibility (14, 34, 35).
During the budding process retroviruses acquire a lipid envelope derived from the plasma membrane of the host cell that contains mainly cellular lipids, viral envelope, and cellular surface proteins (5, 23). Therefore, the envelope composition of retroviral particles reflects in part the cellular origin. However, the parental cells or cell lines from which the HIV-1 virus stocks were harvested do not contribute to the variability in E2 peptide sensitivity. The E2 responsiveness of HIV-1 virus stocks did not change when viruses had been produced either on PBMC or on CEMx174-M7-R5 cells. In addition, we could observe both effects with HIV reporter particles that were harvested exclusively from 293T cells. These HIV reporter particles differ only in the incorporated gp160 envelope. Therefore, the E2 peptide sensitivity seems to be determined by the HIV-1 envelope protein. To analyze the primary structure of the respective envelope proteins, gp160 amino acid sequences from all tested HIV-1 isolates were taken from the GenBank database and aligned using CLUSTAL W software (version 1.83; Swiss Institute of Bioinformatics, Switzerland [http://www.ch.embnet.org/software/clustalw.html]). However, we could not identify any specific mutations or amino acid motifs in gp120 or gp41 that were different in E2-responsive versus nonresponsive HIV strains (data not shown), even though we cannot exclude any structural differences in the respective envelope variants or differences in HIV-1 entry kinetics that might refer to E2 responsiveness. The entry process of HIV-1 is a complex, multistaged process, in which subsequent binding events and conformational changes occur, leading to specific time windows for entry interference. Differences in receptor or coreceptor specificity can lead to diverse dynamics of the HIV-1 entry process, conceivably leading to variations in sensitivity to E2-derived peptides, as described for other known entry inhibitors. For example, for T-20, a fusion inhibitor known to interact with an N-terminal heptad repeat (HR1) within gp41, it has been shown that primary HI viruses exhibit substantial variability in T-20 sensitivity and that different coreceptor specificities and other determinants outside of HR1 can affect this sensitivity (7, 24).
We could demonstrate that GBV-C peptides interfere with very early events of the HIV-1 replication cycle, such as receptor binding to CD4 and the CXCR4 or CCR5 coreceptor or fusion between the viral and cellular membrane. These events in early HIV-1 replication are possibly affected by interaction of the E2-derived peptides with the HIV-1 particle. A direct interaction with receptors of the HIV-1 target cell, as it is described for a variety of small molecule receptor antagonists (30), could be excluded, since preincubation of HIV-1 target cells with HIV-inhibitory E2-peptides and subsequent removal of unbound peptides led to the loss of antiviral activity, whereas the antiviral ability of the peptides could be retained after preincubation and intensive washing of HIV-1 particles. Nevertheless, up to this point we cannot distinguish between the two possibilities that the N-terminal region of GBV-C E2 glycoprotein directly binds to HI virions and sterically hinders the subsequent entry process or whether the HIV-1 virus particle is irreversibly altered by the contact with GBV-C E2, as is described for sCD4 or its derivatives (2, 25).
GBV-C E1 and E2 glycoproteins are believed to be present on virions, and GBV-C virus titers can be observed at up to 107 to 108 genome equivalents per ml of plasma in GBV viremic HIV-1 patients (14, 29). However, for GBV-C no experimental data are available addressing the E2 density on GBV-C particles or the existence of free E2 protein that is not associated with GBV-C RNA. Therefore, up to this point we cannot exclude the possibility that native GBV-C E2 protein is biologically relevant for the GBV-C/HIV-1 interference. Therefore, the further elucidation of the exact mechanism by which GBV-C E2-derived peptides interfere with HIV-1 entry may not only lead to new insights into viral interference mechanisms but may also lead to new therapeutic strategies for the treatment of HIV-1 infections.
ACKNOWLEDGMENTS
This study was supported by grants of the Interdisziplinäres Zentrum für Klinische Forschung Teilprojekt B16 (Y.K. and H.W.), Graduiertenkolleg 1071 Teilprojekt A5 (K.E.), and the Akademie der Wissenschaften und Literatur zu Mainz Project 2 1.223 (H.R.).
We thank Jan Münch and Frank Kirchhoff (University of Ulm, Ulm, Germany) for providing VIR-576, Carsten Münk (University Hospital Düsseldorf, Düsseldorf, Germany) for providing the BlaM-Vpr expression plasmid, and Jutta Eichler and Marek Mössl (Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Germany) and Heinrich Sticht (Department of Biochemistry/Bioinformatics, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Germany) for helpful discussions and technical support.
Viral stocks and CEMx174-M7-R5 cells were obtained through the German National Reference Center for Retroviruses, Institute for Virology, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Germany. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc.; HIV-1 gp120 monoclonal antibody (IgG1 b12) from Dennis Burton and Carlos Barbas; HIV-1 gp120 monoclonal antibody (2G12) and HIV-1 gp41 monoclonal antibody (4E10) from Hermann Katinger; CXCR4 monoclonal antibody (1265) from James Hoxie; cell surface CD4 complex monoclonal B4 from United Biomedical, Inc.; and Bicyclam JM-2987 (a hydrobromide salt of AMD-3100) and T-20 fusion inhibitor from Roche.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Alter H. J. 1997. G-pers creepers, where'd you get those papers? A reassessment of the literature on the hepatitis G virus. Transfusion 37:569–572 [DOI] [PubMed] [Google Scholar]
- 2. Arthos J., et al. 2002. Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein: implications for therapeutic and vaccine strategies. J. Biol. Chem. 277:11456–11464 [DOI] [PubMed] [Google Scholar]
- 3. Birk M., Lindback S., Lidman C. 2002. No influence of GB virus C replication on the prognosis in a cohort of HIV-1-infected patients. AIDS 16:2482–2485 [DOI] [PubMed] [Google Scholar]
- 4. Bjorkman P., et al. 2004. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS 18:877–886 [DOI] [PubMed] [Google Scholar]
- 5. Cantin R., Methot S., Tremblay M. J. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79:6577–6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cavrois M., de Noronha C., Greene W. C. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151–1154 [DOI] [PubMed] [Google Scholar]
- 7. Derdeyn C. A., et al. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358–8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feucht H. H., et al. 1997. Prevalence of hepatitis G viremia among healthy subjects, individuals with liver disease, and persons at risk for parenteral transmission. J. Clin. Microbiol. 35:767–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. George S. L., Xiang J., Stapleton J. T. 2003. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology 316:191–201 [DOI] [PubMed] [Google Scholar]
- 10. Herrera E., Gomara M. J., Mazzini S., Ragg E., Haro I. 2009. Synthetic peptides of hepatitis G virus (GBV-C/HGV) in the selection of putative peptide inhibitors of the HIV-1 fusion peptide. J. Phys. Chem. B 113:7383–7391 [DOI] [PubMed] [Google Scholar]
- 11. Herrera E., et al. 2010. Effect of synthetic peptides belonging to E2 envelope protein of GB virus C on human immunodeficiency virus type 1 infection. J. Med. Chem. 53:6054–6063 [DOI] [PubMed] [Google Scholar]
- 12. Hsu M., et al. 2003. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J. Virol. 77:989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung S., et al. 2007. HIV entry inhibition by the envelope 2 glycoprotein of GB virus C. AIDS 21:645–647 [DOI] [PubMed] [Google Scholar]
- 14. Jung S., et al. 2005. Inhibition of HIV strains by GB virus C in cell culture can be mediated by CD4 and CD8 T-lymphocyte derived soluble factors. AIDS 19:1267–1272 [DOI] [PubMed] [Google Scholar]
- 15. Kramer H. B., et al. 2010. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 6:e1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larios C., et al. 2005. Characterization of a putative fusogenic sequence in the E2 hepatitis G virus protein. Arch. Biochem. Biophys. 442:149–159 [DOI] [PubMed] [Google Scholar]
- 17. Lefrere J. J., et al. 1999. Prevalence of GB virus type C/hepatitis G virus RNA and of anti-E2 in individuals at high or low risk for blood-borne or sexually transmitted viruses: evidence of sexual and parenteral transmission. Transfusion 39:83–94 [DOI] [PubMed] [Google Scholar]
- 18. Lefrere J. J., et al. 1999. Carriage of GB virus C/hepatitis G virus RNA is associated with a slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in coinfected persons. J. Infect. Dis. 179:783–789 [DOI] [PubMed] [Google Scholar]
- 19. Marzi A., et al. 2007. Modulation of HIV and SIV neutralization sensitivity by DC-SIGN and mannose-binding lectin. Virology 368:322–330 [DOI] [PubMed] [Google Scholar]
- 20. Mazzini S., et al. 2007. 3D-Structure of the interior fusion peptide of HGV/GBV-C by 1H NMR, CD, and molecular dynamics studies. Arch. Biochem. Biophys. 465:187–196 [DOI] [PubMed] [Google Scholar]
- 21. Munch J., et al. 2007. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell 129:263–275 [DOI] [PubMed] [Google Scholar]
- 22. Nattermann J., et al. 2003. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS 17:1457–1462 [DOI] [PubMed] [Google Scholar]
- 23. Ott D. E. 2008. Cellular proteins detected in HIV-1. Rev. Med. Virol. 18:159–175 [DOI] [PubMed] [Google Scholar]
- 24. Reeves J. D., et al. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. U. S. A. 99:16249–16254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schon A., et al. 2006. Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. Biochemistry 45:10973–10980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schroter M., Feucht H. H., Schafer P., Zollner B., Laufs R. 1999. GB virus C/hepatitis G virus infection in hemodialysis patients: determination of seroprevalence by a four-antigen recombinant immunoblot assay. J. Med. Virol. 57:230–234 [DOI] [PubMed] [Google Scholar]
- 27. Simons J. N., et al. 1995. Isolation of novel virus-like sequences associated with human hepatitis. Nat. Med. 1:564–569 [DOI] [PubMed] [Google Scholar]
- 28. Stapleton J. T., Williams C. F., Xiang J. 2004. GB virus type C: a beneficial infection? J. Clin. Microbiol. 42:3915–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tillmann H. L., et al. 2001. Infection with GB virus C and reduced mortality among HIV-infected patients. N. Engl. J. Med. 345:715–724 [DOI] [PubMed] [Google Scholar]
- 30. Tilton J. C., Doms R. W. 2010. Entry inhibitors in the treatment of HIV-1 infection. Antivir. Res. 85:91–100 [DOI] [PubMed] [Google Scholar]
- 31. Toyoda H., Fukuda Y., Hayakawa T., Takamatsu J., Saito H. 1998. Effect of GB virus C/hepatitis G virus coinfection on the course of HIV infection in hemophilia patients in Japan. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 17:209–213 [DOI] [PubMed] [Google Scholar]
- 32. Van der Bij A. K., et al. 2005. GB virus C coinfection and HIV-1 disease progression: the Amsterdam Cohort Study. J. Infect. Dis. 191:678–685 [DOI] [PubMed] [Google Scholar]
- 33. Williams C. F., et al. 2004. Persistent GB virus C infection and survival in HIV-infected men. N. Engl. J. Med. 350:981–990 [DOI] [PubMed] [Google Scholar]
- 34. Xiang J., et al. 2004. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1α, MIP-1β, and SDF-1. Lancet 363:2040–2046 [DOI] [PubMed] [Google Scholar]
- 35. Xiang J., McLinden J. H., Chang Q., Kaufman T. M., Stapleton J. T. 2006. An 85-aa segment of the GB virus type C NS5A phosphoprotein inhibits HIV-1 replication in CD4+ Jurkat T cells 2. Proc. Natl. Acad. Sci. U. S. A. 103:15570–15575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiang J., et al. 2001. Effect of coinfection with GB virus C on survival among patients with HIV infection. N. Engl. J. Med. 345:707–714 [DOI] [PubMed] [Google Scholar]
- 37. Xiang J., Wunschmann S., Schmidt W., Shao J., Stapleton J. T. 2000. Full-length GB virus C (hepatitis G virus) RNA transcripts are infectious in primary CD4-positive T cells. J. Virol. 74:9125–9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiang J. H., McLinden J. H., Chang Q., Jordan E. L., Stapleton J. T. 2008. Characterization of a peptide domain within the GB virus C NS5A phosphoprotein that inhibits HIV replication. PLoS One 3:e2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeo A. E., et al. 2000. Effect of hepatitis G virus infection on progression of HIV infection in patients with hemophilia. Ann. Intern. Med. 132:959–963 [DOI] [PubMed] [Google Scholar]
- 40. Yeo A. E., Matsumoto A., Shih J. W., Alter H. J., Goedert J. J. 2000. Prevalence of hepatitis G virus in patients with hemophilia and their steady female sexual partners. Sex. Transm. Dis. 27:178–182 [DOI] [PubMed] [Google Scholar]