Abstract
With the recent demonstration in the RV144 Thai trial that a vaccine regimen that does not elicit neutralizing antibodies or cytotoxic T lymphocytes may confer protection against human immunodeficiency virus type 1 (HIV-1) infection, attention has turned to nonneutralizing antibodies as a possible mechanism of vaccine protection. In the current study, we evaluated the kinetics of the antibody-dependent cell-mediated cytotoxicity (ADCC) response during acute and chronic SIVmac251 infection of rhesus monkeys. We first adapted a flow cytometry-based ADCC assay, evaluating the use of different target cells as well as different strategies for quantitation of activated natural killer (NK) cells. We found that the use of SIVmac251 Env gp130-coated target cells facilitates analyses of ADCC activity with a higher degree of sensitivity than the use of simian immunodeficiency virus (SIV)-infected target cells; however, the kinetics of the measured responses were the same using these different target cells. By comparing NK cell expression of CD107a with NK cell expression of other cytokines or chemokine molecules, we found that measuring CD107a expression is sufficient for evaluating the anti-SIV function of NK cells. We also showed that ADCC responses can be detected as early as 3 weeks after SIVmac251 infection and that the magnitude of this antibody response is inversely associated with plasma viral RNA levels in animals with moderate to high levels of viral replication. However, we also demonstrated an association between NK cell-mediated ADCC responses and the amount of SIVmac251 gp140 binding antibody that developed after viral infection. This final observation raises the possibility that the antibodies that mediate ADCC are a subset of the antibodies detected in a binding assay and arise within weeks of infection.
INTRODUCTION
The findings of the recently reported human immunodeficiency virus type 1 (HIV-1) vaccine trial in Thailand (RV144) have refocused interest on vaccine-elicited antibody responses. In this human vaccine trial, a recombinant canary pox priming immunization followed by an envelope protein boosting immunization generated modest protection against the acquisition of HIV-1 infection (22). Since this vaccine regimen elicited nonneutralizing antibody and CD4+ T cell responses, attention is turning to the possibility that nonneutralizing antibodies may have mediated the protection seen in this clinical trial.
It has been proposed that antibodies that do not neutralize HIV-1 infection of target cells, as measured in conventional assays, may inhibit viral replication through Fc receptor-mediated effector mechanisms (7, 8, 12–14). These mechanisms include opsonization of viral particles for elimination by phagocytic cells or targeting of virus for destruction by antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is an natural killer (NK) cell-mediated destruction of virus-infected cells that are targeted for destruction by antiviral antibodies.
The most formidable obstacle encountered in attempting to establish an ADCC assay is the selection of an appropriate way to express viral antigen on the surface of target cells. Generally accepted targets for ADCC are either virus-infected, mitogen-stimulated peripheral blood mononuclear cells (PBMC) (18) or an immortalized cell line (26). The limitations of using PBMC as targets include donor-to-donor variability in the kinetics of HIV-1 replication and the cost, labor, and inconvenience associated with the preparation of sufficient numbers of activated PBMC for use in assays. The use of an immortalized cell line is simpler and less expensive than using PBMC as targets and facilitates assay standardization. A cell line frequently used for this purpose is CEM-NKr-CCR5, an NK-resistant cell line that stably expresses CD4 and CCR5 (26). CEM-NKr-CCR5 cells can be pulsed with gp120 protein. When gp120 protein has saturated its binding sites on the surfaces of CEM-NKr-CCR5 cells, their expression is relatively stable. This approach to generating target cells allows the analysis of ADCC activity directed solely against determinants of the gp120 molecules.
In the current study, we established a standardized assay for measuring ADCC using the CEM-NKr-CCR5 cell line as target cells. We then used that assay to explore the evolution of ADCC in SIVmac251-infected rhesus monkeys. We also examined virologic and clinical correlates of that immune response.
MATERIALS AND METHODS
EDTA-anticoagulated plasma samples were obtained from rhesus monkeys (Macaca mulatta). All animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals (19) and with the approval of the Institutional Animal Care and Use Committee of Harvard Medical School.
Plasma simian immunodeficiency virus (SIV) RNA levels.
Plasma viral RNA levels were measured by an ultrasensitive branched DNA amplification assay with a detection limit of 125 copies per ml (Siemens Diagnostics, Berkeley, CA).
Antibodies.
The following monoclonal antibodies (MAbs) were used: anti-CD107a allophycocyanin (APC) (H4A3; BD Biosciences), anti-tumor necrosis factor alpha (TNF-α) Alexa Fluor 700 (MAb11; BD Biosciences, San Jose, CA), anti-gamma interferon (IFN-γ) phycoerythrin-Cy7 (PE-Cy7) (B27; BD Biosciences), anti-MIP-1β phycoerythrin (PE) (D21-1351; BD Biosciences), anti-CD3 Pacific Blue (SP34-2; BD Biosciences), anti-CD20 allophycocyanin-Cy7 (APC-Cy7) (L27; BD Biosciences), anti-CD56 peridinium chlorophyll protein-Cy5.5 (PerCP-Cy5.5) (B159; BD Biosciences), anti-CD16 fluorescein isothiocyanate (FITC) (3G8; BD Biosciences), and anti-CD69 phycoerythrin-Texas Red (ECD) (TP1.55.3; BD Biosciences). A Live/Dead Aqua fluorescent reactive dye (Invitrogen) was also used as a viability marker to exclude dead cells.
VSVg envelope-pseudotyped SIVmac239.
We pseudotyped the infectious molecular clone SIVmac239 (a gift from Henrich Gottlinger) with the vesicular stomatitis virus G (VSVg) envelope protein in order to increase first-round infectivity. Briefly, 293T cells were cotransfected with plasmid DNA expressing SIVmac239 and VSVg envelope at a ratio of 1:2, and supernatants were harvested after 48 h.
NK cell-mediated ADCC assay.
An intracellular cytokine staining (ICS)-based assay was used to measure SIV antibody-mediated NK cell cytokine expression and degranulation. ADCC activity is defined as the percentage of CD3− CD20− CD56+ NK cells expressing CD107a or other functional molecules. The following three different target cells were used in this study: SIVmac251 gp130-coated CEM-NKr-CCR5 target cells (AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, Bethesda, MD), VSVg Env-pseudotyped SIVmac239-infected CEM-NKr-CCR5 target cells, or SIVmac251-infected CEM-NKr-CCR5 target cells. SIVmac251 gp130-coated CEM-NKr-CCR5 target cells were prepared by incubation with gp130 derived from SIVmac251 (Woburn, ImmunoDiagnostics) for 1 h at room temperature, followed by washing twice with ice-cold R10. Virus-infected CEM-NKr-CCR5 target cells were prepared by incubation with VSVg Env-pseudotyped SIVmac239 or SIVmac251 at a multiplicity of infection (MOI) of 1 for 7 days. Prior to use in the ADCC assay, target cells were washed, and 250,000 cells were added to each well of a 96-well round-bottom plate. A total of 100 μl of heat-inactivated plasma at a 1:200 dilution was added to each well, and the target and antibody mixture were incubated for 15 min at room temperature. Whole-blood samples from healthy human donors were obtained from a commercial vendor (Research Blood Components). Natural killer cells were isolated using the RosetteSep human NK cell enrichment cocktail (Stem Cell Technologies). A total of 250,000 purified human effector NK cells were added to each well at an effector cell/target cell (E/T) ratio of 1:1. After 6 h of incubation in the presence of monensin and anti-CD107a at 37°C in 5% CO2, cells were washed and stained with antibodies specific for CD3, CD20, CD56, and CD16 and then for the intracellular molecules CD69, IFN-γ, TNF-α, and MIP-1β. Cells were washed and fixed in 1% paraformaldehyde.
Flow cytometric analysis.
Samples were collected on a LSR II instrument (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR). Approximately 200,000 events were collected per sample. Doublets were excluded by forward-scatter (FSC) area versus FSC height. Dead cells were excluded on the basis of their staining with amine reactive dye. NK cells were determined by their lack of expression of CD3 and CD20 and their expression of CD56. CD3− CD20− CD56+ NK cells were evaluated for the expression of CD69, CD16, CD107a, IFN-γ, TNF-α, and MIP-1β. The frequency of total cells producing IFN-γ, TNF-α, and MIP-1β, either individually or in any combination, was determined by FlowJo software.
ELISA.
Binding of plasma to SIV gp140 protein was assessed by enzyme-linked immunosorbent assay (ELISA) as previously described but using SIVmac251.30 gp140 protein to coat plates and a secondary antibody directly conjugated to horseradish peroxidase (HRP) for detection (27). Briefly, 200 ng of the purified recombinant protein was adsorbed onto Reacti-Bind 96-well plates (Pierce, Rockford, IL), followed by blocking and incubation of serially diluted plasma samples. Bound antibody was detected using an HRP-conjugated goat anti-monkey IgG secondary antibody (Rockland Immunochemicals, Gilbertsville, PA). Plates were developed using SureBlue 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Gaithersburg, MD).
RESULTS
Selection of target cells for an ADCC assay and use of the assay to define the evolution of NK cell-mediated ADCC responses following SIVmac251 infection.
We first sought to determine the best target cell population to use for standardizing ADCC assays to monitor SIVmac251-infected rhesus monkeys. We did this in the context of defining the kinetics of the ADCC response through the first 25 weeks of SIVmac251 infection. We compared NK cell-mediated ADCC responses in assays using three different target cells: SIVmac251 gp130-coated CEM-NKr-CCR5 target cells, VSVg-pseudotyped SIVmac239-infected CEM-NKr-CCR5 target cells, and SIVmac251-infected CEM-NKr-CCR5 target cells. We developed a protocol to assay plasma for ADCC activity based on previously described methods, using human blood samples to derive NK cells (6, 16). The human and rhesus monkey Fcγ receptors share a high degree of homology, and utilization of human lymphocytes to assay rhesus monkey plasma for ADCC has previously been validated (6, 23).
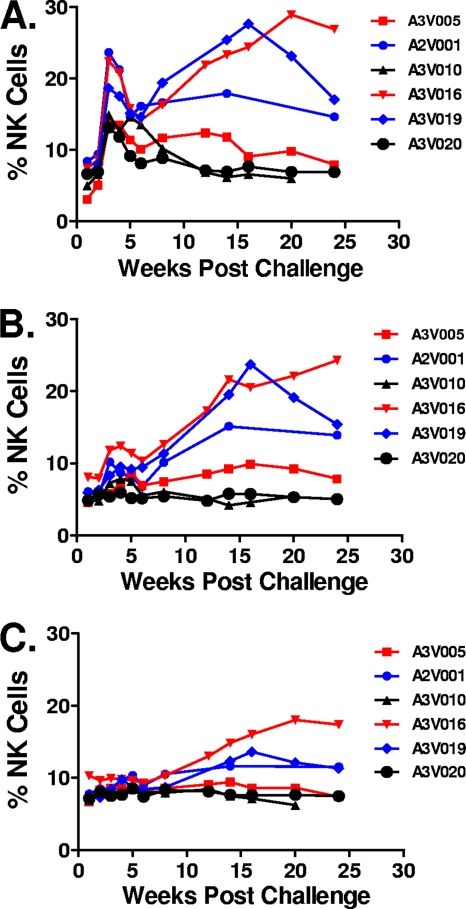
Six Mamu-A*01− rhesus monkeys were infected with SIVmac251 by the intravenous route and followed for 25 weeks (25). ADCC activity was first evaluated using SIVmac251 gp130-coated target cells at the indicated time points after infection (Fig. 1A). ADCC activity detected at day 0 is defined as the background level for each individual animal. The percentage of CD3− CD20− CD56+ NK cells expressing CD107a was first detected at week 2 after viral infection and was maximal at week 3 in all of the monkeys. Variation was observed, however, between monkeys as they reached their steady-state ADCC responses. The magnitudes of the ADCC responses were reduced dramatically by week 8 following infection. They remained at low levels in 2 animals with very high levels of viral replication (A3V010 and A3V020). These 2 rapid progressor monkeys had set point plasma viral RNA levels of approximately 108 copies per ml of plasma, and they died by day 175 postchallenge. In contrast, the antibody-mediated ADCC was maintained at higher levels in the other 4 animals, and these monkeys had moderate to low set point viral loads.
Fig. 1.
NK cell-mediated ADCC responses following SIVmac251 infection measured using 3 different target cell populations. Purified human NK cells were incubated with SIVmac251 gp130-coated CEM-NKr-CCR5 target cells (A), VSVg Env-pseudotyped SIVmac239-infected CEM-NKr-CCR5 target cells (B), or SIVmac251-infected CEM-NKr-CCR5 target cells (C) and a 1:200 dilution of plasma sampled from 6 Mamu-A*01− rhesus monkeys at the indicated time points following SIVmac251 infection. Data are expressed as percentages of CD3− CD20− CD56+ NK cells expressing CD107a.
We then evaluated ADCC activity using VSVg Env-pseudotyped SIVmac239-infected CEM-NKr-CCR5 target cells (Fig. 1B). The percentage of target cells infected by this VSVg-pseudotyped virus was greater than 90%, as determined by staining target cells with plasma sampled from a preselected SIVmac251-infected rhesus monkey, followed by staining with a fluorescein-conjugated goat anti-human IgG. Although the dynamic range of the assay performed using these target cells was less than that observed with the protein-pulsed target cells, a comparable distribution of ADCC responses in the various monkeys was observed. When CEM-NKr-CCR5 cells inoculated with SIVmac251 were used as target cells, less then 40% of them expressed the SIVmac251 envelope. Furthermore, no ADCC responses were detected during acute viral infection, and lower levels of responses were observed during chronic infection using these target cells (Fig. 1C). Nevertheless, the relative levels of ADCC in these monkeys, as measured using these cells, were comparable to those measured using the other target cells. While the protein-coated CEM-NKr-CCR5 cells detected higher levels of ADCC than the infected cells assays during the first 8 weeks of infection, the overall kinetics of the ADCC responses were comparable in assays using all 3 target cell populations.
Comparable NK cell-mediated ADCC activity when measured using different functional molecules.
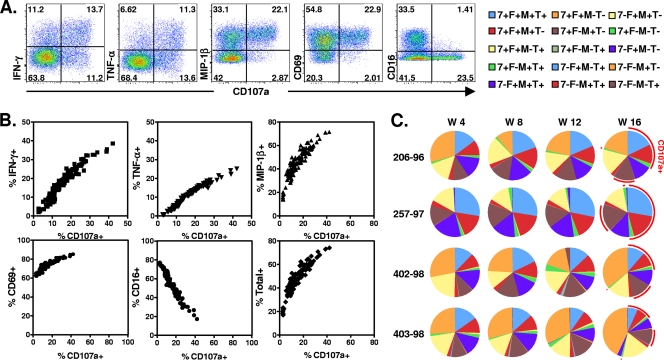
The functional properties of the NK cells involved in ADCC are not well delineated. In fact, NK cells have a variety of effector functions that may contribute to ADCC against SIVmac251, including the production of cytokines and β-chemokines (5, 7). We used polychromatic flow cytometry to assess NK cell production of IFN-γ, TNF-α, and MIP-1β and expression of CD107a in the setting of the ADCC assay to determine whether these properties of NK cells are associated with disease progression in SIVmac251-infected monkeys (Fig. 2). Plasma samples from 52 previously vaccinated rhesus monkeys were obtained 20 weeks following SIVmac251 infection and from another 4 monkeys during acute and chronic phases of SIVmac251 infection. The expression of functional molecules, including CD107a, IFN-γ, TNF-α, MIP-1β, CD69, and CD16, was evaluated in the setting of ADCC assays using their plasma samples, and the percent total was calculated by FlowJo software as the proportion of NK cells producing CD107a, IFN-γ, TNF-α, and MIP-1β, either individually or in any combination (Fig. 2B). A strong positive correlation was observed between the frequency of NK cells expressing CD107a and these NK cells expressing other functional molecules, including the activation-associated molecules CD69, IFN-γ, TNF-α, and MIP-1β, and the frequency of total functional NK cells. A strong negative correlation was detected between the proportion of NK cells producing CD107a and the downregulation of CD16. Similar results were also observed in ADCC assays using VSVg Env-pseudotyped SIVmac239-infected CEM-NKr-CCR5 target cells or SIVmac251-infected CEM-NKr-CCR5 target cells (data not shown). These results suggest that a variety of functions of NK cells (Fcγ receptor IIIA [FcγRIIIa] downregulation, activation, cytokine and chemokine secretion, and degranulation) are triggered simultaneously during NK cell-mediated ADCC.
Fig. 2.
Comparable NK cell-mediated ADCC activities when measured by NK cell production of different functional molecules. Purified human NK cells were incubated with SIVmac251 gp130-coated CEM-NKr-CCR5 target cells and a 1:200 dilution of plasma sampled from 52 previously vaccinated rhesus monkeys at 20 week following SIVmac251 challenge and from another 6 monkeys during the periods of both acute and chronic SIVmac251 infection. The percentage of CD3− CD20− CD56+ NK cells expressing different functional molecules, including CD107a, IFN-γ, TNF-α, MIP-1β, CD69, and CD16, was evaluated, and the percent total was calculated by FlowJo software as the proportion of NK cells producing CD107a, IFN-γ, TNF-α, and MIP-1β either individually or in any combination. (A) The representative flow plots show the proportions of gated CD3− CD20− CD56+ NK cells that became activated (CD69), downregulated (CD16), and degranulated (CD107a) and produced different cytokines and chemokines (IFN-γ, TNF-α, and MIP-1β). (B) The dot plots summarize the correlations between the expression levels of these different functional molecules. (C) Plasmas sampled from 4 Mamu-A*01+ rhesus monkeys at weeks 4, 8, 12, and 16 after SIVmac251 infection were assayed for the functional repertories of the NK cells. Total functional CD3− CD20− CD56+ NK cells were divided into 15 subpopulations based on their production of CD107a, IFN-γ, MIP-1β, and TNF-α either individually or in any combination. Functional profiles were determined by expressing each cytokine response as a proportion of the total functional NK cell response, and data are presented in pie charts.
The functional profiles of the antibody-activated NK cells may be important in the control of disease progression in infected monkeys. We therefore did a complete analysis of the functional repertoires of the NK cells. Total antibody-activated functional NK cells were divided into 15 subpopulations based on their production of CD107a, IFN-γ, MIP-1β, and TNF-α either individually or in any combination. As shown in Fig. 2C, the plasma samples from the same animals induced a similar pattern of NK cell functional responses throughout the course of viral infection. For example, plasma from animal 257-97, the monkey with the lowest viral load, induced the highest frequency of CD107+ NK cell as well as the highest proportion of CD107a+ IFN-γ+ TNF-α+ MIP-1β+ NK cells.
Correlation between NK cell-mediated ADCC responses and plasma viral RNA levels.
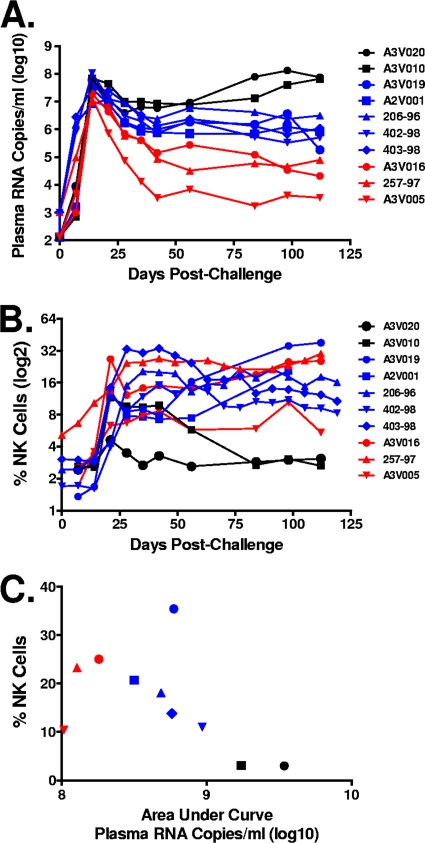
The same 6 Mamu-A*01− monkeys from the previous study as well as another 4 Mamu-A*01+ monkeys were inoculated by the intravenous route with SIVmac251 and monitored for 125 days to evaluate their virologic statuses (20, 25). Plasma virus was initially detected at day 7 after infection and was maximal at day 14 (Fig. 3A). Variation between monkeys in their set point plasma viral RNA levels was observed, as follows: 3 animals had relative low levels of viral replication by day 120 following infection (approximately 104.5 copies per ml of plasma), and 5 animals had moderate levels of viral replication at this time (approximately 106 copies per ml of plasma). Two additional animals had very high levels of viral replication, with set point plasma viral RNA levels of approximately 108 copies per ml of plasma; these animals were dead by day 175 postchallenge. A similar variation between monkeys was also observed when we compared the areas under the concentration-time curves (AUC) of the log viral loads of these animals as a function of time.
Fig. 3.
Correlation between the magnitudes of the NK cell-mediated ADCC responses and the plasma viral RNA levels. Six Mamu-A*01− and 4 Mamu-A*01+ rhesus monkeys were infected with SIVmac251 by the intravenous route and followed for 120 days. (A) Dynamics of plasma viral RNA levels following SIVmac251 infection. (B) Purified human NK cells were incubated with SIVmac251 gp130-coated CEM-NKr-CCR5 target cells and a 1:200 dilution of plasma sampled from these rhesus monkeys at the indicated time points following SIVmac251 infection. Data are expressed as percentages of CD3− CD20− CD56+ NK cells expressing CD107a. (C) The dot plot shows the relationship between the ADCC responses at 100 days after SIVmac251 infection and the AUC of viral RNA levels through day 100 after this virus infection for each monkey.
ADCC activity was assayed using SIVmac251 gp130-coated target cells and plasma sampled at the indicated time points after infection (Fig. 3B). The percentage of CD3− CD20− CD56+ NK cells expressing CD107a was maximal using plasma sampled at week 3 after infection. Variation between monkeys in their steady-state, chronic ADCC responses was observed. The ADCC activity was reduced dramatically in plasma specimens sampled at week 10, and it remained at a low level in the two animals with high, persistent levels of viral replication (A3V010 and A3V020). In contrast, ADCC was maintained at a higher level in plasma samples obtained from 8 animals with moderate to low set point viral loads.
We then evaluated ADCC activity in plasma obtained from the infected monkeys sampled at day 100 after viral infection and the AUC of plasma viral RNA levels through day 100 after SIVmac251 infection in these animals (Fig. 3C). Interestingly, low ADCC activity was observed in animal A3V005, the monkey with the lowest plasma viral RNA levels at day 100 after infection. No ADCC activity was detected in assays using plasma specimens from the 2 animals with very high levels of viral replication. Importantly, a negative correlation between the ADCC activity and the plasma viral RNA levels in the animals with moderate to high levels of viral replication was demonstrated (r = −0.64; P = 0.047).
Association between SIVmac251 gp140 binding antibody titer and NK cell-mediated ADCC responses.
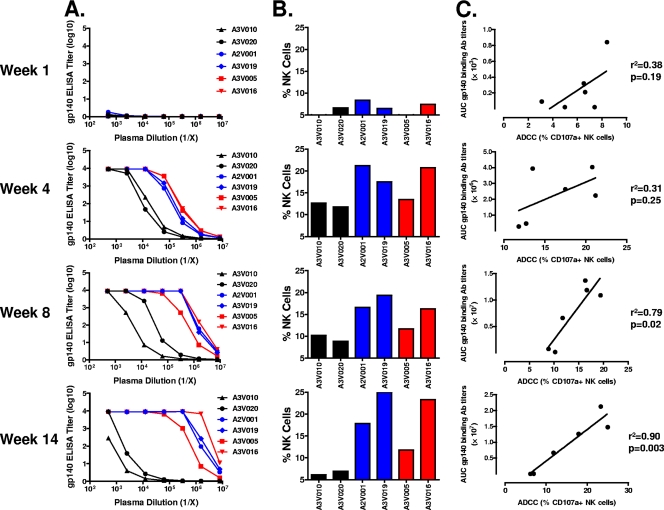
The titers of antibodies against SIVmac251 gp140 protein in the plasma samples collected from 6 Mamu-A*01− monkeys were assessed by ELISA at the 4 indicated time points following SIVmac251 infection (Fig. 4A). ADCC activity was measured in the plasma sampled from these monkeys at the same time points following SIVmac251 infection (Fig. 4B). As expected, negligible binding antibody was observed 1 week after viral infection. By week 4 after infection, plasma samples obtained from all of the animals showed appreciable binding to the protein. However, 2 of the animals, A3V010 and A3V020, had considerably lower plasma binding antibody titers than those seen in the other 4 animals. These 2 animals also had the lowest ADCC activity. At week 8, binding antibody titers in some of the animals increased, while those in others decreased. A3V010 and A3V020 continued to have the lowest binding antibody titers as well as the lowest ADCC activity levels in the cohort of monkeys. At the final time point examined, week 14, both the binding antibody titers and ADCC activity levels for A3V010 and A3V020 were found to have decreased further, whereas both of these antibody activities remained unchanged or increased in the other animals. Importantly, when the ADCC responses were plotted against the AUC of the gp140 binding antibody titers, a strong positive correlation was detected at week 8 and week 14 after SIVmac251 infection (Fig. 4C). These results suggest that there is an association between NK cell-mediated ADCC responses and the titers of SIVmac251 gp140 binding antibodies in these monkeys.
Fig. 4.
Association between the SIVmac251 gp140 binding antibody titers and the NK cell-mediated ADCC responses. (A) Plasma sampled from 6 Mamu-A*01− rhesus monkeys at weeks 1, 4, 8, and 14 after SIVmac251 infection were assayed by ELISA for the level of antibody binding to SIVmac251 gp140. (B) Purified human NK cells were incubated with SIVmac251 gp140-coated CEM-NKr-CCR5 target cells and a 1:200 dilution of plasma sampled from 6 Mamu-A*01− rhesus monkeys at the indicated time points following SIVmac251 infection. Data are expressed as percentages of CD3− CD20− CD56+ NK cells expressing CD107a. (C) The dot plots show the relationship between the ADCC responses and the AUC of the gp140 binding antibody titers.
DISCUSSION
While investigators agree on the potential importance of ADCC as a mechanism for protection against a virus (3, 4, 11, 15, 17), there is no generally accepted, standardized assay to measure ADCC. Assays for measuring HIV-1-specific ADCC must have three components: target cells that express HIV-1 antigens, effector cells such as natural killer (NK) cells, and a source of antibody such as serum, plasma, or monoclonal antibodies. The traditional radioactive chromium release assay is relatively insensitive and labor-intensive (1). Gomez-Roman and colleagues have recently described a fluorescent killing assay that they term a “rapid fluorescent ADCC (RFADCC) assay” (9). This assay measures a fluorescent dye that target cells release when they die. Other groups have explored intracellular cytokine staining-based assays for evaluating NK cell expression of effector molecules following activation by HIV-1 antigens (Ags) and anti-HIV-1 Abs (16, 24). It is not clear whether these different ADCC assays are comparable in what they measure.
The generally accepted target cells for HIV-1 ADCC assays are either virus-infected, mitogen-stimulated human peripheral blood mononuclear cells (PBMC) (18) or CD4+ T cell lines that are resistant to lysis by NK cells in the absence of antibody (26). The major limitation of the PBMC assay is donor-to-donor variability in the kinetics of HIV-1 replication in vitro. An immortalized cell line is easier to work with and less expensive than PBMC, and its use allows for standardization of assays. CEM-NKr-CCR5 is an NK-resistant CD4+ T cell line that stably expresses CCR5 (26). In the current study, we compared NK cell-mediated ADCC responses using 3 different target cells, including SIVmac251 gp130-coated CEM-NKr-CCR5 target cells, VSVg Env-pseudotyped SIVmac239-infected CEM-NKr-CCR5 target cells, or SIVmac251-infected CEM-NKr-CCR5 target cells. Peripheral blood mononuclear cells (PBMC) from uninfected donors that were enriched for NK cells were used as effector cells. The flow cytometry-based assay allowed us to gate on CD3− CD20− CD56+ cells in characterizing the function of NK cells following activation. The results of the present study show that, in contrast to neutralizing antibody activity, antibody-mediated killing of the SIV-infected cells in the presence of NK effector cells is detectable in monkeys as early as 3 weeks after SIVmac251 infection (2, 21). This finding suggests that HIV-1-specific antibody may play a role in the control of viremia during acute infection.
We have shown that SIV Env gp130-coated target cells are more sensitive than VSVg Env-pseudotyped SIVmac239-infected or SIVmac251-infected target cells for detection of ADCC. This can be explained, at least in part, by the percentage of target cells that are expressing SIV antigen. Target cells prepared by SIV Env gp130 coating have saturating amounts of gp130 that bind stably to target cells, with little shedding of antigen over a 6-hour time course—an appropriate length of time for a flow-based ADCC assay. This approach permits the analysis of ADCC activity directed solely against determinants of the gp130 molecule. VSVg-pseudotyped SIVmac239 infection results in a high percentage of infected target cells, as measured by staining target cells with plasma obtained from an SIVmac251-infected rhesus monkey. SIVmac251 infection resulted in the infection of fewer than 40% of target cells. ADCC activity was detected in plasma sampled from monkeys during acute viral infection, and lower-level responses were measured during chronic infection using these target cells. Therefore, SIV Env gp130-coated target cells greatly facilitate analyses of ADCC activity with a high degree of sensitivity and reproducibility. However, a recent study demonstrated that substantial ADCC activity against the Pol and Vpu proteins can be detected in the sera of HIV-infected individuals (24). Therefore, further study of ADCC responses using Pol or Vpu protein-pulsed cell lines would better define the ADCC activity in the plasma specimens of SIVmac251-infected rhesus monkeys. Further, the use of primary cells rather than an immortalized cell line might provide useful data in an ADCC assay since cell lines and primary cells can differ in their expression of receptors and antigen-presenting machinery.
Since ADCC results in the lysis of target cells, we used CD107a as an indictor of the biological activity of the NK cells in the ADCC assays. Although much of the antiviral activity mediated by plasma antibody from infected animals is probably due to the death of the virus-infected cells, it is likely that noncytolytic mechanisms, such as the secretion of soluble molecules by activated NK cells, also play a role in reducing virus replication. HIV-1-specific antibody, in the presence of envelope-expressing target cells, augments chemokine and cytokine release from NK cells (5, 7). Thus, it is likely that cytokines, including IFN-γ and TNF-α, and the chemokine MIP-1β released from NK cells after Fc receptor stimulation were responsible for some of the antiviral activity detected in the ADCC assay. However, the role of cytokine release relative to cytotoxicity in inhibiting virus replication was not ascertained. In the current study, using polychromatic flow cytometry, we were able to simultaneously assess Ab-stimulated NK cell production of IFN-γ, TNF-α, MIP-1β, and CD107a. The results demonstrated a very strong positive correlation between the frequency of NK cells expressing CD107a and NK cell production of other molecules, including the activation-associated molecule CD69, cytokines IFN-γ and TNF-α, and the chemokine MIP-1β. A strong negative correlation was also detected between the proportion of NK cells producing CD107a and the downregulation of CD16. These results suggest that NK cells are able to recognize Ab bound to target cells through FcγRIIIa (CD16) that is expressed on 80 to 90% of peripheral blood NK cells. Following activation, NK cells enter a refractory period during which CD16 molecules are shed from their surfaces (10). This loss of CD16 is thought to prevent chronic stimulation of NK cells and activation-induced cell death. The activated NK cells then produce a variety of functional molecules, including CD107a, IFN-γ, TNF-α, MIP-1β, and CD69. The strong positive correlation between Env-specific ADCC-triggered degranulation and the expression of cytokines and chemokines by activated NK cells suggests that measuring a single molecule expressed by effector NK cells, such as CD107a, is sufficient to evaluate the anti-SIV function of NK cells in the context of ADCC.
ADCC, like cytotoxic T lymphocyte (CTL) activity, mediates the death of infected cells in vitro. It is therefore plausible that ADCC may play a role in vivo in controlling SIV replication during primary infection. In the current study, we found that in contrast to neutralizing activity, antibody directed against infected cells and capable of inhibiting SIV replication in the presence of NK effector cells is detectable in infected monkeys as early as 3 weeks after infection. Furthermore, we found that the magnitude of the antiviral antibody response is inversely associated with plasma SIV RNA levels in monkeys with moderate to high levels of viral replication. These findings suggest that SIV-specific antibody could play a role in the control of viremia during SIVmac251 infection. Interestingly, relative low ADCC activity was observed in monkey A3V005, the animal with the lowest plasma viral RNA levels at day 100 after viral infection. This result is consistent with the possibility that certain levels of antigen may be needed to maintain high ADCC activity after viral infection.
We also evaluated the association between the development of SIVmac251 gp140 binding antibody and the NK cell-mediated ADCC responses in SIV-infected monkeys. We found that binding antibodies to gp140 as measured by ELISA were detectable at 4 weeks after viral infection, coincident with the development of measurable ADCC activity. In addition, the magnitude of this antibody response was associated with the level of ADCC activity. These results are consistent with the possibility that the antibodies that mediated ADCC are a cohort of the antibodies detected in the gp140 protein binding assay, and the titers of these antibodies reflect the immune competence of the infected monkeys.
ACKNOWLEDGMENTS
We thank Michelle Lifton for her technical assistance. We also thank Galit Alter for her generous assistance and advice.
This work was supported in part by funds from the intramural research program of the Vaccine Research Center, NIAID, NIH; Harvard Medical School CFAR grant AI060354; CHAVI grant AI06785; and NIH grant HHSN272201000028C.
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Ahmad R., et al. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21:227–233 [DOI] [PubMed] [Google Scholar]
- 2. Asmal M., et al. 2011. Antibody-dependent cell-mediated viral inhibition emerges after SIVmac251 infection of rhesus monkeys coincident with gp140 binding antibodies and is effective against neutralization-resistant viruses. J. Virol. 85:5465–5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baum L. L., et al. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 157:2168–2173 [PubMed] [Google Scholar]
- 4. Binley J. M., et al. 2000. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270:237–249 [DOI] [PubMed] [Google Scholar]
- 5. Fauci A. S., Mavilio D., Kottilil S. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5:835–843 [DOI] [PubMed] [Google Scholar]
- 6. Forthal D. N., et al. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 80:9217–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forthal D. N., Landucci G., Daar. E. S. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forthal D. N., Moog C. 2009. Fc receptor-mediated antiviral antibodies. Curr. Opin. HIV AIDS 4:388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gomez-Roman V. R., et al. 2006. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 308:53–67 [DOI] [PubMed] [Google Scholar]
- 10. Grzywacz B., Kataria N., Verneris M. R. 2007. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 21:356–359 (Author's reply, 21:359.) [DOI] [PubMed] [Google Scholar]
- 11. Hessell A. J., et al. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104 [DOI] [PubMed] [Google Scholar]
- 12. Holl V., et al. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80:6177–6181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holl V., et al. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koup R. A., et al. 1989. Antigenic specificity of antibody-dependent cell-mediated cytotoxicity directed against human immunodeficiency virus in antibody-positive sera. J. Virol. 63:584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambotte O., et al. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Q., et al. 2009. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J. Virol. 83:8705–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mascola J. R., Montefiori D. C. 2010. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28:413–444 [DOI] [PubMed] [Google Scholar]
- 18. Montefiori D. C., et al. 1998. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J. Virol. 72:1886–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Research Council 1996. Guide for the care and use of laboratory animals. National Academic Press, Washington, DC [Google Scholar]
- 20. Permar S. R., et al. 2008. Potent simian immunodeficiency virus-specific cellular immune responses in the breast milk of simian immunodeficiency virus-infected, lactating rhesus monkeys. J. Immunol. 181:3643–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Permar S. R., et al. 2010. Limited contribution of mucosal IgA to simian immunodeficiency virus (SIV)-specific neutralizing antibody response and virus envelope evolution in breast milk of SIV-infected, lactating rhesus monkeys. J. Virol. 84:8209–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rerks-Ngarm S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 23. Rogers K. A., Scinicariello F., R. Attanasio. 2006. IgG Fc receptor III homologues in nonhuman primate species: genetic characterization and ligand interactions. J. Immunol. 177:3848–3856 [DOI] [PubMed] [Google Scholar]
- 24. Stratov I., Chung A., Kent S. J. 2008. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J. Virol. 82:5450–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Y., Permar S. R., Buzby A. P., Letvin N. L. 2007. Memory CD4+ T-lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection. J. Virol. 81:8009–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trkola A., Matthews J., Gordon C., Ketas T., Moore J. P. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J. Virol. 73:8966–8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]