Abstract
Eight percent of the human genome is composed of human endogenous retroviruses (HERVs), which are thought to be inactive remnants of ancient infections. Previously, we showed that individuals with early HIV-1 infection have stronger anti-HERV T cell responses than uninfected controls. In this study, we investigated whether these responses persist in chronic HIV-1 infection and whether they have a role in the control of HIV-1. Peripheral blood mononuclear cells (PBMCs) from 88 subjects diagnosed with HIV-1 infection for at least 1 year (median duration of diagnosis, 13 years) were tested for responses against HERV peptides in gamma interferon (IFN-γ) enzyme immunospot (ELISPOT) assays. Individuals who control HIV-1 viremia without highly active antiretroviral therapy (HAART) had stronger and broader HERV-specific T cell responses than HAART-suppressed patients, virologic noncontrollers, immunologic progressors, and uninfected controls (P < 0.05 for each pairwise comparison). In addition, the magnitude of the anti-HERV T cell response was inversely correlated with HIV-1 viral load (r2 = 0.197, P = 0.0002) and associated with higher CD4+ T cell counts (r2 = 0.072, P = 0.027) in untreated patients. Flow cytometric analyses of an HLA-B51-restricted CD8+ HERV response in one HIV-1-infected individual revealed a less activated and more differentiated phenotype than that stimulated by a homologous HIV-1 peptide. HLA-B51 tetramer dual staining within this individual confirmed two different T cell populations corresponding to these HERV and HIV-1 epitopes, ruling out cross-reactivity. These findings suggest a possible role for anti-HERV immunity in the control of chronic HIV-1 infection and provide support for a larger effort to design an HIV-1 vaccine that targets conserved antigens such as HERV.
INTRODUCTION
Much of the recent effort in identifying immune correlates of protection in HIV-1 has focused on defining the qualities of a successful T cell response in individuals who maintain low or undetectable viral loads in the absence of treatment, i.e., controllers (1, 6, 9, 27). As a result of these investigations, there have been major advances in the understanding of the immunobiology of HIV-1 infection (30). However, the quest for a fully effective vaccine is still ongoing, and the designs of many vaccine candidates have only varied from each other slightly (31). We have studied an area outside conventional HIV-1 vaccine strategies and explored the potential for a human endogenous retrovirus (HERV)-based HIV-1 vaccine.
Transposable elements make up 45% of the human genome (19). Human endogenous retroelements (which rely on an RNA intermediate before integration into the genome) can be classified into the non-long terminal repeat (LTR) class and the LTR class, represented by endogenous retroviruses (ERV). Many ERV entered the primate germ line as infectious retroviruses at several time points during human evolution (4). Out of the six HERV superfamilies, HERV-K (HML-2) is considered to be the youngest and most transcriptionally active (18). A report in 2008 described the visualization of HERV-K-like viral particles in the plasma of lymphoma patients (8). A clinical study demonstrated the potential utilization of another HERV family in a novel cancer immunotherapy product (33). In a group of patients with metastatic renal cell carcinoma (RCC) who experienced tumor regression after allogeneic hematopoietic stem cell transplant, RCC-reactive donor-derived CD8+ T cells directed against a 10-mer HERV-E peptide were identified. This antigen was expressed in RCC cells but not healthy tissues, suggesting that it could be targeted by a new tumor peptide vaccine or by adoptively infused peptide-specific cytotoxic T lymphocytes (CTLs) (33).
Host cells have developed mechanisms to prevent lentiviral replication, as well as to restrict the movement of retroelements in order to maintain genomic stability. Esnault et al. (11) and others (24) reported that the host protein APOBEC3 restricts endogenous retroelements. The HIV-1 Vif protein has been shown to disable APOBEC3G (13, 32), which could lead to the activation of HERV. In support of this hypothesis, our group and others have demonstrated that HERV-K transcripts can be detected in the plasma of HIV-1-infected individuals, suggesting that HIV-1 infection can alter the biology of HERV-K by enhancing its gene expression (7, 12).
We also previously reported that adults with early HIV-1 infection have detectable ex vivo CD8+ T cell responses to HERV antigens and that these responses are absent in healthy subjects (12). The magnitude of the anti-HERV response is inversely correlated with HIV-1 plasma viral load, suggesting a potential role for these responses in the control of HIV-1 (12). The current study examined the immunological consequences of HERV antigen production and presentation in individuals with chronic HIV-1. We hypothesized that subjects who are able to control HIV-1 in the absence of highly active antiretroviral therapy (HAART) have stronger and more frequent HERV-specific T cell responses than those who are unable to control HIV-1 without HAART. We also hypothesized that responses would be higher in individuals controlling the virus as an apparent consequence of the host response (controllers) than in individuals suppressing the virus as a consequence of antiretroviral treatment. In order to elucidate a possible mechanism of viral control in these individuals, we compared the functionality and phenotype of HERV-, HIV-1-, and cytomegalovirus (CMV)-specific responses. The work outlined in this study has the potential to lead to a vaccination strategy utilizing HERV-specific T cells with the capacity to kill HIV-1-infected cells expressing HERV epitopes. It is envisioned that such a novel vaccine would be part of a combination vaccination approach that includes stimulation of immunity to HIV-1 antigens as well as to HERV.
MATERIALS AND METHODS
Study populations.
Samples of peripheral blood mononuclear cells (PBMCs) were selected from participants in two different San Francisco-based HIV-1-infected cohorts, Options (15) and SCOPE (17), as well as from an HIV-1-infected cohort at the University of Toronto. Samples from HIV-1-negative controls were obtained from 22 individuals who donated blood to the Stanford blood bank. The study was approved by the local institutional review boards (University of California, San Francisco [UCSF] Committee on Human Research and University of Toronto Institutional Review Board), and individuals gave written informed consent. Studies were performed on cryopreserved PBMCs.
PBMC samples were obtained from the following categories of HIV-1-infected individuals: 30 untreated virologic controllers (which included 10 viremic controllers with HIV-1 viral loads between 50 and 2,000 copies/ml and 20 elite controllers with viral loads of <50 to 75 HIV-1 copies/ml), 20 highly active antiretroviral therapy (HAART)-suppressed patients (<50 to 75 HIV-1 copies/ml), and 18 untreated virologic noncontrollers (>2,000 copies/ml). All had a CD4+ T cell count of >250 cells/mm3. A fourth group of untreated HIV-1-infected patients was also tested. These 20 individuals were defined as immunologic progressors, with an HIV-1 viral load of >2,000 copies/ml and CD4+ T cell count of <250 cells/mm3. All 88 patients had been diagnosed with HIV-1 at least 1 year prior to inclusion in this study. There was no significant difference in the median ages of the five groups (including HIV-1-infected and uninfected subjects) or duration of diagnosis among the HIV-1-infected groups (P > 0.05 for all pairwise comparisons). Table 1 describes baseline subject characteristics.
Table 1.
Characteristics of study subjects
| Participant category (n) | Median age (yr [IQR]) | Gendera | Ethnicity and no. of subjectsb | Median duration of HIV-1 diagnosis (yr [IQR]) | Median CD4+ T cell count (cells/mm3 [IQR]) | Median HIV-1 viral load (copies/ml [IQR]) | |
|---|---|---|---|---|---|---|---|
| M | F | ||||||
| Controller (30) | 46.5 (40.5-52) | 23 | 7 | C, 13; L, 4; A, 1; AA, 10; NA, 2 | 13 (6-18) | 794 (569-1,059) | 50 (40-302) |
| HAART-suppressed (20) | 48 (39.5-51) | 17 | 3 | C, 11; L, 5; A, 1; AA, 3 | 13 (9.8-17) | 686 (568-1,069) | 75 (50-75) |
| Virologic noncontroller (18) | 49.5 (38.5-54) | 15 | 3 | C, 9; A, 1; AA, 8 | 14 (8.5-20) | 523 (370.5-744.5) | 37,568 (17,573-69,763) |
| Immunologic progressors (20) | 43 (37-47) | 17 | 3 | C, 10; L, 5; A, 1; AA, 3; NA, 1 | 8 (5-16) | 200 (144.5-231.5) | 150,974 (89,532-230,970) |
| HIV-1 negative (22)c | 49 (44-54.5) | 12 | 8 | C, 8; L, 2; A, 3 | |||
F, female; M, male.
C, Caucasian; L, Latino/Hispanic; A, Asian; AA, African-American; NA, Native American.
Some information about the gender and ethnicity of the HIV-1-negative group was not available.
PBMC preparation and storage.
PBMCs were isolated by standard Ficoll-Hypaque density gradient centrifugation on fresh blood samples and immediately cryopreserved in fetal calf serum (HyClone, Logan, UT) containing 10% dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO) in liquid nitrogen. The cryopreserved cells were stored in liquid nitrogen until they were used.
Peptides and tetramers.
A commercially manufactured set of 28 9- to 13-mer individual HERV peptides with >95% purity (Genscript, Piscataway, NJ) was used to screen individuals for anti-HERV CD8+ T cell responses (see Table S1 in the supplemental material). The HERV peptides were designed using HLA binding prediction software (with a predicted combined binding score of >0.75 according to NetCTL, version 1.2) (20, 29). They included peptides from several HERV families, including HERV-K, HERV-H, and HERV-L. A subset of these peptides was used in our previous publication (12) and is marked with an asterisk in Table S1. A set of overlapping 15-mer HERV-K Gag and Env peptides (JPT Peptide Technologies, Berlin, Germany) was also used to comprehensively map one elite controller's (subject P1's) responses. HIV-1 peptides selected from the Gag, Nef, Env, and Pol proteins (Sigma Aldrich and Invitrogen, Carlsbad, CA) (see Table S2) and a CMV pp65 pool of overlapping 15-mer peptides (JPT Peptide Technologies) were also tested. All HERV peptides were used at a concentration of 100 μg/ml for the initial enzyme-linked immunospot (ELISPOT) screening assays and then titrated down to final concentrations of 1 μg/ml to 10 μg/ml for subsequent ELISPOT assays and flow cytometry experiments. HLA-B51 tetramers were synthesized and provided by the NIH Tetramer Core Facility (Atlanta, GA).
ELISPOT assays.
The ELISPOT assay has been described previously (22). In brief, 96-well plates (Millipore, Billerica, MA) were coated with human monoclonal anti-interferon gamma (IFN-γ) immunoglobulin (Mabtech, Mariemont, OH). After plates were washed and blocked with 10% fetal calf serum, PBMCs were added at a concentration of 105 cells per well. Duplicate wells were prepared for each experimental condition. Spot totals for duplicate wells were averaged, and all spot numbers were normalized to numbers of IFN-γ spot-forming units (SFU) per million PBMCs (SPM). The spot values from medium control wells were subtracted, after which a positive response to a peptide was defined as >50 SPM and >2 times the medium control value. The total magnitude of the HERV T cell response was calculated by adding up all of the individual peptide SPM values.
HLA restriction.
The HLA restriction of one of the most immunogenic HERV peptides was determined using patient-derived PBMCs incubated with HERV peptide-pulsed and unpulsed single-HLA allele transfectant B cells as targets (provided by Lewis Lanier, University of California, San Francisco, CA) in an ELISPOT assay. B cell transfectants at a concentration of 104 cells per well were pulsed with peptide for 1 h at 37°C and then washed three times before being added into a standard ELISPOT assay with PBMCs. Control conditions included PBMCs incubated with HLA-mismatched (B51−) targets with or without peptide pulsing and HLA-matched (B51+) targets with or without peptide pulsing. As an additional control, B cell transfectants with or without peptide pulsing were also tested in the ELISPOT assay without PBMCs.
Cell surface and intracellular staining by flow cytometry.
PBMCs from an HIV-1-infected individual (subject P2) were stimulated with the peptides HERV-K FAFTIPAI ([FI8] from the reverse transcriptase region), HIV-1 TAFTIPSI (TI8), or a CMV pp65 peptide pool for 6 h at 37°C in the presence of anti-CD49d (BD Biosciences, San Jose, CA), Golgi Stop (BD Biosciences), and brefeldin A (Sigma Aldrich). Control conditions included unstimulated cells (negative control) and cells stimulated with anti-CD3 antibody (positive control). After incubation, cells were washed with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline with 1% bovine serum albumin; Sigma Aldrich). To determine phenotype and function of antigen-specific cells, 106 antigen-stimulated PBMCs were surface stained with fluorophore-conjugated antibodies to anti-CD3, -CD4, -CD8, -CD27, and -CD28 (all BD Biosciences). Following cell surface staining, PBMCs were washed, fixed with 1% paraformaldehyde (Polyscience, Niles, IL), and permeabilized with Perm Buffer (BD Biosciences). They were then stained with antibodies against intracellular cytokines IFN-γ and tumor necrosis factor alpha (TNF-α). A second phenotyping (T cell activation) panel consisted of cell surface markers for CD3, CD4, CD8, CD38, and intracellular cytokines IFN-γ and TNF-α. An amine aqua dye (Invitrogen) was also included to discriminate between live and dead cells. Following staining, PBMCs were washed with FACS buffer, fixed in paraformaldehyde, and stored at 4°C until analysis.
An HLA-B51-HERV-K FAFTIPAI-allophycocyanin (APC) tetramer and a B51-HIV-1 TAFTIPSI-phycoerythrin (PE) tetramer (NIH Tetramer Core Facility, Atlanta, GA) were used to stain PBMCs from an HIV-1-infected subject (P2) and a healthy HIV-1-negative control. Each tetramer was used at a dilution of 1:1,000 (based on prior determination of the optimal titration). The tetramer dual staining was carried out at 37°C in the dark for 30 min. The cells were then washed and stained with fluorophore-conjugated anti-CD3, -CD8, -CD14, and -CD19 antibodies (the latter two to exclude monocytes and B cells) and an amine aqua dye to exclude dead cells.
For all flow cytometry experiments, data were acquired with an LSR-II system (Becton Dickinson). At least 100,000 events were collected and analyzed with FlowJo software, version 9.0 (Tree Star, Ashland, OR).
Statistical analysis.
Subject characteristics (age, duration of diagnosis, HIV-1 viral load, and CD4+ T cell count), the total magnitudes of ELISPOT assay responses (in SPM), and the total number of positive responses were compared between groups using the Kruskal-Wallis, Dunn's multiple comparison, and two-sided Mann-Whitney U tests. Linear regression and Spearman correlation analyses were used to measure associations between HIV-1 viral load and magnitude and breadth of the HERV response, HIV-1 viral load and magnitude of the HIV-1 response, the magnitudes of the HIV-1 response and the HERV response, and the magnitude of the HERV response and CD4+ T cell count in HIV-1-infected individuals not on treatment. All tests were conducted using GraphPad Prism, version 4.00 (GraphPad Software, San Diego, CA), with the statistical significance of the findings set at a P value of less than 0.05.
RESULTS
HIV-1 controllers have stronger HERV-specific responses than other chronically infected subjects.
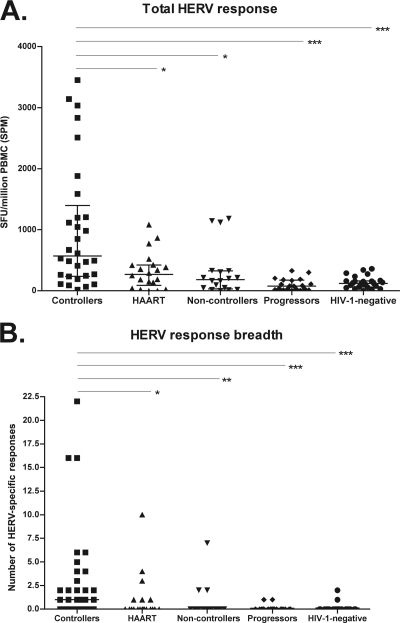
We measured T cell responses against HERV using a set of manufactured peptides selected from three HERV families (HERV-K, HERV-L, and HERV-H). The total magnitude of the HERV-specific T cell response was measured as the sum of all of the single peptide responses for each individual. The median total magnitude of HERV-specific T cell responses among HIV-1-negative subjects was 122.1 SPM (interquartile range [IQR], 62.5 to 165 SPM). Individuals controlling HIV-1 viremia in the absence of treatment (controllers) had a median total response magnitude of 571.4 SPM (IQR, 240 to 1,395 SPM). This was significantly higher than the total responses of the HIV-1-negative subjects (P < 0.0001), the HAART-suppressed group (median, 270.3 SPM; IQR, 87.6 to 422.5 SPM; P < 0.05), the noncontroller group (median, 181.3 SPM; IQR, 32.5 to 327.5 SPM; P < 0.05), and the progressors (median, 77.5 SPM; IQR, 22.5 to 175; P < 0.0001) (Fig. 1A). HIV-1-infected controllers also had a significantly higher number (breadth) of HERV-specific responses than the HIV-1-negative individuals (P < 0.0001), the HIV-1-infected noncontrollers (P < 0.01), progressors (P < 0.0001), and HAART-suppressed patients (P < 0.05) (Fig. 1B).
Fig. 1.
(A) The total HERV response magnitude of HIV-1 controllers was significantly higher than that of HAART-suppressed patients (*, P < 0.05), noncontrollers (*, P < 0.05), progressors (***, P < 0.0001), and HIV-1-negative subjects (***, P < 0.0001), as measured in IFN-γ ELISPOT assays. SFU, spot-forming units; SPM, spot-forming units per million PBMCs. (B) HIV-1 controllers had a significantly higher number of positive HERV-specific T cell responses than HAART-suppressed patients (*, P < 0.05), noncontrollers (**, P < 0.01), progressors (***, P < 0.0001), and HIV-negative subjects (***, P < 0.0001). A positive response was defined as >50 SFU/million PBMC and >2 times the negative-control value (after subtraction of the value of the negative control). P values are shown only for statistically significant pairwise comparisons (derived from the Mann-Whitney test and the Dunn's multiple comparison test as posttests to the Kruskal-Wallis test). Medians with interquartile range are shown for each group.
Untreated HIV-1-infected subjects with preserved CD4+ T cell counts do not differ in the magnitude of their HIV-1 and CMV responses.
In order to characterize the baseline immune function of the HIV-1-infected subjects, responses to an HIV-1 peptide pool (which included Gag, Nef, Env, and Pol sequences) and a CMV pp65 peptide pool were assessed in a subset of individuals for whom adequate PBMC samples were available. There was no significant difference in the CMV pp65 pool response magnitude among the three HIV-1-infected groups with preserved CD4+ T cell counts (P > 0.05 for all pairwise comparisons) (see Fig. S1A in the supplemental material). However, the progressors had a lower median CMV response magnitude than the controllers and HAART-suppressed patients (P < 0.01 and P < 0.05, respectively). The HIV-1 controllers, noncontrollers, and progressors did not differ in their responses to the HIV-1 peptide pool (P > 0.05 for all pairwise comparisons), but the controllers had stronger HIV-1 pool responses than the HAART-suppressed patients (P < 0.05) (see Fig. S1B). Consistent with previous reports using the IFN-γ ELISPOT assay (2), there was no correlation between the magnitude of the HIV-1 pool response and HIV-1 plasma viremia in untreated subjects (r2 = 0.007, P = 0.525) (see Fig. S1C). There was also no significant association between the magnitude of the HIV-1 pool responses and the HERV responses in untreated patients (r2 = 0.033, P = 0.164) (see Fig. S1D).
HERV-specific immunity correlates with control of HIV-1.
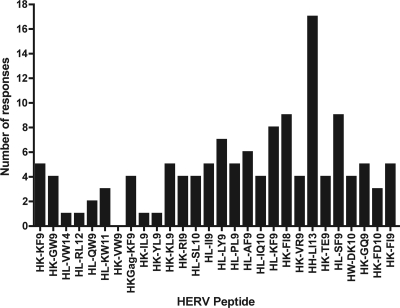
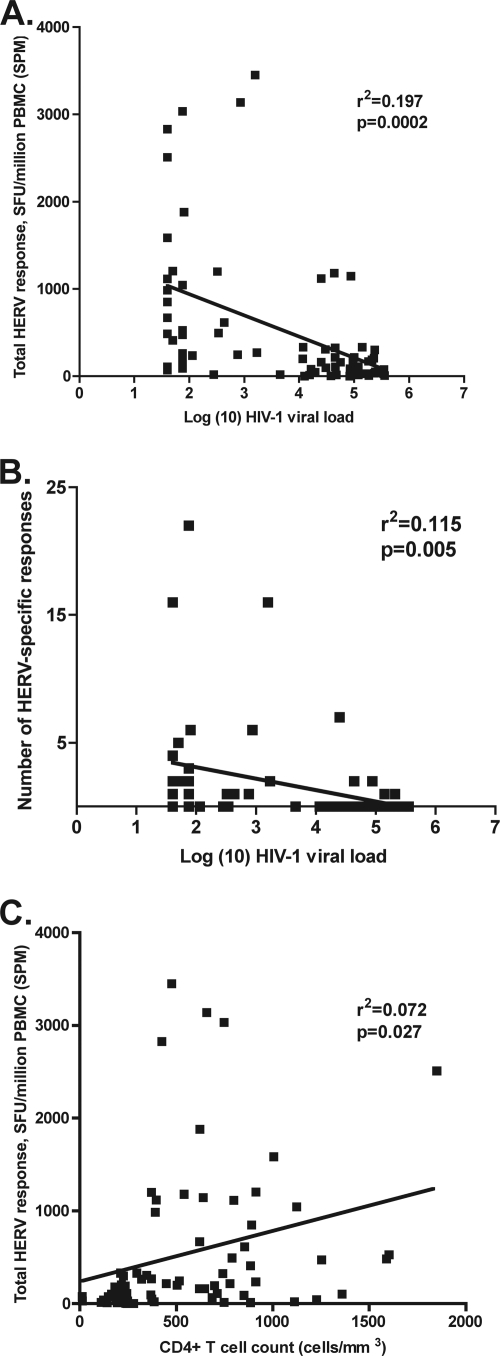
Twenty-seven out of the 28 HERV peptides tested elicited at least one positive response in the HIV-1-infected cohort, with the majority of the peptides eliciting responses from two or more individuals (Fig. 2). There was no difference between the number of “unique” HERV peptides that stimulated a response and the number of immunogenic HERV peptides that shared four or more amino acids in common with an HIV-1 epitope (“similar”; median number of responses elicited by both unique and similar HERV peptides, 4; P = 0.63). In the untreated HIV-1-infected individuals (controllers, noncontrollers, and progressors), the HIV-1 viral load was inversely correlated with the magnitude of the HERV T cell response (r2 = 0.197, P = 0.0002 by linear regression; Spearman r = −0.535, P < 0.0001) (Fig. 3A) and the breadth of HERV T cell response (r2 = 0.115, P = 0.005 by linear regression; Spearman r = −0.339, P = 0.005) (Fig. 3B). There was also a positive correlation between HERV response magnitude and CD4+ T cell count in untreated patients (r2 = 0.072, P = 0.027 by linear regression; Spearman r = 0.269, P = 0.027) (Fig. 3C). The inverse correlation between HERV response magnitude and HIV-1 viral load persisted even when the progressor group (CD4+ T cell count of <250 cells/mm3) was excluded (r2 = 0.091, P = 0.037 by linear regression; Spearman r = −0.302, P = 0.037) (data not shown).
Fig. 2.
The number of positive responses (defined as having a magnitude of >50 SFU/million PBMC and >2 times the magnitude of the negative control after subtraction of the value of the negative control in IFN-γ ELISPOT assays) to each HERV peptide among all study subjects.
Fig. 3.
Both the magnitude (A) and the breadth (B) of the HERV response were significantly inversely correlated with HIV-1 viral load in untreated HIV-1-infected subjects. The HERV response was also positively associated with higher CD4+ T cell counts (C). r2 and P values (by linear regression) are as shown on the figure.
Mapping of responses against HERV-K Gag and Env in an HIV-1-infected controller.
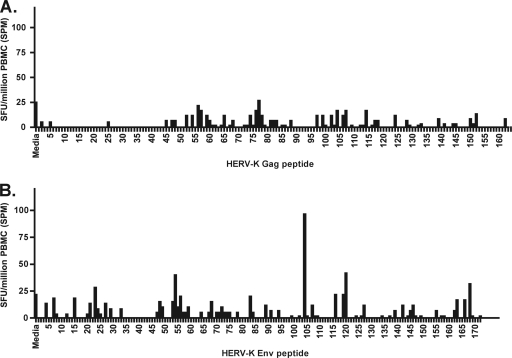
In order to more comprehensively characterize the T cell response against HERV-K (the youngest and most transcriptionally active HERV family), we tested a set of 336 overlapping 15-mer peptides spanning HERV-K Gag and Env in a controller (P1, for whom large numbers of PBMCs were available) who was infected with HIV-1 in 1990 and maintained an undetectable viral load over the following 2 decades. We detected a positive response to an HERV-K Env peptide, CIDSTFNWQHRILLV, at a 2003 time point (Fig. 4) that persisted and increased in magnitude over the next 5 years.
Fig. 4.
Comprehensive mapping of responses to HERV-K Gag (A) and Env (B) in one HIV-1-infected elite controller (P1) using overlapping peptides revealed a response to HERV-K Env peptide 104 (CIDSTFNWQHRILLV).
Characterization of responses against two similar HERV-K and HIV-1 epitopes in a unique subject.
We further investigated one particularly immunogenic peptide from the reverse transcriptase region of HERV-K, FAFTIPAI (FI8). More than one-quarter of the HIV-1-infected controllers that were tested recognized this peptide. One individual (P2) had an especially robust ELISPOT response (580 SPM) to FI8, which titrated to 1 μg/ml (Fig. 5A). In addition, a strong response to an HIV-1 peptide with a similar sequence, TAFTIPSI (TI8), was observed. This subject was infected with HIV-1 in 1999 and maintained a CD4+ T cell count above 400 cells/mm3 and an HIV-1 viral load below 6,000 copies/ml for almost a decade but eventually began HAART in 2007 (Fig. 5B). Longitudinal analysis of this individual's responses to HERV-K FI8, HIV-1 TI8, and an HIV-1 peptide pool showed that all three waned in 2005, just before the patient initiated HAART, whereas the response to a CMV pp65 peptide pool was maintained (Fig. 5C).
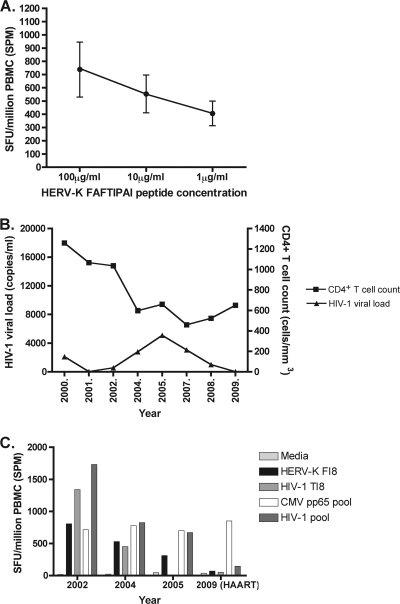
Fig. 5.
(A) The strength of subject P2's T cell response to the HERV-K FAFTIPAI peptide decreased according to the peptide concentration in the ELISPOT assay well. (B) Subject P2's HIV-1 viral load and CD4+ T cell count over the course of 10 years. (C) This individual's response to HERV-K FI8, homologous HIV-1 peptide TI8, and an HIV-1 peptide pool waned just before the subject was put on HAART. In contrast, the CMV pp65 response was maintained over these time points. Note that the response to HIV-1 TAFTIPSI was not tested at the 2005 time point.
In order to determine the HLA restriction of the HERV-K FI8 response in P2 (who was HLA-B51+), we tested the ability of patient-derived PBMCs to recognize single HLA-allele B cell transfectants that had been pulsed with peptide FI8 in an ELISPOT assay. The peptide-pulsed HLA-B51+ B cells were recognized to a greater degree than the unpulsed B cells and HLA-B51− B cells, confirming that P2's response to this epitope is restricted by HLA-B51 (Fig. 6).
Fig. 6.
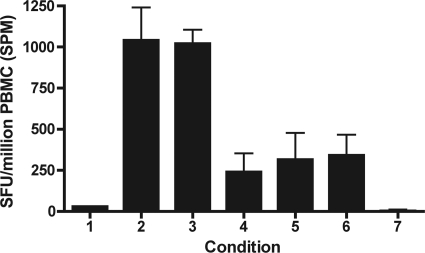
Subject P2's response to peptide HERV-K FI8 was found to be restricted by the HLA-B51 allele in an ELISPOT-based assay using peptide-pulsed and unpulsed HLA-matched and-mismatched B cells as antigen presenting cells. P2's PBMCs were incubated with the following: lane 1, medium; lane 2, HERV-K FI8 peptide; lane 3, HLA-B51+ B cells pulsed with FI8 peptide; lane 4, HLA-B51+ B cells not pulsed with peptide; lane 5, HLA-B51− B cells pulsed with FI8 peptide; lane 6, HLA-B51− B cells not pulsed with peptide. Lane 7 represents all of the above conditions using B cells alone, without subject P2's PBMCs, in the ELISPOT assay.
The HERV-K FAFTIPAI response is more differentiated and less activated than the HIV-1 TAFTIPSI response.
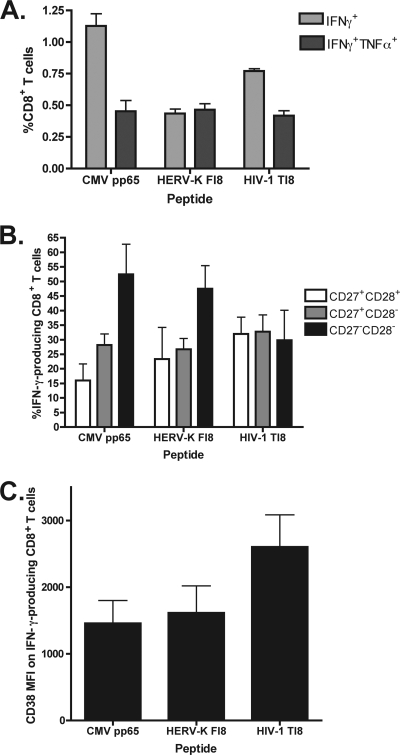
To further characterize P2's responses to the two similar peptides, HERV-K FI8 and HIV-1 TI8, we evaluated the functionality and phenotypes of the CD8+ T cell populations stimulated by these antigens. We also included a CMV pp65 pool in order to examine how these responses differ from one that is maintained long-term and is associated with a controlled chronic viral infection. An intracellular cytokine detection assay was used to analyze the simultaneous production of IFN-γ and TNF-α in antigen-specific cells. The CD8+ population stimulated by HERV-K FI8 had similar proportions of dual-cytokine (IFN-γ and TNF-α)-producing and monofunctional (IFN-γ+) cells, as opposed to the primarily IFN-γ-only-producing population specific to the HIV-1 TI8 epitope (Fig. 7A; see also Fig. S2A in the supplemental material).
Fig. 7.
(A) Of the cytokine-producing cells in the population of CD8+ T cells stimulated by the HERV-K FAFTIPAI (FI8) peptide, the proportion producing both IFN-γ and TNF-α was similar to that producing IFN-γ only, whereas the HIV-1 TAFTIPSI (TI8)-specific population was dominated by IFN-γ production. (B) The HERV-K FI8-specific CD8+ population had a differentiation profile similar to that of the CMV pp65 pool-specific cells (the highest proportion being CD27− CD28−, consistent with a late-differentiation stage) and distinct from that of the HIV-1 TI8-specific cells (most cells being CD27+, consistent with a less mature stage). (C) The HERV-K FI8-specific CD8+ population had a lower level of activation than the HIV-1 TI8-specific CD8+ cells, as measured by CD38 mean fluorescence intensity. Values are means and standard errors of the means.
To assess the differentiation stage of CD8+ T cells that produced cytokines against HERV-K FI8, HIV-TI8, and the CMV pool, three subpopulations based on coexpression of T cell maturation markers CD27 and CD28 were quantified. CD8+ T cells were defined as CD27+ CD28+ (early stage), CD27+ CD28− (intermediate stage), or CD27− CD28− (late stage) (3, 5). The IFN-γ+ population stimulated by HIV-1 TI8 had a higher proportion of cells at an early to intermediate stage of differentiation (CD27+ CD28+ and CD27+ CD28−). In contrast, the IFN-γ-producing cells stimulated by HERV-K FI8 had largely a late-differentiation phenotype (CD27− CD28−). Similarly, the IFN-γ+ CMV-specific population had a late stage of differentiation (Fig. 7B; see also Fig. S2B in the supplemental material). In addition, the HERV-K FI8-specific CD8+ T cells were less activated (as measured by a CD38 mean fluorescence intensity [MFI] of 1,615) than the HIV-1 TI8-specific cells (CD38 MFI, 2,603) and had a level of activation that was comparable to that of the CMV-specific CD8+ population (CD38 MFI, 1,458) (Fig. 7C; see also Fig. S2C).
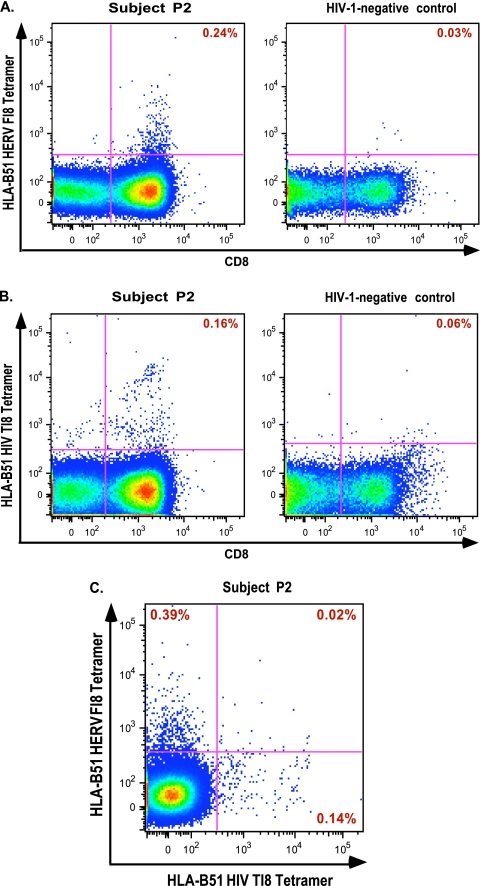
Single-tetramer staining of PBMCs from patient P2 and an HIV-1 uninfected control with either HLA-B51-HERV-K FAFTIPAI-APC (Fig. 8A) or B51-HIV-1 TAFTIPSI-PE (Fig. 8B) showed tetramer-positive CD8+ populations in P2 but not in the control subject. Finally, by performing dual staining with both tetramers, we confirmed that HERV-K FI8 and HIV-1 TI8 stimulated separate CD8+ T cell populations in subject P2. We identified two distinct populations that each stained positive for either the HLA B51-HERV-K FAFTIPAI-APC tetramer or the B51-HIV-1 TAFTIPSI-PE tetramer (Fig. 8C).
Fig. 8.
Single staining with an HLA-B51 HERV-K FI8 tetramer (A) and an HLA-B51 HIV-1 TI8 tetramer (B) in subject P2 and a healthy HIV-1-negative control. (C) Dual tetramer staining revealed two distinct CD8+ T cell populations within subject P2.
DISCUSSION
Human endogenous retroviruses (HERVs) are thought to be inactive elements of our genome, normally held in check by host cellular restriction proteins such as APOBEC3G (11). We hypothesized that the impairment of these controls in HIV-1 infection could expose the immune system to HERV antigens that act as immutable targets for cytotoxic lysis, resulting in the containment of HIV-1 viremia. Previously, our lab has shown that HERV expression occurs in HIV-1-infected cells and that HERV-specific CD8+ T cell responses are stimulated in primary HIV-1 infection (12). These responses are negatively correlated with HIV-1 viral load (12). Here, we extend these observations to include individuals who have long-term chronic HIV-1 infection, focusing on a rare subset of individuals who are able to suppress HIV-1 indefinitely in the absence of combination therapy. The mechanism of control in these HIV-1 controllers remains to be fully defined. Although there is evidence that the HIV-specific T cell response contributes to virologic containment (1, 10), it is clear from a number of studies that other factors are likely to be involved (10, 26). A recent genome-wide association study in a multiethnic cohort of HIV-1 controllers and progressors showed that differences in HLA alleles explain 19% of the variance of host control (27). In order to determine what effect, if any, HERV-specific T cell responses have on viral control, we studied a cohort of HIV-1 controllers and compared their responses to those of untreated virologic noncontrollers and immunologic progressors, patients on HAART, and HIV-1-uninfected controls. While it is likely that robust CD4+ T cell help contributes to strong CD8+ T cell responses, we sought to rule out that it was the primary cause of any observed differential responses to HERV antigens. Therefore, to avoid a confounding effect of generalized immune dysfunction due to a lack of CD4+ T cell help, we performed additional analyses that excluded the immunologic progressor group. Our finding that the magnitude of CMV pp65-specific responses was similar in the controller, HAART-suppressed, and noncontroller groups supports the fulfillment of this criterion.
We found that in chronic HIV-1 infection, controllers have HERV responses with higher magnitude and greater breadth than patients with viral suppression on HAART, virologic noncontrollers, immunologic progressors, and HIV-1-negative subjects. Interestingly, controllers who lack HLA alleles that are associated with protection from HIV-1 disease progression (HLA-B27 and -B57) constituted a large proportion of the subjects with the strongest HERV responses, suggesting that there may be an alternative mechanism of HIV control (such as HERV-specific cytotoxic T cells) in these controllers. There was no difference in the magnitude of the HIV-1-specific responses between the controllers, noncontrollers, and progressors (as defined by IFN-γ production) and no correlation between this measure and HIV-1 plasma viremia. These data are consistent with other studies reporting that neither the magnitude nor the breadth of the HIV-1-specific CD8+ T cell response is associated with a difference in viral load (2). Of note, there was also no significant correlation between the strength of HERV-specific responses and HIV-1-specific responses in untreated patients, suggesting that these are independent variables. In contrast, we discovered that the HERV-specific T cell response magnitude was inversely correlated with HIV-1 viral load (even when the immunologic progressors were excluded), suggesting a potential role for these responses in the control of HIV-1. There was also a positive correlation between HERV response magnitude and CD4+ T cell count in untreated individuals. The group with viral suppression on HAART responded to fewer HERV epitopes and had weaker responses, lending support to the hypothesis that these responses are a cause rather than a consequence of viral control.
Longitudinal analyses of HERV responses in two subjects revealed interesting differences. One individual (P2) had a strong, titratable response to the HERV-K FAFTIPAI epitope. This person was able to contain HIV-1 replication to low levels and maintain normal CD4+ T cell counts for almost a decade. Interestingly, the patient eventually initiated HAART, and we observed a decline in the magnitude of this person's HERV response in the few years preceding this juncture. In contrast, another subject (elite controller P1) with more than 15 years of undetectable HIV-1 viral load and normal CD4+ T cell count without HAART had an increase in his response to a different HERV-K epitope over 5 years. These data are generally consistent with our primary hypothesis that the generation and maintenance of HERV-specific responses may contribute to virus control.
To investigate possible mechanisms of protection of the HERV-specific response, we took advantage of P2's comparably strong ELISPOT responses to two very similar HERV and HIV-1 peptides (HERV-K FI8 and HIV-1 TI8). After defining the HLA restriction (B51) of the HERV response, we compared its functionality and phenotype to those of the HIV-1 epitope and a CMV pp65 peptide pool. We found that within the HERV-K FI8-specific population, there were as many cells producing two cytokines as there were producing just one. In contrast, the HIV response was dominated by monofunctional (IFN-γ-secreting) cells. IFN-γ secretion is a first-line antiviral defense mechanism and promotes the expression of TNF-α receptors on the cell surface, among other functions (28). TNF-α, in turn, has broad, beneficial effects in protective immunity and can kill virally infected target cells by binding to the cell surface receptor and triggering an apoptosis signaling cascade (reviewed in reference 21). The combination of the two cytokines is therefore critical for an effective immune response and has been shown to clear hepatitis B from hepatocytes and lymphocytic choriomeningitis virus (LCMV) from acutely infected mice (14, 23). HIV-1-specific CD8+ T cells that can generate a multifunctional response have been linked to slower disease progression, and polyfunctional T cell responses are associated with chronic control of other viral infections such as CMV, Epstein-Barr virus (EBV), and influenza virus (6). We were surprised to find that even a HERV epitope that shares close homology with an HIV-1 sequence (having six out of eight amino acids in common) could trigger a distinct CD8+ T cell response. Given the inverse correlation between HIV-1 viral load and the HERV response in our cohort, we might expect this pattern to hold true for the total HERV-specific and HIV-1-specific CD8+ T cell population in this individual and others, though this remains to be tested.
The observed differences in functionality of the HERV and HIV-1 responses in our subject hinted that there could also be a divergence in their phenotypic parameters, leading us to examine their differentiation and activation profiles. Dual staining with the HLA-B51 tetramers confirmed that two distinct and specific T cell populations that correspond to each of these antigens exist and also ruled out the potential of a cross-reactive effect. In our previous report (12), we compared the phenotype of CD8+ T cells responding to a CMV pool and to two unique HERV and HIV-1 peptides without a high degree of sequence homology. Unlike the maturationally deficient HIV-1-specific population, the HERV-specific CD8+ T cells were similar to the CMV-specific CD8+ T cells in having a terminally differentiated phenotype that is associated with improved viral control (25). Our present study confirms this even though the two HERV and HIV-1 epitopes were similar and also demonstrates that the HERV response was comparable to the CMV response in its level of activation. The HERV-specific population had a late-differentiation (CD27− CD28−) and low-activation (as measured by CD38 MFI) phenotype, which has previously been associated with greater cytotoxic activity (3). In contrast, the HIV-1-specific population was more immature and had a higher level of activation. A disparity in activation level between HIV-1-specific cells and CMV-specific cells within the same individual was also recently reported by Barbour, et al. (5). A lack of CD38 on the surface of virus-specific T cells has been correlated with control of infections, including HIV-1 and EBV, and has been linked to polyfunctional cytokine production, MIP-1β chemokine secretion, proliferative capacity, and lack of exhaustion as measured by PD-1 expression (6). Conversely, HIV-specific CD8+ T cells from viremic patients tend to be CD38+ PD-1+ and monofunctional (secreting only IFN-γ) (6). While the combination of these measures was not examined in the current study, the lack of CD38 expression on the HERV-specific T cell population suggests that this epitope stimulates cytotoxic T lymphocytes (CTLs) with these favorable characteristics and warrants additional exploration.
Another factor contributing to the ability of HERV-specific CTLs to proliferate and kill target cells may be linked to their level of avidity. As HERVs are encoded in the human genome and therefore represent self-antigens, T cells directed against them may bind to their cognate epitopes with low avidity. This is consistent with the increased peptide concentrations required to generate a response in many of our subjects. Low-avidity T cells (which are more prone to tolerance induction and are stimulated only with high antigen load) have been shown to proliferate better than high-avidity T cells and to lack their replicative defects (16). Therefore, HERV-specific CTLs may play a role in the containment of HIV-1 due to a superior ability to proliferate. Of note, we previously showed that despite being directed against self-antigens, HERV-specific CTLs are not impaired in their ability to kill cells pulsed with their cognate peptide (12). Overall, our present study has identified phenotypic and functional traits of the HERV-specific response that may confer an enhanced ability beyond that of most HIV-specific CTLs to effectively target and lyse infected cells. Our finding that the HIV-1 viral load was lower in patients with stronger and broader HERV responses provides empirical evidence for this.
Our results constitute the discovery of a novel correlate of immune protection from disease progression in chronic HIV-1 infection and have implications for both a better understanding of HIV-1 pathogenesis and a potential new approach to HIV-1 vaccines. While the primary advantage of a therapeutic agent derived from HERVs is their conserved nature, features associated with a superior cytotoxic ability offer an additional benefit to the induction of HERV-specific responses. Our findings show that individuals who can control HIV-1 in the absence of HAART have the greatest HERV responses. This HERV-specific immunity is correlated with the containment of HIV-1 replication and preservation of CD4+ T cell count in untreated subjects and provides strong support for the further investigation of an HERV-based HIV-1 vaccine.
Supplementary Material
Acknowledgements
We thank the NIH Tetramer Core Facility for synthesis of the HLA-B51 tetramers and Lewis Lanier (University of California, San Francisco) for providing single-HLA allele transfectant B cells used for HLA restriction experiments. We acknowledge Neil Sheppard, Peter Loudon, and James Merson of Pfizer for their helpful discussions.
This work was supported by the following funding sources: a Pfizer-sponsored research agreement; NIH grants AI76059 and AI84113; University of California, San Francisco-Gladstone Institute of Virology and Immunology Center for AIDS Research (CFAR), an NIH-funded program (P30 AI027763); and The Fogarty International Center, grant D43 TW00003. This work was supported in part by the Centers for AIDS Research at UCSF (PO AI27763) and the UCSF Clinical and Translational Science Institute (UL1 RR024131). Additional support was provided by NIAID (RO1 AI087145 and K24AI069994), American Foundation for AIDS Research (106710-40-RGRL), the NIH/NIAID CFAR Network of Integrated Clinical Systems (grant 1 R24 AI067039-1), and the Ragon Institute. C.E.O. was funded by Consejo Nacional de Ciencia y Tecnología, Mexico (SALUD-2009-01-115265).
R.B.J., K.E.G., D.F.N., F.M.H., and M.A.O. are listed as inventors on a patent application related to this work.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 27 April 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Addo M. M., et al. 2007. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS One 2:e321.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Addo M. M., et al. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appay V., et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385 [DOI] [PubMed] [Google Scholar]
- 4. Bannert N., Kurth R. 2004. Retroelements and the human genome: new perspectives on an old relation. Proc. Natl. Acad. Sci. U. S. A. 101(Suppl. 2):14572–14579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbour J. D., et al. 2009. High CD8+ T cell activation marks a less differentiated HIV-1 specific CD8+ T cell response that is not altered by suppression of viral replication. PLoS One 4:e4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Betts M. R., et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Contreras-Galindo R., et al. 2006. A new real-time RT-PCR for quantitation of human endogenous retroviruses type K (HERV-K) RNA load in plasma samples: increased HERV-K RNA titers in HIV-1 patients with HAART non-suppressive regimens. J. Virol. Methods 136:51–57 [DOI] [PubMed] [Google Scholar]
- 8. Contreras-Galindo R., et al. 2008. Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer. J. Virol. 82:9329–9336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deeks S. G., Walker B. D. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416 [DOI] [PubMed] [Google Scholar]
- 10. Emu B., et al. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 82:5398–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esnault C., Priet S., Ribet D., Heidmann O., Heidmann T. 2008. Restriction by APOBEC3 proteins of endogenous retroviruses with an extracellular life cycle: ex vivo effects and in vivo “traces” on the murine IAPE and human HERV-K elements. Retrovirology 5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrison K. E., et al. 2007. T cell responses to human endogenous retroviruses in HIV-1 infection. PLoS Pathog. 3:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gramberg T., Sunseri N., Landau N. R. 2009. Accessories to the crime: recent advances in HIV accessory protein biology. Curr. HIV/AIDS Rep. 6:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guidotti L. G., et al. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825–829 [DOI] [PubMed] [Google Scholar]
- 15. Hecht F. M., et al. 2002. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 16:1119–1129 [DOI] [PubMed] [Google Scholar]
- 16. Horton H., et al. 2006. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J. Immunol. 177:7406–7415 [DOI] [PubMed] [Google Scholar]
- 17. Hunt P. W., et al. 2003. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187:1534–1543 [DOI] [PubMed] [Google Scholar]
- 18. Kurth R., Bannert N. 2010. Beneficial and detrimental effects of human endogenous retroviruses. Int. J. Cancer 126:306–314 [DOI] [PubMed] [Google Scholar]
- 19. Lander E. S., et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860–921 [DOI] [PubMed] [Google Scholar]
- 20. Larsen M. V., et al. 2007. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics 8:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makedonas G., Betts M. R. 2006. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin. Immunopathol. 28:209–219 [DOI] [PubMed] [Google Scholar]
- 22. Meiklejohn D. A., et al. 2004. ELISPOT cell rescue. J. Immunol. Methods 288:135–147 [DOI] [PubMed] [Google Scholar]
- 23. Mestan J., et al. 1986. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature 323:816–819 [DOI] [PubMed] [Google Scholar]
- 24. Muckenfuss H., et al. 2006. APOBEC3 proteins inhibit human LINE-1 retrotransposition. J. Biol. Chem. 281:22161–22172 [DOI] [PubMed] [Google Scholar]
- 25. Northfield J. W., et al. 2007. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ TEMRA cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J. Virol. 81:5759–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereyra F., et al. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571 [DOI] [PubMed] [Google Scholar]
- 27. Pereyra F., et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramana C. V., et al. 2000. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J. 19:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rammensee H., Bachmann J., Emmerich N. P., Bachor O. A., Stevanovic S. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219 [DOI] [PubMed] [Google Scholar]
- 30. Saag M., Deeks S. G. 2010. How do HIV elite controllers do what they do? Clin. Infect. Dis. 51:239–241 [DOI] [PubMed] [Google Scholar]
- 31. Sekaly R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stopak K., de Noronha C., Yonemoto W., Greene W. C. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell 12:591–601 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi Y., et al. 2008. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J. Clin. Invest. 118:1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.