Abstract
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the brain caused by JC virus (JCV). To assess the role of CD4+ and CD8+ T-cells against JCV in the clinical outcome of PML and PML in the setting of immune reconstitution inflammatory syndrome (IRIS), we tested gamma interferon (IFN-γ) response by enzyme-linked immunosorbent spot (ELISpot) and intracellular cytokine staining (ICS) in 117 subjects, including 66 PML patients with different clinical outcomes. Both assays were concordant and demonstrated that the cellular immune response against JCV is associated with better clinical outcome. PML survivors had an early CD8+ T-cell response more frequently than PML progressors (100% versus 27.3%; P = 0.001), while only a trend was observed for the early CD4+ T-cell response between these two groups (80% versus 45.5%; P = 0.18). Although IRIS itself was more frequent in the PML survivor group, there was no difference in IFN-γ-producing CD4+ and CD8+ T-cells between IRIS and non-IRIS PML patients, suggesting that T-cells expressing other cytokines likely have a role in the immunopathogenesis of IRIS. ELISpot and ICS assays are useful prognostic markers of PML evolution and may help in the clinical management of these patients.
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the brain caused by JC virus (JCV). A majority of people sustain primary infection with JCV during childhood, after which the virus remains quiescent in healthy individuals throughout life. However, reactivation of JCV may occur in the setting of severe immunosuppression, such as in patients with HIV/AIDS, those treated with immunomodulatory medications, and organ transplant recipients. Reactivation may lead to PML (28), although it has also been described in the setting of minimal or occult immunosuppression (14).
PML may also manifest itself or worsen during immune reconstitution inflammatory syndrome (IRIS). PML with IRIS (PML-IRIS) is defined by an inflammatory reaction within PML lesions, which can be associated with paradoxical worsening or development of new neurological dysfunction in the setting of recovery of the immune system (29). Although PML-IRIS was first recognized in HIV infection, it has also been observed in HIV-negative persons following discontinuation of immunosuppressive or immunomodulatory medications, such as in multiple sclerosis patients with natalizumab-induced PML (22, 32). In the setting of HIV disease, a lower CD4+ T-cell nadir is considered a risk factor for the development of IRIS (26), but the pathophysiology of IRIS is poorly understood. After patients start combined antiretroviral therapy (cART), there is an initial and rapid increase in CD4+ T-cells and more specifically a release of memory T-cells from recovering lymphoid tissues (reviewed in reference 16), but the role of CD4+ T-cells in the setting of PML-IRIS has not been previously studied. In the absence of a biomarker, IRIS is defined based on clinical and radiological grounds. Furthermore, symptoms associated with IRIS may vary in intensity (16), and thus treatment of PML-IRIS with corticosteroids to dampen the ongoing inflammation in the brain remains a matter of debate (29).
We and others have investigated the cellular immune response against JCV using assays of proliferation in response to JCV antigen, measuring the function of CD4+ T-cells (13), or using JCV peptide stimulation for measurement of CD8+ T-cells by tetramer staining or 51Cr release assays (8, 18). These studies showed that early detection of JCV-specific CD8+ lymphocytes is associated with better control of PML (9) and longer survival (25). However, the 51Cr release assay is cumbersome, and the tetramer staining assay is limited by the HLA restriction of the selected epitope. We, therefore, chose to use the gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) and intracellular cytokine staining (ICS) assays for the measurement of the cellular immune response to JCV. These assays are commonly used to detect and quantify the cellular immune response of a host against a virus in the blood and to monitor this response in vaccine trials. They are based on the release of IFN-γ by T-cells after recognition of their cognate antigens. IFN-γ upregulates several genes, which have antiviral, apoptotic, and immunomodulatory functions, through the JAK/STAT pathway (reviewed in reference 23). While ELISpot measures both CD4+ and CD8+ antigen-specific T-cells, ICS makes the distinction between antigen-specific CD4+ and CD8+ T-cell responses. However, a major limitation in the study of the cellular immune response to JCV is the very low frequency of specific T-cells and the difficulty in detecting them in peripheral blood ex vivo (21). To further characterize the cellular immune response to JCV and to differentiate the role of CD4+ and CD8+ T-cells, we performed ELISpot and ICS in PML patients with various clinical outcomes, including those with IRIS, and in control subjects after stimulation of peripheral blood mononuclear cells (PBMC) with JCV peptides in vitro.
MATERIALS AND METHODS
Subjects.
All subjects participating in this study signed informed consent according to the local institutional review board (IRB) guidelines and to the guidelines of the IRB of the Beth Israel Deaconess Medical Center.
A total of 117 study subjects were enrolled in this study and were divided into 5 different groups: 1, 6 PML-early (PML-E) patients, who were within 1 year of neurological symptom onset; 2, 18 PML progressors (PML-P), who died within 1 year of symptom onset; 3, 42 PML survivors (PML-S), who survived more than 1 year after symptom onset; 4, 10 HIV+ controls who did not have PML; 5, 41 healthy HIV-negative control subjects (HC).
PML diagnosis was established on the basis of either uni- or multifocal progressive neurological disease with typical magnetic resonance imaging (MRI) findings and positive brain biopsy (histology-confirmed PML) or positive JCV DNA PCR in cerebrospinal fluid (CSF) (laboratory-confirmed PML) (6). We also tested 11 patients who had clinical and radiological findings consistent with PML but no demonstration of JCV in the CSF and for whom brain biopsy was not performed. These were considered possible PML cases (3 progressors and 8 survivors), but they were excluded from all the primary analyses (6).
Patients with immune reconstitution inflammatory syndrome (IRIS) were defined as patients who presented with a paradoxical development of PML or developed an inflammatory reaction at a site of a previously diagnosed PML lesion(s) in the setting of recovery of the immune system. This was accompanied by contrast enhancement on MRI. In HIV+ patients, immune reconstitution was furthermore characterized by an increase of CD4+ T-cell count and decrease of HIV plasma viral load.
Peptide library.
VP1 is the major capsid protein of JCV. To detect JCV VP1-specific T-cells with gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) and intracellular cytokine staining (ICS) assays, we use a JCV VP1 protein library, which consists of 97 15-amino-acid (aa) peptides, which overlap by 11 aa, spanning the entire VP1 protein. The peptides are divided into four sequential pools, A to D as follows: pool A, p1 to p93 (n = 24); pool B, p97 to p157 (n = 24); pool C, p161 to p253 (n = 24); and pool D, p257 to p341 (n = 25). This overlapping peptide library allowed us to detect JCV-specific CD4+ or CD8+ T-cells regardless of the HLA alleles of the study subjects.
PBMC preparation and culture.
Thirty milliliters of heparinized blood was collected from each study subject. The tubes were spun at 1,400 rpm for 5 min, and plasma was removed. The remaining blood fraction was mixed 1:1 with Dulbecco's phosphate-buffered saline (D-PBS). Peripheral blood mononuclear cells (PBMC) were separated by Ficoll-Paque gradient centrifugation, washed, and resuspended in R-12 (RPMI 1640–12% fetal calf serum [FCS]) media to a concentration of 3.5 × 106 cells/ml. PBMC (7 × 106) were plated without peptides to serve as the negative control or stimulated with each overlapping peptide pool (A to D) in R-12 medium and after 72 h were supplemented with 25 U/ml interleukin-2 (IL-2) and incubated for 10 to 14 days (4). Prior to the ELISpot and ICS assays, the PBMC were incubated in IL-2-free medium for 16 to 24 h.
ELISpot assay.
ELISpot assays were done as previously described (19). Briefly, a multiscreen 96-well plate (Millipore) was prepared with diluted purified anti-human IFN-γ monoclonal antibody (Ab) (0.5 μg/ml) (B27; BD Pharmingen). After being counted with a Guava automated cell counter, 100,000 cells per well were plated in the presence of 50 μl of peptide dilution at a 2-μg/ml final concentration. These cells were used to measure the absolute patient's response. In addition, 100,000 cells per well were stimulated with phytohemagglutinin (PHAM) (10 μg/ml) and used as positive control and 100,000 cells per well were not restimulated (either with one of the peptide pools or with PHAM) and were used to measure the baseline IFN-γ secretion. Each condition was tested in triplicate. After incubation overnight at 37°C, the cells were washed and incubated with rabbit polyclonal anti-human IFN-γ–biotin (Biosource) for 2 h at 37°C. After the plate was washed again, 100 μl of streptavidin (Southern Biotechnology; dilution of 20 μl in 10 ml ELISpot reagent buffer) was added to each well and the plate was incubated for 45 min at room temperature. The plate was then washed three times with D-PBS–Tween 20 and three times with D-PBS. Subsequently, 100 μl of nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate chromogen (Pierce) was added to each well and the plate was developed for 7 min. We then air dried the plate for 24 h before analyzing it on the ELISpot plate reader (Hitech Instruments) using Image-Pro Plus image-processing software (version 4.1) (Media Cybernetics, Des Moines, IA). The results were quality controlled by a dedicated ELISpot analyst.
The ELISpot results were reported after subtraction of the baseline IFN-γ secretion from the absolute patient's response. A test was considered positive when the number of spot-forming units (SFU) was three times greater than the baseline, had a coefficient of variability (CV) less than 70% in triplicate wells, and was greater than 50 per 106 cells after subtraction of the baseline.
ICS assay.
Intracellular cytokine staining was done as previously described (19). Briefly, after being counted with the Guava automated cell counter, 106 cells, resuspended in 100 μl, were added to 100 μl of a JCV peptide dilution, serving as the absolute patient's response. As for the ELISpot, 106 cells were added to PHAM (10 μg/ml) to obtain a positive control and 106 cells were not restimulated but also went through the ICS to obtain the baseline IFN-γ secretion. All cells were incubated for 1 h at 37°C. Subsequently, 50 μl of diluted 1% monensin (Golgistop; BD Biosciences) was added, followed by incubation for 5 h at 37°C. The reaction was stopped at 4°C overnight. The next day cells were washed and stained with surface marker antibodies for CD8 (BD Biosciences; clone SK1) and CD4 (BD Biosciences; clone L200). Subsequently, cells were fixed with 200 μl Cytofix/Cytoperm (BD Biosciences), washed, and stained with IFN-γ-specific antibody (BD Biosciences; clone B27) and CD3 antibody (BD Biosciences; clone SK-7). Cells were fixed with 1.5% formaldehyde-PBS before samples were analyzed with a FACSCalibur apparatus (BD Biosciences) and FlowJo software (Tree Star), gating on CD3+ and then CD4+ and CD8+ cells. Approximately 500,000 events were collected per sample. Results were expressed as % IFN-γ-producing CD4+ or CD8+ T-cells.
The ICS results were reported after subtraction of the baseline IFN-γ secretion for each pool from the absolute patient's response. A test was considered positive when the percentage of IFN-γ-producing CD4+ or CD8+ T-cells was equal to or greater than twice the baseline IFN-γ secretion.
Statistical analysis.
Prism 4 for MacIntosh was used for statistical analysis, unless otherwise noted, and for creating graphs. Student's t test was done to compare ages and CD4+ T-cell counts in the PML-P and PML-S groups. The first ELISpot and ICS were performed on the initial blood sample obtained after PML symptom onset. For the PML-E and PML-P groups, the first blood draw occurred within 6 months of symptom onset. However, within the PML-S group, some patients had their first blood draw more than 6 months after symptom onset. The PML survivor group was thus divided into two different groups, one tested within 6 months of symptom onset and the other tested more than 6 months after symptom onset. In each group, ELISpot and ICS results were scored as either positive or negative, according to the criteria mentioned above. We used Fisher's exact test to determine statistical significance between the ELISpot and ICS results in the PML-P and PML-S groups. Concordance rates were calculated by counting the concordant ELISpot and ICS results obtained from individual blood samples (either both positive or both negative) and comparing this number to the total number of ELISpot and ICS results that were obtained simultaneously on individual blood samples. ICS results were also scored as positive or negative for CD4+ and CD8+ T-cells in each group. Fisher's exact test was used to determine statistical significance for the CD4+ and CD8+ T-cell responses between the PML-P and PML-S groups.
For the ELISpot assay, the magnitude of the response was measured as the number of SFU/106 cells for peptide pool A through pool D. Subsequently, the mean response was calculated for each subject in each group. The Mann-Whitney test was done to determine statistical significance between the different groups.
For the ICS assay, the magnitude of the CD4 response was measured as the percentage of IFN-γ-producing CD4+ T-cells versus the total CD4+ T-cells in pools A through D. Again, a mean response for pools A to D was calculated for each subject in each group. The Mann-Whitney test was done to determine statistical significance between the different groups. The same analysis was performed for the CD8+ T-cell response.
Analysis similar to that outlined above was done to compare IRIS samples to non-IRIS samples. The Mann-Whitney test was done to determine statistical significance between the two groups.
A linear mixed-effects model with linear and quadratic times using SAS software was used to examine the longitudinal responses of PML survivors and IRIS patients.
RESULTS
Subject characteristics.
Study subject characteristics are presented in Table 1. Of 66 PML patients, 32 (48.5%) were HIV+. HIV+ patients accounted for 25/42 (59.5%) PML survivors (PML-S) and 6/18 (33.3%) PML progressors (PML-P) (Fisher's exact test, P = 0.09). PML-S tended to be younger than PML-P (median age, 47.5 years versus 60.5 years; P = 0.06). The entire group of HIV+ PML-S had higher CD4+ T-cell counts than HIV+ PML-P (median, 334/μl versus 24/μl; P = 0.009), but when only PML-S CD4+ T-cell counts measured within 6 months of symptom onset were compared to CD4+ T-cell counts of PML-P measured within 6 months of symptom onset, the difference between the two groups became less pronounced (median, 223.5/μl versus 24/μl; P = 0.03). The CD4+ T-cell counts of HIV-negative PML-S or PML-P patients were not significantly different (median, 253/μl versus 468/μl; P = 0.65). In the PML-E group, all patients but one were HIV negative and their median CD4+ T-cell count was 532.5/μl. The HIV+ control group had a median CD4+ T-cell count of 579/μl.
Table 1.
Study subject characteristics
| Characteristic | Result for groupa: |
||||
|---|---|---|---|---|---|
| PML-E | PML-P | PML-S | HIV+ | HC | |
| Total no. of patients (no. of HIV+ patients) | 6 (1) | 18 (6) | 42 (25) | 10 (10) | 41 (0) |
| No. (%) of IRIS patients | 3 (50) | 1 (5.6) | 14 (33.3) | NAc | NA |
| Age (yr) range (median) | 20–62 (52) | 36–83 (60.5) | 27–84 (47.5) | 30–70 (45.5) | 20–53 (27) |
| No. of males/females | 2/4 | 13/5 | 30/12 | 9/1 | 19/22 |
| HIV+ CD4+ count/μlb | 33 (n = 1) | 4–61 (24) (n = 5) | 98–1,335 (334) (n = 21) | 15–1,062 (579) (n = 9) | NA |
| Range of HIV− CD4+ counts/μl (median) | 528–537 (532.5) (n = 2) | 41–1,644 (468) (n = 9) | 80–943 (253) (n = 11) | NA | NA |
| HIV VLd (copies/ml)b | 20,700 (n = 1) | Ue–1.2 × 106 (42,842) (n = 5) | U-75,200 (U) (n = 21) | U–126,000 (94) (n = 10) | NA |
| Treatmentsf (no. of patients) | Mi (3), Mi + Me (2) | Mi (9), Mi + Me (5) | Mi (21), Mi + Me (7) | NA | NA |
E, early; P, progressor; S, survivor; HIV+, HIV+ patients; HC, healthy controls.
For n > 1, ranges (medians) are given.
NA, not applicable.
VL, viral load.
U, undetectable.
Mi, mirtazapine; Me, mefloquine.
Other underlying disorders in the HIV-negative patients are presented in Table 2. Treatment consisted of combined antiretroviral therapy (cART) in all HIV+ patients, except for two HIV+ patients in the PML-S group and one patient in the PML-P group. If immunosuppressive medications were given, these were decreased or stopped after diagnosis of PML in HIV-negative patients. Once PML diagnosis was established, 3 PML-E patients, 9 PML-P patients, and 21 PML-S patients received mirtazapine (15 to 45 mg) at bedtime (10). Two PML-E, five PML-P, and seven PML-S patients also received mefloquine, 250 mg/day for 3 days and 250 mg/week thereafter, for up to 6 months (3). There was no significant difference in mirtazapine and/or mefloquine treatment between PML progressors and survivors.
Table 2.
Underlying disorders in HIV-negative patients
| Disorder | No. of patients in group: |
||
|---|---|---|---|
| PML-E | PML-P | PML-S | |
| Hematological malignanciesa | 2 | 4 | 5 |
| Other malignanciesb | 2 | ||
| Other hematological diseasec | 1 | 3 | 5 |
| Autoimmune diseased | 1 | 3 | 3 |
| Transplante | 2 | ||
| Otherf | 1 | 2 | |
Chronic lymphocytic leukemia, non-Hodgkin lymphoma, acute myeloid leukemia, chronic myeloid leukemia, or abdominal B-cell lymphoma.
Brain stem glioma or breast carcinoma.
Idiopathic CD4+ or CD8+ T-cell lymphocytopenia, Waldenstrom macroglobulinemia, common variable immunodeficiency, polycythemia vera, Good's syndrome, or lymphomatosis granulomatosis.
Multiple sclerosis treated with natalizumab, lupus, dermatomyositis, rheumatoid arthritis, or sarcoid.
Kidney or lung transplant.
Alcoholic cirrhosis or unknown.
IRIS was present in 3/6 (50%) PML-E, in 1/18 (5.6%) PML-P, and in 14/42 (33.3%) PML-S. IRIS was thus more frequent in PML survivors than progressors (P = 0.03).
ICS is more sensitive than ELISpot for detection of JCV-specific T-cells after in vitro stimulation.
We first analyzed the rate of concordance between the first ELISpot and ICS assays among subjects of each group; assays were performed using the same blood sample after in vitro stimulation (Table 3). An example of ICS results in PML survivors is shown in Fig. 1. Among all groups, ICS more often tended to have positive results than ELISpot. The rate of concordance between the ICS and ELISpot assays varied between the groups (Table 3). PML progressors more often had discordant results than the other groups. In PML progressors, the ELISpot was positive in only 16.7% of cases compared to 45.5% for the ICS assays. By contrast, the concordance rate of both assays for PML survivors was 77.8%, and ICS tended to be always more sensitive than ELISpot. Interestingly, the rate of concordance between the two assays was stable over time in PML survivors and was similar to that for the healthy control group.
Table 3.
JCV-specific T-cell responses by ELISpot and ICS assays
| Result | No. (%) of patients with result/total no. tested for groupa: |
|||||
|---|---|---|---|---|---|---|
| PML-E, <6 mo | PML-P, <6 mo | PML-S, <6 mo | PML-S, >6 mo | HIV+ | HC | |
| ELISpot + | 3/3 (100) | 2/12 (16.7) | 13/17 (76.5) | 17/21 (81.0) | 7/9 (77.8) | 29/36 (80.6) |
| ICS + | 4/5 (80) | 5/11 (45.5) | 10/10 (100) | 12/12 (100) | 9/9 (100) | 34/39 (87.2) |
| ELISpot/ICS +/+ or −/−b | 2/2 (100) | 5/8 (62.5) | 7/9 (77.8) | 7/9 (77.8) | 6/8 (75) | 28/34 (82.4) |
Times are from PML onset.
Concordance rate for ELISpot and ICS results, performed on the same blood sample.
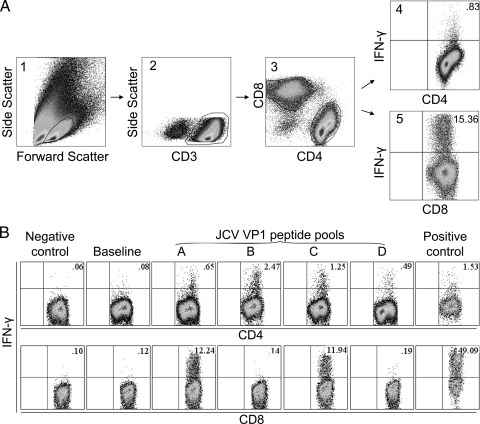
Fig. 1.
Gamma interferon (IFN-γ) response in CD4+ and CD8+ T-cells of 3 PML survivors after stimulation with JC virus peptides. (A) Gating strategy on peripheral blood cells of one PML survivor. Lymphocytes stimulated with JCV peptide pool D are selected by forward and side scatter in panel 1. The CD3+ T-cells are delineated in panel 2, and CD4+ and CD8+ T-cells are then gated in panel 3. IFN-γ-expressing CD4+ and CD8+ T-cells are shown in the upper right quadrants of panels 4 and 5, and their percentages are indicated. (B) IFN-γ response in CD4+ and CD8+ T-cells of 2 PML survivors after stimulation with JC virus peptides. The percentages of IFN-γ-expressing CD4+ T-cells of one patient (upper row) and CD8+ T-cells of another patient (lower row) are indicated in each dot plot. PBMC were stimulated with 97 overlapping 15-mer peptides covering the entire JCV VP1 protein, divided into pools A to D, for 10 to 14 days, rested in serum free-media, and then restimulated with the same pools for 6 h during the ICS assay. Negative control consisted of cells stimulated with no peptide at any given time point. Positive control consisted of cells which were stimulated with phytohemagglutinin (PHAM) during the intracellular cytokine staining. Some cells were stimulated with pools A to D at the time of going into culture but not restimulated at the time of ICS. The IFN-γ secretion of these cells measured during the ICS was considered the baseline IFN-γ secretion for that specific pool. In this example, only the baseline IFN-γ secretion for pool B is shown. The CD4+ T-cell response in each pool was at least twice the baseline and considered positive in all pools, while the CD8+ T-cell response was positive for pools A and C.
PML progressors are less likely than survivors to have a detectable cellular immune response against JCV measured by ELISpot and ICS.
We subsequently compared the results for PML-P and PML-S in the first ELISpot and ICS assays performed in each group (Table 3). The PML-P group had positive ELISpot results less frequently than the PML-S group within 6 months of or 6 months after diagnosis. Both sets of results were statistically significant (16.7% versus 76.5% and 81% [P = 0.003 and 0.0006]). A similar observation was made for ICS results when comparing the PML-P group to the PML-S groups tested either within 6 months of or more than 6 months after disease onset (45.5% versus 100% and 100% [P = 0.012 and 0.0046]).
Early JCV-specific CD8+ T-cell responses, but not CD4+ T-cell responses, are significantly different between PML survivors and progressors.
We then analyzed the results of the first ICS assay based on the CD4+ and CD8+ T-cell responses (Table 4). As expected from the overall responses, the PML-P group had positive responses less frequently than the PML-S group for both CD4 and CD8. Indeed, only 27.3% of the PML-P group had a CD8+ T-cell response versus 100% of the PML-S group within 6 months of PML onset and 91.7% of the PML-S group more than 6 months after PML onset (P = 0.001 and 0.003). However, 45.5% of the PML-P group still had a CD4+ T-cell response compared to 80% of the PML-S group within 6 months of PML onset and 91.7% of the PML-S group more than 6 months after PML onset (P = 0.18 and 0.03). From a calculation of odds ratios, PML survivors were 4.8 times more likely to have an early CD4+ response and 22.2 times more likely to have both early CD4+ and CD8+ responses than PML progressors. While both CD4+ and CD8+ responses are more frequently detected in PML survivors, only the CD8+ T-cell responses were significantly different between PML survivors and progressors early after disease onset.
Table 4.
JCV-specific CD4+ and CD8+ T-cell responses in ICS assay
| Response | No. +/no. tested (%) for groupa: |
|||||
|---|---|---|---|---|---|---|
| PML-E, <6 months | PML-P, <6 months | PML-S, <6 months | PML-S, >6 months | HIV+ | HC | |
| CD4+ | 3/5 (60) | 5/11 (45.5) | 8/10 (80) | 11/12 (91.7) | 7/9 (77.8) | 31/39 (79.5) |
| CD8+ | 4/5 (80) | 3/11 (27.3) | 10/10 (100) | 11/12 (91.7) | 7/9 (77.8) | 25/39 (64.1) |
Times are from PML onset.
The magnitude of the cellular immune response is lower in PML progressors than survivors.
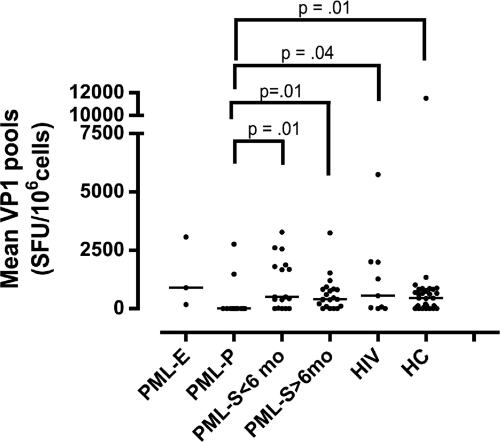
We subsequently examined the magnitude of the T-cell response by the ELISpot for the different groups (Fig. 2). The total cellular immune response measured by the first ELISpot assay was weaker in the PML-P group than in the PML-S group <6 months after disease onset (P = 0.01), the PML-S group >6 months after disease onset (P = 0.01), the HIV control group (P = 0.04), and the HC group (P = 0.01) (Fig. 2).
Fig. 2.
Measurement of the cellular immune response against JC virus by enzyme-linked immunosorbent spot (ELISpot) assay. Results are for the first ELISpot assays in 3 early PML patients (PML-E), 12 PML progressors (PML-P), 17 PML survivors (PML-S) within 6 months of PML onset (<6 mo), 21 PML survivors 6 months after PML onset (>6 mo), 9 HIV control subjects (HIV+), and 36 healthy control subjects (HC). Each dot represents the mean response of a patient to pool A through pool D in the first ELISpot assay. SFU, spot-forming units. The bars indicate the median results within the respective groups.
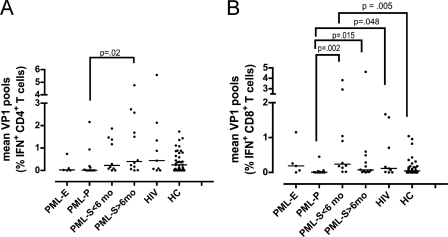
The magnitude of the T-cell response measured by ICS is shown in Fig. 3 A and B. The CD4+ T-cell response was weaker in the PML-P group than in the PML-S group >6 months after disease onset (P = 0.02) (Fig. 3A). In addition, the PML-P group had lower CD8+ T-cell responses than the PML-S group <6 months after disease onset (P = 0.002), the PML-S group >6 months after disease onset (P = 0.015), and the HIV control group (P = 0.048) (Fig. 3B). The PML-S group <6 months after disease onset actually showed stronger CD8+ T-cell responses than the healthy control group (P = 0.005).
Fig. 3.
Measurement of the cellular immune response against JC virus by ICS assay. Shown are CD4+ (A) and CD8+ (B) T-cell responses in the first ICS assay in 5 early PML patients (PML-E), 11 PML progressors (PML-P), 10 PML survivors (PML-S) within 6 months of PML onset (<6 mo), 12 PML survivors 6 months after PML onset (>6 mo), 9 HIV control subjects (HIV+), and 39 healthy control subjects (HC). Each dot represents the mean CD4+ or mean CD8+ T-cell response of a patient to pool A through pool D in the first ICS assay. The mean result is expressed as the percentage of gamma interferon-producing CD4+ T-cells versus the total CD4+ T-cells or the percentage of gamma interferon-producing CD8+ T-cells versus the total CD8+ T-cells. The bars indicate the median results for the respective groups.
The cellular immune response against JCV is stable over time in PML survivors.
We then examined longitudinally the T-cell response in each patient in the PML-S group who was tested several times after disease onset, within 6 days to 12 years after disease onset. Of 25 PML-S patients with longitudinal ELISpot data, 17 (68%) kept their original positive or negative result over time; this was the case for 18/20 (90%) patients with longitudinal ICS data. Because of the nature of the disease, there was not enough longitudinal data to analyze for the PML-P group. When the magnitudes of the ELISpot and ICS assays were examined with a linear mixed-effects model with linear and quadratic times, it was found that the cellular immune response remained stable over time in the PML survivor group (data not shown).
IRIS is not associated with a stronger Th1 immune response among PML patients.
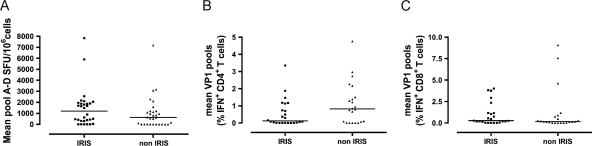
We then examined the immune responses in PML patients with and without IRIS. Thirty ELISpot IRIS samples and 24 ICS IRIS samples of 14 PML survivors, 1 PML progressor, and 3 PML early patients were collected during the occurrence of IRIS over a time period of 20 to 486 days (median, 137 days) after the onset of PML symptoms. Eight IRIS patients were HIV+. These samples were compared to 30 ELISpot samples and 22 ICS samples of 2 PML-early patients, one PML progressor, and 13 PML survivors who never experienced IRIS. Sampling was performed within 488 days of onset of PML symptoms. Four non-IRIS patients were HIV+. There was no difference in the magnitude of the ELISpot or ICS CD4+ and CD8+ T-cell responses between the two groups (Fig. 4 A, B, and C). Examination of the longitudinal ELISpot responses in 10 patients with IRIS and longitudinal CD4+ and CD8+ ICS responses in 9 patients with IRIS showed no increase in the immune responses at the height of IRIS. These results suggest that IRIS is not driven by a change in the Th1 immune response mediated by IFN-γ.
Fig. 4.
Measurement of the cellular immune response against JC virus by ELISpot and ICS assays in PML and PML-IRIS patients. Thirty ELISpot assays and CD4+ and CD8+ T-cell responses in 24 ICS assays in 18 IRIS patients were matched to non-IRIS patients by HIV status, CD4 count, and time interval since disease onset. Each dot represents the mean ELISpot (A), mean CD4+ T-cell response (B), and mean CD8+ T-cell response (C) of a patient to pool A through pool D. For the ELISpot, the mean result is expressed as SFU per 106 cells. Bars are the median results for the respective groups. For the ICS, the mean result is expressed as the percentage of gamma interferon-producing CD4+ or CD8+ T-cells versus the total CD4+ or CD8+ T-cells. Bars are the median results for the respective groups.
When all analyses were repeated, including those for the 11 possible PML cases (total of 77 patients), the results were similar and most trends and differences were accentuated by the increased sample size (data not shown).
DISCUSSION
We used a combination of two IFN-γ-based immunological assays, ELISpot and ICS, to characterize the cellular immune response to JCV in patients with PML with various clinical outcomes. Since the frequency of JCV-specific T-cells is very low in peripheral blood and cannot be measured readily ex vivo (21), we chose to perform these assays after stimulation in vitro with JCV peptides (15). Therefore, we used stringent criteria to determine the cutoff of our assays. We observed that the concordance rate for the two assays was high in PML survivors, where it reached that observed in healthy controls, while this was only 62.5% in PML progressors. This discrepancy likely is due to the fact that ICS is more sensitive than ELISpot and is, therefore, able to detect a minute number of JCV-specific T-cells present in the blood of PML progressors. Indeed, when we broke down the results of the ICS into CD4+ and CD8+ T-cells, it became apparent that the improved response observed in PML progressors was caused by enhanced detection of JCV-specific CD4+ T-cells by ICS (45.5%), despite their low CD4 counts.
These results confirm our previous studies highlighting the importance of JCV-specific CD8+ T-cells in disease containment and survival of PML (8, 9, 18, 25). Indeed, the difference in detection of CD8+ T-cells between PML progressors (27.3%) and survivors (100%) by ICS performed early after disease onset was highly significant and constitutes a valuable prognostic marker of disease evolution. Conversely, almost half of PML progressors had detectable JCV-specific CD4+ T-cells, which did not prevent their fatal outcome.
JCV PCR on CSF has a sensitivity that may be as low as 58% in HIV+ PML patients on cART (24). Therefore, individuals considered to have possible PML as a diagnosis of exclusion based solely on clinical and radiological criteria reflect the real clinical setting of PML patients (6). Indeed, we obtained similar results when we repeated all our immunological analyses including these cases.
These data expand on those reported in previous studies (13, 17). Our results confirm those of Gasnault et al., who reported detection of JCV-specific CD4+ T-cells in 9/10 (90%) PML survivors using a proliferation assay. However, in patients with active PML, we found different results: in the previous study, none of 14 patients had JCV-specific CD4+ T-cells, whereas we could detect these cells in 3/5 (60%) early PML patients and 5/11 (45.5%) PML progressors. The difference may in part be due to the higher sensitivity of the ICS than the proliferation assay. An additional explanation might be that Gasnault et al. used purified virus to stimulate T-cells while we used a JCV VP1 peptide library, which might potentially influence the number and repertoire of the epitopes recognized by T-cells. Moreover, unlike Khanna et al., we used only cells that had never been frozen and adopted a stringent threshold commonly accepted in immunological studies to determine the cutoff of our ELISpot assay. Because of the low frequency of JCV-specific T-cells in circulating blood (21), we also expanded these T-cells in vitro, which allowed us to measure robust responses.
CD8+ T-cells have also been reported to have a critical role in control of other viruses. For example, of patients who received a bone marrow transplant, those who developed a cytotoxic cytomegalovirus (CMV)-specific CD8+ T-cell response did not get CMV pneumonia, while 6/10 patients who did not develop a response died of CMV pneumonia (27). Similarly, all newly hepatitis C virus-infected subjects with no detectable CD8+ T-cell response developed persistent infection (7). In our study, the magnitude of the CD8+ T-cell response was significantly lower in the PML progressors than in the PML survivors. However, these results also emphasize the fact that, although the cytotoxic response is important for the clinical outcome of PML, it is not enough to prevent it. The antiviral role of CD4+ T-cells has also been observed for other viruses (12, 30). Our study confirms the important role of CD4+ T-cells in successfully fighting viral disease, since PML survivors were 4.8 times more likely to have a detectable CD4+ T-cell response than PML progressors. However, almost half of PML progressors had a CD4+ T-cell response which was not sufficient to lead to a favorable clinical outcome. Furthermore, in the PML survivor group tested within 6 months of symptom onset, all patients who had a CD4+ response also had a CD8+ response.
Longitudinal follow-up in PML survivors demonstrated that the T-cell response against JCV mediated by both CD4+ and CD8+ T-cells remained stable over up to 12 years. This is remarkable since PML was clinically and radiologically inactive and burnt out in those cases, which suggests that for PML, unlike HIV (reviewed in reference 11), persistent antigenic stimulation is not necessary to maintain cellular immunity over time. This finding may explain why development of new lesions is extremely rare in PML survivors. Indeed, using tetramer staining and 51Cr release assays, we observed that 24/25 (95%) PML patients who were still alive 5 to 15 years after disease onset had detectable JCV-specific CD8+ T-cells (20).
Interestingly, equal proportions of PML progressors and survivors received the investigational medications mirtazapine and/or mefloquine. JCV uses the 5HT2a serotonin receptor to enter into astroglial cells. Mirtazapine, a common antidepressant medication, is a 5HT2a receptor blocker, which decreases viral entry into these cells in vitro (10). Mefloquine, used for treatment of malaria, was shown to inhibit JCV replication in vitro (3). Although our conclusions are limited by the observational nature of the study, neither of these drugs appeared to influence the clinical outcome.
One of our objectives was to study the immunopathogenesis of IRIS. Indeed, others have shown that patients with tuberculosis (TB) and IRIS have higher numbers of TB-specific CD4+ T-cells (2). However, in our study, there was no difference in the JCV-specific CD4+ T-cell response between PML survivors with IRIS and without IRIS. Furthermore, we did not see a rise in IFN-γ-producing CD4+ or CD8+ immune response at the height of IRIS, even though PML-IRIS is characterized histologically by infiltration of the brain parenchyma by CD8+ T lymphocytes (31), indicating that what happens locally in the brain is not necessarily mirrored in the blood. Both of these findings suggest that measuring IFN-γ-producing Th1 T-cells alone is not sufficient and that T-cells expressing other cytokines likely have a role in the immunopathogenesis of IRIS. Indeed, pretreatment levels of CD8+ CD25+cells, a regulatory T-cell subset, are increased in HIV+ patients developing IRIS when started on cART (5). Recently, the importance of Th17 proinflammatory T-cells in IRIS occurring in HIV+ patients has been noted (1). Since we tested the CD4+ and CD8+ T-cell responses in PML patients when IRIS was already present, preclinical changes in T-cell function leading to IRIS could not be evaluated (16). Further longitudinal studies are needed to decipher the immunopathogenesis of IRIS in PML.
ACKNOWLEDGMENTS
This study was supported in part by a grant from Harvard University CFAR and NIH grant P30 AI60354. I.J.K. was supported by NIH grants R01 and R56 NS 041198, R01 NS 047029, and K24 NS 060950, and S.G. was supported by NIH grant T32 CA09031-32 and a Belgian-American Foundation grant. S.G. is a fellow of the Clinical Investigator Training Program, Beth Israel Deaconess Medical Center—Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co. L.N. is supported by The Harvard Clinical and Translational Science Center (NIH Award UL1 RR 025758).
We have no conflicting financial interests.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Bonham S., Meya D. B., Bohjanen P. R., Boulware D. R. 2008. Biomarkers of HIV immune reconstitution inflammatory syndrome. Biomark Med. 2:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourgarit A., et al. 2006. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 20:F1–F7 [DOI] [PubMed] [Google Scholar]
- 3. Brickelmaier M., et al. 2009. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob. Agents Chemother. 53:1840–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y., et al. 2009. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cianchetta-Sivori M., Raso S., Fernandez-Guerrero M., Gorgolas M., Garcia R. 2007. Do CD8(+)CD25(+) cells predict immune reconstitution syndrome in HIV-positive patients who begin HAART? AIDS 21:2347–2349 [DOI] [PubMed] [Google Scholar]
- 6. Cinque P., Koralnik I. J., Clifford D. B. 2003. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J. Neurovirol. 9(Suppl. 1):88–92 [DOI] [PubMed] [Google Scholar]
- 7. Cox A. L., et al. 2005. Comprehensive analyses of CD8+ T-cell responses during longitudinal study of acute human hepatitis C. Hepatology 42:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du Pasquier R. A., et al. 2003. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1p36 in patients with proven or possible progressive multifocal leukoencephalopathy. J. Virol. 77:11918–11926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du Pasquier R. A., et al. 2004. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain 127:1970–1978 [DOI] [PubMed] [Google Scholar]
- 10. Elphick G. F., et al. 2004. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306:1380–1383 [DOI] [PubMed] [Google Scholar]
- 11. Ford E. S., Puronen C. E., Sereti I. 2009. Immunopathogenesis of asymptomatic chronic HIV infection: the calm before the storm. Curr. Opin. HIV AIDS 4:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gamadia L. E., et al. 2003. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T-cells in protection against CMV disease. Blood 101:2686–2692 [DOI] [PubMed] [Google Scholar]
- 13. Gasnault J., et al. 2003. Critical role of JC virus-specific CD4 T-cell responses in preventing progressive multifocal leukoencephalopathy. AIDS 17:1443–1449 [DOI] [PubMed] [Google Scholar]
- 14. Gheuens S., Pierone G., Peeters P., Koralnik I. J. 2010. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J. Neurol. Neurosurg. Psychiatry 81:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goonetilleke N., et al. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T-cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson T., Nath A. 2010. Neurological complications of immune reconstitution in HIV-infected populations. Ann. N. Y. Acad. Sci. 1184:106–120 [DOI] [PubMed] [Google Scholar]
- 17. Khanna N., et al. 2009. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J. Virol. 83:4404–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koralnik I. J., et al. 2002. Association of prolonged survival in HLA-A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J. Immunol. 168:499–504 [DOI] [PubMed] [Google Scholar]
- 19. Letvin N. L., et al. 2007. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J. Virol. 81:12368–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lima M. A., Bernal-Cano F., Clifford D. B., Gandhi R. T., Koralnik I. J. 2010. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. J. Neurol. Neurosurg. Psychiatry 81:1288–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lima M. A., et al. 2007. Frequency and phenotype of JC virus-specific CD8+ T lymphocytes in the peripheral blood of patients with progressive multifocal leukoencephalopathy. J. Virol. 81:3361–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linda H., et al. 2009. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N. Engl. J. Med. 361:1081–1087 [DOI] [PubMed] [Google Scholar]
- 23. Maher S. G., Romero-Weaver A. L., Scarzello A. J., Gamero A. M. 2007. Interferon: cellular executioner or white knight? Curr. Med. Chem. 14:1279–1289 [DOI] [PubMed] [Google Scholar]
- 24. Marzocchetti A., et al. 2005. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J. Clin. Microbiol. 43:4175–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marzocchetti A., et al. 2009. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 73:1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCombe J. A., et al. 2009. Neurologic immune reconstitution inflammatory syndrome in HIV/AIDS: outcome and epidemiology. Neurology 72:835–841 [DOI] [PubMed] [Google Scholar]
- 27. Reusser P., Riddell S. R., Meyers J. D., Greenberg P. D. 1991. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78:1373–1380 [PubMed] [Google Scholar]
- 28. Tan C. S., Koralnik I. J. 2010. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 9:425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan K., Roda R., Ostrow L., McArthur J., Nath A. 2009. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 72:1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urbani S., et al. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44:126–139 [DOI] [PubMed] [Google Scholar]
- 31. Vendrely A., et al. 2005. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol. 109:449–455 [DOI] [PubMed] [Google Scholar]
- 32. Wenning W., et al. 2009. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N. Engl. J. Med. 361:1075–1080 [DOI] [PubMed] [Google Scholar]