Abstract
Plasticity at excitatory glutamatergic synapses in the central nervous system is believed to be critical for neuronal circuits to process and encode information allowing animals to perform complex behaviors such as learning and memory. In addition, alterations in synaptic plasticity are associated with human diseases including Alzheimer's, epilepsy, chronic pain, drug addiction, and schizophrenia. Long-term potentiation (LTP) and depression (LTD) in the hippocampal region of the brain are two forms of synaptic plasticity that increase or decrease, respectively, the strength of synaptic transmission by postsynaptic AMPA-type glutamate receptors. Both LTP and LTD are induced by activation of NMDA-type glutamate receptors but differ in the level and duration of Ca2+ influx through the NMDA receptor and the subsequent engagement of downstream signaling by protein kinases including PKA, PKC, and CaMKII and phosphatases including PP1 and calcineurin-PP2B (CaN). This review addresses the important emerging roles of the A-kinase anchoring protein (AKAP) family of scaffold proteins in regulating localization of PKA and other kinases and phosphatases to postsynaptic multi-protein complexes that control NMDA and AMPA receptor function during LTP and LTD.
Introduction to excitatory synaptic plasticity
Excitatory synapses in the central nervous have the remarkable ability to modify the strength of synaptic transmission in response to prior activity. This activity-dependent control of synaptic strength is known as synaptic plasticity and can involve both rapid and long-lasting modifications to either presynaptic or postsynaptic function. Synaptic plasticity plays important roles during normal postnatal development and learning and memory, as well as in disease states such as Alzheimer's, epilepsy, mental retardation, and drug addiction. Various forms of both short and long-term synaptic plasticity have been documented in most major brain regions, with long-term forms of synaptic plasticity receiving the most attention as they relate to learning and memory. Long-term synaptic plasticity has been most extensively studied in the hippocampus, a brain region important for spatial learning and formation of new declarative memories. Within the hippocampus, postsynaptic mechanisms of induction and expression of excitatory synaptic plasticity are best characterized for the synapses between the Schaffer collateral axons of CA3 pyramidal neurons and the dendrites of CA1 pyramidal neurons.
At CA1 synapses, there are two predominant postsynaptic ionotropic glutamate receptors subtypes that function as ligand-gated cation channels and are distinguished by their activation by different synthetic agonists; N-methyl-D-aspartate receptors (NMDAR) and α-amino-3-hydroxy-5-methylisooxazole-4-propionic acid receptors (AMPAR) (reviewed in (Bleakman and others 2007; Dingledine Borges Bowie and Traynelis 1999)). Both of these receptors are heterotetrameric assemblies with NMDARs containing two NR1 subunits that bind the co-agonist glycine or D-serine and two NR2A-D subunits (also known as GluNA-D) that bind glutamate. In the hippocampus most synaptic NMDARs contain NR1 with NR2A or NR2B subunits and are permeable to Na+, K+, and Ca2+. AMPARs contain two pairs of two GluR1-4 subunits (also known as GluA1-4) which all bind glutamate. Most AMPARs in the hippocampus are composed of GluR1/2 or GluR2/3 with a small population of GluR1/1 homomeric channels (reviewed in (Bleakman and others 2007; Dingledine Borges Bowie and Traynelis 1999; Kumar Bacci Kharazia and Huguenard 2002)). Due to mRNA editing, the GluR2 subunit contains an Arg instead of a Gln, which is present in all other GluR subunits, within the pore region; this Arg makes any GluR2-containing receptor impermeable to Ca2+ and causes it to display a linear current-voltage relationship. In contrast, GluR2-lacking AMPARs, such as GluR1 homomers, are permeable to Ca2+ and display inward rectification due intracellular blockage of the pore by polyamines at depolarized positive membrane potentials. GluR1/1 receptors do not contribute to basal synaptic transmission under most conditions; however, they transiently exchange in and out of synapses and can be recruited to synapses during synaptic plasticity and after excitotoxic insults such as ischemia (reviewed in (Liu and Zukin 2007)).
Two prominent forms of long-term synaptic plasticity in the CA1 region of the hippocampus are NMDAR-dependent long-term potentiation (LTP) and long-term depression (LTD) (reviewed in (Malenka and Bear 2004)). Under basal conditions, AMPARs regulate the majority of fast excitatory synaptic transmission, however, during long-term plasticity, NMDARs become essential for the modulation of synaptic transmission through coincident detection of presynaptic glutamate release and postsynaptic depolarization that removes a pore-blocking Mg2+ ion allowing influx of Ca2+ through the receptor (Figure 1A). This influx of Ca2+ activates a number of downstream signaling pathways including, prominently, protein kinases such as Ca2+-calmodulin dependent protein kinases I and II (CaMKI, CaMKII), the cAMP dependent protein kinase (PKA), and protein kinase C (PKC) and protein phosphatases such as protein phosphatases 1 (PP1), 2A (PP2A), and 2B (PP2B-calcineurin-(CaN)). These protein kinases and phosphatases in turn regulate AMPAR activity by modulating channel properties and postsynaptic localization directly through receptor phosphorylation and indirectly through other protein substrates many of which have yet to be identified. In addition, these same kinases and phosphatases trigger corresponding changes in postsynaptic dendritic spine structure with LTP promoting spine formation and enlargement and LTD favoring spine shrinkage and elimination (Figure 1A) (reviewed in (Tada and Sheng 2006))
Figure 1. Mechanisms of Postsynaptic Plasticity.
A) Regulation of NMDAR-induced LTP and LTD in dendritic spines. LTD induces spine shrinkage, AMPAR dephosphorylation, and AMPAR removal from spines through protein phosphatase activity. LTP induces spine growth, AMPAR phosphorylation, and AMPAR recruitment to spines through protein kinase activity. B) Regulation of AMPAR-GluR1 subunit phosphorylation and trafficking to and from synapses during LTP and LTD. These trafficking events may be similar for both GluR1/2 and GluR1/1 receptors. During LTP, AMPARs may be secreted to the extrasynaptic membrane directly in spines or to regions of dendrite shafts near spines (not depicted). Likewise, during LTD AMPAR endocytosis may occur both within spines and on dendrite shafts (not depicted).
High frequency presynaptic stimulation (HFS) induces NMDAR-dependent LTP by increasing synaptic AMPAR number and activity, whereas, low frequency stimulation (LFS) induces NMDAR-dependent LTD, by decreasing AMPAR activity and number (reviewed in (Derkach Oh Guire and Soderling 2007; Malenka and Bear 2004; Shepherd and Huganir 2007)). More specifically, during NMDAR-induced LTP brief but strong elevations in postsynaptic Ca2+ promote phosphorylation regulated insertion of AMPARs containing GluR1 into extrasynaptic or perisynaptic plasma membrane sites either in dendritic shafts or within spines adjacent to the postsynaptic density (PSD). These perisynaptic receptors are then incorporated into the PSD after lateral movement within the plasma membrane (Bats Groc and Choquet 2007; Kennedy Davison Robinson and Ehlers 2010; Passafaro Piech and Sheng 2001; Petrini and others 2009; Yang Wang Frerking and Zhou 2008; Yudowski and others 2007) (Figure 1B). These LTP inserted receptors are predominantly GluR1/2 receptors but may also be GluR1/1 homomeric receptors at certain developmental ages (Lu and others 2007; Plant and others 2006) but see also (Adesnik and Nicoll 2007)). PKA activation and phosphorylation of GluR1-Ser845 may participate in this process of AMPAR-trafficking by either stabilizing receptors in the extrasynaptic/perisynaptic plasma membrane and/or promoting recycling in endosomes (Ehlers 2000; Esteban and others 2003; Man Sekine-Aizawa and Huganir 2007; Oh Derkach Guire and Soderling 2006; Snyder and others 2005; Yang Wang Frerking and Zhou 2008). Thus, PKA may promote LTP by stabilizing recycling and/or plasma membrane pools of GluR1-AMPARs that are then further recruited to the membrane and synapse by Ca2+ stimulation of CaMKII, CaMKI, or PKC driven trafficking events and GluR1 phosphorylation on Ser818 and Ser831 (Barria Derkach and Soderling 1997; Barria Muller Derkach Griffith and Soderling 1997; Boehm and others 2006; Esteban and others 2003; Guire Oh Soderling and Derkach 2008; Hayashi and others 2000; Lee and others 2003; Oh Derkach Guire and Soderling 2006) (Figure 1B). In addition, single AMPAR channel activity may also be increased during LTP through PKA phosphorylation of Ser845 to increase channel open probability and CaMKII/PKC phosphorylation of Ser831 to increase single channel conductance (Banke and others 2000; Barria Derkach and Soderling 1997; Barria Muller Derkach Griffith and Soderling 1997; Benke Luthi Isaac and Collingridge 1998; Derkach Oh Guire and Soderling 2007). Accordingly, mice that have both GluR1 Ser831 and 845 mutated to Ala have impaired HFS induced LTP in acute hippocampal slices and show an inability to increase the strength of LTP in response to β-adrenergic receptor activation of cAMP-PKA signaling (Hu and others 2007; Lee and others 2003). In addition, single GluR1 Ser845 and Ser818, but not Ser 831, to Ala mutations inhibit LTP in cultured hippocampal slices (Boehm and others 2006; Esteban and others 2003; Lee and others 2003). However, mice with only Ser831 or Ser845 to Ala knock-in mutations still show normal HFS-LTP, indicating some redundancy and overlap in the functional roles of these different GluR1 phosphorylation sites during LTP in vivo (Lee Takamiya He Song and Huganir 2010).
In contrast, NMDAR-induced LTD is triggered by prolonged but low-level Ca2+ elevations that promote GluR1 Ser845 dephosphorylation and decreases in synaptic AMPAR activity and number (Figures 1A, 1B). Interestingly, PKA may be required to induce LTD in part through phosphorylation of Ser845 which can be dephosphorylated by PP1, PP2A, and CaN. (Banke and others 2000; Kameyama Lee Bear and Huganir 1998; Lee Kameyama Huganir and Bear 1998; Lee and others 2003; Lu and others 2008; Tavalin and others 2002). However, PKA may also phosphorylate other targets acutely during LTD induction (Lu and others 2007). Importantly, in mice were GluR1 Ser845 is mutated to Ala with or without Ser831 mutation, LTD is impaired (Lee and others 2003; Lee Takamiya He Song and Huganir 2010). During LTD, it is thought that there is a rapid dephosphorylation of Ser845 by CaN and PP1/PP2A followed by an un-tethering of AMPARs from the PSD and then CaN-dependent endocytic removal of AMPARs from the extrasynaptic plasma membrane (Beattie and others 2000; Carroll Beattievon Zastrow and Malenka 2001; Ehlers 2000; Lee Kameyama Huganir and Bear 1998; Smith Gibson and Dell'Acqua 2006; Tavalin and others 2002) (Figure 1B). In particular, pharmacological induction of LTD with bath application of NMDA (chemical or cLTD) induces both dephosphorylation of GluR1-Ser845, and endocytosis of AMPARs through CaN dependent pathways in cultured neurons and acute hippocampal slices (Beattie and others 2000; Ehlers 2000; Lee Kameyama Huganir and Bear 1998; Smith Gibson and Dell'Acqua 2006). One possible role of GluR1 Ser845 phosphorylation during LTD may be to stabilize a perisynaptic pool of GluR1 homomers that can rapidly exchange in and out of the synapse which are then removed by endocytosis during LTD (He and others 2009).
Thus, taken together this information suggests that NMDAR control of AMPAR phosphorylation, activity and trafficking regulates long-term changes in synaptic strength. However, these NMDAR-induced changes in synaptic strength depend on the activation of what are inherently broad specificity kinases and phosphatases such as PKA and CaN that have many different cellular functions both inside and outside of the nervous system. Thus, it is important to understand how the signaling functions of these kinases and phosphatases are adapted and specialized to control highly coordinated postsynaptic signaling events. The leading models explaining how postsynaptic signaling pathways become specialized during LTP and LTD are all increasingly focusing on mechanisms that target kinases, phosphatases, and other signaling proteins to multi-protein complexes that organize localized signaling networks near both upstream activators and downstream targets within dendritic spines and the PSD. This concise localization within spines allows for multi-functional activity of the enzymes toward multiple substrates, yet also promotes more restricted, precise spatio-temporal control of specific substrate phosphorylation events.
Scaffolding proteins
At the core of multi-protein signaling networks are scaffolding, anchoring, and adaptor proteins that bind receptors, second messenger-generating enzymes, kinases, and phosphatases. This review will focus on the organization of postsynaptic cAMP and Ca2+ second messenger signaling networks by A-kinase anchoring protein (AKAP) family scaffold proteins. AKAPs are a diverse group of functionally related proteins that anchor PKA as well as other signaling proteins to coordinate signal transduction at different subcellular locations (Figure 2A, 2B). Between mammals and lower multi-cellular eukaryotes including Drosophila and C. elegans, there have been over 50 AKAPs identified. AKAPs have little or no primary sequence similarity, yet all contain a convergent short region of secondary structure consisting of an amphipathic α-helical motif that binds with high affinity to the N-terminus of the RI and/or RII subunit dimer in the PKA R2C2 holoenzyme (reviewed in (Carnegie Means and Scott 2009; Wong and Scott 2004)) (Figure 2A). Originally AKAPs were characterized solely based on their ability to anchor PKA; however, AKAPs are now recognized for their ability to form multi-protein complexes in a variety of subcellular regions where they integrate cAMP signaling with other pathways. In particular, AKAP signaling complexes have emerged as important regulators of glutamate receptors and a variety of voltage-gated ion channels in neurons and other excitable cells (Figure 2B).
Figure 2. Subcellular targeting of PKA and other signaling proteins by AKAPs.
A) Left, schematic representation of different converging signaling pathways leading to adenylyl cyclase production of cAMP and activation of the PKA holoenzyme. Right, schematic regulation of the AKAP-anchored PKA holoenzyme. B) Examples of AKAP organized glutamate receptor and ion channel signaling complexes. Top, AKAP79/150 organized signaling complexes. Bottom, Yotiao, MAP2, and AKAP15/18 organized signaling complexes.
AKAP79/150 (human 79/rodent 150; also AKAP5) is a postsynaptic scaffolding protein that can serve as a signal integrator binding a number of signaling, scaffolding, receptor, and ion channel proteins involved in long-term synaptic plasticity (reviewed previously in (Dell'Acqua and others 2006; Wong and Scott 2004)). AKAP79/150 was first determined to anchor PKA by binding the RII regulatory subunit dimer near the AKAP C-terminus (Carr Stofko-Hahn Fraser Cone and Scott 1992). (Figures 3A and 4). AKAP79/150 is localized within neurons through a unique targeting domain near N-terminus. Within this domain, AKAP79/150 has three distinct basic targeting sub-domains (Figure 4) that collectively interact with cortical F-actin, the acidic phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2), and cadherin cell adhesion molecules to target the AKAP to dendrite plasma membranes and the PSD in dendritic spines. (Dell'Acqua Faux Thorburn Thorburn and Scott 1998; Gomez Alam Smith Horne and Dell'Acqua 2002; Gorski Gomez Scott and Dell'Acqua 2005). This N-terminal region also promotes AKAP79/150 binding to KCNQ2/3 K+ channels that carry M-current (Figure 2B), which is regulated by PIP2, Ca2+-calmodulin and AKAP-anchored PKC phosphorylation in neurons (Bal Zhang Hernandez Zaika and Shapiro 2010; Hoshi and others 2003). Accordingly, AKAP79/150 binds to PKC through an anchoring site also located within the A sub-domain of this basic N-terminal targeting domain (Figure 4) (Klauck and others 1996). Recently, several adenyly cyclase (AC) isoforms have also been shown to bind AKAP79/150 through the B sub-domain within the targeting domain (Efendiev and others 2010; Willoughby and others 2010).
Figure 3. The AKAP79/150-MAGUK postsynaptic signaling complex in regulation of AMPA receptor phosphorylation during synaptic plasticity.
A) AKAP79/150 can be linked to both AMPA and NMDA receptors through MAGUK scaffolding proteins. Influx of Ca2+ through the NMDAR activates CaN, PKC, and PKA activity to regulate postsynaptic substrate phosphorylation including AMPAR-GluR1. B) Regulation of the AMPAR endocytosis and translocation of AKAP79/150 from the PSD during LTD. Ca2+ influx through the NMDAR activates AKAP-anchored CaN, resulting in dephosphorylation of the GluR1-Ser845 and AMPAR endocytosis. Activation of CaN along with PLC also promotes depolymerization of spine actin to promote subsequent translocation of AKAP-PKA complexes away from the postsynaptic membrane in dendritic spines. This delayed movement of AKAP79/150-PKA away from spines may prevent re-phosphorylation and recycling of AMPARs during LTD.
Figure 4. Domain Organization of the AKAP79/150 Signaling Scaffold.
Amino acid numbering is given for human AKAP79 from 1 at the N-terminus to 427 at the C-terminus. The locations of the various mapped binding sites and the indicated binding partners are shown. See the text for more details. The repetitive sequence unique to rodent AKAP150 which gives it a highe molecular weight (150 kDa vs. 79kDa for human) is inserted near residue 315 between the MAGUK binding and the CaN anchoring domains.
AKAP79/150 anchors the phosphatase CaN just N-terminal to the PKA anchoring site through a PxIxIT-type docking motif which is similar to those found in other CaN binding partners such as the transcription factor NFAT (Coghlan and others 1995; Dell'Acqua Dodge Tavalin and Scott 2002; Oliveria Dell'Acqua and Sather 2007; Oliveria Gomez and Dell'Acqua 2003). Located between the targeting domain and CaN anchoring domains, AKAP79/150 has an internal domain that binds to the src-homology 3 (SH3) and guanylate kinase (GK) domains of the membrane-associated guanylate kinase (MAGUK) scaffolding proteins PSD-95 and SAP97 (Colledge and others 2000; Robertson Gibson Benke and Dell'Acqua 2009). This interaction links AKAP79/150 through the PSD-95, Discs-large, Zona-occludens 1 (PDZ) domains in the MAGUKs to the C-termini of NMDARs and AMPARs which serve as substrates for PKA, PKC, and CaN (Figure 3A). Finally, at the extreme C-terminus of the AKAP is a modified leucine zipper (LZ) motif (Figure 4) that promotes interaction with the Cav1.2 pore forming subunit of the L-type voltage-gated calcium channel, another substrate target of PKA, PKC, and CaN (Oliveria Dell'Acqua and Sather 2007) (Figure 2B).
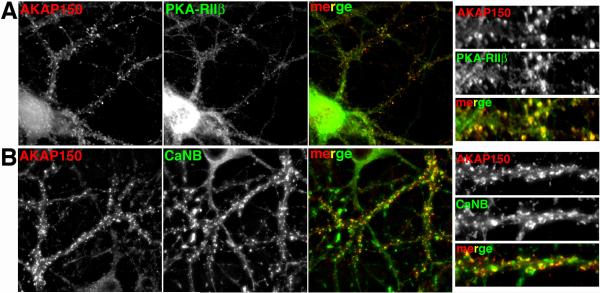
The organization of this postsynaptic assembly suggests that AKAP79/150 is a master scaffolding protein that links F-actin, PIP2, MAGUKs, cadherins, kinases and phosphatases, together with glutamate receptors and ion channels to regulate activity dependent signaling processes at synapses. In particular, AKAP79/150 anchors both PKA and CaN, potentially poising the two enzymes to act antagonistically against each other to control AMPAR synaptic strength during NMDAR-induced LTP and LTD. Accordingly, AKAP150, PKA and CaN all show punctate co-localized immunostaining on dendritic spines in cultured rat hippocampal neurons (Figure 5).
Figure 5. AKAP79/150 is co-localized with PKA and CaN in hippocampal neuron dendrites.
A) Immunofluorescent staining for rat AKAP150 (red) and the PKA-RIIβ regulatorysubunit (green) shows punctate co-localization (yellow in merge panels) along hippocampal neurons dendrites including in dendritic spines. Right hand panels are magnifications of dendrites. Prominent localization of PKA-RIIβ independent of AKAP150 is also seen in the cell body and the interior of dendrite shafts where it may bind other AKAPs including MAP2. B) Immunofluorescent staining for AKAP150 (red) and CaNB regulatory subunit (green) shows punctate co-localization (yellow in merge panels) along hippocampal neurons dendrites including in dendritic spines. Right hand panels are magnifications of dendrites. Prominent localization of CaNB independent of AKAP150 is also seen in the cell body and in likely axonal/presynaptic punctate along dendrites that are in most cases closely opposed to the sites of dendritic co-localization (yellow) between AKAP150 and CaN. See (Gomez Alam Smith Horne and Dell'Acqua 2002) for the original report of these findings.
AKAP regulation of AMPAR and NMDAR activity
The first studies implicating AKAP-anchoring of PKA in modulating AMPAR channel activity, used a short peptide known as Ht31 to acutely disrupt PKA anchoring in cells; Ht31 is derived from the conserved PKA-R subunit binding amphipathic helix motif common to the AKAP family (Carr Hausken Fraser Stofko-Hahn and Scott 1992). Following introduction of Ht31 or the C-subunit catalytic inhibitor peptide PKI through the recording electrode, extrasynaptic agonist evoked-AMPAR/kainate currents in cultured hippocampal neurons were decreased in a non-additive manner (Rosenmund and others 1994). Additionally, postsynaptic infusion of Ht31 peptide reduced the amplitude of AMPAR miniature excitatory postsynaptic currents (mEPSCs). These results provided the first evidence that AKAP anchored PKA activity was critical for regulating AMPAR activity in both extrasynaptic and synaptic membrane pools in hippocampal neurons.
A number of years later, reconstitution studies in heterologous cell expression systems and additional work in cultured neurons, helped establish that AKAP79/150 was the primary AKAP responsible for PKA localization to postsynaptic spines (see Figure 6) and for PKA regulation of AMPAR activity. In addition, CaN phosphatase activity anchored to AKAP79/150 was shown to be responsible for the observed decreases in AMPAR currents seen when PKA anchoring was disrupted or PKA activity was inhibited. By reconstituting AKAP79/150-MAGUK-GluR1 complexes in HEK cells, it was shown that repetitive application of agonist leads to a time-dependent Ca2+-dependent-decrease in AMPAR currents that is controlled by GluR1 Ser845, GluR1 binding to SAP97, and both the AKAP79/150 PKA and CaN anchoring sites (Dell'Acqua Dodge Tavalin and Scott 2002; Hoshi Langeberg and Scott 2005; Tavalin and others 2002). This functional data taken together with biochemical analysis of GluR1 Ser845 phosphorylation (Colledge and others 2000) provided evidence that AKAP79/150 anchored PKA promoted phosphorylation of Ser845 and that CaN dephosphorylation was responsible for the down-regulation of GluR1 currents. Thus, this AMPAR regulation seen in heterologous cells is very similar to mechanisms proposed for LTD in hippocampal neurons that involve GluR1 Ser845 dephosphorylation and AMPAR endocytosis (Figure 1B).
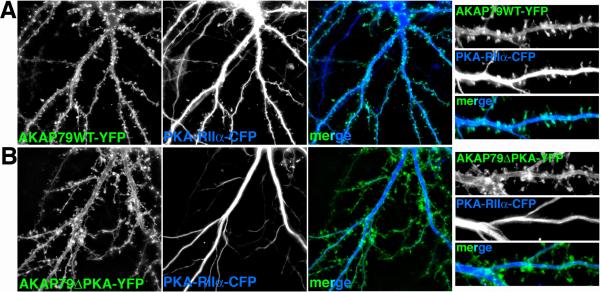
Figure 6. AKAP79/150 PKA anchoring controls dendritic spine localization of PKA.
A) Co-expression of AKAP79WT-YFP (green) and PKA-RIIα-CFP (blue) in rat hippocampal neurons leads to co-localization (turquoise in merge panels) of PKA-RII in spines with the AKAP79. B) However, co-expression of the 1-360 truncation mutant AKAP79ΔPKA-YFP (green) with PKA-RIIα-CFP (blue) fails to target PKA-RII to spine leading to its retention in the interior of dendrite shafts likely through anchoring to MAP2. Right hand panels are magnifications of dendrites. See (Smith Gibson and Dell'Acqua 2006) for the original report of these findings.
Accordingly, parallel studies in hippocampal neurons found that Ca2+ and CaN activity are required for the down-regulation of AMPAR currents seen following inhibition of PKA activity or disruption of PKA anchoring with Ht31 (Tavalin and others 2002). In addition, disruption of PKA anchoring with Ht31 induces internalization of GluR1 in hippocampal neurons through a CaN-dependent mechanism and mimics and occludes LTD induction in hippocampal slices (Snyder and others 2005). Using RNAi to suppress expression of endogenous AKAP150 in rat hippocampal neurons in combination with replacement by human AKAP79 mutants, LTD-like AMPAR current down-regulation was shown to be modulated by the level of anchored PKA activity and to be completely dependent on CaN anchored to AKAP79/150 (Hoshi Langeberg and Scott 2005). These data strongly support the model that AKAP79/150 forms a complex that dually anchors both PKA and CaN, and poises the two enzymes for opposing roles in regulating AMPAR activity in neurons (Figure 3A). More recent work using reconstituted HEK cell expression indicate that AKAP79/150-anchored PKC is also capable of regulating GluR1 currents through phosphorylation of Ser831; however, whether this form of regulation also occurs in neurons awaits further investigation (Tavalin 2008). In addition, while AKAP79/150 is also linked to NMDARs through PSD-95 binding (Colledge and others 2000) it is not known whether AKAP79/150 anchored PKA, PKC or CaN also participate in modulation of NMDAR currents in neurons.
Another postsynaptic AKAP that may play an important role in regulating NMDA receptors is Yotiao, a ~200 kDA protein derived from alternative splicing of a much larger AKAP known as AKAP350/450 (also AKAP9). Yotiao was first identified through a yeast 2-hybrid screening using the NR1 subunit of the receptor as bait (Lin and others 1998) and then subsequently identified as an AKAP protein through functional screening for PKA-RII binding (Westphal and others 1999). Yotiao forms a phosphatase-kinase signaling complex with the NR1-1A subunit splice variant of NMDARs (Figure 2B). The associated complex is similar to AKAP79/150 in that it binds both PKA and a phosphatase, but in this case, Yotiao binds PP1 instead of CaN-PP2B. The resulting activity of Yotiao on the NMDAR is somewhat similar to AKAP79/150 regulation of AMPAR activity; NMDAR currents are enhanced by activation of anchored PKA and opposed by the activity of the anchored phosphatase PP1. However, they differ in that PP1 has high basal activity in the Yotiao signaling complex, unlike CaN in the AKAP79/150 complex, which is activated by Ca2+ elevations. Interestingly, Yotiao has also been implicated in assembly of a similar PKA/PP1 signaling complex that regulates KCNQ1 K+ channels in the heart (Figure 2B). Mutations in KCNQ1 and Yotiao that disrupt formation of this complex have been linked to some forms of long QT syndrome, an inherited cardiac arrhythmia leading to sudden death (Chen and others 2007; Marx and others 2002). Recent studies indicate that Yotiao, like AKAP79/150, also binds and regulates several AC isoforms in brain (Piggott Bauman Scott and Dessauer 2008). Thus, both AKAP79/150 and Yotiao may scaffold the entire AC-cAMP-PKA pathway with different sets of PKA substrates in neurons. Overall, these results demonstrate that anchoring complexes may be inherently similar; yet can use different enzymatic components to modulate different receptors through an antagonistic balance between kinases and phosphatases.
AKAP79/150 involvement in LTP and LTD
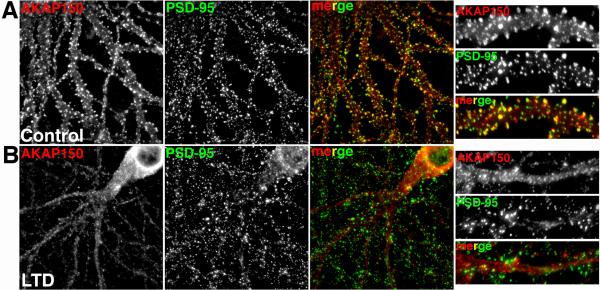
With AKAP79/150 having been shown to regulate AMPAR activity by anchored PKA and CaN involving an LTD-like mechanism, it was suspected that this signaling complex also plays an important role in the regulation of long-term synaptic plasticity in vivo. Initial work characterizing the targeting of AKAP79/150 to dendritic spines determined that localization of the AKAP is regulated through its interaction with the actin cytoskeleton (Gomez Alam Smith Horne and Dell'Acqua 2002). When F-actin is disrupted, spine localization of AKAP79/150 is lost and co-localization and co-precipitation of the AKAP with PSD-95 is disrupted. Importantly, activation of NMDA receptors with glutamate or NMDA (cLTD conditions) also results in CaN-dependent reorganization of dendritic F-actin leading to redistribution of the AKAP79/150 away from PSD-95 in dendritic spines (Gomez Alam Smith Horne and Dell'Acqua 2002) (Figures 3B and 7). Furthermore, AKAP79/150 binding to postsynaptic cadherins is disrupted by NMDA stimulation with AKAP79/150 translocating away from the cadherins that remain in spines (Gorski Gomez Scott and Dell'Acqua 2005). Later studies showed that cLTD treatment in cultured neurons and acute hippocampal slices result in a persistent redistribution of not only AKAP79/150 (Figure 7), but also PKA-RII from postsynaptic membranes to the cytoplasm with CaN localization showing much smaller changes (Smith Gibson and Dell'Acqua 2006). The cLTD-induced removal of AKAP79/150 and PKA from the postsynaptic fractions is coincident with but slightly delayed in time from GluR1 Ser845 dephosphorylation and endocytosis, which are detected within 3-5 minutes of NMDA exposure. In this same study, fluorescence resonance energy transfer (FRET) imaging of AKAP79 binding to PKA-RII in cultured hippocampal neurons was used to demonstrate that NMDA stimulation causes redistribution of AKAP-PKA complexes from dendritic spines and plasma membranes to the cytoplasm of dendrite shafts and the soma (Figure 8).
Figure 7. AKAP79/150 postsynaptic co-localization with PSD-95 is disrupted following chemical induction of LTD in hippocampal neurons.
A) Immunofluorescent staining for rat AKAP150 (red) and the PSD-95 (green) shows punctate co-localization (yellow in merge panels) along hippocampal neurons dendrites including in dendritic spines in control neurons. B) AKAP150 staining declusters and redistributes away from spines and PSD-95 puncta toward dendrite shafts and the soma 30 minutes following a chemical LTD induction stimulus (25 μM NMDA, 3 minutes). Right hand panels are magnifications of dendrites. See (Smith Gibson and Dell'Acqua 2006) for the original report of these findings.
Figure 8. FRET imaging reveals redistribution of AKAP79-PKA anchoring from dendritic spines to dendrite shafts following chemical induction of LTD in living hippocampal neurons.
A) Diagram showing design of the FRET imaging approach used to detect changes in the localization of AKAP79-PKA anchoring within hippocampal neurons. B) Corrected images of sensitized FRET emission from PKA-RIIα-YFP at 535 nM upon excitation of AKAP79-CFP at 436 nm (pseudocolor scale: blue=no FRET to red=high FRET) for dendrites from control untreated neurons or 15 minutes after chemical LTD induction (25 μM NMDA, 3 minutes). See (Oliveria Gomez and Dell'Acqua 2003; Smith Gibson and Dell'Acqua 2006) for the original reports using this FRET imaging method.
The studies described above indicated that AKAP79/150 targeting to spines is coordinated with changes in AMPAR-trafficking and spine structure associated with LTD. Accordingly, cLTD activation of NMDARs stimulates phospholipase C (PLC) cleavage of PIP2 in spines which is required along with CaN activation for depolymerization of spine actin and translocation of AKAP79/150 from spines (Horne and Dell'Acqua 2007)(Figure 3B). Inhibition of PLC also prevents cLTD-induced decreases in spine PSD-95 levels and AMPAR internalization. Consistent with AKAP79/150 having positive effects on both spine structure and AMPAR postsynaptic localization and function, overexpression of AKAP79 in developing cultured neurons increases spine size, AMPAR synaptic localization, and AMPAR mEPSC amplitudes (Robertson Gibson Benke and Dell'Acqua 2009). Interestingly, these effects of AKAP79 expression on spine maturation do not require PKA or CaN anchoring but do require the MAGUK binding domain, indicating that just physical linkage between AKAP79/150 and MAGUKs may play some roles in controlling postsynaptic structure and function.
In sum, it appears that cLTD activation of NMDARs disrupts AKAP79/150-MAGUK-glutamate receptor complexes within the synaptic membrane via multiple signaling pathways that combine to coordinate removal of the AKAP and its anchored pool of PKA from the spines with structural changes in spine actin and functional changes in AMPARs. Initially activation of anchored CaN in the AKAP-MAGUK complex could promote rapid dephosphorylation and endocytosis of AMPARs, with the later removal of AKAP79/150-PKA complexes from the PSD preventing PKA rephosphorylation of AMPAR-GluR1 subunits to ensure continuing endocytosis and prevent delivery of recycled receptors back to the plasma membrane (Figure 3B). Recent studies in cultured neurons do indeed support a role for both AKAP-MAGUK binding and CaN anchoring in NMDA-induced AMPAR endocytosis (Bhattacharyya Biou Xu Schluter and Malenka 2009).
Plasticity in AKAP150 mutant mice
These many studies over a number of years support a model where AKAP79/150 is coordinately regulating activity of the GluR1 subunit in the AMPAR through the opposing actions of PKA and CaN; however, more direct evidence showing the importance of this AKAP in controlling LTP and LTD in vivo has awaited the more recent characterization of AKAP150 mutant mice (Tunquist and others 2008; Weisenhaus and others 2010). In hippocampal sections from AKAP150 -/- mice, PKA-RII localization to dendrites is strongly reduced and localization to the cell body layer is increased compared to wild-type mice although hippocampal anatomy is normal (Figure 9A). Accordingly, PKA levels in synaptic membrane fractions are also strongly reduced in these knock-out mice (Figure 9B)(Weisenhaus and others 2010). In cultured hippocampal neurons from an independently generated AKAP150 -/- mouse line, PKA-RII localization to dendritic spines is eliminated, whereas in wild-type mice AKAP150 is co-localized with PKA in spines (Tunquist and others 2008). These data from two different AKAP150 -/- lines strongly support previous results with expression of AKAP79 wild-type versus a PKA binding deficient mutant in neuronal cultures showing that PKA is targeted to dendritic spines predominantly through AKAP79/150 anchoring with PKA localization in dendrite shafts likely being through anchoring to MAP2 (Figure 6) (Smith Gibson and Dell'Acqua 2006). Recent analyses of a MAP2 mutant mouse indicate that this abundant dendrite shaft localized AKAP may also participate in regulating PKA localization to dendrites and in PKA modulation of LTP (Zhong and others 2009). However, these MAP2 mutant mice have large alterations in dendritic structure making it difficult to assess the precise role of MAP2 in controlling postsynaptic PKA signaling (Khuchua and others 2003).
Figure 9. Deficits in postsynaptic PKA localization and hippocampal synaptic plasticity in AKAP150 mutant mice.
A) Immunohistochemical (IHC) staining for PKA-RIIα shows decreased PKA localization in CA1 dendritic regions and increased PKA localization in the cell body layer (red arrows) in AKAP150 knockout (KO) and D36 mice compared to WT. B) PKA-RIIα immunoblotting shows decreased PKA levels in synaptic membrane pellet (P) fractions and increased PKA levels in cytoplasmic supernatant (S) fractions in AKAP150 KO and D36 mice compared to WT. AKAP150 immunoblotting confirms lack of AKAP150 expression in KO mice and normal expression and distribution in D36 mice compared to WT. C) AKAP150 D36 mice but not AKAP150 KO mice exhibit reduced CA1 hippocampal LTP at 7-12 weeks of age and D) LTD at ~2 weeks (10-14 days) of age compared to WT littermate mice. Shown are plots of the average field EPSP initial slope measured 55-60 minutes after induction of LTD (1Hz, 15 min) or LTP (100Hz, 1 sec) as a % of the baseline initial slope (100%) measure 5 minutes prior to induction. WT and KO mice show ~45% potentiation with LTP and ~35% depression with LTD, while D36 mice show only ~15% potentiation with LTP and ~10% depression with LTD. *p<0.05 by ANOVA. Reproduced and adapted from (Weisenhaus and others 2010).
While LTP is normal in AKAP150 -/- mice at 8 weeks of age as compared to wild-type controls, LTD is significantly impaired in these mice. In line with this plasticity data, it was demonstrated that these AKAP150 -/- mice show modest deficiencies in spatial memory retention (Tunquist and others 2008). There was no detection of up-regulation of the expression of MAP2 or any other AKAP in AKAP150 -/- animals. Nonetheless, deletion of the entire AKAP150 protein and its many functions may result in opposing changes that largely cancel each other out (for instance removal of both PKA and CaN anchoring) or other indirect compensatory responses that minimize the observed phenotypes. In order to eliminate these unintended compensatory mechanisms that are inherent when using null animals, an AKAP150 D36 knock-in mouse was generated to selectively remove the PKA anchoring site by truncation of the last 36 residues of the protein (Lu and others 2007; Lu and others 2008; Weisenhaus and others 2010). In these D36 animals, like AKAP-/- animals, PKA localization to hippocampal dendrites and synaptic fractions is strongly reduced (Figure 9AB) (Lu and others 2008; Weisenhaus and others 2010). Despite these decreases in PKA dendritic localization, basal CA1 AMPAR transmission appears to be normal for both AKAP150 -/- and D36 mice across a range of ages. However, in two-week old D36 mice, LTD in the CA1 hippocampal area is strongly reduced with around 10% LTD remaining, but AKAP150 -/- mice at this age have normal levels of LTD (Figure 9D) (Lu and others 2008; Weisenhaus and others 2010). The level of LTD impairment in D36 slices was shown to be similar to that achieved with PKA inhibitors in control slices suggesting a complete loss of PKA contributions to LTD in the D36 mouse (Lu and others 2008).
Using D36 mice, it was also determined that there is an age-dependent requirement for AKAP-anchored PKA in LTP at ~8 weeks of age but not 4 weeks of age that is not apparent in subsequent analysis of AKAP150 null animals (Figure 9C) (Lu and others 2008; Weisenhaus and others 2010). Importantly, reversal learning deficits were found in adult D36 but not AKAP150 -/- mice in this study (Weisenhaus and others 2010). Interestingly, in wild-type slices using pharmacologic inhibitors, both PKA activity and GluR2-lacking Ca2+ permeable AMPARs (likely GluR1/1) were found to be required for LTP in ~8 week-old mice but not in 4 week-old mice. The mechanisms of insertion of Ca2+-permeable AMPARs in LTP may also involve removal of GluR2-containing receptors and are likely to be quite complex and require signaling in addition to PKA involving PKC, CaMKI, and CaMKII (Guire Oh Soderling and Derkach 2008; Plant and others 2006; Yang Wang and Zhou 2010).
The LTD results for D36 mice could fit with recent characterization of GluR1 Ser845 to Ala mice showing that this phosphorylation site helps stabilize a small perisynaptic pool of GluR1/1 homomers that do not contribute to basal transmission but rapidly exchange in and out of the synapse and are dephosphorylated and endocytosed during LTD (He and others 2009). While immunoblotting analyses of the basal levels of GluR1 Ser845 phosphorylation in hippocampal tissue from AKAP150 -/- mice found a decrease, no decrease has been detected in D36 mice (Lu and others 2007; Tunquist and others 2008). However, this Ser845 immunoblotting was done for total cell extracts and would likely miss a decrease in Ser845 phosphorylation especially if it was associated with the specific loss of a perisynaptic pool of GluR1 receptors. Nonetheless, overall these data suggest that AKAP150 anchored PKA is important for LTD induction in young animals and for LTP induction in older animals with regulation of GluR1 likely being involved in both cases. Due to the more pleiotropic nature of the complete AKAP150 knockout, the specific involvement of AKAP150-anchored PKA in LTP and reversal learning was only found using the more selective knock-in approach. Thus, it remains to be determined whether the LTD deficits and modest memory retention deficits independently reported for ~8 week-old AKAP150 -/- mice (Tunquist and others 2008) are more related to loss of PKA anchoring, CaN anchoring, PKC anchoring or some other process. To this end, we have recently developed an AKAP150 homozygous knock-in mouse expressing a deletion of the CaN binding region which we are in the process of characterizing to test the role of anchored CaN in AMPAR regulation during LTD/LTP and in learning and memory.
Other targets of AKAP79/150 in plasticity?
As briefly mentioned above, AMPARs may not be the only postsynaptic targets of AKAP79/150 and phosphorylation regulation in neuronal plasticity. The transmembrane AMPA receptor regulator proteins (TARPs) in many ways function as accessory subunits for AMPARs by modifying channel biophysical properties, altering receptor pharmacology, and controlling receptor trafficking to the plasma membrane and the PSD (Chen and others 2000; Tomita and others 2005; Tomita Sekiguchi Wada Nicoll and Bredt 2006). In particular, the C-termini of TARPs can bind to the PDZ domains of PSD-95 to favor AMPAR retention in the PSD (Bats Groc and Choquet 2007). The C-terminal domain of TARP2 (also γ2 and Stargazin) is phosphorylated by PKA, PKC and CaMKII and can be dephosphorylated by CaN and PP1/PP2A (Tomita Stein Stocker Nicoll and Bredt 2005). These phosphorylation sites likely coordinate regulation of PSD-95 binding with trafficking of AMPARs to and from the PSD during LTP and LTD (Bats Groc and Choquet 2007; Opazo and others 2010; Stein and Chetkovich 2010; Tomita Stein Stocker Nicoll and Bredt 2005). Although, it is not known if AKAP79/150 anchored PKA, PKC or CaN regulate TARP phosphorylation.
All the mechanisms of LTP and LTD discussed above do not require transcription or translation; however, gene expression is required for NMDAR-dependent LTP to persist for more than 1-2 hours and establish late-LTP. L-type calcium channel Ca2+ influx also contributes to synaptic plasticity, especially by regulating gene expression in late-LTP (Moosmang and others 2005). As mentioned above, in the C-terminal tail of AKAP79/150 is the LZ sequence that interacts with C-terminus of Cav1.2 L-channels (Figure 4)(Oliveria Dell'Acqua and Sather 2007). AKAP79/150 anchored PKA and CaN bi-directionally regulate L-channel activity in hippocampal neurons with PKA enhancing L-channel currents and CaN strongly opposing this enhancement (Oliveria Dell'Acqua and Sather 2007). Influx of Ca2+ through the L–type channel also activates AKAP79/150 anchored CaN to promote dephosphorylation of the transcription factor NFAT which translocates from dendrites to the nucleus to regulate gene expression (Graef and others 1999; Oliveria Dell'Acqua and Sather 2007). Thus, AKAP79/150 regulation of the L-channels could impact late-LTP by regulating gene expression. Regulation of activity-dependent gene expression and late-LTP has not yet been analyzed in AKAP150 mutant mice. Finally, AKAP79/150 interacts with potassium channels that control intrinsic membrane excitability and action potential firing properties including the KCNQ2/3 M-current channels which are targets of PKC and KV4.2 A-current channels which are targets of PKA and CaN (Bal Zhang Hernandez Zaika and Shapiro 2010; Hoshi and others 2003; Lin Sun Wikenheiser Kung and Hoffman 2010). Thus, signaling processes organized by AKAP79/150 in LTP and LTD may coordinate changes in postsynaptic AMPAR activity with changes in voltage-gated dendritic channels controlling gene expression and membrane excitability.
Additional future directions
While this review has focused on postsynaptic signaling by AKAPs and PKA in synaptic plasticity, there are well-characterized forms of presynaptic plasticity, such as LTP at hippocampal mossy fiber synapses onto CA3 neurons and cerebellar parallel fiber synapses onto Purkinje neurons that also require PKA signaling in the presynaptic neuron. In addition, analysis of mice over-expressing an Ht31 peptide transgene in either presynaptic CA3 or postsynaptic CA1 neurons demonstrated that some forms of late-LTP at CA1 synapses can be blocked solely by disrupting PKA anchoring in presynaptic neurons (Nie McDonough Huang Nguyen and Abel 2007). However, very little is know about the identify or signaling functions of presysnaptic AKAPs that may target PKA to substrates that regulate synaptic vesicle trafficking or release such as synapsin or RIM proteins. This area of presynaptic AKAP-PKA signaling is definitely an area ripe for future investigation.
Modifications to excitatory synapses in the ventral tegmental area, nucleus accumbens, and prefrontal cortex are thought to be important for drug addiction. Repeated exposure to drugs of abuse such as cocaine and amphetamine results in alterations in plasticity at these synapses that contribute to the effects of craving and withdrawal associated with addiction (Hyman and Malenka 2001). Importantly, activation of cAMP-PKA coupled D1 dopamine receptors leading to changes in L-channel and AMPAR activity has been implicated in the plastic changes associated with exposure to addictive drugs. AKAP79/150 is also expressed in these brain regions (Glantz Amat and Rubin 1992) and with AKAP79/150 emerging as an important regulator of AMPARs and L-channels in hippocampal neurons, it makes this signaling scaffold a likely player in the molecular and cellular processes leading to drug addiction. Finally, the potential for AKAP signaling complexes being involved in aberrant kinase and phosphatase signaling mechanisms in neurodegenerative disease such as Alzheimer's, developmental disabilities such as Down syndrome, mental health disorders such as schizophrenia, and neuronal injuries such as in chronic pain are all also major areas for future investigation.
Acknowledgement of Grant Support
R01NS040701 and R01MH080291 to M.L.D. J.L.S. is supported by T32AA007464.
References
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27(17):4598–602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal M, Zhang J, Hernandez CC, Zaika O, Shapiro MS. Ca2+/calmodulin disrupts AKAP79/150 interactions with KCNQ (M-Type) K+ channels. J Neurosci. 2010;30(6):2311–23. doi: 10.1523/JNEUROSCI.5175-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20(1):89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272(52):32727–30. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–5. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53(5):719–34. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3(12):1291–300. doi: 10.1038/81823. others. [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393(6687):793–7. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci. 2009;12(2):172–81. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Lodge D, Monaghan DT, Jane DE, Nisenbaum ES. Sibley DR, Hanin I, Kuhar M, Skolnick P, editors. Ionotropic glutamate receptors. 2007.
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51(2):213–25. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61(4):394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J Biol Chem. 1992;267(19):13376–82. [PubMed] [Google Scholar]
- Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992;267(24):16816–23. [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2(5):315–24. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–43. doi: 10.1038/35050030. others. [DOI] [PubMed] [Google Scholar]
- Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104(52):20990–5. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267(5194):108–11. doi: 10.1126/science.7528941. others. [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27(1):107–19. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315-360. J Biol Chem. 2002;277(50):48796–802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. Embo J. 1998;17(8):2246–60. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85(7):627–33. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8(2):101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Efendiev R, Samelson BK, Nguyen BT, Phatarpekar PV, Baameur F, Scott JD. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J Biol Chem. 2010;285(19):14450–8. doi: 10.1074/jbc.M110.109769. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28(2):511–25. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6(2):136–43. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Glantz SB, Amat JA, Rubin CS. cAMP signaling in neurons: patterns of neuronal expression and intracellular localization for a novel protein, AKAP 150, that anchors the regulatory subunit of cAMP-dependent protein kinase II beta. Mol Biol Cell. 1992;3(11):1215–28. doi: 10.1091/mbc.3.11.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci. 2002;22(16):7027–44. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Gomez LL, Scott JD, Dell'Acqua ML. Association of an A-kinase-anchoring protein signaling scaffold with cadherin adhesion molecules in neurons and epithelial cells. Mol Biol Cell. 2005;16(8):3574–90. doi: 10.1091/mbc.E05-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401(6754):703–8. doi: 10.1038/44378. others. [DOI] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28(23):6000–9. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+- permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106(47):20033–8. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne EA, Dell'Acqua ML. Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J Neurosci. 2007;27(13):3523–34. doi: 10.1523/JNEUROSCI.4340-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7(11):1066–73. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6(6):564–71. doi: 10.1038/nn1062. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131(1):160–73. doi: 10.1016/j.cell.2007.09.017. others. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21(5):1163–75. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141(3):524–35. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuchua Z, Wozniak DF, Bardgett ME, Yue Z, McDonald M, Boero J. Deletion of the N-terminus of murine map2 by gene targeting disrupts hippocampal ca1 neuron architecture and alters contextual memory. Neuroscience. 2003;119(1):101–11. doi: 10.1016/s0306-4522(03)00094-0. others. [DOI] [PubMed] [Google Scholar]
- Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271(5255):1589–92. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22(8):3005–15. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21(5):1151–62. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112(5):631–43. doi: 10.1016/s0092-8674(03)00122-3. others. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol. 2010;103(1):479–89. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci. 1998;18(6):2017–27. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sun W, Wikenheiser AM, Kung F, Hoffman DA. KChIP4a regulates Kv4.2 channel trafficking through PKA phosphorylation. Mol Cell Neurosci. 2010;43(3):315–25. doi: 10.1016/j.mcn.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–34. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. Embo J. 2007;26(23):4879–90. doi: 10.1038/sj.emboj.7601884. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y. AKAP150-anchored PKA activity is important for LTD during its induction phase. J Physiol. 2008;586(Pt 17):4155–64. doi: 10.1113/jphysiol.2008.151662. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104(9):3579–84. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295(5554):496–9. doi: 10.1126/science.1066843. others. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25(43):9883–92. doi: 10.1523/JNEUROSCI.1531-05.2005. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie T, McDonough CB, Huang T, Nguyen PV, Abel T. Genetic disruption of protein kinase A anchoring reveals a role for compartmentalized kinase signaling in theta-burst long-term potentiation and spatial memory. J Neurosci. 2007;27(38):10278–88. doi: 10.1523/JNEUROSCI.1602-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281(2):752–8. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55(2):261–75. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Gomez LL, Dell'Acqua ML. Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. J Cell Biol. 2003;160(1):101–12. doi: 10.1083/jcb.200209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P. CaMKII Triggers the Diffusional Trapping of Surface AMPARs through Phosphorylation of Stargazin. Neuron. 2010;67(2):239–252. doi: 10.1016/j.neuron.2010.06.007. others. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4(9):917–26. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63(1):92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott LA, Bauman AL, Scott JD, Dessauer CW. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A. 2008;105(37):13835–40. doi: 10.1073/pnas.0712100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9(5):602–4. doi: 10.1038/nn1678. others. [DOI] [PubMed] [Google Scholar]
- Robertson HR, Gibson ES, Benke TA, Dell'Acqua ML. Regulation of postsynaptic structure and function by an A-kinase anchoring protein-membrane-associated guanylate kinase scaffolding complex. J Neurosci. 2009;29(24):7929–43. doi: 10.1523/JNEUROSCI.6093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368(6474):853–6. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Smith KE, Gibson ES, Dell'Acqua ML. cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci. 2006;26(9):2391–402. doi: 10.1523/JNEUROSCI.3092-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280(17):16962–8. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EL, Chetkovich DM. Regulation of stargazin synaptic trafficking by C-terminal PDZ ligand phosphorylation in bidirectional synaptic plasticity. J Neurochem. 2010;113(1):42–53. doi: 10.1111/j.1471-4159.2009.06529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16(1):95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ. AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J Biol Chem. 2008;283(17):11445–52. doi: 10.1074/jbc.M709253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22(8):3044–51. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435(7045):1052–8. doi: 10.1038/nature03624. others. [DOI] [PubMed] [Google Scholar]
- Tomita S, Sekiguchi M, Wada K, Nicoll RA, Bredt DS. Stargazin controls the pharmacology of AMPA receptor potentiators. Proc Natl Acad Sci U S A. 2006;103(26):10064–7. doi: 10.1073/pnas.0603128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45(2):269–77. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A. 2008;105(34):12557–62. doi: 10.1073/pnas.0805922105. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenhaus M, Allen ML, Yang L, Lu Y, Nichols CB, Su T. Mutations in AKAP5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS One. 2010;5(4):e10325. doi: 10.1371/journal.pone.0010325. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285(5424):93–6. doi: 10.1126/science.285.5424.93. others. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Masada N, Wachten S, Pagano M, Halls ML, Everett KL. AKAP79/150 interacts with AC8 and regulates Ca2+-dependent cAMP synthesis in pancreatic and neuronal systems. J Biol Chem. 2010;285(26):20328–42. doi: 10.1074/jbc.M110.120725. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5(12):959–70. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci U S A. 2008;105(32):11388–93. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Zhou Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc Natl Acad Sci U S A. 2010;107(26):11999–2004. doi: 10.1073/pnas.0913004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27(41):11112–21. doi: 10.1523/JNEUROSCI.2465-07.2007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Sia GM, Sato TR, Gray NW, Mao T, Khuchua Z. Subcellular dynamics of type II PKA in neurons. Neuron. 2009;62(3):363–74. doi: 10.1016/j.neuron.2009.03.013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]