Abstract
Sepsis is primarily a disease of the aged, with increased incidence and mortality occurring in aged hosts. Heat shock protein (HSP) 70 plays an important role in both healthy aging and the stress response to injury. The purpose of this study was to determine the role of HSP70 in mediating mortality and the host inflammatory response in aged septic hosts. Sepsis was induced in both young (6–12week old) and aged (16–17 month old) HSP70−/− and wild type (WT) mice to determine if HSP70 modulated outcome in an age-dependent fashion. Young HSP70−/− and WT mice subjected to cecal ligation and puncture (CLP), Pseudomonas aeruginosa pneumonia or Streptococcus pneumoniae pneumonia had no differences in mortality, suggesting HSP70 does not mediate survival in young septic hosts. In contrast, mortality was higher in aged HSP70−/− mice than aged WT mice subjected to CLP (p=0.01), suggesting HSP70 mediates mortality in sepsis in an age-dependent fashion. Compared to WT mice, aged septic HSP70−/− mice had increased gut epithelial apoptosis and pulmonary inflammation. In addition, HSP70−/−mice had increased systemic levels of TNF-α, IL-6, IL-10 and IL-1β compared to WT mice. These data demonstrate that HSP70 is a key determinant of mortality in aged but not young hosts in sepsis. HSP70 may play a protective role in an age-dependent response to sepsis by preventing excessive gut apoptosis and both pulmonary and systemic inflammation.
INTRODUCTION
Heat shock proteins (HSPs) are a highly evolutionarily conserved group of molecules that play an important role in the host response to a wide variety of stresses, including infection, injury, oxidative damage, hypoxia, and thermal stress. HSP70 is the most extensively studied member of the family (1,2), and has been shown to have an important role in numerous diseases. HSP70 modifies the host response to injury by increasing resistance to numerous inflammatory mediators including oxidants and TNF-α (3–6). HSP70 also inhibits NF-κB activation, altering pro-survival protein and cytokine expression (7,8). In addition, immune cell receptors capture HSPs released from necrotic cells or HSP-containing exosomes (9,10), and receptor engagement by HSP70 increases dendritic cell production of TNF-α, IL-1β, IL-6, and chemokines (11–13). HSP70 has been implicated in the pathophysiology of sepsis, a disease that kills over 200,000 people annually in the United States (14–17).
HSP70 levels are decreased in both animal and human studies in sepsis. Cecal ligation and puncture (CLP), a model of fecal peritonitis, impairs pulmonary HSP70 levels (18), and expressing HSP70 in the lungs via adenoviral vector decreases pulmonary histologic abnormalities, prevents alveolar type II cell proliferation and improves short-term survival following CLP (19,20). Giving parenteral glutamine following CLP improves survival, an effect which is mediated through HSP70 since the survival benefit is not seen in HSP70−/− mice (21,22). In addition, HSP70 induction is impaired in lymphocytes in septic patients (23). Parenteral glutamine supplementation increases serum HSP70 in critically ill surgical patients and is associated with a decreased ICU length of stay (24). Genetic variants of HSP70 have also been associated with the development of septic shock in patients (25,26).
Sepsis is a disease that primarily affects the aged. More than 60% of septic patients are older than 65 years of age, and 80% of deaths from the disease occur in aged patients (14,27). Animal studies suggest that the pathophysiology of sepsis is different depending upon a host’s age, as aged mice have significantly increased mortality, an altered inflammatory response, and increased apoptosis when subjected to CLP, burn injury or endotoxemia (28–36). Gut epithelial apoptosis is increased in both animal models and human autopsy studies of sepsis (37–39) and this is further disproportionately increased in aged, septic mice (35). This may be significant since preventing sepsis-induced gut apoptosis improves survival in murine models of sepsis (40–42). The inflammatory response is also increased in aged septic mice (29,30,32–34,43,44).
The relationship between HSP70 and aging is complex. HSP70 is induced in response to both intrinsic and extrinsic stress. Since aging theory postulates that lifespan is causally linked to the ability of a host to resist these stresses, the ability of HSP70 to respond to and resist these stresses may lead to increased lifespan (45–47). Supporting this theory is evidence that lifelong systemic overexpression of HSP-encoding genes (including HSP70) increases lifespan in invertebrate animals (48,49). Possible mechanisms for this include alterations in the host’s immune or apoptotic response (45,50). In higher animals, age-related HSP increases or decreases have been reported for both tissue-specific and disease-specific states (45,51–54).
While there has been extensive research into a) HSP70 and sepsis, b) sepsis and aging, and c) HSP70 and aging, the complex interactions between HSP70, sepsis, and aging have not previously been studied. This study sought to determine if there was an age-dependent role of HSP70 in sepsis. In light of the importance of gender on HSP70 expression (55,56), outcomes from critical illness (57–59) and life expectancy with normal aging (60), the interaction of the three variables was studied in both male and female mice.
MATERIALS AND METHODS
Animals
HSP70−/− mice (which have germline deletions of both HSP70.1 and 70.3) were generated by backcrossing 129/SvJ × C57Bl/6 HSP70−/− mice (61) onto a 129/SvJ background. To determine the lifespan of both knockout and WT control animals, unmanipulated mice were aged to 25 months. Based upon the lifespan tables generated (see results), subsequent experiments on septic mice were performed on 6–12 week old mice (young) or 16–17 month old mice (aged). All mice were maintained on 12 hour light/dark cycles with free access to food and water at all times. Experiments were performed in accordance with National Institutes of Health guidelines and were approved by both the Washington University and Emory University Animal Studies Committees.
Sepsis Models
CLP was performed as a model of polymicrobial, intraabdominal sepsis (62). Under isoflurane anesthesia, a midline abdominal incision was performed, the cecum exteriorized, and then ligated approximately 10–15% distal to the ileocecal valve. The cecum was then punctured once with a 30 gauge needle and gently squeezed to extrude some stool. The cecum was returned to the abdomen, and the abdominal wall was closed. Mice were given a subcutaneous injection of 1 mL 0.9% NaCl to compensate for third-space fluid losses. In addition, animals were given 50 mg/kg/day of imipenem/cilastatin for broad spectrum antibiotic coverage. Animals were either sacrificed at 24 hours or followed 7 days for survival. Of note, aged WT and HSP70−/− male mice, aged female HSP70−/− mice, and young male HSP70−/− mice were operated on at the same time but are broken down into three different comparisons in the manuscript based upon a prehoc determination (aged male HSP70−/− vs. aged male WT, aged male HSP70−/− vs. aged female HSP70−/− , and aged male HSP70−/− vs. young male HSP70−/− mice).
To assess whether HSP70 had an effect on survival in an alternate model of sepsis, experiments were also performed using pneumonia as a model of monomicrobial, intrapulmonary sepsis. Pneumonia was induced via direct intratracheal injection of either Pseudomonas aeruginosa (ATCC 27853) or Streptococcus pneumoniae (strain 99.55, capsular subtype 6A) (63,64). P. aeruginosa was placed in trypticase soy broth with constant shaking overnight. The resulting culture was centrifuged at 6000g, washed twice with 0.9% NaCl, and re-suspended to a density of 0.2 A600nm. S. pneumoniae was placed on 5% blood agar plates overnight, washed, and re-suspended to an absorbance of 1.0 A600nm. Under isoflurane anesthesia, a midline cervical incision was made, and either 20 μl of P. aeruginosa (4 × 106 CFU) or 60 μl S. pneumoniae (2–4 ×107CFU) diluted in 0.9% NaCl was introduced into the trachea with a 29-gauge syringe. Following incision closure, mice were given a subcutaneous injection of 1 mL 0.9% NaCl to compensate for third-space fluid losses. Mice receiving Streptococcus pneumoniae also received a single dose of ceftriaxone at a dose of 50 mg/kg. Both of these models were designed to have approximately 50% mortality in young mice.
Intestinal epithelial apoptosis
Apoptotic cells in the intestinal epithelium were quantified in 100 contiguous well-oriented crypt-villus units per animal using two complimentary techniques: H&E-staining and active caspase-3 staining (65). Apoptotic cells were identified on H&E-stained sections by morphologic criteria where cells with characteristic nuclear condensation and fragmentation were considered to be apoptotic. For active caspase-3 staining, intestinal sections were deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide for 10 minutes. Slides were then placed in Antigen Decloaker (Biocare Medical, Concord, CA) and heated in a pressure cooker for 45 minutes to facilitate antigen retrieval. Sections were then blocked with 20% goat serum (Vector Laboratories, Burlingame, CA), and incubated with rabbit polyclonal anti-active caspase-3 (1:100; Cell Signaling Technology, Beverly, MA) overnight at 4°C. Sections were then incubated with goat anti-rabbit biotinylated secondary antibody (1:200; Vector Laboratories) for 30 minutes at room temperature, followed by Vectastain Elite ABC reagent (Vector Laboratories) for 30 minutes at room temperature. Slides were developed with diaminobenzidine, and counterstained with hematoxylin.
Cytokine analysis
Peritoneal fluid was obtained by lavaging the peritoneum with 5ml of sterile 0.9% NaCl and then centrifuged to obtain a supernatant used for cytokine analysis. Whole blood was collected retro-orbitally and centrifuged at 5000 rpm for 5 minutes in serum separator tubes. Both peritoneal and plasma cytokine concentrations were evaluated using a Bio-Plex Pro Mouse Cytokine Standard Group I Kit (Biorad, Hercules, CA) per the manufacturer’s protocol. All samples were run in duplicate.
Western Blots
Left lung tissue was removed, snap frozen, and stored at −80°C. Protein lysates were prepared from frozen tissue by homogenizing in a 5x volume of ice-cold homogenization buffer (66). Lysates were centrifuged at 10,000 rpm at 4°C for 5 minutes and the supernatant was collected. The Bradford protein assay was used to determine total protein concentration, and protein samples (40μg) were then heated at 95°C for 5 minutes after being added to an equal volume of 2x Laemmli buffer. Samples were run on polyacrylamide gels (Bio-Rad; Hercules, CA) for 45 minutes at 180V and then transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad) for 2 hours at 80V. Membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma-Aldrich, Inc.) at room temperature for 30 minutes. Membranes were then incubated overnight at 4°C with rabbit anti-HSP90 polyclonal antibody (1:4000 Stressgen, Victoria BC, Canada), rabbit anti-HSP25 polyclonal antibody (1:4:000 Stressgen), or rabbit anti-β-actin antibody (1:2000 Cell Signaling Technology). Membranes were washed and incubated for 1 hour at room temperature with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:1000 Cell Signaling Technology) and developed via a chemiluminescent system (Pierce; Rockford, IL). Proteins were detected following exposure to x-ray film.
Myeloperoxidase assay
The pulmonary vasculature was perfused with 1 ml PBS, and lungs were snap frozen and stored at −80°C. Right lower lobe sections were thawed, weighed, and homogenized in 4 ml of 20 mM potassium phosphate buffer with 0.5 g/dl hexadecyltrimethyl ammonium bromide. This was sonicated for 90 seconds and then incubated for 2 hours at 60o C. Samples were then centrifuged and 100 μl of supernatant placed into 2.9 ml of 50 mM potassium phosphate buffer (pH 6.0) with 0.167 mg/ml O-dianisidine and 0.0005% hydrogen peroxide. Absorbance was measured for 3 minutes at 460 nm. MPO activity per gram of protein was calculated using the rate of change in absorbance over 3 minutes and the protein content of the sample.
Statistics
Data were analyzed using the statistical software program Prism 4.0 (GraphPad, San Diego, CA) and are presented as mean ± SEM. Survival studies were analyzed using the Log-Rank test. All other data were tested for Gaussian distribution using the Shapiro-Wilk normality test. If data were found to have Gaussian distributions, comparisons were performed using the Student’s t-test. If not, comparisons were performed using the Mann Whitney test. A p value of <0.05 was considered to be statistically significant.
RESULTS
HSP70 has no effect on mortality in young septic mice
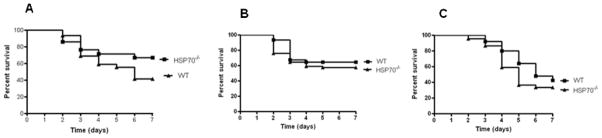
Young male HSP70−/− mice and WT controls were subjected to three different models of sepsis (CLP, Pseudomonas aeruginosa pneumonia and Streptococcus pneumoniae pneumonia) and followed 7 days for survival (Fig. 1). There were no statistically significant differences in survival between HSP70−/− and WT mice in any model.
FIGURE 1.
Germline deletion of HSP70 has no impact on survival on young mice in three different mouse models of sepsis. Male 6 week old HSP70−/− mice and WT controls were subjected to (A) CLP (n=21–29/group), (B) P. aeruginosa pneumonia (n=31–71/group or (C) S. pneumoniae pneumonia (n=25–44/group). There were no differences in survival between HSP70−/− and WT mice in any model although there was a nonsignificant trend toward improved survival in HSP70−/− mice after CLP.
HSP70 prevents mortality in aged septic mice
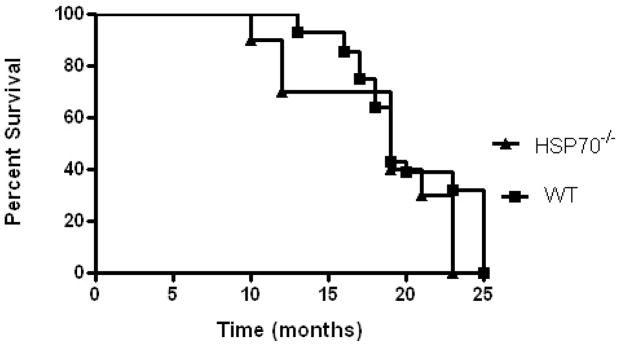
Unmanipulated male and female HSP70−/− and WT mice were first followed until they died or became moribund and were sacrificed to determine their lifespans. Approximately 65–75 percent of unmanipulated HSP70−/− and WT mice survived to 16–17 months, a survival percentage similar to that expected for 70–75 year old humans (Fig. 2) (67). As such, 16–17 month mice were used in subsequent experiments examining the role of HSP70 in aging and sepsis.
FIGURE 2.
HSP70−/− (n=10) and WT (n=28) animals were followed daily until they died or were determined to be moribund and were sacrificed. Approximately 65–75 percent of unmanipulated HSP70−/− and WT mice survived to 16–17 months. Based upon this, experiments in aged animals were performed on 16–17 month old mice.
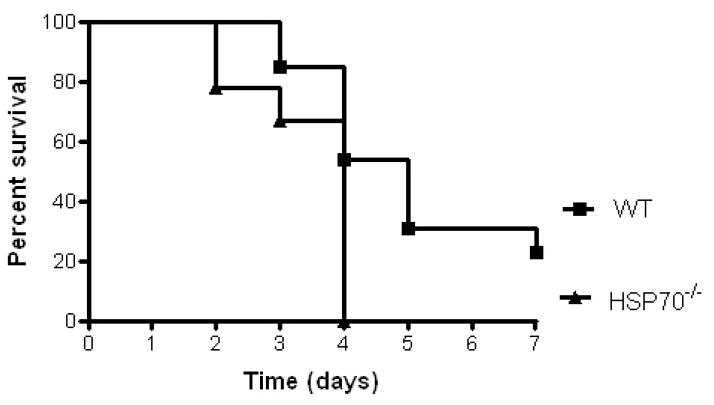
Aged male HSP70−/− and WT mice were then subjected to CLP and followed for survival. Survival was increased in WT mice compared to HSP70−/− mice (Fig. 3).
FIGURE 3.
Mortality is higher in aged septic HSP70−/− mice than aged septic WT mice. Aged male HSP70−/− and WT mice were subjected to 1x30 CLP, given fluids and antibiotics and followed for survival seven days after their septic insult. Survival was increased in WT mice compared to HSP70−/− mice (n=9–13/group, p=0.01).
HSP70 prevents sepsis-induced gut epithelial apoptosis and systemic inflammation in aged male mice
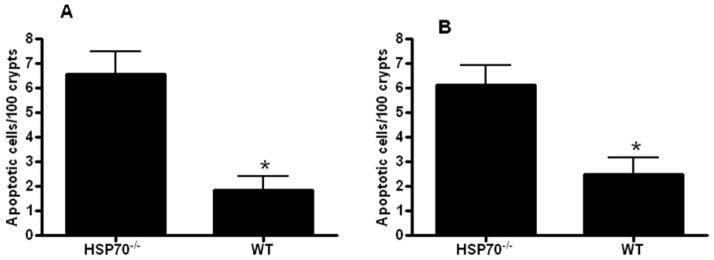
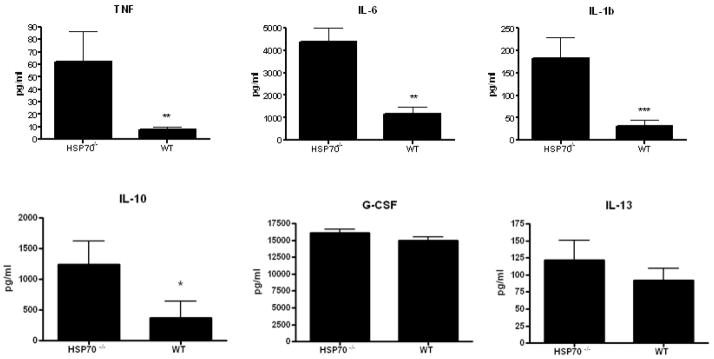
A different cohort of aged male HSP70−/− and WT mice were sacrificed 24 hours after CLP. Gut epithelial apoptosis was increased in HSP70−/− mice compared to WT mice whether assayed by active caspase 3 or H&E staining (Fig. 4). The systemic inflammatory response was markedly increased in HSP70−/− mice with elevated TNF-α, IL-6, IL-10 and IL-1β compared to WT mice (Fig. 5). Although there were differences in local peritoneal cytokines between the mice, these were less pronounced than the differences seen in plasma cytokines (Fig. 6).
FIGURE 4.
Gut epithelial apoptosis is increased in aged septic HSP70−/− mice compared to aged septic WT mice. Apoptosis was quantitated in 100 contiguous crypts by both active caspase 3 (A) and H&E staining (B). (n=14–18/group, p=0.001 and 0.004 respectively).
FIGURE 5.
The systemic inflammatory response is increased in aged septic HSP70−/−mice compared to aged septic WT mice. Systemic levels of TNF-α, IL-6, IL-1β, and IL-10 were all increased in HSP70−/− mice (n=13–15/group, p=0.008, 0.01, 0.0008, 0.03 respectively).
FIGURE 6.
Comparison of the local inflammatory response in aged septic HSP70−/− mice and aged septic WT mice. Levels of TNF-α, IL-6, and G-CSF were higher in the peritoneal fluid of HSP70−/− mice (n=10–12/group, p=0.02, 0.04, 0.01 respectively).
HSP70 decreases sepsis-induced pulmonary inflammation
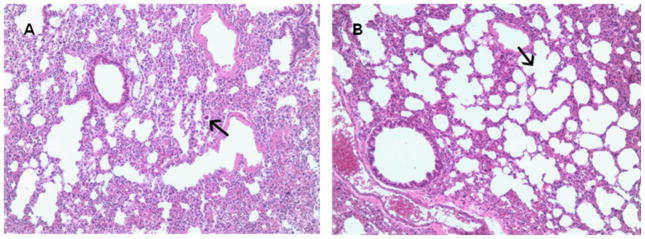
In light of data demonstrating that pulmonary administration of HSP70 improves pulmonary dysfunction in CLP-induced ARDS (19), pulmonary pathology and neutrophil infiltration were examined in HSP70−/− and WT mice. Pulmonary inflammation was more severe in HSP70−/− mice compared to WT mice as judged by a pathologist blinded to sample identity (Fig. 7A, B). In contrast, no significant differences were noted between animals in the myeloperoxidase assay (data not shown). To determine whether knocking out HSP70 caused compensatory changes in other heat shock proteins, HSP 25 and 90 protein levels were examined in aged male HSP70−/− and WT mice. No differences in HSP 25 or 90 were noted, regardless of whether an animal expressed HSP70 (data not shown).
FIGURE 7.
Pulmonary inflammation is increased in aged septic HSP70−/− mice compared to aged septic WT mice. Using a lung inflammation score ranging from 1 to 4, HSP70−/−mice (a) had an average score of 2.4 ± 0.4 with hypercellular alveoli, including an inflammatory infiltrate with some larger inflammatory-type cells (arrow). In contrast, WT mice (b) had a score of 1.7 ± 0.3 with more open, regularly spaced alveoli (arrow) and a relative paucity of inflammatory cells (n=6–7 animals/group). Representative micrograph at 100x is shown.
Gender alters mortality in aged but not young HSP70−/− mice
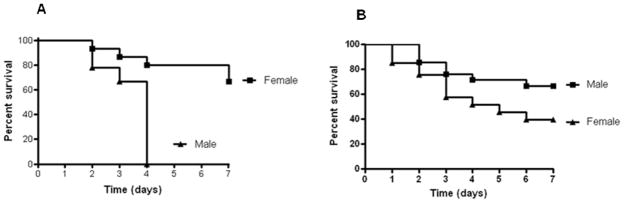
Both young and aged male and female HSP70−/− were subjected to CLP and followed for survival. Survival was markedly increased in aged female HSP70−/− mice compared to aged male HSP70−/− mice (Fig. 8A). In contrast, there were no differences in mortality between young male and female HSP70−/− mice (Fig. 8B).
FIGURE 8.
Mortality is higher in aged male septic HSP70−/− mice than aged female septic HSP70−/− mice (A, n= 9–15, p=0.005). In contrast, no statistically significant differences were detected between young male and female HSP70−/− mice subjected to CLP (B, n=21–33/group) although there was a non-significant trend (p=0.06) toward lower mortality in male mice.
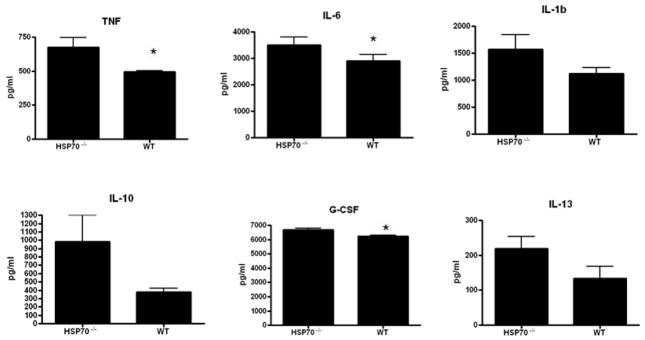
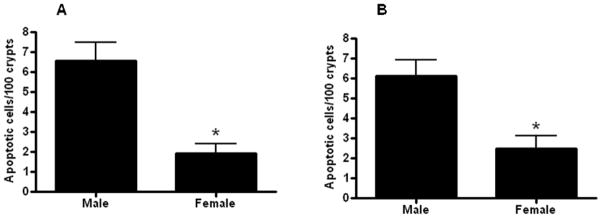
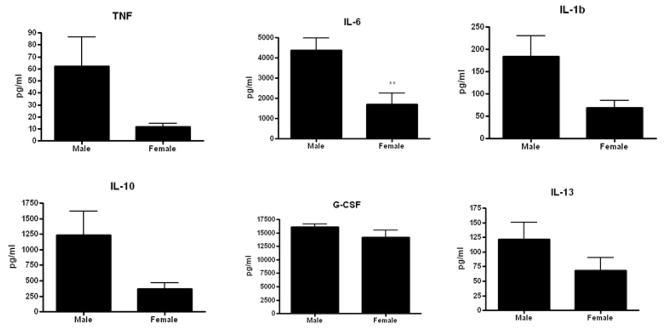
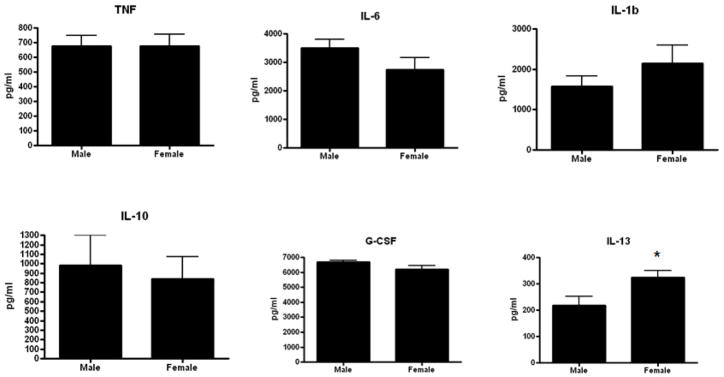
Gut epithelial apoptosis was increased in aged male HSP70−/− mice compared to aged female HSP70−/− mice whether assayed by active caspase 3 or H&E staining (Fig. 9). However, this was not accompanied by statistically significant changes in either the systemic or local inflammatory response (Fig. 10, 11). Despite the differences in mortality, the only statistically significant cytokine differences between aged male and female HSP70−/− mice were elevated plasma IL-6 and diminished peritoneal IL-13 in male mice.
FIGURE 9.
Gut epithelial apoptosis is increased in aged male septic HSP70−/− mice compared to aged female septic HSP70−/− mice Apoptosis was quantitated in 100 contiguous crypts by both active caspase 3 (A) and H&E staining (B). (n=12–14/group, p=0.0008 and 0.004 respectively).
FIGURE 10.
Comparison of the systemic inflammatory response in aged male and female septic HSP70−/− mice. Although there were trends towards higher levels in male mice in most cytokines evaluated, the only statistically significant difference was a higher level of IL-6 in male mice (n=12–15/group, p=0.004).
FIGURE 11.
Comparison of the local inflammatory response in aged male and female septic HSP70−/− mice. Levels of peritoneal cytokines were generally similar in both groups, with the only statistically significant difference being a lower level of IL-13 in male mice (n=10–12/group, p=0.03).
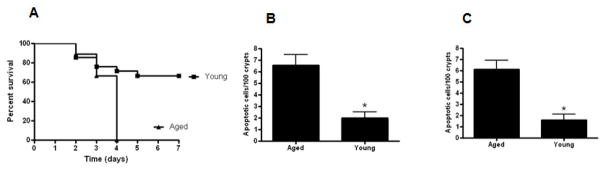
Aged male HSP70−/− mice have higher mortality than young male HSP70−/− mice
Young and aged male HSP70−/− were subjected to CLP and followed for survival. Survival was higher in young male HSP70−/− mice compared to aged male HSP70−/− mice (Fig. 12A). The increased mortality seen in aged septic male HSP70−/− mice was associated with increased gut epithelial apoptosis whether assayed by active caspase 3 or H&E staining (Fig. 12B, C). However, this was not accompanied by statistically significant changes in either the systemic or local inflammatory response with the exception of decreased plasma G-CSF in aged mice (data not shown).
FIGURE 12.
Mortality is higher in aged male HSP70−/− mice than young male HSP70−/−mice subjected to CLP (A, n= 9–21, p=0.002). Gut epithelial apoptosis is increased in aged male HSP70−/− mice compared to young male HSP70−/− mice subjected to CLP. Apoptosis was quantitated in 100 contiguous crypts by both active caspase 3 (B) and H&E staining (C). (n=8–14/group, p=0.002 and 0.001 respectively).
DISCUSSION
This study shows that HSP70 mediates mortality in aged mice subjected to sepsis but not in young mice given the same insult. This suggests that alterations in HSP70 may be one of the reasons that survival is lower in aged patients who become septic compared to younger patients with the same disease. Possible mechanisms for how HSP70 modulates mortality in aged septic hosts include preventing gut epithelial apoptosis and preventing both pulmonary and systemic inflammation.
This study gives new insights into the interactions between HSP70, aging, and gender in survival from sepsis. A major finding is that survival is worse in aged septic HSP70−/− mice compared to WT mice, but no significant differences were found between young septic HSP70−/− mice and WT mice in 3 models of sepsis. This suggests HSP70 plays a role in mediating mortality in aged but not young mice. These findings are complimentary to findings that tissue-specific expression of HSP70 in young mice in the lungs improves 48 hour survival following CLP (no longer term survival experiments have been published) (19,20) or that giving parenteral glutamine (which upregulates HSP70) improves survival after CLP in young mice (21,22). Taken together, the results suggest that while a basal amount of HSP70 is not necessary for an appropriate host response to infection in a young animal, a supranormal amount may be adaptive. In contrast, a basal amount of HSP70 appears to be required for an aged host to respond to a septic insult, possibly due to the accumulation of life stresses that occur prior to injury onset.
The fact that there was greater pulmonary inflammation in aged septic HSP70−/−mice than WT is consistent with work by Deutschman and Weiss showing that lung-specific delivery of HSP70 prevents ARDS following lethal CLP as well as studies demonstrating glutamine administration increases pulmonary HSP70 and prevents lung injury. However, this study also yields possible new mechanisms through which HSP70 might modulate mortality in aging and sepsis. Aged septic HSP70−/− mice had increased gut epithelial apoptosis and increased systemic inflammation compared to aged septic WT mice. Since prevention of gut apoptosis improves survival in both CLP and pneumonia (40,41), it is plausible that the increase in gut apoptosis seen in aged, septic HSP70−/− mice is responsible for the increased mortality seen in these animals. Similarly, an altered inflammatory response has been repeatedly implicated as one of the central causes of mortality from sepsis (68,69). The inflammatory response is also increased in aged septic mice (29,30,32–34,43,44). It is therefore possible that when the inflammatory changes of HSP deletion are superimposed upon the already abnormal host milieu in aging and sepsis, this plays a role in mediating the increased mortality seen in aged, septic HSP70−/− mice.
Our finding that mortality is similar between young septic HSP70−/− and WT mice differs from published findings by Wischmeyer et al (70) which found increased mortality in HSP70−/− mice following CLP. Possibilities for these seemingly contradictory results include multiple differences in study design including whether antibiotics were used (none vs. imipenem), the type of anesthesia used (ketamine and xylazine vs. inhaled isoflurane), and the size of injury (2×22 CLP vs. 1×30 CLP). While we do not rule out the possibility that HSP70 plays an important role in modulating mortality under differential experimental conditions, the fact that we saw no statistically significant differences in survival between young HSP70−/− and WT mice in three distinct models of sepsis strengthens the results presented herein.
In addition to the main finding of this manuscript that HSP70 modulates mortality in sepsis in aged but not young animals, we also examined the importance of both gender and age in isolation by comparing a) aged male and female septic HSP70−/− mice and b) aged and young septic HSP70−/− mice. Although this is the first report of aged HSP70−/−mice, the findings that both male mice and aged mice had higher mortalities is consistent with existing literature demonstrating both are risk factors in the development and outcome from sepsis (58,71). Interestingly, despite the associated increase in gut apoptosis in septic male aged mice compared to either septic female aged mice or septic male young mice, there were minimal difference in either systemic or peritoneal cytokines. Thus gut apoptosis is increased in mice with higher mortality in all comparisons studied (aged male HSP70−/− vs. aged male WT, aged male HSP70−/− vs. aged female HSP70−/−, aged male HSP70−/− vs. young male HSP70−/−). In contrast, the systemic inflammatory response is significantly different only in aged male HSP70−/− vs. aged male WT mice. This suggests that the differences in the inflammatory response are specifically due to the presence or absence of HSP while differences in gut apoptosis may more generally reflect increased mortality rather than a specific response to alterations in HSP levels, despite the known anti-apoptotic effect of HSP in the gastrointestinal tract (72).
This study has several limitations. First, we cannot conclude that unmanipulated HSP70−/− and WT mice have similar lifespans and thus cannot know if there is an impact of HSP70 deletion on survival independent of sepsis. Although there was no statistically significant difference detected between animals followed for lifespan (p value = 0.2 in figure 2), this could be an artifact of the relatively small number of knockout mice (n-10). Next, although septic aged male HSP70−/− have increased mortality and both increased gut apoptosis and inflammation, we cannot conclude that this correlation is causative without further experiments. Consistent with this, the results do not provide a central mechanism to explain our findings. Since the NFkB pathway is closely linked to sepsis, apoptosis and inflammation, future experiments examining both aging HSP70 expression and NFkB activation would be important to perform. Further, over the five years it took to perform the study, two different surgeons performed the CLP experiments (KWM, ACF). While all experiments were performed in a blinded, paired fashion (such that an equal number of experimental mice and control mice were operated on and followed for survival), we cannot rule out that differences in surgical technique affected our results, such as the higher than would be expected mortality in young septic HSP70−/− and WT animals. Additionally, female mice were operated on throughout the estrous cycle, potentially altering hormonal effects on survival. Also, pulmonary pathology was not assayed in female or young mice. Bacterial load was also not evaluated in either the blood or peritoneal fluid in young or aged HSP70−/− and WT mice. Mice were assayed at only a single timepoint after the onset of sepsis, and it is impossible to know what differences exist between mice at times other than 24 hours following the onset of sepsis. Finally, while survival studies were performed in three different models of sepsis in young mice, studies in aged mice were performed using only a single model (CLP), so it is unclear if the results are generalizable to other models of sepsis.
Despite these limitations, our data indicates that HSP70 is an important mediator in survival in aged septic mice. Since the increased mortality seen in aged septic HSP70−/−mice compared to matched WT mice was not seen in young knockout mice compared to WT mice in three models of sepsis, the effect of HSP70 appears to be age specific. Whether the difference in mortality is mediated by increased gut apoptosis, increased pulmonary or systemic inflammation or a mechanism not identified here should be the focus of future study.
Acknowledgments
This work was supported by funding from the National Institutes of Health (GM66202, GM072808, GM08795, GM044118, GM082008, AG025286, DK52574)
Reference List
- 1.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De MA. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- 3.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 4.Jaattela M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993;177:231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De MA. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278:35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- 9.Saito K, Dai Y, Ohtsuka K. Enhanced expression of heat shock proteins in gradually dying cells and their release from necrotically dead cells. Exp Cell Res. 2005;310:229–236. doi: 10.1016/j.yexcr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 11.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 12.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 13.Lehner T, Bergmeier LA, Wang Y, Tao L, Sing M, Spallek R, van der Zee R. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. EurJ Immunol. 2000;30:594–603. doi: 10.1002/1521-4141(200002)30:2<594::AID-IMMU594>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chen HW, Chou FP, Lue SI, Hsu HK, Yang RC. Evidence of multi-step regulation of HSP72 expression in experimental sepsis. Shock. 1999;12:63–68. doi: 10.1097/00024382-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Wizorek JJ, Coopersmith CM, Laramie JM, Tong A, Stromberg PE, Hotchkiss RS, Buchman TG, Cobb JP. Sequence makes a difference: paradoxical effects of stress in vivo. Shock. 2004;22:229–233. doi: 10.1097/01.shk.0000133593.55400.ca. [DOI] [PubMed] [Google Scholar]
- 17.Oberbeck R, Deckert H, Bangen J, Kobbe P, Schmitz D. Dehydroepiandrosterone: a modulator of cellular immunity and heat shock protein 70 production during polymicrobial sepsis. Intensive Care Med. 2007;33:2207–2213. doi: 10.1007/s00134-007-0851-4. [DOI] [PubMed] [Google Scholar]
- 18.Weiss YG, Bouwman A, Gehan B, Schears G, Raj N, Deutschman CS. Cecal ligation and double puncture impairs heat shock protein 70 (HSP-70) expression in the lungs of rats. Shock. 2000;13:19–23. doi: 10.1097/00024382-200013010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromberg Z, Raj N, Goloubinoff P, Deutschman CS, Weiss YG. Enhanced expression of 70-kilodalton heat shock protein limits cell division in a sepsis-induced model of acute respiratory distress syndrome. Crit Care Med. 2008;36:246–255. doi: 10.1097/01.CCM.0000295473.56522.EF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med. 2005;33:1206–1213. doi: 10.1097/01.ccm.0000166357.10996.8a. [DOI] [PubMed] [Google Scholar]
- 22.Singleton KD, Wischmeyer PE. Glutamine's protection against sepsis and lung injury is dependent on heat shock protein 70 expression. AmJ Physiol Regul Integr Comp Physiol. 2007;292:R1839–R1845. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder S, Lindemann C, Hoeft A, Putensen C, Decker D, von Ruecker AA, Stuber F. Impaired inducibility of heat shock protein 70 in peripheral blood lymphocytes of patients with severe sepsis. Crit Care Med. 1999;27:1080–1084. doi: 10.1097/00003246-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 2005;31:1079–1086. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 25.Kee C, Cheong KY, Pham K, Waterer GW, Temple SE. Genetic variation in heat shock protein 70 is associated with septic shock: narrowing the association to a specific haplotype. IntJ Immunogenet. 2008;35:465–473. doi: 10.1111/j.1744-313X.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 26.Waterer GW, ElBahlawan L, Quasney MW, Zhang Q, Kessler LA, Wunderink RG. Heat shock protein 70-2+1267 AA homozygotes have an increased risk of septic shock in adults with community-acquired pneumonia. Crit Care Med. 2003;31:1367–1372. doi: 10.1097/01.CCM.0000063088.86079.03. [DOI] [PubMed] [Google Scholar]
- 27.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 28.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83:1493–1501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009;37:1018–1023. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hyde SR, Stith RD, McCallum RE. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immun. 1990;58:619–624. doi: 10.1128/iai.58.3.619-624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- 35.Turnbull IR, Buchman TG, Javadi P, Woolsey CA, Hotchkiss RS, Karl IE, Coopersmith CM. Age disproportionately increases sepsis-induced apoptosis in the spleen and gut epithelium. Shock. 2004;22:364–368. doi: 10.1097/01.shk.0000142552.77473.7d. [DOI] [PubMed] [Google Scholar]
- 36.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Husain KD, Coopersmith CM. Role of intestinal epithelial apoptosis in survival. Curr Opin Crit Care. 2003;9:159–163. doi: 10.1097/00075198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N EnglJ Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 41.Coopersmith CM, Chang KC, Swanson PE, Tinsley KW, Stromberg PE, Buchman TG, Karl IE, Hotchkiss RS. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30:195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 42.Stromberg PE, Woolsey CA, Clark AT, Clark JA, Turnbull IR, McConnell KW, Chang KC, Chung CS, Ayala A, Buchman TG, Hotchkiss RS, Coopersmith CM. CD4+ lymphocytes control gut epithelial apoptosis and mediate survival in sepsis. FASEB J. 2009;23:1817–1825. doi: 10.1096/fj.08-119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez CR, Karavitis J, Palmer JL, Faunce DE, Ramirez L, Nomellini V, Kovacs EJ. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators Inflamm. 2010:475139. doi: 10.1155/2010/475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tower J. Hsps and aging. Trends Endocrinol Metab. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van RH. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Exp Gerontol. 2007;42:153–159. doi: 10.1016/j.exger.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Swindell WR. Heat shock proteins in long-lived worms and mice with insulin/insulin-like signaling mutations. Aging (Albany NY) 2009;1:573–577. doi: 10.18632/aging.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516:53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]
- 50.Singh V, Aballay A. Heat shock and genetic activation of HSF-1 enhance immunity to bacteria. Cell Cycle. 2006;5:2443–2446. doi: 10.4161/cc.5.21.3434. [DOI] [PubMed] [Google Scholar]
- 51.Njemini R, Bautmans I, Lambert M, Demanet C, Mets T. Heat shock proteins and chemokine/cytokine secretion profile in ageing and inflammation. Mech Ageing Dev. 2007;128:450–454. doi: 10.1016/j.mad.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Visala RD, Boyle GM, Parsons PG, Watson K, Jones GL. Influence of ageing, heat shock treatment and in vivo total antioxidant status on gene-expression profile and protein synthesis in human peripheral lymphocytes. Mech Ageing Dev. 2003;124:55–69. doi: 10.1016/s0047-6374(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 53.Blake MJ, Fargnoli J, Gershon D, Holbrook NJ. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. AmJ Physiol. 1991;260:R663–R667. doi: 10.1152/ajpregu.1991.260.4.R663. [DOI] [PubMed] [Google Scholar]
- 54.Heydari AR, Takahashi R, Gutsmann A, You S, Richardson A. Hsp70 and aging. Experientia. 1994;50:1092–1098. doi: 10.1007/BF01923466. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Otaka M, Odashima M, Tamaki K, Takada M, Izumi Y, Shibuya T, Sakamoto N, Itoh H, Watanabe S. Correlation of heat shock protein expression to gender difference in development of stress-induced gastric mucosal injury in rats. J Clin Biochem Nutr. 2010;47:64–73. doi: 10.3164/jcbn.10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fekete A, Vannay A, Ver A, Rusai K, Muller V, Reusz G, Tulassay T, Szabo AJ. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. AmJ Physiol Renal Physiol. 2006;291:F806–F811. doi: 10.1152/ajprenal.00080.2006. [DOI] [PubMed] [Google Scholar]
- 57.Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock. 2009;31:227–237. doi: 10.1097/SHK.0b013e31818347e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel TR, Dombrovskiy VY, Carson JL, Graham AM, Lowry SF. Postoperative Sepsis in the United States. Ann Surg. 2010;252:1065–1071. doi: 10.1097/SLA.0b013e3181dcf36e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 60.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 61.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, III, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 63.McConnell KW, McDunn JE, Clark AT, Dunne WM, Dixon DJ, Turnbull IR, Dipasco PJ, Osberghaus WF, Sherman B, Martin JR, Walter MJ, Cobb JP, Buchman TG, Hotchkiss RS, Coopersmith CM. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med. 2010;38:223–241. doi: 10.1097/CCM.0b013e3181b4a76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM, Dominguez JA, Perrone EE, Dunne WM, Hotchkiss RS, Buchman TG, Linehan DC, Coopersmith CM. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit Care Med. 2010;38:886–893. doi: 10.1097/CCM.0b013e3181c8fdb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW, Barrett TA, Ayala A, Perl M, Buchman TG, Coopersmith CM. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol Histopathol. 2007;22:623–630. doi: 10.14670/hh-22.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30:36–42. doi: 10.1097/shk.0b013e31815D0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. Natl Vital Stat Rep. 2010;58:1–132. [PubMed] [Google Scholar]
- 68.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N EnglJ Med. 2010;363:87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russell JA. Management of sepsis. N EnglJ Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 70.Singleton KD, Wischmeyer PE. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. AmJ Physiol Lung Cell Mol Physiol. 2006;290:L956–L961. doi: 10.1152/ajplung.00466.2005. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe E, Buchman TG, Hirasawa H, Zehnbauer BA. Association between lymphotoxin-alpha (tumor necrosis factor-beta) intron polymorphism and predisposition to severe sepsis is modified by gender and age. Crit Care Med. 2010;38:181–193. doi: 10.1097/CCM.0b013e3181bc805d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudeja V, Vickers SM, Saluja AK. The role of heat shock proteins in gastrointestinal diseases. Gut. 2009;58:1000–1009. doi: 10.1136/gut.2007.140194. [DOI] [PMC free article] [PubMed] [Google Scholar]