Abstract
To determine if the addition of a behavioral intervention during alcohol detoxification would facilitate initiation of subsequent care, we randomized 150 detoxification patients to receive: treatment as usual (TAU), a Motivation Enhancement Therapy (MET) intervention, or a Peer-delivered Twelve Step Facilitation (P-TSF) intervention. The main outcome was the initiation of any type of subsequent care (i.e., professional treatment or self-help) within 30 and 90 days of discharge. Other outcomes included: alcohol and drug use, completion of subsequent professional treatment, and readmission for detoxification. The mean age of the participants was 45 years; 65% were men, and 84% were white. At the 30-day follow-up, there was no significant difference among the groups in the rate of initiation of any type of subsequent care (82%, 74%, and 82% respectively, p = 0.617); however, the MET group had significantly more patients initiate subsequent inpatient treatment by the 90-day follow-up compared to the P-TSF group (31% and 61%, p = 0.007) and a greater proportion of MET participants completed subsequent inpatient treatment compared to both TAU and P-TSF. There were no differences in drinking-related outcomes (e.g., number of days before first drink, percent days abstinent) between the groups. We conclude that MET during detoxification may provide additional benefits in terms of initiating and maintaining patients in aftercare inpatient treatment programs.
Keywords: alcoholism, detoxification, interventions, therapeutics, outcome assessment
INTRODUCTION
The usual treatment for those with alcohol dependence involves the initiation of abstinence and participation in rehabilitation services. For those with the most severe forms of alcohol dependence, treatment may begin with the medical management of the alcohol withdrawal syndrome in a controlled inpatient setting (i.e., “detoxification”). The primary goals of inpatient alcohol detoxification are to: 1) prevent the complications of alcohol withdrawal (e.g., seizures, delirium), 2) retain the individual in treatment (i.e., avoid a discharge “against medical advice”), 3) initiate a period of abstinence, and 4) link the patient to professional alcohol rehabilitation treatment services and/or mutual self-help programs (e.g., Alcoholics Anonymous) following hospital discharge.1 Medications (e.g., naltrexone) can also be started following detoxification that may assist in maintaining abstinence.2
Although the pharmacological management of the alcohol withdrawal syndrome is effective, the psychosocial outcomes of inpatient detoxification have been disappointing.3–5 One study for example, observed no differences in post-detoxification behavioral outcomes (e.g., treatment utilization, abstinence) between individuals who were detoxified and those who had not undergone detoxification. 6 Other studies suggest that following inpatient detoxification, many patients are not linked to rehabilitation treatment services following discharge and subsequently return to drinking within a few weeks.3, 7–10
To help improve psychosocial interventions performed during detox, authorities have recommended that treatment should specifically focus on fostering long-term treatment planning and utilization,5, 11 rather than only making a client’s “intake assessment.”12 In particular, patient engagement with treatment is a factor that has been associated with increased abstinence in continuing care.13 To this end, a variety of additional interventions are thought to be helpful to link patients to specific post-detoxification treatment services and to promote abstinence or at least reduce drinking and other drug-related behaviors.14–18 For example, one group of investigators found that patients who received Motivational Enhancement Therapy (MET) sessions attended a greater number of self-help group meetings on average (p = .02) than patients who received “standard” treatment. 18 In a non-randomized prospective study, hospitalized patients who received a Twelve Step Facilitation visit from a peer (P-TSF), were more likely to remain abstinent (p = 0.012) and initiate either rehabilitation treatment or self-help (p < 0.001) than in a concurrent control group.19 In an observational study, patients who received P-TSF during inpatient detoxification were more likely (p = 0.05) to report attending self-help group meetings following hospital discharge than a group of patients who did not receive P-TSF.20
Despite these findings, alcohol detoxification treatment has changed very little over the past 2 to 3 decades.21 Furthermore, there is a lack of randomized controlled trials to guide the development of new evidence-based pharmacological or psychological practices for the improvement of the interventions currently used during detoxification. As a result, clinicians have little to guide them on how the current standard-of-care for alcohol detoxification might be improved in regards to facilitation of engagement in subsequent rehabilitation services.
Therefore, objective of this study was to determine if the addition of a behavioral intervention (i.e., MET or P-TSF) to treatment as usual (TAU) provided to alcohol dependent patients hospitalized for the management of alcohol withdrawal (i.e., “detoxification”) would lead to an increase in the rate of initiation of any type of subsequent rehabilitation services (i.e., initiation of professional rehabilitation treatment or attending meetings of mutual self-help programs) within 30 and 90 days of hospital discharge. Other outcomes were evaluated at the 90-day follow-up and included alcohol and drug use, completion of subsequent professional treatment, and readmission for detoxification. It was hypothesized that MET or P-TSF interventions will be superior to TAU only conditions, in terms of attaining aftercare initiation and other outcomes following hospitalization for alcohol detoxification treatment.
METHODS
The Institutional Review Board (IRB) at the sponsoring institution and the Office of the Medical Director of the hospital approved the study protocol. A Certificate of Confidentiality (AA-149-2006) was obtained from Department of Health and Human Services. The study was registered with the Food and Drug Administration at www.ClinicalTrails.gov (NCT00513708).
Participants
The 150 study participants were recruited from patients who had been hospitalized for the medical management of the alcohol withdrawal syndrome. Potential participants were approached for inclusion into the study if they were at least 18 years old, could understand English, resided in the metropolitan area, had physician consent for study entry, and were able to give informed consent. Patients were excluded if they had previously refused to participate or if they were homeless, enrolled in a methadone maintenance program, under the custody of law enforcement officials or unable to provide informed consent (e.g., due to cognitive impairment from a medical condition or a psychotic mental illness).
Setting
Participant recruitment occurred in an 18-bed inpatient alcohol and drug detoxification unit of a 550-bed public tertiary-care teaching hospital that has 1,200 to 1,400 admissions per year. It is staffed by 4 physicians, a nurse practitioner, 6 chemical dependency counselors, and a varying number of nurses and other support staff.
Procedure
Two of the investigators (L.M.F. and H.L.B.) screened the inpatient census for eligibility 6 days per week (Monday through Saturday). Following informed consent, another investigator (E.M.F.) assigned the participant to one of 3 intervention arms using a 2:2:2 block randomization procedure. This same investigator also arranged for and coordinated the delivery of the interventions. The other investigators, the participants, and the clinical staff members were not informed (i.e., were “masked”) of the treatment condition; however, participants often revealed their intervention assignment to the research assistants at the time of follow-up data collection.
The same 2 investigators who had recruited the participant also collected the follow-up data. Follow-up data were collected via a telephone interview on or after 7 days, 30 days, and 90 days following hospital discharge. Those who were not able to be located via the telephone for follow-up were mailed a written data collection instrument that was to be returned to the study office. Participants were compensated by $10 for each of the three follow-ups, with the possible total of $30 received for participation.
Interventions
Treatment as Usual
The TAU group served as the “active comparator” control. Following admission to the detoxification unit, patients received an initial evaluation by an addiction medicine physician or addiction psychiatrist, and a chemical dependency counselor. These evaluations served to guide medical management during detoxification and discharge planning. Psychosocial assessments were also conducted to arrange for appropriate alcohol rehabilitation aftercare treatment. During hospitalization, patients were required to attend one-hour group therapy sessions twice daily regarding maintenance of abstinence, treatment options, nutrition, relaxation techniques, prevention of HIV and relapse prevention. Individual counseling sessions, family sessions, and self-help meetings were available, but not required. A benzodiazepine was typically used as monotherapy for the management of alcohol withdrawal. Dosages were administered based on the clinical assessment of withdrawal risk and severity. Other medications (e.g., antiemetics, analgesics) were also used as needed for symptoms. The typical length of stay was 3 to 5 calendar days. At the time of discharge patients were referred to rehabilitation treatment services based on the recommendation of the physician and chemical dependency counselor, the wishes of the patient, and guidelines, if any, imposed by the patient’s health insurance.
Motivation Enhancement Therapy
Those randomized to the experimental MET intervention group received TAU and a one-on-one, 45- to 60-minute motivational interview based on a standard protocol.22 The goal of MET was to promote behavioral change by providing the patient with feedback and focusing on eliciting and strengthening commitment to change. Self-reported problems were recoded in the participants’ own words on a paper feedback form under pre-printed headings: “Interpersonal,” “Job/School,” “Legal/DWI,” “Medical,” “Psychological,” “Housing,” and “Other.” The participants’ goals for each of these headings were also recorded on the same form. The participants’ plans for immediate treatment (e.g., counseling, self-help, medications) following hospital discharge were recorded on the feedback sheet along with the participant’s specific treatment plans (e.g., “be admitted for a 28-day rehab”) should a relapse occur. Compared to TAU, the MET intervention placed a larger emphasis on eliciting specific commitments for aftercare and relapse plans. The participant received a copy of the feedback report, and they were also given an interpretational handout to take home and keep for future reference.
Peer-delivered Twelve Step Facilitation
Those randomized to the experimental P-TSF intervention group received TAU and a 45- to 60-minute visit by volunteers who were “recovering from alcoholism” and familiar with making “12th-Step calls.” These interventions are detailed in the chapter entitled “Working with Others” in “The Big Book” of AA.23 The peers would give some “practical advice,” encourage the participant to take action, and offer the friendship and fellowship that can be found in AA. Printed informational materials about AA and a schedule of AA meetings were given to each participant.
Fidelity Monitoring Procedures
Several procedures were used to determine whether participants actually received the intervention as planned. Fidelity to the intervention was monitored by one of the members of the study team (E.M.F.) throughout the enrollment period via: 1) regular meetings with the MET interventionists and telephone calls to the P-TSF interventionists prior to each intervention that served to reinforce study procedures, 2) forms that were completed by the interventionists after each intervention to verify adherence to the study protocol, and 3) one of the investigators (E.M.F.) conducted a face-to-face “fidelity check” with each of the patients based on a treatment checklist to assess the adherence to the procedures of the intervention.
Baseline Measurements
Participant Characteristics
These data were collected on paper forms designed for this study and included: participant demographics (age, gender, and race), co-morbid conditions (chronic pain, psychiatric problems), socioeconomic characteristics (education, occupation, and employment), family history of alcohol or drug problems, and criminal history (arrest record, convictions, time spent in jail or prison).
Alcohol and Drug Use
A written check-list of the criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition24 was used to confirm the diagnosis of alcohol dependence. Information about the recent use of alcohol and other drugs was assessed with the Time Line Follow Back (TLFB) procedure.25, 26 In this study, the TLFB was used to determine the percent days abstinent (PDA) and days to first use.
Problems Related to Drinking
The Short Inventory of Problems (SIP) was used to assess negative consequences related to alcohol dependence.27 It is the 15-item abbreviated version of the Drinker Inventory of Consequences.28
Involvement with Self-Help Programs
The 9-item Alcoholics Anonymous Affiliation Scale (AAAS) was used as a measure participant involvement in AA.29
Readiness to Change Behavior
The 19-item Stage of Change Readiness and Treatment Eagerness Scale short form (SOCRATES) was used to assess the motivation for change in the participants.30
Biological Data
The severity of withdrawal symptoms and signs was originally assessed by a counselor in the emergency department prior to admission to the detoxification unit by using the Clinical Institute Withdrawal Assessment, Alcohol-revised (CIWA-Ar)31 and recorded in the medical record. As part of the usual clinical care, the expired breath alcohol concentration (BAC) was measured using an AlcoSensor IV (Intoximeters, Inc., St. Louis, Missouri) in the emergency department prior to admission to the detoxification unit. Urine for toxicology was collected from the patients on the detoxification unit, which was used to test for the presence of opiates, cocaine (i.e., benzolecgonine), cannabinoids, and benzodiazepines by immunoassay as determined with a COBAS Integra 800 system (Roche Diagnostics, Indianapolis, Indiana). A presumptive positive test was reported by the hospital laboratory for a single compound or the combined reactivity of a parent compound and/or its metabolites at a concentration of 300 ng/mL for benzodiazepines, opiates, and cocaine, and 50 ng/mL for cannabinoids. Values for admission alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were obtained from medical records and were expressed as Units/Liter.
Outcome Measurements
The primary outcome was the initiation of mutual self-help meeting attendance (i.e., at least one meeting) or professional outpatient counseling (i.e., at least one session), or an admission to either an inpatient or a residential rehabilitation facility within 30 days of hospital discharge. Secondary 90-day outcomes included: initiation of treatment services during the 90-day follow-up (as opposed to just 30-day), the number of mutual self-help program meetings attended, self-reported drinking behaviors (i.e., total 90-day abstinence, time to first drink, time to first heavy drinking day, and PDA) and drug use, completion of any outpatient or inpatient treatment program, and the readmission to the detoxification unit. Associated qualitative data was collected on factors related to recovery such as employment status, arrests, home life, access to and utilization of health care services (e.g., primary care, hospitalizations), and use of medication to promote abstinence (i.e., disulfiram, naltrexone, or acamprosate).
Data Analyses
Data analyses were performed on a de-identified dataset by investigators (U.J., G.G.H., and L.A.), who were not involved with data collection, on an intent-to-treat basis unless otherwise specified. Categorical variables were compared using the Fisher exact test. Means and standard deviations (SD) were calculated for continuous measures and comparisons were conducted using one-way analysis of variance. Bivariate post hoc analyses were performed with Fisher exact test or independent sample t-tests as appropriate. Alpha criterion was set at p < 0.05. Overall event free survival (i.e., total abstinence from alcohol and all drugs) was calculated according to the Kaplan-Meier method. To generate and analyze longitudinal models for 90-day binary and count outcomes (e.g., completion of aftercare, PDA), we used Generalized Estimating Equations (GEE). Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc, Chicago IL) and Stata version 9 (StataCorp LP, College Station, TX).
To calculate statistical power, we estimated that 20% of patients in the TAU group would initiate aftercare treatment within 30 days of hospital discharge and that 40% of those assigned to either intervention group would initiate treatment based on our previous work with this population.32 For the outcomes expressed as continuous variables (e.g., PDA), we estimated that there would be a 25 point mean difference between the TAU group and an experimental intervention group, and we also predicted that we would observe a pooled within-group standard deviation of about 40 (effect size = 0.65). The calculation of the sample size was based on these assumptions. We estimated that this study had a power of 0.80, using an alpha of 0.05 to detect differences of this magnitude if at least 40 participants in each group (i.e., an 80% follow-up rate) were available for follow-up.
RESULTS
Participant Recruitment and Flow
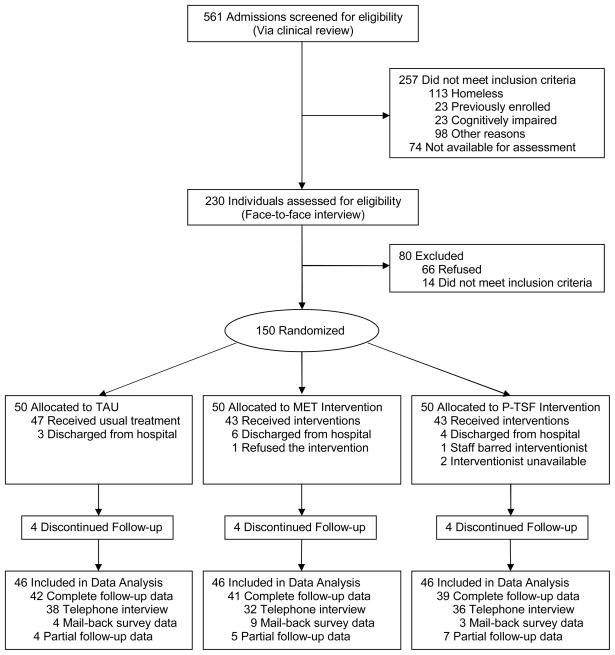
The participant flow is summarized in Figure 1. Between July 9, 2007 and April 11, 2008, there were a total of 561 admissions for the medical management of alcohol withdrawal. Of those, 257 did not meet inclusion criteria and 74 were not available for assessment (65 because they had left the hospital before they could be evaluated and 9 during a time when enrollment was suspended due to an adverse event). The remaining 230 individuals were assessed by one of two investigators (L.M.F or H.L.B.) during a face-to-face interview and 150 (65.2%) agreed to undergo randomization and participate in the study. After randomization, 42, 41, and 39 participants completed follow-up in the TAU, MET, and P-TSF groups, respectively. Partial data were collected from other participants such that 46 (92%) were included in the final data analysis in each of the 3 groups. Partial data collection occurred due to incomplete telephone follow-up interviews (e.g., sometimes a participant was “too tired” to answer all the questions), there were missing data from the mail in survey, or because only a limited amount of follow-up data could be abstracted from hospital records (e.g., when a participant presented to the Emergency Department during the follow-up interval). In addition, 8 individuals (5%) did not complete inpatient treatment for the management of alcohol withdrawal (i.e., “detoxification”) – 3 in the TAU group left hospital early “against medical advice,” 2 in the MET group left early (1 “against medical advice” and 1 by “administrative discharge”), and 3 in the P-TSF group also left “against medical advice.” As a result, not all of the baseline data were collected from these participants.
Figure 1.
Participant flow diagram
Baseline Data
Participant characteristics are summarized in Table 1. The three groups did not significantly differ at baseline on any demographic, psychological or biological variable, alcohol/drug use, treatment history, or criminal history. The MET group had statistically significant lower mean CIWA-Ar scores (p = 0.016) than the other 2 groups. Participant scores on the psychological measures (i.e., SIP, AAAS, and SOCRATES) suggest numerous alcohol-related problems and low involvement in AA, which is typical of individuals seeking treatment for an alcohol use disorder.33
Table 1.
Baseline Participant Characteristics
| Characteristic a | TAU (N=50) | MET (N=50) | P-TSF (N=50) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 46.58±9.92 | 44.96±10.75 | 44.48±12.36 | 0.611 |
| Male gender | 30 (60) | 35 (70) | 33 (66) | 0.572 |
| White race | 44 (88) | 39 (78) | 43 (86) | 0.353 |
| Married | 19 (38) | 15 (30) | 19 (38) | 0.627 |
| High school graduate | 36 (72) | 36 (72) | 44 (88) | 0.088 |
| Employed | 34 (68) | 34 (68) | 36 (72) | 0.882 |
| Unskilled laborer b | 19 (41) | 16 (39) | 16 (33) | 0.714 |
| Owns a home | 18 (36) | 23 (46) | 21 (42) | 0.593 |
|
| ||||
| Alcohol Use | ||||
| Age of first use | 14.86±4.64 | 14.60±4.96 | 15.88±7.02 | 0.488 |
| Years of alcohol use | 28.00±10.51 | 24.50±12.24 | 25.38±14.17 | 0.342 |
| # drinking days in prior 30 days | 23.32±9.07 | 25.54±6.36 | 23.78±9.43 | 0.380 |
| Admission breath alcohol % | .0487±.070 | .085±.123 | .070±.099 | 0.185 |
| Admission CIWA-Ar score | 10.78±6.69 | 7.94±6.864 | 11.70±6.56 | 0.016c |
| Initial AST | 70.98±71.00 | 92.29±93.28 | 88.90±98.29 | 0.441 |
| Initial ALT | 58.31±54.96 | 61.2±52.51 | 60.25±50.82 | 0.962 |
|
| ||||
| Drug Use | ||||
| Currently smoking tobacco | 28 (56) | 34 (68) | 31 (62) | 0.466 |
| 30-day non-heroin opioid | 11 (22) | 12 (24) | 10 (20) | 0.890 |
| 30-day benzodiazepine | 7 (14) | 5 (10) | 10 (20) | 0.363 |
| 30-day cocaine | 10 (20) | 13 (26) | 5 (10) | 0.116 |
| 30-day marijuana | 12 (24) | 6 (12) | 12 (24) | 0.223 |
| Lifetime heroin | 6 (12) | 6 (12) | 10 (20) | 0.426 |
| Lifetime IV drug | 3 (6) | 5 (10) | 10 (20) | 0.085 |
| Admission Toxicology Results b | ||||
| Opiate positive | 3 (7) | 5 (11) | 3 (7) | 0.672 |
| Cocaine positive | 6 (13) | 8 (18) | 6 (14) | 0.787 |
| Cannabinoid positive | 9 (20) | 4 (9) | 6 (14) | 0.360 |
|
| ||||
| Prior Treatment | ||||
| Detoxification admission | 30 (60) | 36 (72) | 29 (58) | 0.291 |
| Inpatient rehabilitation | 23 (46) | 25 (50) | 23 (46) | 0.899 |
| Outpatient rehabilitation | 28 (57) | 39 (78) | 31 (62) | 0.720 |
| AA attendance | 34 (68) | 40 (80) | 38 (76) | 0.411 |
|
| ||||
| Criminal History | ||||
| DUI/DWI arrests | 19 (38) | 22 (44) | 22 (44) | 0.782 |
| Other arrests | 16 (32) | 14 (28) | 17 (34) | 0.805 |
| Misdemeanor convictions | 14 (28) | 15 (30) | 16 (32) | 0.880 |
| Felony convictions | 5 (10) | 5 (10) | 9 (18) | 0.381 |
| Any jail time | 17 (34) | 16 (32) | 20 (40) | 0.684 |
| Months of prior jail timed | 10.35±20.67 | 12.67±24.65 | 30.53±67.25 | 0.334 |
|
| ||||
| Psychological Measures | ||||
| SIP | 12.38±2.50 | 11.70±3.52 | 12.44±2.54 | 0.360 |
| AAAS | 1.94±2.05 | 2.85±2.66 | 2.89±2.55 | 0.088 |
| SOCRATES | ||||
| Taking Steps | 34.94±4.27 | 34.66±5.17 | 35.54±4.24 | 0.598 |
| Recognition | 32.00±3.67 | 31.94±5.28 | 31.94±5.22 | 0.997 |
| Ambivalence | 14.32±3.68 | 13.50±4.97 | 12.56±4.79 | 0.153 |
Abbreviations: AA, Alcoholics Anonymous; AAAS, The Alcoholics Anonymous Affiliation Scale; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CIWA-Ar, Clinical Institute Withdrawal Assessment-Alcohol revised; DUI/DWI, Driving under the influence/Driving while intoxicated; IV, Intravenous; MET, Motivational enhancement therapy; P-TSF, Peer-delivered twelve step facilitation; SIP, The Short Inventory of Problems; SOCRATES, The Stage of Change Readiness and Treatment Eagerness Scale; TAU, Treatment as usual.
Data are presented as n (%) or mean ± standard deviation.
These items contain some missing data.
CIWA-Ar scores were significantly lower in MET compared to P-TSF (p < 0.01) and UC (p < 0.05).
For those who had served time in jail.
Data Analyses
Primary Outcome
The rates of rehabilitation services initiation are summarized in Table 2. Exceeding pre-study assumptions, over half of the participants in each group initiated some sort of professional rehabilitation treatment and about two-thirds attended at least one meeting of a mutual self-help group by the 30-day follow-up; however, across the 3 groups there was not a significant difference in the rates for the initiation of any kind of rehabilitation service (i.e., professional outpatient counseling, inpatient, or residential treatment services or self-help meeting attendance) either as a group or as an individual service. At the 90-day follow-up, those assigned to P-TSF were less likely to initiate inpatient rehabilitation (e.g., a “28-day” program) following hospitalization as compared to participants assigned to the MET group. Moreover, the participants assigned to the MET group were more likely to report having completed inpatient rehabilitation during the 90-day follow-up than those assigned to TAU or P-TSF.
Table 2.
Outcomes: Rehabilitation Services Utilization and Substance Use
| Outcomea | TAU (N=46) | MET (N=46) | P-TSF (N=46) | p-value |
|---|---|---|---|---|
| Primary 30-day Outcome | ||||
| Initiated any serviceb (%) | 36/44 (82) | 36/44 (82) | 32/43 (74) | 0.617 |
|
| ||||
| 30-day Treatment Service Initiation | ||||
| Admitted for inpatient treatment (%) | 16/44 (36) | 16/44 (36) | 10/43 (23) | 0.320 |
| Initiated outpatient counseling (%) | 14/44 (32) | 11/44 (25) | 15/43 (35) | 0.591 |
| Admitted to a residential facility (%) | 1/44 (2) | 1/44 (2) | 2/43 (5) | 0.759 |
| Initiated AA meeting attendance (%) | 29/44 (66) | 29/44 (66) | 28/43 (65) | 0.996 |
|
| ||||
| 90-day Treatment Service Utilization | ||||
| Initiated any serviceb (%) | 39/42 (93) | 37/40 (93) | 35/39 (90) | 0.859 |
| Admitted for inpatient treatment (%) | 19/42 (45) | 25/41 (61) | 12/39 (31) | 0.025d |
| Initiated outpatient counseling (%) | 29/42 (69) | 28/41 (68) | 28/40 (70) | 0.986 |
| Admitted to a residential facility (%) | 4/42 (10) | 3/42 (7) | 5/39 (13) | 0.905 |
| Initiated AA meeting attendance (%) | 33/42 (79) | 34/42 (81) | 30/39 (77) | 0.689 |
| # of AA meetings attended ± SD (n) | 29.2±27.6 (43) | 32.2±33.6 (42) | 36.5±39.5 (43) | 0.610 |
| Readmitted to detoxification unit (%) | 7/43 (16) | 6/41 (15) | 4/39 (10) | 0.720 |
|
| ||||
| 90-day Treatment Completion | ||||
| Completed inpatient treatment (%) | 15/42 (36) | 21/41 (51) | 9/39 (23) | 0.033d |
| Completed outpatient counseling (%) | 5/42 (12) | 1/41 (2) | 2/40 (5) | 0.194 |
| Completed any treatmentc 90 days (%) | 18/42 (43) | 23/43 (54) | 11/39 (28) | 0.068 |
|
| ||||
| 90-day Substance Use | ||||
| Alcohol relapse (%) | 23/42 (55) | 25/43 (58) | 20/41 (49) | 0.685 |
| # of drinking days ± SD (n) | 10.5±16.8 (42) | 15.0±24.8 (42) | 17.8±27.9 (37) | 0.382 |
| # days before 1st drink ± SD (n) | 26.7±22.2 (24) | 31.7±21.3 (28) | 22.5±19.0 (20) | 0.350 |
| Heavy drinking (%) | 17/42 (41) | 20/40 (50) | 15/40 (38) | 0.497 |
| PDA ± SD (n) | 88.3±18.7 (42) | 81.8±28.0 (44) | 81.3±30.5 (39) | 0.396 |
| Drug relapse (%) | 7/42 (17) | 13/42 (31) | 10/40 (25) | 0.308 |
| # of days before 1st drug use ± SD (n) | 52.0±28.9 (7) | 39.4±23.9 (11) | 10.7±12.1 (9) | 0.003e |
Abbreviations: AA, Alcoholics Anonymous; MET, Motivational enhancement therapy; PDA, Percent days abstinent; P-TSF, Peer-delivered twelve step facilitation; TAU, Treatment as usual.
Note: “Initiated” indicates being admitted to/attending treatment program (e.g., not just scheduled treatment) or attending at least 1 AA meeting.
Outcomes are expressed as number positive/total number of participants (percentage) or mean ± standard deviation (number of subjects).
Includes inpatient, outpatient, or residential treatment and AA meeting attendance (a participant may have initiated one or more types).
Includes inpatient, outpatient, and residential treatment (a participant may have completed one or more types).
Initiation and completion of inpatient at 90 days significantly lower in P-TSF compared to MET (p = 0.007 and p = 0.009, respectively).
# of days before 1st drug use significantly lower in P-TSF compared to MET (p = 0.022) and TAU (p = 0.003).
Secondary Outcomes
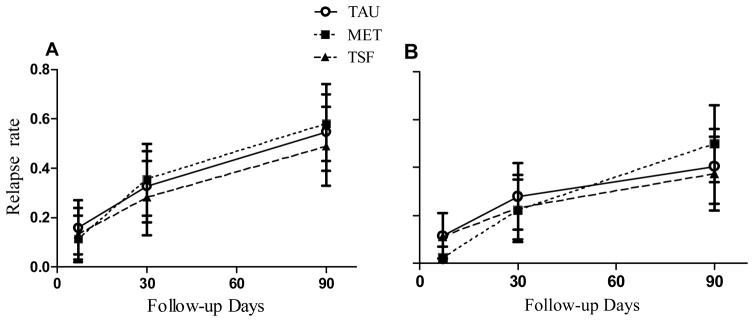
The other outcomes are also summarized in Table 2. There were no significant difference across the 3 groups related to 90-day alcohol or drug use except that those assigned to the P-TSF group relapsed to drug use sooner than those assigned to the other 2 groups. The mean number of drinking days remained below 18 days and the PDA were above 80% for all 3 groups. The TAU group had the lowest drug relapse at 17%, but this was not significantly different than the MET (31%) or the P-TSF (25%) groups. Figure 2 depicts the percentage of participants in each group that relapsed to alcohol (2A) and heavy alcohol (2B).
Figure 2.
Relapse rates for drinking (A) and heavy drinking (B)
Seventeen participants were readmitted for alcohol detoxification within the 90-day follow-up, including 7 in the TAU group (16%), 6 in the MET group (15%), and 4 in the P-TSF group (10%). Four participants were arrested within the 90-day follow-up, one each in the TAU and P-TSF groups and 2 in the MET group. A total of 17 participants reported being given a prescription for a medication to promote continued abstinence from alcohol at the time of hospital discharge; 3 received a prescription for naltrexone, 6 for disulfiram, and 8 for acamprosate. At the 90-day follow-up one participant reported receiving a prescription for disulfiram and another participant received a prescription for acamprosate from their primary care physician following hospitalization.
Ancillary Analyses
Using GEE models to determine which baseline characteristics might be associated with initiation of aftercare rehabilitation services, we observed that being “white” (76.8% vs. 23.2%, OR = 4.08, 95%CI = 1.20–13.81, p = 0.024) and having no history of IV drug abuse (7.1% vs. 92.9%, OR = 0.16, 95%CI = 0.027–0.99, p = 0.049) was associated with participating with inpatient treatment at any time during the 90 days following discharge from detoxification. Similarly, we observed that being “white” (OR = 4.65, 95%CI = 1.04–20.89, p = 0.045), having greater than a high school education (OR = 3.50, 95%CI = 1.23–9.63, p = 0.015), and not ever using IV drugs (OR = 0.16, 95%CI = 0.03–0.87, p = 0.034) was associated with participating with outpatient treatment at any time during the 90 days following discharge from detoxification.
We also examined which of these same participant baseline characteristics were associated with relapse to drinking and with the amount of drinking. In one model, we observed cocaine use at baseline (52.4% vs. 42.6%, OR = 0.11, 95%CI = 0.031–0.40, p < 0.001) decreased the likelihood of relapse; but having a prior non-DUI/DWI arrest record increased the likelihood of relapse (54.4% vs. 45.6%, OR = 3.15, 95%CI = 1.13–8.81, p = 0.028). We also noted that the PDA during the 90-day follow-up period of those with a high school education or less was less than the PDA of those with more than a high school education (76.6 PDA vs. 86.2 PDA, mean difference = 9.6 PDA, 95%CI = 1.75–23.84, p = 0.024).
Adverse Events and Complications
There was one adverse event reported to the IRB during follow-up. Specifically, a participant expressed suicidal ideation during one of the telephone interviews for follow-up data collection and was referred for counseling. The recruitment of additional participants was suspended for approximately 2 weeks until a protocol could be developed and approved by the IRB to address any future similar adverse events. After the study personnel received training related to the new adverse event protocol, participant recruitment resumed.
During the intervention fidelity interviews and the one-week follow-up interviews, the investigators noted that a few participants had difficulty recalling the details of their hospital stay without prompting.
DISCUSSION
This study was designed to determine whether the addition of either a MET or a P-TSF intervention provided to individuals with alcohol dependence in an inpatient setting (i.e., “detoxification”) would lead to greater initiation of subsequent rehabilitation services and less alcohol use following discharge as compared to TAU. Among the participants with outcome data, 79% initiated some kind of treatment (i.e., outpatient counseling, inpatient rehabilitation, or AA meetings) for their alcohol use disorder. Although there was no differences at the 30-day follow-up, those assigned to the MET group had significantly more participants initiate subsequent inpatient rehabilitation by the 90-day follow-up compared to the P-TSF group and more of these participants completed rehabilitation by the 90-day follow-up. Nevertheless, there were no significant differences for the rates of relapse to alcohol use (about 50%) or illicit drug use (about 25%) among the 3 groups at any follow-up interval.
Limitations
There are several reasons why the effects of these interventions are limited. First, it is possible that sampling biases can influence the observed clinical outcomes. The goals of detoxification (i.e., initiation of abstinence and linkage to subsequent rehabilitation services) were met for the majority of these participants. The favorable outcomes regarding completion of detoxification and linkage to rehabilitation services observed among the participants of this prospective study contrast with our previous retrospective observations with this population in which 21% did not complete detoxification (i.e., left early “against medical advise”) and in which 56% initiated rehabilitation services.32 However, the results of the current study are similar to another prospective study with this population in which 72% initiated rehabilitation and 58% remained totally abstinent by the time of the 30-day follow-up.7 The major difference between these retrospective and prospective studies was sample selection. The retrospective studies evaluated all patients, whereas the prospective studies included only those who were willing and able to provide informed consent and excluded certain “high-risk” sub-populations (e.g., those who were homeless). These sampling biases could limit the generalizability of our findings.
Second, since the services provided by TAU are extensive and past studies have demonstrated favorable outcomes associated with TAU, it could be that the effects of any additional brief intervention may be negligible. Nevertheless, we observed a modest effect for the MET intervention compared to the P-TSF. Participants assigned to MET were more likely to initiate inpatient treatment by the time of the 90-day follow-up, but not by the time of the 30-day follow-up. These MET participants were also more likely than both TAU and P-TSF participants to have completed inpatient rehabilitation by the 90-day follow-up (See Table 2). This is noteworthy because the MET interventionists were trained specifically to work with the study participants to develop a plan that would include seeking treatment with an inpatient rehabilitation program should a relapse to alcohol or illicit drug use occur during outpatient treatment. Also, developing a formalized relapse and aftercare plan was not consistently done by the staff of the detoxification (i.e., it was not a part of TAU). However, this study was not designed to assess specific aspects of MET that would promote initiation and completion of subsequent inpatient treatment. This limitation could be addressed by a study that focuses specifically on relapse planning.
Third, some patients receiving treatment for alcohol withdrawal sometimes demonstrate problems with memory. Study participants appeared to have some trouble processing the information provided by the MET or the P-TSF interventionist. For example, the study staff noticed that sometimes a participant would not recall the intervention taking place until prompted. Second, the severity the participants’ alcohol dependence, as evidenced by their scores on the various measures (e.g., CIWA, SIP) and by their extensive treatment history, could have limited the effectiveness of any brief intervention. We also noted several other barriers to aftercare treatment (e.g., memory problems among the participants, health insurance company policies and procedures, time delays in making appointments at treatment centers). Therefore, rather than focusing on interventions during detoxification, patient needs would be better served with coordination of care after hospital discharge
Finally, it may be that the interventions were effective, but the study was underpowered to detect the effect given the large variance observed. We recruited treatment-seeking volunteers who have some motivation to improve; they were seeking treatment and were willing to cooperate with a research study. It could be that the non-volunteers or patients who are not seeking treatment specifically for an alcohol use disorder (e.g. individuals hospitalized for alcohol-related injuries) might be more amenable to the interventions.19, 34
Conclusions
We conclude that MET during detoxification may provide additional benefits in terms of initiating and maintaining patients in aftercare inpatient treatment programs. Although it is plausible that MET increased the likelihood of initiating and completing inpatient due to a larger emphasis on formalizing aftercare and relapse plans, further research is needed to confirm this. However, additional behavioral interventions during detoxification showed little to no effect on the abstinence from alcohol following discharge. Other ways to improve outcomes following detoxification are needed. Future studies should investigate the effect of other types of interventions that might be useful including: initiation of pharmacotherapy to promote abstinence at the time of hospital discharge, or enhancing “case-management” services during a period of time immediately following hospital discharge.
Acknowledgments
This study was supported, in part, by a grant (K23 AA 015616) from the National Institute on Alcohol Abuse and Alcoholism (R.D.B., L.M.F., U. J., and L.A.) and by a grant from the University of Buffalo Interdisciplinary Research Fund (H.L.B. and E.M.F.). The authors are grateful for the help of the interventionists Renee Cadzow, Eric Holet, Renee Kee, Elizabeth McLean-Plunkett and Andrea Nikischer; the chemical dependency counselors of the Erie County Medical Center; the volunteers from the recovery community; and Gerard Connors, Alan Hutson, Robert Rychtarik and Andy Danzo, for their assistance with the manuscript.
References
- 1.Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- 2.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence; the COMBINE study; a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 3.Foster JH, Marshall EJ, Peters TJ. Outcome after in-patient detoxification for alcohol dependence: A naturalistic comparison of 7 versus 28 days stay. Alcohol Alcohol. 2000;35(6):580–586. doi: 10.1093/alcalc/35.6.580. [DOI] [PubMed] [Google Scholar]
- 4.Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 5.Millery M, Kleinman BP, Polissar NL, Millman RB, Scimeca M. Detoxification as a gateway to long-term treatment: assessing two interventions. J Subst Abuse Treat. 2002;23(3):183–190. doi: 10.1016/s0740-5472(02)00246-5. [DOI] [PubMed] [Google Scholar]
- 6.Mattick RP, Hall W. Are detoxification programmes effective? Lancet. 1996;347(8994):97–100. doi: 10.1016/s0140-6736(96)90215-9. [DOI] [PubMed] [Google Scholar]
- 7.Blondell RD, Smith SJ, Canfield MC, Servoss TJ. Abstinence and initiation of treatment following inpatient detoxification. Am J Addict. 2006;15(6):462–467. doi: 10.1080/10550490600998815. [DOI] [PubMed] [Google Scholar]
- 8.Foster JH, Marshall EJ, Peters TJ. Predictors of relapse to heavy drinking in alcohol dependent subjects following alcohol detoxification-the role of quality of life measures, ethnicity, social class, cigarette and drug use. Addict Biol. 1998;3:333–343. doi: 10.1080/13556219872146. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida M, Alterman AI, McLellan AT, et al. Comparative effectiveness and costs of inpatient and outpatient detoxification of patients with mild-to-moderate alcohol withdrawal syndrome. N Engl J Med. 1989;320(6):358–365. doi: 10.1056/NEJM198902093200605. [DOI] [PubMed] [Google Scholar]
- 10.Mark TL, Dilonardo JD, Chalk M, Coffey RM. Trends in inpatient detoxification services, 1992–1997. J Subst Abuse Treat. 2002;23(4):253–260. doi: 10.1016/s0740-5472(02)00271-4. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman BP, Millery M, Scimeca M, Polissar NL. Predicting long-term treatment utilization among addicts entering detoxification: The contribution of help-seeking models. J Drug Issues. 2002;32(1):209–230. [Google Scholar]
- 12.Smart RG, Finley J, Funston R. The effectiveness of post-detoxication referrals: effects on later detoxication admissions, drunkenness and criminality. Drug Alcohol Depend. 1977;2(3):149–155. doi: 10.1016/0376-8716(77)90022-9. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer JA, Harris AH, Cronkite RC, Turrubiartes P. Treatment staff’s continuity of care practices, patients’ engagement in continuing care, and abstinence following outpatient substance-use disorder treatment. J Stud Alcohol Drugs. 2008;69(5):747–756. doi: 10.15288/jsad.2008.69.747. [DOI] [PubMed] [Google Scholar]
- 14.Katz EC, Chutuape MA, Jones H, Jasinski D, Fingerhood M, Stitzer M. Abstinence incentive effects in a short-term outpatient detoxification program. Exp Clin Psychopharmacol. 2004;12(4):262–268. doi: 10.1037/1064-1297.12.4.262. [DOI] [PubMed] [Google Scholar]
- 15.Moos RH. Theory-based active ingredients of effective treatments for substance use disorders. Drug Alcohol Depend. 2007;88(2–3):109–121. doi: 10.1016/j.drugalcdep.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moos RH. Theory-based processes that promote the remission of substance use disorders. Clin Psych Rev. 2007;27(5):537–551. doi: 10.1016/j.cpr.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moos RH, Moos BS. Treated and untreated alcohol-use disorders: course and predictors of remission and relapse. Eval Rev. 2007;31(6):564–584. doi: 10.1177/0193841X07306749. [DOI] [PubMed] [Google Scholar]
- 18.Schilling RF, El-Bassel N, Finch J, Roman R, Hanson M. Motivational interviewing to encourage self-help participation following alcohol detoxification. Res Soc Work Pract. 2002;12:711–730. [Google Scholar]
- 19.Blondell RD, Looney SW, Northington AP, Lasch ME, Rhodes SB, McDaniels RL. Can recovering alcoholics help hospitalized patients with alcohol problems? J Fam Pract. 2001;50(5):447. [PubMed] [Google Scholar]
- 20.Blondell RD, Behrens T, Smith SJ, Greene BJ, Servoss TJ. Peer support during inpatient detoxification and aftercare outcomes. Addict Disord Treat. 2008;7(2):77–86. [Google Scholar]
- 21.Myrick H, Brady KT, Malcolm R. New developments in the pharmacotherapy of alcohol dependence. Am J Addict. 2001;10 (Suppl):3–15. doi: 10.1080/10550490150504092. [DOI] [PubMed] [Google Scholar]
- 22.Miller WR, Rollnick S. Motivational interviewing: preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]
- 23.Alcoholics Anonymous. 4. Alcoholics Anonymous World Services, Inc; New York: 2001. [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington D.C: American Psychiatric Association; 1994. [Google Scholar]
- 25.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 26.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 27.Forcehimes AA, Tonigan JS, Miller WR, Kenna GA, Baer JS. Psychometrics of the Drinker Inventory of Consequences (DrInC) Addict Behav. 2007;32(8):1699–1704. doi: 10.1016/j.addbeh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Miller WR, Tonigan JS, Longabaugh R. Project MACTH Monograph Series. Vol. 4. Rockville, MD: NIAAA; 1995. The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. [Google Scholar]
- 29.Humphreys K, Kaskutas LA, Weisner C. The Alcoholics Anonymous Affiliation Scale: development, reliability, and norms for diverse treated and untreated populations. Alcohol Clin Exp Res. 1998;22(5):974–978. doi: 10.1111/j.1530-0277.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychol Addict Behav. 1996;10(2):81–89. [Google Scholar]
- 31.Foy A, March S, Drinkwater V. Use of an objective clinical scale in the assessment and management of alcohol withdrawal in a large general hospital. Alcohol Clin Exp Res. 1988;12(3):360–364. doi: 10.1111/j.1530-0277.1988.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 32.Blondell RD, Amadasu A, Servoss TJ, Smith SJ. Differences among those who complete and fail to complete inpatient detoxification. J Addict Dis. 2006;25(1):95–104. doi: 10.1300/J069v25n01_12. [DOI] [PubMed] [Google Scholar]
- 33.Project MATCH Research Group. Project MATCH (Matching Alcoholism Treatment to Client Heterogeneity): rationale and methods for a multisite clinical trial matching patients to alcoholism treatment. Alcohol Clin Exp Res. 1993;17(6):1130–1145. doi: 10.1111/j.1530-0277.1993.tb05219.x. [DOI] [PubMed] [Google Scholar]
- 34.Gentilello LM, Donovan DM, Dunn CW, Rivara FP. Alcohol interventions in trauma centers. Current practice and future directions. JAMA. 1995;274(13):1043–1048. [PubMed] [Google Scholar]