Abstract
Gabapentin (GBP; Neurontin) and pregabalin (PGB; Lyrica, S-(+)-3-isobutylgaba) are used clinically to treat several disorders associated with excessive or inappropriate excitability, including epilepsy; pain from diabetic neuropathy, postherpetic neuralgia, and fibromyalgia; and generalized anxiety disorder. The molecular basis for these drugs' therapeutic effects are believed to involve the interaction with the auxiliary α2δ subunit of voltage-sensitive Ca2+ channel (VSCC) translating into a modulation of pathological neurotransmitter release. Glutamate as the primary excitatory neurotransmitter in the mammalian central nervous system contributes, under conditions of excessive glutamate release, to neurological and psychiatric disorders. This study used enzyme-based microelectrode arrays to directly measure extracellular glutamate release in rat neocortical slices and determine the modulation of this release by GBP and PGB. Both drugs attenuated K+-evoked glutamate release without affecting basal glutamate levels. PGB (0.1–100 μM) exhibited concentration-dependent inhibition of K+-evoked glutamate release with an IC50 value of 5.3 μM. R-(−)-3-Isobutylgaba, the enantiomer of PGB, did not significantly reduce K+-evoked glutamate release. The decrease of K+-evoked glutamate release by PGB was blocked by the l-amino acid l-isoleucine, a potential endogenous ligand of the α2δ subunit. In neocortical slices from transgenic mice having a point mutation (i.e., R217A) of the α2δ-1 (subtype) subunit of VSCC, PGB did not affect K+-evoked glutamate release yet inhibited this release in wild-type mice. The results show that GBP and PGB attenuated stimulus-evoked glutamate release in rodent neocortical slices and that the α2δ-1 subunit of VSCC appears to mediate this effect.
Introduction
Several neurological and psychiatric disorders characterized by excessive or dysfunctional neurotransmitter release are routinely treated with gabapentin [GBP; Neurontin, 1-(aminomethyl)cyclohexaneacetic acid] and pregabalin [PGB; Lyrica, S-(+)-3-isobutylgaba, S-(+)-4-amino-3-(2-methylpropyl)butanoic acid] (Dooley et al., 2007). Although multiple mechanisms of action have been proposed historically to account for the preclinical and clinical profiles of these drugs, there is increasing evidence for a significant role of the auxiliary α2δ subunit of voltage-sensitive Ca2+ channel (VSCC) (Taylor et al., 1998, 2007).
The binding of these ligands to the α2δ subunit is believed to be the source of their efficacy in treating epilepsy; pain from diabetic neuropathy, postherpetic neuralgia, and fibromyalgia; and generalized anxiety disorder. With the recent availability of transgenic mice with point mutations of the α2δ subunit (i.e., α2δ-1 and α2δ-2 subtypes) (Bian et al., 2006, 2008), preclinical experiments can be designed to test for altered neurochemical and behavioral effects of GBP and PGB (Field et al., 2006).
In the present study, we used enzyme-based microelectrode arrays (MEAs) that have micrometer-sized platinum recording sites with sampling rates of >1 Hz. These MEAs were developed in response to the limitations of other techniques or devices used to measure neurotransmitters (e.g., microdialysis/perfusate sampling coupled to high-performance liquid chromatography) (Barnes et al., 1988; Shinohara et al., 1998; Burmeister et al., 2000). A drawback of microdialysis or perfusate sampling techniques is that they sample from a large area (Borland et al., 2005) and at relatively slow (minutes) sampling rates. Given the rapid nature of neurotransmission of chemical messengers such as glutamate, faster sampling rates and smaller sampling areas should be beneficial. Enzyme-coated MEAs have been characterized extensively in the brains of anesthetized and behaving animals to measure glutamate (Burmeister et al., 2002; Binns et al., 2005; Day et al., 2006; Nickell et al., 2006; Rutherford et al., 2007; Parikh et al., 2010) but not in brain slices.
We used these MEAs to directly measure K+-evoked (extracellular) glutamate release in rat neocortical slices and to determine the modulation of this release by GBP and PGB. As the primary excitatory neurotransmitter in the mammalian central nervous system, glutamate often has been associated with a variety of pathological conditions (Meldrum, 2000), several of which are responsive to α2δ ligands such as PGB. A reduction of excessive glutamate release by α2δ ligands conceivably translates into clinically relevant therapeutic effects, especially considering the experimental evidence supporting a relationship between α2δ-subunit binding and the modulation of processes subserving neurotransmitter release (Dooley et al., 2007).
An additional aspect of this study was to assess the effects of PGB on K+-evoked (extracellular) glutamate release in neocortical slices from wild-type and α2δ-1 mutant mice. The α2δ-1 transgenic mice have a point mutation (namely, R217A) that markedly reduces [3H]GBP and [3H]PGB binding in central nervous system regions (e.g., neocortex) known to preferentially express the α2δ-1 protein (Bian et al., 2006; Field et al., 2006).
Materials and Methods
Animals.
Male rats [Sprague-Dawley, 2–8 weeks old; Harlan, Indianapolis, IN] and male mice [wild-type and mutant α2δ-1 R217A (Bian et al., 2006; Field et al., 2006), 2–5 months old; Charles River Laboratories, Inc., Wilmington, MA] were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility according to the standards outlined in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Animals were under a 12-h light/dark cycle, had ad libitum access to food and water, and were maintained for a minimum of 5 days before euthanasia by decapitation. The brains were removed by blunt dissection and placed in ice-cold buffer until slice preparation. The glutamate recordings occurred during the light phase of the light/dark cycle. All of the experimental protocols were approved by the Animal Care and Use Committee of the University of Kentucky.
Glutamate Release Measurements.
Neocortical slices from rats and mice were prepared using standard protocols (Hascup et al., 2007). In brief, coronal slices (0.35–0.4 mm in thickness), including the frontal and parietal areas exhibiting relatively high [3H]GBP and [3H]PGB binding (Bian et al., 2006), were maintained for at least 1 h at room temperature in artificial cerebrospinal fluid [aCSF; 124 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.5 mM MgCl2, 26 mM NaHCO3, 1.4 mM NaH2PO4, and 10 mM d-glucose, saturated with 95% O2/5% CO2, pH 7.2–7.4] before the start of an experiment. The slices were transferred to immersion-style chambers (i.e., one slice per chamber) and superfused at a rate of 1.5 to 2.0 ml/min with aCSF (31–33°C). Each chamber was fitted with an Ag/AgCl reference electrode.
Ceramic-based MEAs (four platinum sites in a row, 50 × 150 μm each) were assembled, coated with Nafion, and subsequently coated with three layers of a 1% glutamate oxidase (Associates of Cape Cod, East Falmouth, MA)/1% bovine serum albumin/0.125% glutaraldehyde enzyme solution. Coated MEAs were allowed to cure for a minimum of 2 days before use. Enzyme-based MEAs measure glutamate through the enzymatic breakdown of glutamate to yield a reporter molecule of hydrogen peroxide that is subsequently oxidized on the platinum recording surface to yield an oxidation current. The MEAs then were calibrated (in vitro) with glutamate in a phosphate-buffered solution (pH 7.4) at 31–34°C to 1) generate a standard response curve (sensitivity >2 pA/μM); 2) determine the limit of detection (≥3 times the signal-to-noise ratio; <2.0 μM); and 3) assess the selectivity for glutamate relative to an endogenous electroactive compound, ascorbic acid (>30:1). A MEA or MEA/micropipette assembly was lowered into the neocortical slice, and extracellular glutamate levels were measured once basal glutamate levels stabilized for at least 10 min.
Test substances were delivered through the superfusion system for a minimum of 15 min before slice stimulation unless stated otherwise. The slices were stimulated twice (S1, S2) with high K+ by one of two methods to evoke glutamate release: 1) direct, local application of 70 mM K+ solution [70 mM KCl, 79 mM NaCl, and 2.5 mM CaCl2, pH 7.0–7.4] to depolarize the local glutamatergic network via pressure ejection or 2) superfusion of 70 mM K+ [i.e., an increase of KCl in aCSF with a corresponding decrease of NaCl (59 mM) to maintain iso-osmolarity] to depolarize the whole slice. Slices were allowed to recover for a minimum of 20 min between stimulations. For local application, glass micropipettes (inside tip diameter of 10–15 μm) were formed from stock (1 mm o.d., 0.58 mm i.d.; A-M Systems, Everett, WA) and attached, and the tip was centered over the MEA recording site at a tip-to-tip distance of 70 to 110 μm. The 70 mM K+ solution was applied at 1-min intervals until at least two to five reproducible glutamate responses were recorded. Delivery of solution volumes (i.e., 12.5–400 nl over 0.1–3.0 s) was controlled by a pressure-ejection system (2–12 psi; Picospritzer II, Parker Hannifin, Mayfield Heights, OH) and monitored using a stereomicroscope fitted with a reticule (Gerhardt and Palmer, 1987). Extracellular glutamate levels were measured at 1 Hz using constant potential amperometry (+0.7 V versus Ag/AgCl reference) controlled by a FAST16 electrochemical recording system (Quanteon, LLC, Nicholasville, KY) and analyzed offline by customized Excel-based software.
Calculations and Statistics.
Glutamate release amplitudes were calculated from the difference between maximal K+-evoked glutamate release values and basal values. Values given are X ± S.E. (n ≥ 6). In one set of experiments with PGB, a concentration-effect curve with the corresponding IC50 value was calculated by nonlinear regression (Prism 4.0; GraphPad Software Inc., San Diego, CA). If appropriate, the results were analyzed using the t statistic for group means or one- or two-way analysis of variance followed by post hoc comparisons using Dunnett's or Bonferroni multiple comparison statistic (InStat 3.0; GraphPad Software Inc.). The minimal level of significance was p ≤ 0.05 (two-tail criterion).
Materials.
Substances were either commercially available (Sigma-Aldrich, St. Louis, MO) or donated [i.e., GBP, PGB, and R-(−)-3-isobutylgaba (R-IBG) (Pfizer, Inc., New York, NY)]. Test compounds were dissolved directly in aCSF.
Results
The effects of GBP and PGB on resting glutamate levels were evaluated in initial experiments. Neither drug (0.1–100 μM) altered basal glutamate levels in neocortical slices (data not shown).
In the brains of anesthetized animals, delivery of high K+ to stimulate glutamate release has been performed with local, pressure-ejected administration (Burmeister et al., 2002; Day et al., 2006; Quintero et al., 2007; Stephens et al., 2009). Meanwhile, studies in brain slices permit the use of two methods of delivering high K+ solutions to activate neural networks: local delivery and superfusion. Local stimulation, using pressure delivery, produces a comparable stimulus to those used in previous studies with anesthetized animals. An additional benefit of brain slice recordings is the flexibility to also use superfusion of high K+ to evoke release. This sustained depolarization resembles prolonged or excessive excitability—a condition that characterizes some neurological disorders such as anxiety. We used both stimulation methods here to characterize the effects of α2δ subunit ligands and to compare glutamate measurements with MEAs to those of previous studies.
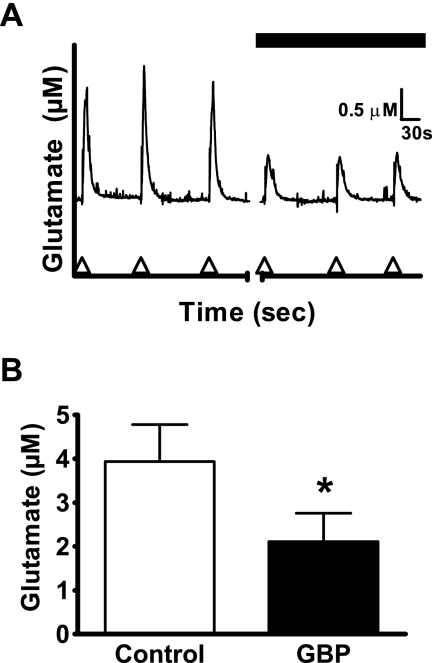
The repeated pressure-ejection delivery of 70 mM K+ solution yielded similar-sized glutamate signals with a mean amplitude of 3.9 ± 0.8 μM (Fig. 1). In the presence of GBP (100 μM), the mean amplitude was decreased significantly by 46% to 2.1 ± 0.7 μM (Fig. 1).
Fig. 1.
Effect of GBP (100 μM) on K+-evoked glutamate release from rat neocortical slices. A, glutamate release evoked by repeated pressure-ejection delivery of 70 mM K+ solution (arrowheads on abscissa) in the absence (first three traces) and presence of GBP (last three traces). B, amplitude of K+-evoked glutamate release (as derived from A) was decreased by GBP. Values given are X ± S.E. (n = 6). The paired t statistic gave t(5) = 2.930 (p = 0.0326). A significant difference from the control value is indicated by an asterisk (*, p ≤ 0.05).
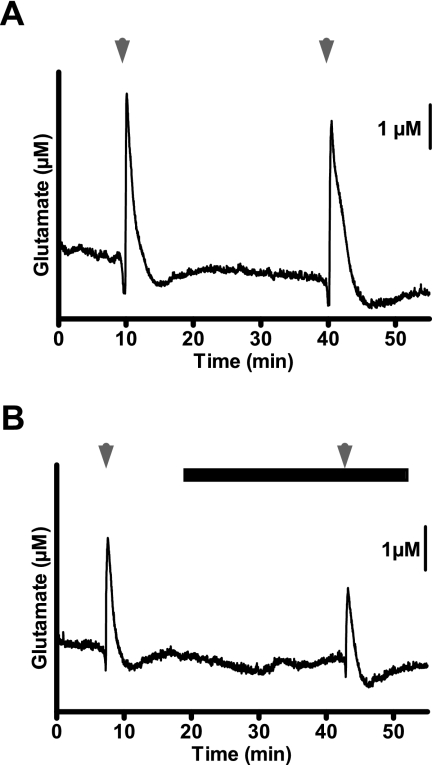
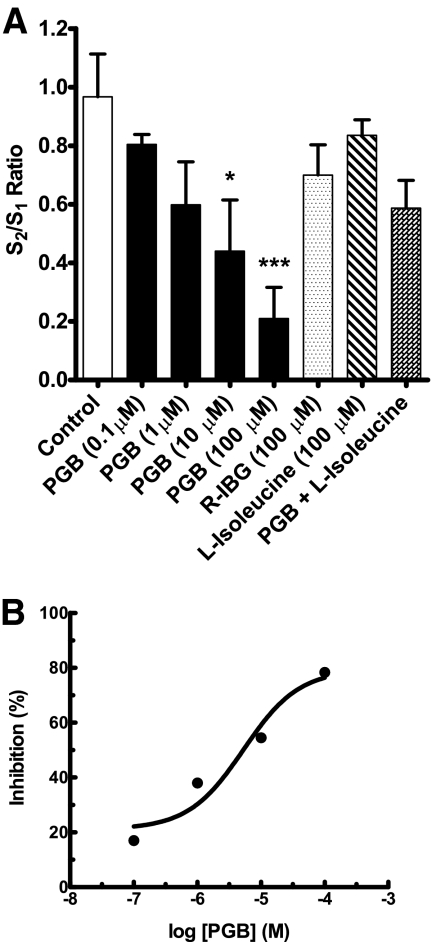
Because GBP was confirmed to modulate K+-evoked glutamate release, the more recently developed α2δ ligand PGB was chosen for testing in additional experiments. PGB (100 μM) attenuated pressure-ejection delivery of 70 mM K+ solution (5.7 ± 1.5 μM glutamate, pre-PGB versus 1.7 ± 1.2 μM glutamate, post-PGB; t(4) = 3.59, p = 0.023). We then transitioned to a paradigm of using repeated stimulation with superfused 70 mM K+ (S1, S2). The S2/S1 ratio of control glutamate signals in rat neocortical slices was 0.97 (Figs. 2A and 3A); this ratio was markedly reduced by 78% to 0.21 by PGB (100 μM) (Figs. 2B and 3A). Other S2/S1 ratios for PGB include 0.80 (nonsignificant, 14% inhibition) at 0.1 μM, 0.60 (nonsignificant, 38% inhibition) at 1 μM, and 0.44 (54% inhibition) at 10 μM (Fig. 3A); an IC50 value of 5.3 μM was determined from the concentration-effect relationship (Fig. 3B). The enantiomer of PGB, R-IBG (100 μM), gave an S2/S1 ratio of 0.70 (nonsignificant, 28% inhibition), contrasting sharply with the effect (78% inhibition) of an equimolar concentration of PGB (Fig. 3A).
Fig. 2.
Effect of PGB (100 μM) on K+-evoked glutamate release in rat neocortical slices. A, detection of glutamate release by MEAs after repeat superfusion with 70 mM K+ (arrowheads; S1, S2) for 50 s. B, PGB (closed bar), present 15 min before S2, attenuated glutamate release.
Fig. 3.
Effects of PGB (0.1–100 μM), R-(−)-3-isobutylgaba (100 μM), and l-isoleucine (100 μM) to inhibit K+-evoked glutamate release in rat neocortical slices. A, concentration–effect relationship of PGB and inactivity of R-(−)-3-isobutylgaba, l-isoleucine, and PGB (100 μM) and l-isoleucine (100 μM) combination after repeat superfusion with 70 mM K+ (S1, S2) for 50 s. Substances were present 15 min before S2. Values given are X ± S.E. (n = 7). Analysis of variance of S2/S1 values for control and PGB concentrations gave F(4,30) = 5.17 (p = 0.003). A significant difference from the control value is indicated by an asterisk (*, p ≤ 0.05 and ***, p ≤ 0.001). The S2/S1 ratios obtained for the other substances, including the PGB and l-isoleucine combination, were not significantly different from the control value. B, the transformed data from A depict inhibition (%) by PGB relative to the mean control S2/S1 ratio of 0.97 normalized to 1.0; the corresponding IC50 value was 5.3 μM.
PGB and the endogenous amino acid l-isoleucine are substrates for the system l-amino acid transporter, and both have a similar nanomolar affinity for the α2δ ligand binding site on the α2δ subunit. The S2/S1 ratio associated with the K+-evoked glutamate signals in the presence of l-isoleucine (100 μM) was 0.84 (nonsignificant, 13% inhibition), yet this compound reduced the effect (78% inhibition) of PGB (100 μM) as indicated by the S2/S1 ratio of 0.59 (nonsignificant, 39% inhibition) (Fig. 3A).
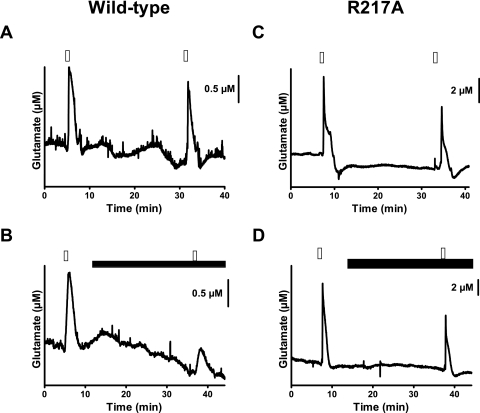
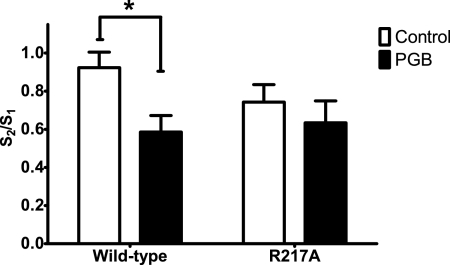
An action of GBP and PGB at the α2δ-1 subtype rather than the α2δ-2 subtype has been proposed to account for the therapeutic effects of these drugs (Bian et al., 2006, 2008; Field et al., 2006). With neocortical slices from the wild-type and α2δ-1 transgenic mice (Bian et al., 2006; Field et al., 2006), PGB (100 μM) significantly decreased K+-evoked glutamate signals (Fig. 4, A and B) in wild-type mice by 36% [S2/S1 ratio of 0.92 (control) versus 0.59 (PGB); n = 13 and 14, respectively] (Fig. 5); in the α2δ-1 transgenic mice, the glutamate signals were unchanged by this drug [S2/S1 = 0.74 (control) versus 0.63 (PGB); n = 9 each] (Fig. 4, C and D).
Fig. 4.
Effects of PGB (100 μM) on K+-evoked glutamate release in neocortical slices from wild-type mice and transgenic mice having a point mutation (i.e., R217A) of the VSCC α2δ-1 subunit. Glutamate release was assessed with MEAs after repeated superfusion with 70 mM K+ (S1, S2) for 50 s. Slices from wild-type mice were treated as control (A) and exposed to PGB (15 min before S2) (B). Meanwhile, slices from transgenic mice (R217A) were treated as control (C) and exposed to PGB (15 min before S2) (D). Open bars, 70 mK K+; closed bars, PGB.
Fig. 5.
The point mutation R217A prevents PGB from attenuating K+-evoked glutamate release in slices. The S2/S1 ratio derived from repeated K+ superfusion of slices was decreased by PGB. A main effect of PGB treatment was identified with a two-way analysis of variance [F(1,41) = 5.57 (p = 0.023)], and a post hoc Bonferroni test revealed a significant effect (p < 0.05) of PGB only in slices from wild-type animals. A significant difference from the control value is indicated by an asterisk (*, p ≤ 0.05). Values given are X ± S.E.
Discussion
Aberrant glutamate neurotransmission is linked to a variety of neurological and psychological disorders. Thus, identifying mechanisms that could modulate abnormal glutamate release may provide an avenue for developing new therapeutics for modulating glutamate signaling. Here, we used enzyme-based MEAs to directly measure extracellular glutamate and observed that both GBP and PGB attenuated the K+-evoked glutamate release.
GBP has been used as an antiepileptic, but its precise mechanism of action is unknown (Taylor et al., 2007). To help to address this, we stimulated the neural network and measured synaptic spillover of glutamate using a technique that we have used previously in anesthetized animals of locally delivering high K+ solution to evoke depolarization and produce a release of glutamate (Burmeister et al., 2002; Day et al., 2006). We observed in these brain slices an attenuation of glutamate release by GBP after locally delivering high K+, an effect on neurotransmitter release that is repeatedly observed after stimulus delivery (Dooley et al., 2000a,b, 2002).
PGB is related structurally to GBP but with greater reported efficacy in clinical studies (Taylor et al., 2007). Using these MEAs, we used a whole slice superfusion of high K+ to stimulate neurotransmitter release similar to Dooley et al. (2000b). We have observed that this type of depolarization evokes glutamate release that is calcium dependent (J. E. Quintero and G. A. Gerhardt, unpublished observations). The dose-dependent inhibition of K+-evoked glutamate release by PGB resulted in an IC50 value of 5.3 μM compared with an IC50 value of 11.8 μM of K+-evoked [3H]norepinephrine release (Dooley et al., 2002).
The magnitude of the attenuation of evoked glutamate release was larger than those reported in some studies that have examined the effect of GBP or PGB on stimulus-evoked neurotransmission (Dooley et al., 2000a,b, 2002; Brown and Randall, 2005). One apparent factor that may influence the effectiveness of these compounds is the type of stimulus, such that GBP and PGB may exert an effect on the prolonged, depolarization-induced neurotransmitter release that more closely resembles hyperexcitability as found in pathological states rather than normal physiological neurotransmission (Dooley et al., 2000a, 2007; Maneuf et al., 2001). In addition, some of the GBP or PGB effects on stimulus-evoked neurotransmitter release may have been diluted in studies on slices or synaptic endings where the whole chamber perfusate is sampled (Dooley et al., 2000a; Fink et al., 2000, 2002) versus the limited focal area sampled by the 50 × 150 μm size of these MEAs in slices. For example, with the high-resolution technique of whole-cell patch clamp in cortical slices, GBP and PGB show an effect as high as ∼80% on parameters related to glutamate neurotransmission (Cunningham et al., 2004). Nonetheless, the effect of GBP and PGB on neurotransmitter release remains controversial given reports that GBP and PGB have no effect on K+-evoked glutamate release from human synaptosomes (Brawek et al., 2009) or that the GBP and PGB effect may be linked to the trafficking of calcium channels to the cell surface (Hendrich et al., 2008; Mich and Horne, 2008; Bauer et al., 2009; Thorpe and Offord, 2010). The full effect of these ligands may result from both altering calcium channel trafficking and more rapid modulation of synaptic function (Taylor, 2009).
Meanwhile, the pharmacological effects of PGB are stereoselective, and this was borne out by the nonsignificant changes to the S2/S1 ratio with the enantiomer R-IBG. The α amino acids l-isoleucine and l-leucine have been proposed as potential endogenous ligands for the α2δ subunit (Thurlow et al., 1993). Although l-isoleucine did not produce a significant change to K+-evoked glutamate release, l-isoleucine did inhibit the PGB attenuation of K+-evoked glutamate release similar to what had been described previously with l-isoleucine and GBP in cortical brain slices (Cunningham et al., 2004). A more complex role in neurotransmission and GBP and PGB effectiveness may be the case for these α amino acids where these endogenous ligands may act as “positive modulators required for full functionality of the α2δ subunit” (Hendrich et al., 2008).
In a previous study, Wang et al. (1999) showed that the arginine at position 217 in the α region of the α2δ subunit is critical for GBP binding. Subsequently, in a R217A knockin mouse that was developed, [3H]GBP and [3H]PGB binding to neocortical membranes was greatly reduced in R217A mice compared with that in wild-type mice (Bian et al., 2006; Field et al., 2006). Accordingly, in slices from R217A mice, we concluded that a functional α2δ subunit is necessary for PGB to attenuate K+-evoked glutamate release. Although the mean S2/S1 ratio (0.74) in slices from the R217A mice was lower than the S2/S1 ratio in slices from the wild-type mice (0.92), the means were not significantly different. However, we cannot rule out the possibility of a change in excitability properties of the neurons and synapses in these animals given that the α2δ subunit is critical for normal synapse formation or function (Eroglu et al., 2009).
In summary, we showed that GBP and PGB can modulate stimulus-evoked glutamate release in rat neocortical brain slices and that the α2δ subunit of VSCC is involved in the inhibitory effects of these ligands. This ability to modulate excitatory neurotransmitter release may explain, in part, the efficacy of these molecules in the clinic. The application of the slice recording methodology coupled to the MEA recording technology establishes a new means to better assess drugs' mechanisms of action by the direct measurement of neurotransmitter release.
This work was supported by the National Institutes of Health National Institute on Aging [Grant AG00242]; and Pfizer, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.178384.
- GBP
- gabapentin
- PGB
- pregabalin
- VSCC
- voltage-sensitive Ca2+ channel
- MEA
- microelectrode array
- aCSF
- artificial cerebrospinal fluid
- R-IBG
- R-(−)-3-isobutylgaba.
Authorship Contributions
Participated in research design: Quintero, Dooley, Pomerleau, Huettl, and Gerhardt.
Conducted experiments: Quintero.
Contributed new reagents or analytic tools: Gerhardt.
Performed data analysis: Quintero, Dooley, Pomerleau, and Gerhardt.
Wrote or contributed to the writing of the manuscript: Quintero, Dooley, Pomerleau, Huettl, and Gerhardt.
Other: Gerhardt directed research efforts.
References
- Barnes S, Leighton GE, Davies JA. (1988) A novel superfusion chamber for the measurement of endogenous glutamate release from cerebellar slices. J Neurosci Methods 23:57–61 [DOI] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, et al. (2009) The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 29:4076–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian F, Hannah D, Campbell B. (2008) Calcium channel alpha2-delta type 2 subunit is the major binding protein for pregabalin in cerebellum and septum: an ex vivo autoradiographic study in wild-type and genetically modified mice. Neuroscience 2008; 2008 Nov 15–19; Washington, DC Program No. 845.813, Society for Neuroscience, Washington, DC [Google Scholar]
- Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. (2006) Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res 1075:68–80 [DOI] [PubMed] [Google Scholar]
- Binns BC, Huang Y, Goettl VM, Hackshaw KV, Stephens RL., Jr (2005) Glutamate uptake is attenuated in spinal deep dorsal and ventral horn in the rat spinal nerve ligation model. Brain Res 1041:38–47 [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. (2005) Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods 146:149–158 [DOI] [PubMed] [Google Scholar]
- Brawek B, Löffler M, Weyerbrock A, Feuerstein TJ. (2009) Effects of gabapentin and pregabalin on K+-evoked 3H-GABA and 3H-glutamate release from human neocortical synaptosomes. Naunyn Schmiedebergs Arch Pharmacol 379:361–369 [DOI] [PubMed] [Google Scholar]
- Brown JT, Randall A. (2005) Gabapentin fails to alter P/Q-type Ca2+ channel-mediated synaptic transmission in the hippocampus in vitro. Synapse 55:262–269 [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. (2000) Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem 72:187–192 [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. (2002) Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J Neurosci Methods 119:163–171 [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. (2004) Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci 20:1566–1576 [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. (2006) Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem 96:1626–1635 [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan CM, Meder WP, Whetzel SZ. (2002) Preferential action of gabapentin and pregabalin at P/Q-type voltage-sensitive calcium channels: inhibition of K+-evoked [3H]-norepinephrine release from rat neocortical slices. Synapse 45:171–190 [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan CM, Pugsley TA. (2000a) Stimulus-dependent modulation of [(3)H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther 295:1086–1093 [PubMed] [Google Scholar]
- Dooley DJ, Mieske CA, Borosky SA. (2000b) Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett 280:107–110 [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. (2007) Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci 28:75–82 [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. (2009) Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, et al. (2006) Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA 103:17537–17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Göthert M. (2002) Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 42:229–236 [DOI] [PubMed] [Google Scholar]
- Fink K, Meder W, Dooley DJ, Göthert M. (2000) Inhibition of neuronal Ca(2+) influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol 130:900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt GA, Palmer MR. (1987) Characterization of the techniques of pressure ejection and microiontophoresis using in vivo electrochemistry. J Neurosci Methods 22:147–159 [DOI] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister J, Gerhardt GA, Michael AC, et al. (2007) Second-by-second measures of L-glutamate and other neurotransmitters using enzyme-based microelectrode arrays, in Electrochemical Methods for Neuroscience (Michael AC, Borland LM. eds) pp 407–450, CRC Press, Boca Raton, FL: [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. (2008) Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA 105:3628–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Maneuf YP, Hughes J, McKnight AT. (2001) Gabapentin inhibits the substance P-facilitated K(+)-evoked release of [(3)H]glutamate from rat caudial trigeminal nucleus slices. Pain 93:191–196 [DOI] [PubMed] [Google Scholar]
- Meldrum BS. (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130:1007S–1015S [DOI] [PubMed] [Google Scholar]
- Mich PM, Horne WA. (2008) Alternative splicing of the Ca2+ channel beta4 subunit confers specificity for gabapentin inhibition of Cav2.1 trafficking. Mol Pharmacol 74:904–912 [DOI] [PubMed] [Google Scholar]
- Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. (2007) Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging 28:1737–1748 [DOI] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. (2010) Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci 30:3518–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JE, Day BK, Zhang Z, Grondin R, Stephens ML, Huettl P, Pomerleau F, Gash DM, Gerhardt GA. (2007) Amperometric measures of age-related changes in glutamate regulation in the cortex of rhesus monkeys. Exp Neurol 208:238–246 [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. (2007) Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J Neurochem 102:712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. (1998) Circadian release of amino acids in the suprachiasmatic nucleus in vitro. Neuroreport 9:137–140 [DOI] [PubMed] [Google Scholar]
- Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. (2011) Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol Aging 32:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP. (2009) Mechanisms of analgesia by gabapentin and pregabalin–calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain 142:13–16 [DOI] [PubMed] [Google Scholar]
- Taylor CP, Angelotti T, Fauman E. (2007) Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 73:137–150 [DOI] [PubMed] [Google Scholar]
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. (1998) A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 29:233–249 [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Offord J. (2010) The alpha2-delta protein: an auxiliary subunit of voltage-dependent calcium channels as a recognized drug target. Curr Opin Investig Drugs 11:761–770 [PubMed] [Google Scholar]
- Thurlow RJ, Brown JP, Gee NS, Hill DR, Woodruff GN. (1993) [3H]gabapentin may label a system-L-like neutral amino acid carrier in brain. Eur J Pharmacol 247:341–345 [DOI] [PubMed] [Google Scholar]
- Wang M, Offord J, Oxender DL, Su TZ. (1999) Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. Biochem J 342:313–320 [PMC free article] [PubMed] [Google Scholar]