Abstract
Previous studies from our laboratory and others have implicated a critical role of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in opioid tolerance and dependence. Translational research targeting the CaMKII pathway is challenging, if not impossible, because of a lack of selective inhibitors. We discovered in a preliminary study that haloperidol, a butyrophenone antipsychotic drug, inhibited CaMKII, which led us to hypothesize that haloperidol can attenuate opioid tolerance and dependence by inhibiting CaMKII. The hypothesis was tested in two rodent models of opioid tolerance and dependence. Pretreatment with haloperidol (0.2–1.0 mg/kg i.p.) prevented the development of morphine tolerance and dependence in a dose-dependent manner. Short-term treatment with haloperidol (0.06–0.60 mg/kg i.p.) dose-dependently reversed the established morphine-antinociceptive tolerance and physical dependence. Correlating with behavioral effects, pretreatment or short-term treatment with haloperidol dose-dependently inhibited morphine-induced up-regulation of supraspinal and spinal CaMKIIα activity. Moreover, haloperidol given orally was also effective in attenuating morphine-induced CaMKIIα activity, antinociceptive tolerance, and physical dependence. Taken together, these data suggest that haloperidol attenuates opioid tolerance and dependence by suppressing CaMKII activity. Because haloperidol is a clinically used drug that can be taken orally, we propose that the drug may be of use in attenuating opioid tolerance and dependence.
Introduction
Opioids are highly efficacious analgesic drugs. However, repeated use of these drugs leads to the development of tolerance and dependence, thereby limiting their effectiveness and usage. The mechanisms underlying opioid tolerance and dependence are not entirely understood. Studies from our laboratory and others have begun to unravel a critical role of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in opioid tolerance and dependence (Wang and Wang, 2006). CaMKII is a multifunctional, Ca2+/calmodulin-activated protein kinase that was originally discovered in the brain (Schulman and Greengard, 1978). It has since been shown to be a critical mediator of neuronal plasticity and play a key role in long-term potentiation, learning and memory (Lee, 2006; Wayman et al., 2008; Redondo et al., 2010). Long-term treatment with morphine has been shown to increase CaMKII activity in vivo (Wang et al., 2003; Liang et al., 2004; Tang et al., 2006a). Supraspinal and spinal inhibition of CaMKII not only prevented but also reversed opioid-antinociceptive tolerance and physical dependence in several rodent models (Wang et al., 2003; Tang et al., 2006a). These data support a critical role of CaMKII in the development and maintenance of opioid tolerance and dependence. Furthermore, inhibiting CaMKIIα by chemical inhibitors, small interfering RNA, and gene deletion methods attenuated opioid-induced hyperalgesia, a clinical and experimental phenomenon that is highly relevant for tolerance (Chen et al., 2010). Therefore, targeting CaMKII or its signaling pathways may provide potential targets of pharmacological intervention for alleviating opioid tolerance or dependence.
Searching for selective chemical inhibitors of CaMKII has not been very successful, because it is difficult to specifically inhibit a protein kinase without affecting a closely related isoform. Here, we have focused our efforts on clinically used drugs that may inhibit CaMKII. Haloperidol belongs to the typical antipsychotic drug class. These drugs are thought to block dopamine D2 receptors, although, similar to most central nervous system drugs, the exact mechanism of action is not entirely understood. The interactions between the dopamine and opioid systems have been studied extensively (e.g.,Unterwald and Cuntapay, 2000). In fact, many of these studies have used typical antipsychotic drugs to block the dopamine activity. However, these drugs also may have other actions (Tang et al., 2006b; Chen et al., 2009). In this study, we tested the hypothesis that haloperidol can inhibit CaMKII and attenuate opioid-antinociceptive tolerance and physical dependence in two rodent models.
Materials and Methods
Morphine sulfate was provided by the National Institutes of Health National Institute on Drug Abuse (Bethesda, MD). Haloperidol, naloxone, and other chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO). Male ICR mice (25 ± 5 g; Harlan Laboratories, Indianapolis, IN) were kept on a 14/10-h light/darkness cycle (5:00 AM on and 7:00 PM off) and provided food and water ad libitum before experimental procedures. Mice were randomly divided into experimental groups according to a computer-generated randomization list. Behavioral tests were performed by an experimenter blinded to specific group and treatment information. All experiments procedures were performed in accordance with the policies and recommendations of the National Institutes of Health guidelines for the handling and use of laboratory animals after approval by the University of Illinois Institutional Animal Care and Use Committee.
Tail-Flick Test.
The tail-flick test was used to determine basal nociception and morphine antinociception as described previously (Wang et al., 2001; Tang et al., 2006a). In brief, One third of the distal portion of mouse tail was immersed into a water bath maintained at 52°C, and the latency of a quick tail-flick response was recorded. Morphine-antinociception was evaluated 30 min after a test dose of morphine (10 mg/kg s.c. unless otherwise stated) and is expressed as the percentage of maximal possible effect (MPE). MPE% = 100% × (postdrug latency − predrug latency)/(cutoff − predrug latency). A 12-s cutoff time was used to prevent tissue damage.
Acute Opioid Tolerance and Dependence.
To induce acute opioid tolerance and dependence, mice were treated with morphine sulfate (100 mg/kg s.c., time 0) (Yano and Takemori, 1977; Bilsky et al., 1996; Tang et al., 2006a). Morphine tolerance and dependence developed within hours and peaked at approximately 4 to 6 h (Shukla et al., 2006). Control mice received an equal volume of saline. Tolerance was assessed by monitoring reduced antinociception of a test dose of morphine (10 mg/kg s.c., given at 4.5 h) by use of the tail-flick test. Before the injection of the test dose of morphine, baseline tail-flick latency was re-established. In all mice, tail-flick latencies had returned to normal values at that time. To examine opioid dependence, morphine- or saline-pretreated mice were challenged with naloxone (10 mg/kg i.p.). Mice were immediately placed into glass cylinders, and the number of vertical jumps was recorded for 15 min (Tang et al., 2006a). The presence of the physical dependence of morphine was indicated by a significant number of naloxone-precipitated jumps compared with that of saline-treated mice.
To prevent morphine tolerance and dependence, haloperidol (0.06–0.60 mg/kg i.p.) was given 30 min before the induction dose of morphine (100 mg/kg s.c.). To reverse morphine tolerance and dependence, haloperidol (0.06–0.60 mg/kg i.p.) was given 30 min before the test dose of morphine (10 mg/kg) or naloxone.
Chronic Opioid Tolerance and Dependence.
To induce chronic opioid tolerance and dependence, mice were treated with morphine (10 mg/kg s.c., given at 8:00 AM and 6:00 PM) for 5 days (Herz and Teschemacher, 1973). Control mice received an equal number and volume of injections with saline. Morphine tolerance and naloxone-precipitated withdrawal were evaluated as described above. Haloperidol (0.1–1.0 mg/kg i.p. or p.o.) was given 30 min before the test dose of morphine or naloxone.
Rotarod Test.
To examine whether haloperidol may cause locomotor impairment, experiments using a rotarod test were conducted as described previously (Chen et al., 2010). Mice were placed on a 1.25 inch-diameter rod powered by a motor with adjustable speeds (Model series 8; IITC, Woodland Hills, CA). On day 1, mice were trained to remain on a fixed speed (4 rpm) for 60s. On the next day, mice were retrained at the same speed. Those mice that failed to stay on the rotarod for 60 s were eliminated from further study (approximately 10% of total animals). Thirty minutes later, baseline was obtained by placing mice on an accelerating rotarod (4–40 rpm over 300 s). The latency to fall from the rotarod was recorded. Mice were then treated with haloperidol (1.0 mg/kg i.p.) or saline and retested 1, 2, and 4 h later for the duration to stay on the accelerating rotarod (4–40 rpm over 300 s).
Western Blotting Analysis.
Spinal and supraspinal CaMKIIα expression and activity were determined in naive and drug-treated mice using the Western blotting method, as we have described previously (Tang et al., 2006a; Luo et al., 2008; Chen et al., 2010; He et al., 2010). For consistency, tissues from frontal cortex and lumbar spinal sections were used to represent supraspinal and spinal samples. Tissues were homogenized in ice-cold radioimmunoprecipitation assay buffer, and solubilized samples (60-μg protein) were separated by 12% SDS-polyacrylamide gel electrophoresis. After electrotransfer onto polyvinylidene difluoride membrane, the membrane was probed with a rabbit anti-Thr286-pCaMKIIα antibody (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or a mouse anti-CaMKIIα antibody (1:1000; Santa Cruz Biotechnology, Inc.) at room temperature for 3 h, followed by incubation with horseradish peroxidase-conjugated donkey anti-rabbit (for pCaMKIIα) or anti-mouse (for CaMKIIα) secondary antibody (1:1000; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The specificities of these antibodies have been characterized in transgenic mice with CaMKIIαT286A point mutation and in mice that were treated with small interfering RNA for CaMKIIα (Chen et al., 2010). An enhanced chemiluminescence detection system (ECL; Thermo Fisher Scientific, Waltham, MA) was applied for detection. The membrane was then stripped and reprobed with a mouse anti-β-actin antibody (1:10,000; Sigma-Aldrich) followed by a horseradish peroxidase-conjugated anti-mouse secondary antibody (1:1000; GE Healthcare) and developed as above. ECL signals were detected by a ChemiDoc system and analyzed using the Quantity One program (Bio-Rad, Hercules, CA). Ratios of the optical densities of CaMKIIα or pCaMKIIα to those of β-actin were calculated for each sample.
Statistical Analysis.
All data are presented as mean ± S.E.M. ED50 values were obtained from dose-response curves using the method of Tallarida and Murray (1987). Comparisons between groups were analyzed using Student's t test (two groups) or a two-way repeated measure analysis of variance followed by post hoc analyses using Dunnett's t test (multiple groups). Statistical significance was established at 95% confidence limit.
Results
Prevention of Acute Opioid Tolerance and Dependence by Haloperidol.
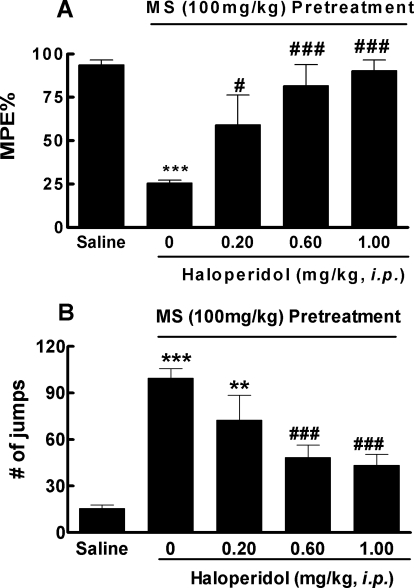
We first investigated whether haloperidol could prevent the development of opioid tolerance and dependence. In the first series of experiments, an acute model of opioid tolerance and dependence was used. Mice received an induction dose of morphine (100 mg/kg s.c.) and were found 4.5 h later to exhibit significantly reduced antinociception (25.5 ± 4.0% MPE versus 93.6 ± 6.4% MPE in saline-pretreated mice, p < 0.001) by a test dose of morphine (10 mg/kg s.c.), indicative of the development of acute opioid tolerance (Fig. 1A). In mice pretreated with haloperidol (1 or 0.6 mg/kg i.p.) 30 min before the induction dose of morphine, morphine-antinociception remained largely intact (90.2 ± 14.1 and 81.6 ± 27.0%, respectively; not significantly different from the saline group). Even at the lowest dose used, haloperidol (0.2 mg/kg i.p.) was able to partially prevent morphine-antinociceptive tolerance (59.0 ± 38.7% MPE, p < 0.05 versus morphine alone) (Fig. 1A). These data demonstrated that haloperidol dose-dependently blocked the development of acute morphine tolerance. ED50 was estimated to be 0.2 ± 0.1 mg/kg.
Fig. 1.
Prevention of acute opioid tolerance (A) and dependence (B) by haloperidol. Separate groups of six mice received haloperidol (0.2, 0.6, and 1.0 mg/kg i.p.) or equal volume of saline 30 min before administration of morphine (100 mg/kg s.c.). Saline mice only received intraperitoneal injections of saline. Four hours later, all groups received a test dose of morphine (10 mg/kg s.c.). The antinociception was determined by the tail-flick assay 30 min later. A, development of morphine tolerance was prevented by haloperidol in a dose-dependent manner. B, development of morphine dependence, as revealed by naloxone (10 mg/kg i.p.)-precipitated withdraw jumping, was also prevented by haloperidol in a dose-dependent manner. Data are expressed in mean ± S.E.M. **, p < 0.01; ***, p < 0.001 compared with the saline group; #, p < 0.05; ###, p < 0.001 compared with the morphine (MS) group.
The acute model can also be used to study opioid dependence, as revealed by challenging morphine-treated mice with naloxone (10 mg/kg i.p.). These mice produced 99 ± 14 jumps. Pretreatment with haloperidol (0.2, 0.6, and 1.0 mg/kg i.p.) reduced the numbers of naloxone-precipitated withdrawal jumps to 72 ± 16, 48 ± 8 (p < 0.001), and 43 ± 7 (p < 0.001), respectively. Therefore, haloperidol prevented morphine physical dependence in a dose-dependent manner.
Effects of Haloperidol on Basal Nociception, Morphine Antinociception, and Locomotor Activity.
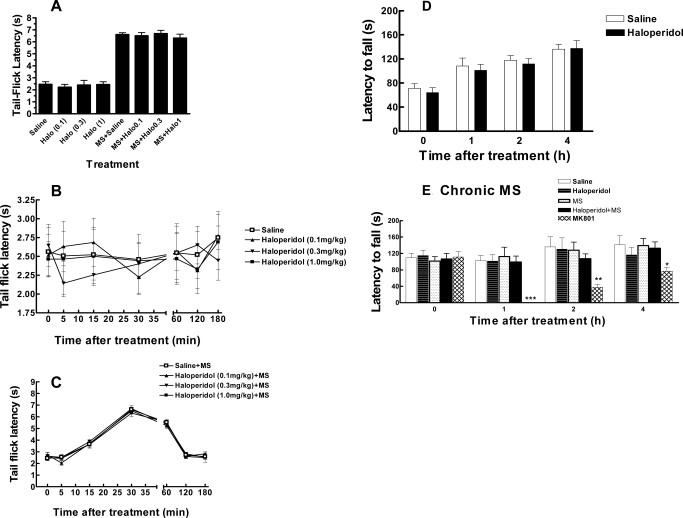
To investigate whether haloperidol itself produced antinociception in naive mice or affected the antinociceptive effect of morphine, haloperidol (1.0 mg/kg i.p.) was either given alone or coadministered with a submaximal dose of morphine (3.0 mg/kg s.c.). The latter was used to avoid the ceiling effect so further enhancement of morphine antinociception could be detected. Haloperidol (0.1–1 mg/kg i.p.) by itself neither produced any antinociception effect compared with the saline group (Fig. 2A) nor altered morphine antinociception (Fig. 2A). The observation was further supported by monitoring the effect of these drugs for 3 h (Fig. 2, B and C).
Fig. 2.
Effects of haloperidol on basal antinociception and morphine antinociception (A–C) and locomotor activity in naive (D) and morphine-tolerant (E) mice. A, separate groups of six mice received haloperidol (Halo; 0.1, 0.3, or 1 mg/kg i.p.) or an equal volume of saline 30 min before the tail-flick test (left columns). Additional groups of six mice were pretreated with saline or haloperidol (0.1, 0.3, or 1 mg/kg i.p.) 30 min before a test dose of morphine (MS; 3 mg/kg s.c.) for antinociception test. Haloperidol at these doses (0.1–1 mg/kg) did not by itself produce antinociception or alter morphine antinociception. B, time course for the effect of haloperidol (0.1, 0.3, or 1 mg/kg i.p.) and saline in the tail-flick test. C, time course for the effect of haloperidol (0.1, 0.3, or 1 mg/kg i.p.) on antinociception produced by morphine (3 mg/kg s.c.). A to C, n = 6/group. D, effect of haloperidol on locomotor activity in naive mice. For the rotarod test, mice were trained to remain on a fixed speed (4 rpm) rotarod for 60 s. On the next day, mice were retrained, and baseline was obtained by placing mice on an accelerating rotarod (4–40 rpm over 300 s). Mice were then treated with haloperidol (1.0 mg/kg i.p.) or saline and retested 1, 2, and 4 h later. The latency to fall off of the rotarod was recorded. n = 6/group. No significant difference was identified. E, effect of haloperidol on locomotor activity in morphine-tolerant mice. Mice received a twice daily injection of morphine (10 mg/kg) to induce tolerance. Mice were trained on day 4, and the rotarod test was tested on day 5 as described above. Mice were treated with saline, haloperidol (1 mg/kg i.p.), haloperidol (1 mg/kg i.p.), and morphine (10 mg/kg s.c.), or MK801 (1 mg/kg i.p.). The latter is an NMDA receptor antagonist that is known to impair locomotor activity and was used as a positive control. n = 6 for each test group and 12 for the saline group. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the saline group.
Another potential confounding factor is that haloperidol may impair locomotor activity in animals. To address this possibility, we tested the effect of haloperidol on locomotor activity using a rotarod test (Chen et al., 2010). After two training sessions (24 h apart), mice were tested for their ability to stay on an accelerating rotarod at 0, 1, 2, and 4 h after the intraperitoneal administration of haloperidol (1.0 mg/kg) or saline. Haloperidol (1.0 mg/kg i.p.) did not affect the locomotor activity (p > 0.05, compared with the saline group) (Fig. 2D). These data suggested that the effect of haloperidol at doses of up to 1 mg/kg on morphine tolerance and dependence was not attributed to direct antinociceptive activity, interfering with morphine antinociception or impairment of locomotor activity. Haloperidol at a higher dose (3 mg/kg) seemed to cause sedation in ICR mice.
Reversal of Acute Opioid Tolerance and Dependence by Haloperidol.
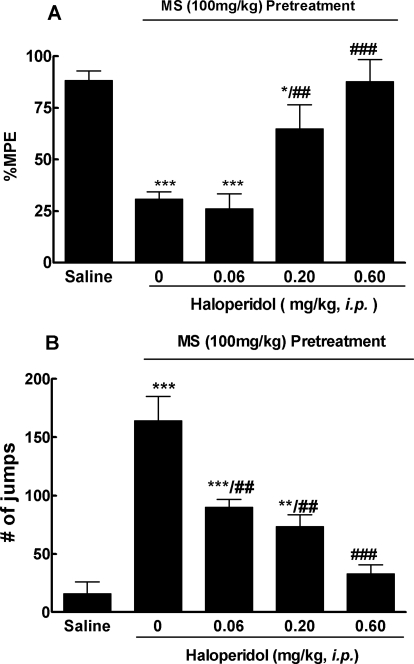
We next investigated whether short-term treatment with haloperidol was able to reverse the already established morphine-antinociceptive tolerance and physical dependence in the same acute mouse model. Haloperidol (0.60 mg/kg i.p.) completely reversed morphine tolerance (87.7 ± 10.7% MPE versus 30.7 ± 3.6% by the morphine group, p < 0.001), whereas at a lower dose (0.20 mg/kg i.p.), it showed a partial effect (64.8 ± 11.7% MPE). At the lowest dose used (0.06 mg/kg i.p.), it was ineffective (26.1 ± 7.2% MPE) (Fig. 3A).
Fig. 3.
Reversal of acute opioid tolerance (A) and dependence (B) by haloperidol. Separate groups of six mice received morphine (100 mg/kg s.c.) or an equal volume of saline. Four hours later, haloperidol (0.06, 0.20, and 0.60 mg/kg i.p.) or saline was given to these mice. Thirty minutes later, all groups received a test dose of morphine (10 mg/kg s.c.) for the antinociception test. A, the established morphine-antinociceptive tolerance was reversed by haloperidol in a dose-dependent manner. B, development of morphine dependence, as revealed by 10 mg/kg i.p. naloxone-precipitated withdraw jumping, which was also reversed by haloperidol in a dose-dependent way. Data are expressed as mean ± S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the saline group; ##, p < 0.01; ###, p < 0.001 compared with the morphine (MS) alone group.
Five hours after the induction dose of morphine, naloxone (10 mg/kg i.p.)-precipitated withdrawal jumping was evaluated. Haloperidol (0.06–0.60 mg/kg i.p.) dose-dependently attenuated the number of withdrawal jumps (p < 0.001 compared with the morphine group; Fig. 3B). At the highest dose (0.60 mg/kg), haloperidol was able to completely suppress the withdrawal jumping. The drug at lower doses (0.06 and 0.20 mg/kg) also significantly reduced the number of withdrawal jumps (Fig. 3B).
Effect of Haloperidol on Brain and Spinal CaMKIIα Activity in Acute Opioid Tolerance and Dependence.
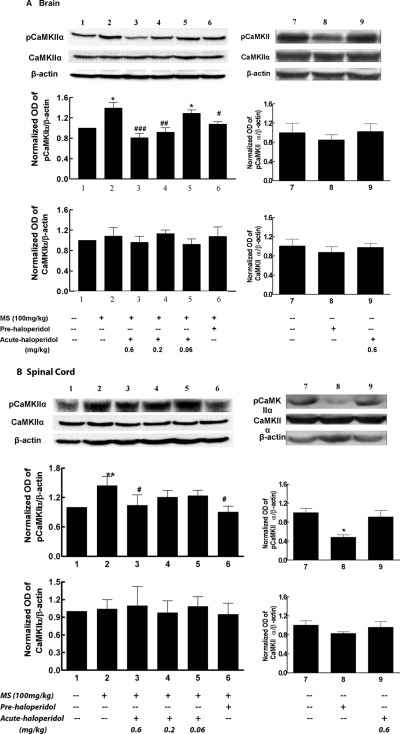
To identify the potential cellular mechanism of haloperidol in opioid tolerance and dependence, we examined CaMKIIα expression and activity in mice treated with morphine and different doses of haloperidol. Treatment with haloperidol for 0.5 to 5 h did not change the expression of CaMKIIα. However, spinal CaMKIIα activity was inhibited 30 min after the treatment with haloperidol. After morphine exposure, CaMKIIα activity was significantly increased in the brain and spinal cord of morphine-treated mice (Fig. 4). Pretreatment with haloperidol (0.6 mg/kg i.p.) effectively prevented morphine-induced CaMKIIα activation in the brain (Fig. 4A) and spinal cord (Fig. 4B) (lane 6, both p < 0.05 compared with morphine group), correlating with its effect in preventing the development of tolerance to and dependence on morphine. Acute treatment with haloperidol (0.6 mg/kg i.p.) significantly reversed morphine-induced CaMKIIα activation in the brain and spinal cord. Haloperidol at a lower dose (0.2 mg/kg) attenuated supraspinal, but not spinal, activation of CaMKIIα by morphine. At the lowest dose (0.06 mg/kg), haloperidol did not affect CaMKIIα activity. No statistical difference was found for CaMKIIα expression between control (saline) and treated (morphine or morphine plus haloperidol) mice in the brain or spinal cord (Fig. 4). Therefore, short-term haloperidol treatment dose-dependently reversed morphine-induced CaMKIIα activation, which was in agreement with dose-dependent reversal of morphine tolerance and dependence. Furthermore, the increased CaMKIIα activity was largely attributed to increased activation but not expression of the kinase.
Fig. 4.
Effect of haloperidol on supraspinal (A) and spinal (B) CaMKIIα activity in opioid-tolerant and -dependent mice. Frontal cortex (A) and spinal lumbar section (B) samples were taken to determine CaMKIIα activity. For acute haloperidol effect, mice were pretreated with morphine (100 mg/kg) or saline at time 0, followed by haloperidol (0.6 mg/kg) or saline at time = 4.5 h, with samples taken at time = 5 h. For the pretreatment effect, mice received morphine (100 mg/kg) and haloperidol (0.06, 0.2, 0.6 mg/kg) or saline at time 0, and samples were taken at time = 5 h. The activated CaMKIIα and total CaMKIIα were determined by the Western blotting method using antibodies specific for Thr286-pCaMKIIα and CaMKIIα, respectively. Histogram data, expressed as mean ± S.E.M., were constructed from the representative figure shown and three other experiments (n = 4). *, p < 0.05; **, p < 0.01 compared with the saline group; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 compared with the morphine (MS) alone group.
Reversal of Chronic Opioid Tolerance and Dependence by Haloperidol.
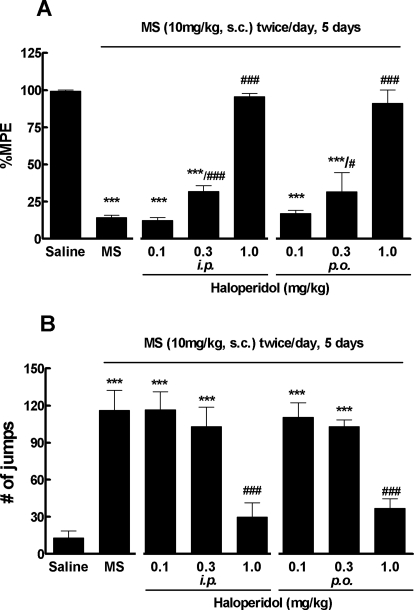
Given the promising effect of haloperidol in acute morphine tolerance and dependence, we further investigated the drug in chronic models of morphine tolerance and dependence. Mice received morphine sulfate (10 mg/kg s.c.) or saline every 12 h. Morphine tolerance quickly developed and reached the peak around day 5 (Tang et al., 2006a; He et al., 2010) when the test dose of morphine (10 mg/kg) produced significantly reduced antinociception (14.1 ± 3.8% MPE), indicative of the presence of opioid tolerance (Fig. 5A). Short-term treatment with haloperidol (1.0 mg/kg i.p.) completely reversed morphine tolerance; the test dose of morphine remained highly active and produced significant antinociception (95.5 ± 5.3% MEP, p < 0.001; Fig. 5A). The drug at a lower dose (0.3 mg/kg i.p.) partially attenuated morphine-antinociceptive tolerance (31.7 ± 9.7% MPE), whereas it was ineffective (12.3 ± 4.5% MPE) at the lowest dose (0.1 mg/kg i.p.). Likewise, haloperidol at the highest dose (1.0 mg/kg i.p.) completely blocked naloxone-precipitated withdrawal jumping (Fig. 5B), although it did have significant effect on the lower doses (0.3 and 0.1 mg/kg i.p.).
Fig. 5.
Reversal of chronic morphine tolerance and dependence by haloperidol in mice. Separate groups of six mice received morphine (10 mg/kg s.c.) or an equal volume of saline twice daily for 5 days. Haloperidol dose-dependently reversed the established morphine-antinociceptive tolerance (A) and naloxone (10 mg/kg i.p.)-precipitated withdrawal jumping (B). Data are expressed in mean ± S.E.M. ***, p < 0.001 compared with the saline group; #, p < 0.05; ###, p < 0.001 compared with the morphine (MS) alone group.
Although haloperidol (1.0 mg/kg i.p.) was found not to impair locomotor activity in naive mice, it was not known whether the drug had a different effect on opioid-tolerant mice. Therefore, we further determined the effect of haloperidol in the rotarod test in morphine-tolerant mice (10 mg/kg, twice daily for 5 days). After two training sessions (on days 4 and 5), mice were tested for their ability to stay on an accelerating rotarod at 0, 1, 2, and 4 h after the intraperitoneal administration of saline, haloperidol (1.0 mg/kg), haloperidol (1.0 mg/kg), and morphine (10 mg/kg s.c.) or MK801 [1.0 mg/kg; (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate]. MK801, an NMDA receptor antagonist that is known to impair rotarod performance, was used as a positive control. Unlike MK801, haloperidol (1.0 mg/kg) did not affect the locomotor activity (Fig. 2E). These data suggested that the effect of haloperidol on chronic morphine tolerance and dependence was not the result of impaired locomotor activity.
Because haloperidol is effective when taken orally in humans, we further examined whether haloperidol administered orally was effective in reversing chronic morphine tolerance and dependence. On day 5, haloperidol (0.1–1.0 mg/kg, gastric gavage) was administered 30 min before the test dose of morphine (10 mg/kg s.c.). As expected, haloperidol administered orally dose-dependently attenuated both morphine-antinociceptive tolerance (Fig. 5A) and morphine physical dependence (Fig. 5B).
Effect of Haloperidol on CaMKIIα Activity in Mice Chronically Tolerant to and Dependent on Morphine.
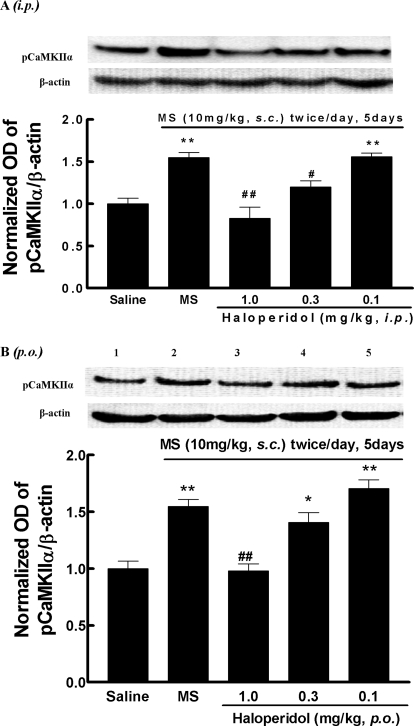
To correlate behavioral effect with biochemical inhibition of CaMKIIα by haloperidol, brain (frontal cortex) samples were taken from mice that have been treated chronically with morphine (10 mg/kg s.c., twice daily, for 5 days) and acutely with haloperidol (approximately 0.1–1.0 mg/kg i.p. or p.o.). Haloperidol (1.0 mg/kg) either given intraperitoneally (Fig. 6A) or orally (Fig. 6B) completely blocked morphine-induced activation of CaMKIIα (lane 3). At the second highest dose (0.3 mg/kg, lane 4), haloperidol partially blocked CaMKIIα activation after intraperitoneal administration (Fig. 6A, p < 0.01 compared with the morphine group), but the effect was not significant when given orally (Fig. 6B). At 0.1 mg/kg, haloperidol did not alter CaMKIIα activation by morphine by either route of administration.
Fig. 6.
Effect of haloperidol on brain CaMKIIα activity in opioid-tolerant and -dependent mice (chronic model). Separate groups of three mice received morphine (10 mg/kg s.c.) or an equal volume of saline twice per day for 5 days. Thirty minutes before the last injection of morphine on day 5, mice were treated with haloperidol (A) (approximately 0.1–1.0 mg/kg i.p.), haloperidol (approximately 0.1–1.0 mg/kg p.o.) (B), or saline. Brain samples were taken 30 min after the last injection of morphine or saline. The activated CaMKIIα and total CaMKIIα were determined by the Western blotting method using antibodies specific for Thr286-pCaMKIIα and CaMKIIα, respectively. Histogram data, expressed as mean ± S.E.M., were constructed from the representative figure shown and three other experiments. *, p < 0.05; **, p < 0.01 compared with the saline group; #, p < 0.05; ##, p < 0.01 compared with the morphine (MS) alone group.
Discussion
In this study, we demonstrated that haloperidol disrupted opioid-antinociceptive tolerance and physical dependence in two rodent models. The behavioral effect of haloperidol seemed to correlate with its inhibitory effect on CaMKIIα in opioid-tolerant and -dependent state. These data are in agreement with our previous studies that have suggested a critical role of CaMKIIα and its signaling pathways in opioid tolerance and dependence (Wang et al., 2003; Tang et al., 2006a).
It has been demonstrated that CaMKIIα can phosphorylate the NMDA receptor (McGlade-McCulloh et al., 1993; Lau and Huganir, 1995), leading to enhanced NMDA receptor activity and influx of Ca2+ through the channels (Kitamura et al., 1993). The latter, in turn, results in more activation of calmodulin (Klee et al., 1980; Shifman et al., 2006) and autophosphorylation of CaMKIIα at position Thr286 (Fukunaga et al., 1992; Strack and Colbran, 1998). This positive feedback between CaMKIIα and NMDA receptor can serve as one mechanism for sustained activation of CaMKIIα and NMDA receptors in opioid tolerance and dependence. NMDA receptor activation is a key mechanism promoting opioid tolerance and dependence (Trujillo and Akil, 1991; Noda and Nabeshima, 2004).
As a major serine/threonine protein kinase, CaMKIIα is expected to have many downstream effectors in addition to the NMDA receptor. Of these, the μ-opioid receptor (μOR) is arguably the most relevant for opioid tolerance and dependence. CaMKII has been reported to modulate desensitization of μOR in cells (Mestek et al., 1995; Koch et al., 1997). This was further supported by the findings that μOR and CaMKIIα are colocalized in dorsal root ganglion neurons and in the superficial laminae of the spinal cord dorsal horn (Brüggemann et al., 2000). CaMKIIα has also been reported to phosphorylate glycosylated phosducin-like protein after long-term treatment with morphine (Sánchez-Blázquez et al., 2008).
The typical antipsychotic drug haloperidol has been used clinically for decades. Its antipsychotic effect is thought to be mediated by blocking the dopamine D2 receptor (Leucht et al., 2008). Haloperidol is approximately 40 times more selective to the D2 receptor than it is to the D1 receptor (Leucht et al., 2008). A high level of D2 receptor occupancy was found after a relatively low dose of haloperidol in humans (Kapur et al., 1996).
The interaction between the dopamine and opiate systems is well recognized (Unterwald and Cuntapay, 2000). Considerable evidence suggests that dopamine activity affects the opioid system by modulating opiate peptide transcripts (Morris and Hunt, 1991), synthesis (Voorn et al., 1994), release, and biotransformation (Hong et al., 1985; Terashvili et al., 2008) (Li et al., 1986). In contrast, opioids modulate the dopamine system by several mechanisms, such as dopamine synthesis (Alper et al., 1980), release (Di Chiara and Imperato, 1988; Devine et al., 1993), biotransformation (Yonehara and Clouet, 1984), and activity of dopaminergic neurons (Walker et al., 1987; Johnson and North, 1992).
In addition, haloperidol has been reported to potentiate antinociception of morphine in the rat, possibly by acting as a σ-receptor antagonist (Chien and Pasternak, 1995). This would raise the possibility that haloperidol could have disrupted opioid tolerance by merely potentiating opioid analgesia. However, we found that haloperidol inhibited CaMKII activity in morphine-treated mice. Previous studies using chemical inhibitors and genetic manipulation, which are not expected to affect σ-receptor activity, demonstrated that inhibition of CaMKII led to diminished opioid tolerance and dependence (Fan et al., 1999; Tang et al., 2006a). Moreover, we found that haloperidol (up to 1 mg/kg) did not interfere with morphine antinociception, which may indicate that such an action is dose-dependent and may also differ among animal species (or strain). We found obvious signs of sedation in mice that were treated with haloperidol at 3 mg/kg; therefore, we did not pursue further studies using doses higher than 1 mg/kg. Haloperidol (up to 1 mg/kg) did not by itself produce antinociception effect, which was in agreement with a previous report (Chien and Pasternak, 1995).
On the other hand, haloperidol has also been shown to antagonize the effects of opioids in other experimental settings (Cowan et al., 1986; Cheido and Idova, 2007). Whereas haloperidol increased the biosynthesis and release of endogenous opioid peptides from the myenteric plexus (Milanés et al., 1985), the drug is also known to down-regulate μOR in certain brain regions (Mavridis and Besson, 1999; Bower et al., 2000). Some of these discrepancies again could be attributed to the different doses used. However, these results were largely interpreted by its direct effect of blocking the dopamine D2 receptor. In fact, some of the pharmacological effects of haloperidol may be attributed to its actions at CaMKII. For example, it has been previously demonstrated that morphine-conditioned place preference was reversed by the treatment of a high dose of haloperidol (Manzanedo et al., 2001). The effect may also be explained by its inhibition of CaMKII. Furthermore, CaMKII can positively regulate the D2 receptor signaling (Greenstein et al., 2007) and expression (Takeuchi et al., 2002).
The D2 dopamine agonists enhanced the ability of MK801 to attenuate the development of morphine tolerance and dependence (Verma and Kulkarni, 1995). However, the physical signs of opioid withdrawal were not altered in mice lacking the D2 dopamine receptor (Maldonado et al., 1997), indicating that attenuation of opioid dependence by haloperidol was not attributed to blocking the D2 receptor.
In summary, we found that haloperidol prevented and reversed morphine-induced CaMKIIα activation, antinociceptive tolerance, and physical dependence. These data not only provide additional support for the role of CaMKIIα in opioid tolerance and dependence but also is a step forward in the direction of translational application, especially because the drug was effective when given orally. These data raise the possibility of applying haloperidol to prevent or treat opioid dependence and to improve pain treatment by attenuating opioid tolerance and opioid-induced hyperalgesia.
This work was supported in part by the National Institutes of Health National Heart, Lung and Blood Institute [Grant HL098141]; and the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant AT003647]. Y.C. was supported by a University of Illinois predoctoral scholarship.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175539.
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- NMDA
- N-methyl-d-aspartate
- μOR
- the μ-opioid receptor
- MPE
- maximal possible effect
- MK801
- (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate.
Authorship Contributions
Participated in research design: Yang, Chen, Tang, and Wang.
Conducted experiments: Yang, Chen, and Tang.
Performed data analysis: Yang, Chen, and Wang.
Wrote or contributed to the writing of the manuscript: Yang, Chen, and Wang.
Other: Wang acquired funding for the research.
References
- Alper RH, Demarest KT, Moore KE. (1980) Morphine differentially alters synthesis and turnover of dopamine in central neuronal systems. J Neural Transm 48:157–165 [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Bernstein RN, Wang Z, Sadée W, Porreca F. (1996) Effects of naloxone and d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J Pharmacol Exp Ther 277:484–490 [PubMed] [Google Scholar]
- Bower CM, Hyde TM, Zaka M, Hamid EH, Baca SM, Egan MF. (2000) Decreased mu-opioid receptor binding in the globus pallidus of rats treated with chronic haloperidol. Psychopharmacology (Berl) 150:260–263 [DOI] [PubMed] [Google Scholar]
- Brüggemann I, Schulz S, Wiborny D, Höllt V. (2000) Colocalization of the mu-opioid receptor and calcium/calmodulin-dependent kinase II in distinct pain-processing brain regions. Brain Res Mol Brain Res 85:239–250 [DOI] [PubMed] [Google Scholar]
- Cheido MA, Idova GV. (2007) The differential contribution of dopamine D(1) and D (2) receptors to mu-opioidergic immunomodulation. Neurosci Behav Physiol 37:721–724 [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. (2009) Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther 330:650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang C, Wang ZJ. (2010) Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci 30:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. (1995) Sigma antagonists potentiate opioid analgesia in rats. Neurosci Lett 190:137–139 [DOI] [PubMed] [Google Scholar]
- Cowan A, Rance MJ, Blackburn TP. (1986) In vivo studies on delta opioid receptors. NIDA Res Monogr 75:473–476 [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther 266:1236–1246 [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Opposite effects of μ and κ opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080 [PubMed] [Google Scholar]
- Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. (1999) Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol 56:39–45 [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Soderling TR, Miyamoto E. (1992) Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neurons. J Biol Chem 267:22527–22533 [PubMed] [Google Scholar]
- Greenstein R, Novak G, Seeman P. (2007) Amphetamine sensitization elevates CaMKIIbeta mRNA. Synapse 61:827–834 [DOI] [PubMed] [Google Scholar]
- He Y, Yang C, Kirkmire CM, Wang ZJ. (2010) Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 30:10251–10258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A, Teschemacher H. (1973) Development of tolerance to the antinociceptive effect of morphine after intraventricular injection. Experientia 29:64–65 [DOI] [PubMed] [Google Scholar]
- Hong JS, Yoshikawa K, Kanamatsu T, Sabol SL. (1985) Modulation of striatal enkephalinergic neurons by antipsychotic drugs. Fed Proc 44:2535–2539 [PubMed] [Google Scholar]
- Johnson SW, North RA. (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S, Zipursky R. (1996) High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiatry 153:948–950 [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Miyazaki A, Yamanaka Y, Nomura Y. (1993) Stimulatory effects of protein kinase C and calmodulin kinase II on N-methyl-d-aspartate receptor/channels in the postsynaptic density of rat brain. J Neurochem 61:100–109 [DOI] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Richman PG. (1980) Calmodulin. Annu Rev Biochem 49:489–515 [DOI] [PubMed] [Google Scholar]
- Koch T, Kroslak T, Mayer P, Raulf E, Höllt V. (1997) Site mutation in the rat mu-opioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J Neurochem 69:1767–1770 [DOI] [PubMed] [Google Scholar]
- Lau LF, Huganir RL. (1995) Differential tyrosine phosphorylation of N-methyl-d-aspartate receptor subunits. J Biol Chem 270:20036–20041 [DOI] [PubMed] [Google Scholar]
- Lee HK. (2006) Synaptic plasticity and phosphorylation. Pharmacol Ther 112:810–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht C, Kitzmantel M, Chua L, Kane J, Leucht S. (2008) Haloperidol versus chlorpromazine for schizophrenia. Cochrane Database Syst Rev:CD004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Li X, Clark JD. (2004) Increased expression of Ca2+/calmodulin-dependent protein kinase II alpha during chronic morphine exposure. Neuroscience 123:769–775 [DOI] [PubMed] [Google Scholar]
- Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. (2008) Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 325:267–275 [DOI] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. (1997) Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature 388:586–589 [DOI] [PubMed] [Google Scholar]
- Manzanedo C, Aguilar MA, Rodríguez-Arias M, Miñarro J. (2001) Effects of dopamine antagonists with different receptor blockade profiles on morphine-induced place preference in male mice. Behav Brain Res 121:189–197 [DOI] [PubMed] [Google Scholar]
- Mavridis M, Besson MJ. (1999) Dopamine-opiate interaction in the regulation of neostriatal and pallidal neuronal activity as assessed by opioid precursor peptides and glutamate decarboxylase messenger RNA expression. Neuroscience 92:945–966 [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. (1993) Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362:640–642 [DOI] [PubMed] [Google Scholar]
- Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. (1995) The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci 15:2396–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanés MV, Vargas ML, Martínez JA, Pérez D, Brugger AJ. (1985) Effects of chronic treatment with atypical neuroleptics on the biosynthesis and release of opioid peptides in guinea-pig ileum. Regul Pept 10:319–327 [DOI] [PubMed] [Google Scholar]
- Morris BJ, Hunt SP. (1991) Proenkephalin mRNA levels in rat striatum are increased and decreased, respectively, by selective D2 and D1 dopamine receptor antagonists. Neurosci Lett 125:201–204 [DOI] [PubMed] [Google Scholar]
- Noda Y, Nabeshima T. (2004) Opiate physical dependence and N-methyl-d-aspartate receptors. Eur J Pharmacol 500:121–128 [DOI] [PubMed] [Google Scholar]
- Redondo RL, Okuno H, Spooner PA, Frenguelli BG, Bito H, Morris RG. (2010) Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci 30:4981–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Muñoz M, Montero C, de la Torre-Madrid E, Garzón J. (2008) Calcium/calmodulin-dependent protein kinase II supports morphine antinociceptive tolerance by phosphorylation of glycosylated phosducin-like protein. Neuropharmacology 54:319–330 [DOI] [PubMed] [Google Scholar]
- Schulman H, Greengard P. (1978) Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature 271:478–479 [DOI] [PubMed] [Google Scholar]
- Shifman JM, Choi MH, Mihalas S, Mayo SL, Kennedy MB. (2006) Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by calmodulin with two bound calciums. Proc Natl Acad Sci USA 103:13968–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Tang L, Wang ZJ. (2006) Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci Lett 404:266–269 [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. (1998) Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 273:20689–20692 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Miyamoto E, Fukunaga K. (2002) Activation of the rat dopamine D2 receptor promoter by mitogen-activated protein kinase and Ca2+/calmodulin-dependent protein kinase II pathways. J Neurochem 83:784–796 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. (1987) Manual of Pharmacological Calculations with Computer Programs, pp 26–31, Springer Verlag, New York [Google Scholar]
- Tang L, Shukla PK, Wang LX, Wang ZJ. (2006a) Reversal of morphine antinociceptive tolerance and dependence by the acute supraspinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 317:901–909 [DOI] [PubMed] [Google Scholar]
- Tang L, Shukla PK, Wang ZJ. (2006b) Trifluoperazine, an orally available clinically used drug, disrupts opioid antinociceptive tolerance. Neurosci Lett 397:1–4 [DOI] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Schwasinger ET, Hung KC, Hong JS, Tseng LF. (2008) (+)-Morphine attenuates the (−)-morphine-produced conditioned place preference and the mu-opioid receptor-mediated dopamine increase in the posterior nucleus accumbens of the rat. Eur J Pharmacol 587:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87 [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Cuntapay M. (2000) Dopamine-opioid interactions in the rat striatum: a modulatory role for dopamine D1 receptors in delta opioid receptor-mediated signal transduction. Neuropharmacology 39:372–381 [DOI] [PubMed] [Google Scholar]
- Verma A, Kulkarni SK. (1995) Role of D1/D2 dopamine and N-methyl-d-aspartate (NMDA) receptors in morphine tolerance and dependence in mice. Eur Neuropsychopharmacol 5:81–87 [DOI] [PubMed] [Google Scholar]
- Voorn P, Docter GJ, Jongen-Rêlo AL, Jonker AJ. (1994) Rostrocaudal subregional differences in the response of enkephalin, dynorphin and substance P synthesis in rat nucleus accumbens to dopamine depletion. Eur J Neurosci 6:486–496 [DOI] [PubMed] [Google Scholar]
- Walker JM, Thompson LA, Frascella J, Friederich MW. (1987) Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol 134:53–59 [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr., Lai J, Porreca F. (2001) Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 21:1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Tang L, Xin L. (2003) Reversal of morphine antinociceptive tolerance by acute spinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. Eur J Pharmacol 465:199–200 [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Wang LX. (2006) Phosphorylation: a molecular switch in opioid tolerance. Life Sci 79:1681–1691 [DOI] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Silva A, Soderling TR. (2008) Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59:914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano I, Takemori AE. (1977) Inhibition by naloxone of tolerance and dependence in mice treated acutely and chronically with morphine. Res Commun Chem Pathol Pharmacol 16:721–734 [PubMed] [Google Scholar]
- Yonehara N, Clouet DH. (1984) Effects of delta and mu opiopeptides on the turnover and release of dopamine in rat striatum. J Pharmacol Exp Ther 231:38–42 [PubMed] [Google Scholar]