Abstract
Tramadol is an unscheduled atypical analgesic that acts as an agonist at μ-opioid receptors and inhibits monoamine reuptake. Tramadol can suppress opioid withdrawal, and chronic administration can produce opioid physical dependence; however, diversion and abuse of tramadol is low. The present study further characterized tramadol in a three-choice discrimination procedure. Nondependent volunteers with active stimulant and opioid use (n = 8) participated in this residential laboratory study. Subjects were trained to discriminate between placebo, hydromorphone (8 mg), and methylphenidate (60 mg), and tests of acquisition confirmed that all volunteers could discriminate between the training drugs. The following drug conditions were then tested during discrimination test sessions: placebo, hydromorphone (4 and 8 mg), methylphenidate (30 and 60 mg), and tramadol (50, 100, 200, and 400 mg). In addition to discrimination measures, which included discrete choice, point distribution, and operant responding, subjective and physiological effects were measured for each test condition. Both doses of hydromorphone and methylphenidate were identified as hydromorphone- and methylphenidate-like, respectively. Lower doses of tramadol were generally identified as placebo, with higher doses (200 and 400 mg) identified as hydromorphone, or opioid-like. The highest dose of tramadol increased ratings on the stimulant scale, but was not significantly identified as methylphenidate-like. Tramadol did not significantly increase subjective ratings associated with reinforcement. Taken together, these results extend previous work with tramadol as a potential medication for the treatment of opioid dependence and withdrawal, showing acute doses of tramadol exhibit a profile of effects similar to opioid agonists and may have abuse liability in certain populations.
Introduction
Tramadol is an unscheduled atypical analgesic marketed as Ultram (Ortho-McNeil-Janssen Pharmaceutical, Titusville, NJ) and as generic. Tramadol exerts its analgesic effects in humans and animals through activation of two different systems; it is an agonist at μ-opioid receptors and inhibits monoamine reuptake, specifically serotonin and norepinephrine (Raffa et al., 1992; Desmeules et al., 1996; for review see Grond and Sablotzki, 2004; Ide et al., 2006). The racemic form of tramadol has affinity for μ-opioid receptors but is less potent than morphine (Raffa et al., 1992). A metabolite formed after first-pass metabolism, O-desmethyltramadol (M1), possesses a higher affinity for μ-opioid receptors compared with tramadol and probably contributes to its analgesic effects (Hennies et al., 1988; Raffa et al., 1992; Gillen et al., 2000).
Consistent with this unique pharmacological profile, tramadol exhibits some opioid agonist-like effects, but has lower abuse potential than typical opioid analgesics (Zacny, 2005, Epstein et al., 2006). For example, tramadol maintained lower rates of self-adminstration compared with lefetamine, morphine, and remifentanil in monkeys and rats (Yanagita, 1978, O'Connor and Mead, 2010), but retains analgesic effects (Raffa et al., 1992; Ide et al., 2006). These preclinical findings are consistent with results from clinical laboratory studies suggesting that tramadol does not produce significant morphine-like effects (Preston et al., 1991; Cami et al., 1994; Adams et al., 2006; Lofwall et al., 2007). Although chronic tramadol administration has the ability to produce physical dependence in the laboratory (Yanagita, 1978, Lanier et al., 2010), epidemiological and postmarketing surveillance of tramadol reports low abuse and diversion since its introduction in the United States in 1994 (Cicero et al., 1999, 2005, Woody et al., 2003; Inciardi et al., 2006).
Modest opioid agonist activity in an oral product is desirable for opioid withdrawal treatment. Ideally, a medication would exhibit enough efficacy to relieve opioid withdrawal symptoms, but not enough to support significant abuse or regulatory scheduling that would limit clinical availability. Because it exhibits some opioid agonist characteristics but with lower abuse liability compared with full μ-opioid receptor agonists, tramadol may be a useful therapeutic agent for opioid dependence. In opioid-dependent volunteers maintained on morphine, tramadol suppressed spontaneous opioid withdrawal induced by placebo substitution and did not significantly increase subject-rated effects of feeling high, drug liking, or drug effect (Carroll et al., 2006; Lofwall et al., 2007). In methadone-maintained volunteers, acute tramadol challenges failed to elicit significant morphine-like effects or precipitate withdrawal symptoms (Cami et al., 1994). Taken together, these data suggest tramadol may be useful in treating opioid withdrawal.
The current study used a drug discrimination paradigm to expand on prior human laboratory studies examining the effects of tramadol in experienced drug users. Drug discrimination is a behavioral tool that is useful for distinguishing a test drug from other drug classes, as well as for distinguishing activity at different opioid receptor systems (Herling and Woods, 1981, Young et al., 1984; Kamien et al., 1993; Dykstra et al., 1997). In prior studies acute tramadol elicited only modest opioid agonist-like subjective effects, suggesting possible nonopioidergic mechanisms. In animal drug discrimination procedures, tramadol fully substituted for morphine in morphine-trained rats, and this effect was attenuated with the opioid receptor antagonist naloxone (Ren and Zheng, 2000). Filip et al. (2004) reported an enhancement of tramadol discrimination after reboxetine, a norepinephrine reuptake inhibitor, and milnacipram, a serotonin and norepinephrine reuptake inhibitor, but not the selective serotonin reuptake inhibitors fluoxetine or venlafaxine. Although these compounds did not substitute for tramadol in tramadol-trained rats, these data suggest norepinephrine, and possibly serotonin, may play a role in the discriminative stimulus effects of tramadol.
To investigate further the pharmacological profile of tramadol the discriminative and subjective effects of tramadol were examined in humans. Because tramadol exerts activity at both opioid and monoamine systems, nondependent volunteers with recent sporadic opioid and stimulant use were trained to discriminate placebo, hydromorphone (HM), and methylphenidate (MPH) in a three-choice discrimination procedure (e.g., Preston et al., 1987; Jones et al., 1999). Doses of hydromorphone, methylphenidate, and tramadol were then tested. It was hypothesized that volunteers would successfully acquire the discrimination and that higher doses of tramadol would be identified primarily as an opioid agonist, but engender less opioid agonist-like subjective effects compared with hydromorphone.
Materials and Methods
Subjects
Participants were volunteers with current sporadic opioid and stimulant use (including cocaine in all subjects), but they were not physically dependent on opioids or stimulants (Table 1). Eight male volunteers completed the study. Females were enrolled; however, none completed the protocol. Participants underwent routine medical screening that included a medical history, physical examination, EKG, chemistry, hematology, urine drug testing, and routine medical urinalysis testing (e.g., specific gravity, pH, etc.). Medical staff not involved in the study as investigators reviewed all results, and all subjects were found to be without significant medical problems. The Structured Clinical Interview for the DSM-IV was completed to ensure volunteers were not physically dependent on substances (except caffeine and nicotine). In addition, participants were monitored drug-free for 48 h after residential unit admission to ensure there was no evidence of physical dependence on drugs other than caffeine and nicotine.
TABLE 1.
Demographics
Values are means (±S.E.M.) for continuous measures, except where otherwise indicated.
| Variable | Total (n = 8) |
|---|---|
| Age (years) | 40.3 (±2.3) |
| Sex (male) | 8 |
| Race (white) | 5 |
| Education (years) | 12.4 (±0.4) |
| Opioid use | |
| Years since first opioid use | 15.8 (±2.3) |
| Days of opioid use in last 30 days | 12.8 (±2.7) |
| Intravenous users | 4 |
| Lifetime use (years) | 4.9 (±1.3) |
| Cocaine use | |
| Years since first cocaine use | 17.5 (±4.3) |
| Days of cocaine use in last 30 days | 8 (±1.8) |
| Intravenous users | 0 |
| Lifetime use (years) | 6.8 (±2.1) |
| Other drugs used in last 30 daysa | |
| Alcohol | 7 |
| Cannabis | 2 |
| Benzodiazepines | 2 |
Number of volunteers that used each drug.
Pregnancy and significant medical or psychiatric illness (e.g., insulin-dependent diabetes, schizophrenia) were exclusionary. Individuals seeking treatment were not enrolled in the study and were assisted in referral to community-based treatment programs. The Institutional Review Board approved the study, and all volunteers gave written informed consent and were paid for their participation.
Study Setting
Participants lived on a closed, 14-bed residential unit for the duration of the study. Breathalyzer testing for alcohol was completed on the day of admission and randomly at least twice weekly. In addition, urine samples were collected at admission and daily throughout the study and tested intermittently for the presence of illicit drugs using an EMIT system (Olympus AU400; Syva Co., San Jose, CA). No evidence of unauthorized alcohol or drug abuse was detected during the study. Participants did not have access to caffeinated beverages and were allowed to smoke cigarettes ad libitum, except 30 min before and during experimental sessions.
Drugs
Drugs were encapsulated in red/white capsules and filled with lactose. Each volunteer received four red/white capsules on each session day. Lactose-filled capsules served as placebo. During training sessions and tests of acquisition, volunteers received placebo, hydromorphone (8 mg), and methylphenidate (60 mg). During discrimination sessions, volunteers received placebo, hydromorphone (4 and 8 mg), methylphenidate (30 and 60 mg), and tramadol (50, 100, 200, and 400 mg). Compounds were obtained from commercial sources: hydromorphone (Abbott Laboratories, Abbott Park, IL), methylphenidate (Novartis Consumer Health, East Hanover, NJ), and tramadol (PriCara, Raritan, NJ).
All drug administration was double-blind. Each participant was assigned three arbitrary letters that corresponded to each training drug condition (placebo, 8 mg of hydromorphone, 60 mg of methylphenidate). Letters varied across volunteers, but remained unchanged for each volunteer throughout participation. Capsule administration occurred at 9:00 AM on each session day, which was 30 min before the start of postdrug assessments and 90 min before the start of discrimination assessments.
General Methods
After volunteers completed informed consent, they were admitted and oriented to the residential unit. Volunteers were informed that the purpose of the study was to examine the effects of tramadol and that they would be required to discriminate between placebo, an opioid, and a stimulant. Examples of each of these were given and participants were told during test sessions that they might experience no effects, opioid agonist effects, stimulant effects, or other effects. Volunteers were instructed to attend closely to the effects of each letter-coded drug. They were informed that correct identification of the administered drug by letter code would result in a monetary bonus. All volunteers had a practice session for familiarization with study procedures and measures; these data were not included in the analyses. Volunteers were permitted to eat a light breakfast (e.g., toast and juice) 45 min before sessions, but then they were allowed only water until session end.
There were three phases for each volunteer, although staff and participants were aware of only two phases (the second and third phases were indistinguishable). Daily sessions were conducted weekdays (Monday–Friday).
Discrimination Training (Phase 1)
The purpose of this phase was to train subjects to identify each condition by letter code. In random order each participant received at least two exposures to each training drug condition: placebo, hydromorphone (8 mg), and methylphenidate (60 mg). During training exposures, volunteers were informed which letter they were receiving immediately before drug administration and were again informed of the letter code at session end.
Test of Acquisition (Phase 2)
After training sessions, acquisition of discrimination was tested. The purpose of this phase was to test whether volunteers could identify each training drug condition by the correct letter code. Each volunteer received at least two exposures of each training drug condition in randomized order. Subjects were not informed of the letter code of the drug before drug administration. At each session end, volunteers were informed of the letter code of the administered drug condition and whether they had earned a monetary bonus for correctly identifying the drug by letter code. The criterion for acquisition of the discrimination was at least 67% correct responses for the combined drug conditions and at least one correct response for each drug condition.
Discrimination Test Sessions (Phase 3)
During this phase, doses of hydromorphone (4 and 8 mg), methylphenidate (30 and 60 mg), tramadol (50, 100, 200, and 400 mg), and placebo were tested in a random order. These sessions were conducted in the same manner as the test of acquisition sessions (phase 2), except that no feedback on letter code was provided on discrimination test days. Test of acquisition sessions (i.e., feedback about the letter code given after the session was completed) were interspersed with discrimination test sessions.
Experimental Sessions
Subject-Rated and Physiological Effects.
Subject-rated effects and pupil diameter were collected 15 min before capsule administration, which was used for baseline, and at 30, 60, 90, 120, and 150 min after capsule administration. Pupil diameter was measured using a Neuroptics Pupilometer (Neuroptics Inc., Irvine, CA).
At each time point, volunteers completed three computer questionnaires rating the subjective effects of the drug condition administered: 1) visual analog scales (VAS), 2) an adjective rating scale, and 3) a pharmacological class questionnaire. On VAS items, volunteers placed an arrow along a 100-point line anchored with “not at all” and “extremely” to indicate the degree of effect produced by the drug condition. Participants rated drug effects as high, like, good effects, bad effects, sick, desire for cocaine now, similar to opioid, and similar to stimulant. In addition, participants rated the degree to which each drug condition was similar to each of the training drugs, as identified by letter code (e.g., similar to drug X; similar to drug Y). Volunteers rated adjectives on a five-point scale from 0 (no effect) to 4 (extremely). The adjective list constituted a 16-item opioid agonist scale (carefree, coasting, drive, drunken, dry mouth, energetic, friendly, good mood, heavy or sluggish feeling, nervous, nodding, pleasant sick, relaxed, skin itchy, talkative/soapboxing, turning of stomach) and a 27-item stimulant scale [confused, craving for cocaine, difficulty concentrating, dizzy/lightheaded, drug effect, excited, fearful, feel a thrill, feeling of power, fidgety, headache, hungry, irritable, jittery, nausea, numbness, restless, seeing/hearing things, shaky (hands), sleepy, stimulated, suspicious, sweating, thirsty, tingling, tired, tremor]. On the pharmacological class questionnaire, volunteers indicated which drug class was most similar to the drug condition they received that day. Ten drug classes were listed with descriptive labels and examples of each: placebo, opiates, phenothiazines, barbiturates, antidepressants, opiate antagonists, hallucinogens, benzodiazepines, stimulants, and other.
Discrimination Procedures.
Discrimination was assessed at 90 and 120 min after capsule administration using three procedures: 1) discrete choice, 2) point distribution, and 3) operant responding. During discrete choice, volunteers chose the letter of the training drug that they thought they received. In point distribution, volunteers distributed 50 points among the three training drug letters depending on how certain they were of the identity of the administered drug. Lastly, volunteers emitted operant responses on computer keys that corresponded to the training letters, on a fixed interval 1-s schedule for 8.5 min. Points were earned for responses on each training drug. Payments during phases 2 and 3 for test of acquisition sessions were based on the accuracy of responses. The maximum possible payment for discrimination tasks was $10/session. On discrimination test days, payments were based on an average of the payments received for test of acquisition sessions.
Data Analysis
Data from the eight subjects who completed the protocol were included in the final analysis. To preserve testing in a random order, some volunteers received more than one exposure to a test drug during discrimination testing (phase 3). For data analysis purposes only the first exposure to each test drug was included, with the exception of sessions repeated because of malfunctions. To encompass the peak effects for each training drug condition, data from only the 120-min time point were used for analysis of the three discrimination measures. Peak effects for each session were determined for subjective and physiological measures. For most measures, the reported value was a peak increase; however, the peak increase and peak decrease were analyzed for pupil diameter. Means for the discrimination measures and peak effects for subjective and physiological measures were both analyzed using a repeated-measures regression model with an exchangeable covariance structure and an effect of drug condition. Pairwise comparisons were examined using a conservative one-step procedure, Tukey's honestly significant difference. Peak placebo effects were compared with each drug condition. In addition, all tramadol conditions were compared with hydromorphone (4 and 8 mg) and methylphenidate (30 and 60 mg).
Results
Test of Acquisition.
All eight volunteers correctly identified each drug condition at least once during the test of acquisition with at least 67% correct responses for all drug conditions (phase 2). Four volunteers completed the initial six tests of acquisition session with at least five correct responses of six. The remaining volunteers received one to three additional exposures to drug conditions that were initially incorrect (range: 6–9 training sessions in phase 1; range: 6–9 test sessions in phase 2). Additional test of acquisition sessions were randomly interspersed throughout discrimination test sessions (phase 3). Overall, volunteers correctly identified placebo 87% of the time, methylphenidate (60 mg) 87.5% of the time, and hydromorphone (8 mg) 90% of the time.
Discrimination Test Sessions.
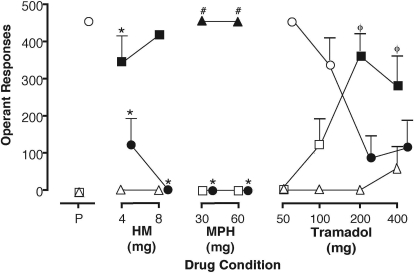
Results from phase 3 (range: 9–14 test sessions in phase 3) for operant responses, point distribution, and discrete choice data are shown for the 120-min time point (Table 2). Figure 1 illustrates results from the operant responses. Placebo was associated only with placebo-appropriate responding. Hydromorphone was associated with significantly higher rates of hydromorphone-appropriate responding, whereas both doses of methylphenidate were associated with significantly higher methylphenidate-appropriate responding, compared with placebo (Fig. 1). Higher doses of tramadol were associated with decreased placebo-appropriate responding and increased hydromorphone-appropriate responding, compared with lower doses. Tramadol was generally not associated with methylphenidate-appropriate responding (with the exception of the 400-mg dose).
TABLE 2.
Summary of discrimination measures
Numbers shown for operant responses and point distribution (maximum total = 50) are means (S.E.M.). F ratio indicates a statistically significant overall regression (P < 0.0001, df = 8, 56). Lack of variability for discrete choice precluded statistical analysis. Numbers for discrete choice are mean percentage for each drug condition. All data are from the 120-min time point.
| F | Placebo | HM (mg) |
MPH (mg) |
Tramadol (mg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 30 | 60 | 50 | 100 | 200 | 400 | |||
| Operant responses | ||||||||||
| Placebo | 16.4 | 455.5 (9.6) | 116.6 (76.4)a | 0.0 (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 464.1 (4.2)b,c,d,e | 341.3 (74.8)b,c,d,e | 86.6 (62.0)a | 115.6 (75.7)a |
| HM | 14.3 | 0.0 (0.0) | 341.3 (74.7)a | 453.4 (9.0)a | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0)b,c | 118.1 (77.3)b,c | 367.0 (60.2)a,d,e | 282.9 (83.4)a,d,e |
| MPH | 102.7 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 465.4 (4.7)a | 465.8 (3.0)a | 0.0 (0.0)d,e | 0.0 (0.0)d,e | 0.0 (0.0)d,e | 59.9 (59.9)d,e |
| Point distribution | ||||||||||
| Placebo | 17.2 | 50.0 (0.0) | 11.9 (7.8)a | 0.0 (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 50.0 (0.0)b,c,d,e | 37.5 (8.2)b,c,d,e | 9.4 (6.6)a | 12.5 (8.2)a |
| HM | 15.0 | 0.0 (0.0) | 38.1 (7.8)a | 50.0 (0.0)a | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0)b,c | 12.5 (8.2)b,c | 38.3 (6.5)a,d,e | 31.3 (9.1)a,d,e |
| MPH | 109.0 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 50.0 (0.0)a | 50.0 (0.0)a | 0.0 (0.0)d,e | 0.0 (0.0)d,e | 0.0 (0.0)d,e | 6.3 (6.3)d,e |
| Discrete choice | ||||||||||
| Placebo | 100 | 25 | 0 | 0 | 0 | 100 | 75 | 25 | 25 | |
| HM | 0 | 75 | 100 | 0 | 0 | 0 | 25 | 75 | 63 | |
| MPH | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 12 | |
p < 0.05 vs. placebo.
p < 0.05 vs. 4 mg HM.
p < 0.05 vs. 8 mg HM.
p < 0.05 vs. 30 mg MPH.
p < 0.05 vs. 60 mg MPH.
Fig. 1.
Reinforced operant responses for placebo (circles), hydromorphone (squares), and methylphenidate (triangles) during discrimination test sessions. Data points are means (+S.E.M.) for eight volunteers based on one administration of each test condition for each volunteer. For clarity, the placebo data points have been shifted rightward to avoid overlapping symbols. Closed symbols indicate a significant difference compared with placebo. *, p < 0.05 versus tramadol (50 and 100 mg); #, p < 0.05 versus tramadol (50, 100, 200, and 400 mg); φ, p < 0.05 versus methylphenidate (30 and 60 mg).
A similar pattern of results was observed for the point distribution and discrete choice tasks, although the lack of variability precluded statistical analysis for the discrete choice data (Table 2). For these discrimination tasks, hydromorphone was identified as hydromorphone 75 to 100% of the time, whereas methylphenidate was identified as methylphenidate on 100% of occasions. As the dose of tramadol increased, volunteers identified it as hydromorphone on the majority of occasions (63–75%). The highest dose of tramadol was identified as methylphenidate or placebo 25 and 12% of the time, respectively (Table 2).
Subjective and Physiological Effects.
On the pharmacological class questionnaire, volunteers identified placebo as placebo on 100% of occasions (Table 3). Doses of hydromorphone were predominantly identified as an opioid agonist on 75% (4 mg) and 100% (8 mg) of occasions, whereas both doses of methylphenidate were identified as a stimulant on 100% of occasions. Lower doses of tramadol were generally identified as placebo on 100% (50 mg) and 75% (100 mg) of occasions. As the dose or tramadol increased, identifications as placebo decreased. Higher doses of tramadol (200 and 400 mg) were primarily identified as an opioid agonist (63%). The remaining identifications for these doses were split between placebo and stimulant (Table 3).
TABLE 3.
Pharmacological class questionnaire
Numbers shown are percentages of drug identifications made for each dose condition at the 120-min time point, from a total of eight subjects.
| Placebo | HM (mg) |
MPH (mg) |
Tramadol (mg) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 30 | 60 | 50 | 100 | 200 | 400 | ||
| Placebo | 100 | 25 | 0 | 0 | 0 | 100 | 75 | 37 | 25 |
| Opioid Agonist | 0 | 75 | 100 | 0 | 0 | 0 | 25 | 63 | 63 |
| Stimulant | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | 12 |
VAS Ratings of Similarity.
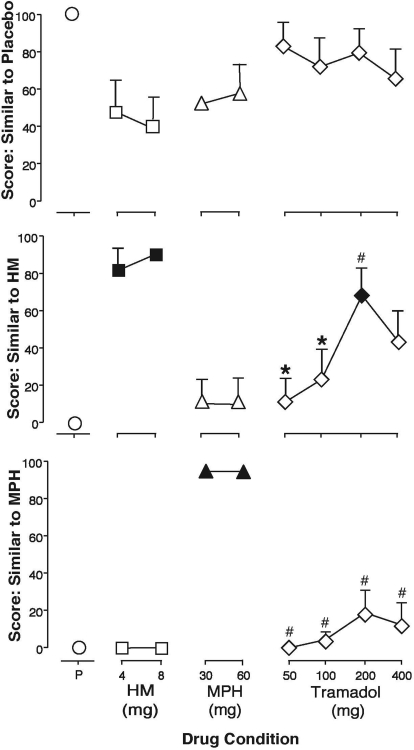
Volunteers rated how similar test doses were to each of the training drugs, as identified by letter code, and to a drug class (i.e., opioid, stimulant) on a 100-point visual analog scale (Table 4). Generally, volunteers rated placebo as most similar to placebo (Fig. 2). Compared with placebo, both doses of hydromorphone (4 and 8 mg) and one dose of tramadol (200 mg), but not methylphenidate, were rated significantly similar to hydromorphone (Fig. 2) and opioid (Table 4). Methylphenidate (30 and 60 mg), but not hydromorphone or tramadol, was rated significantly similar to methylphenidate and stimulant compared with placebo (Fig. 2; Table 4). Subject ratings of similarity of each drug test condition were consistent between training drugs identified by letter code and drug class.
TABLE 4.
Peak change from baseline analysis
Numbers shown are mean (S.E.M.) peak maximum change from baseline for each dose condition, with the exception of pupils that show mean (S.E.M.) nadir maximum change from baseline (n = 8).
| F | Placebo | HM (mg) |
MPH (mg) |
Tramadol (mg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 30 | 60 | 50 | 100 | 200 | 400 | |||
| VAS | ||||||||||
| Similar to placebo | 2.1 | 100.0 (0.0) | 50.0 (18.9) | 40.1 (16.7) | 56.3 (17.5) | 62.5 (18.3) | 87.5 (12.5) | 75.0 (16.4) | 83.9 (12.5) | 69.0 (16.2) |
| Similar to HM | 9.4** | 0.0 (0.0) | 86.9 (12.4)a | 100.0 (0.0)a | 12.5 (12.5) | 13.4 (12.4) | 12.5 (12.5)b,c | 25.0 (16.4)b,c | 72.0 (14.5)a,d,e | 46.0 (16.6) |
| Similar to MPH | 47.3** | 0.4 (0.4) | 0.0 (0.0) | 0.0 (0.0) | 100.0 (0.0)a | 100.0 (0.0)a | 0.0 (0.0)d,e | 4.5 (4.5)d,e | 19.5 (12.9)d,e | 12.8 (12.5)d,e |
| Similar to opioid | 8.9** | 0.0 (0.0) | 62.9 (15.9)a | 85.3 (9.9)a | 12.5 (12.5) | 7.4 (6.4) | 12.5 (12.5)b,c | 7.4 (5.3)b,c | 50.0 (14.9)a | 37.8 (14.8)c |
| Similar to stimulant | 21.1** | 0.4 (0.4) | 0.0 (0.0) | 0.9 (0.9) | 79.9 (13.4)a | 85.5 (9.5)a | 0.0 (0.0)d,e | 0.0 (0.0)d,e | 18.3 (12.7)d,e | 13.1 (12.4)d,e |
| Like | 4.4** | 0.0 (0.0) | 29.0 (11.1) | 44.1 (11.8)a | 11.3 (4.2) | 23.3 (7.0) | 1.3 (1.3)c | 6.1 (4.9)c | 14.9 (8.3) | 23.8 (11.8) |
| Bad effects | 3.6* | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.1) | 20.6 (12.0) | 26.0 (11.7)a | 0.0 (0.0)e | 0.0 (0.0)e | 2.3 (1.2) | 3.1 (2.5) |
| Good effects | 5.3** | 0.0 (0.0) | 24.3 (8.3) | 47.5 (11.9)a | 13.0 (4.6) | 24.5 (6.2) | 0.4 (0.4)c | 7.1 (5.4)c | 13.6 (8.2)c | 20.4 (10.8) |
| High | 4.7** | 0.0 (0.0) | 19.8 (5.8) | 29.0 (6.5)a | 22.1 (9.1) | 37.1 (9.7)a | 0.4 (0.4)c,e | 5.8 (3.5)e | 10.5 (4.6) | 17.6 (7.6) |
| Drug effect | 5.0** | 0.0 (0.0) | 18.8 (5.2) | 31.6 (6.4)a | 25.8 (9.5) | 42.9 (10.0)a | 0.4 (0.4)c,e | 4.4 (3.0)e | 18.6 (7.5) | 19.4 (8.2) |
| Subject Adjectives | ||||||||||
| Opioid agonist scale | 2.3* | 1.4 (0.7) | 2.9 (1.0) | 4.8 (2.1) | 2.1 (0.5) | 3.4 (1.2) | 0.4 (0.4)c | 1.6 (0.7) | 2.1 (1.0) | 3.8 (1.8) |
| Stimulant scale | 8.6** | 1.4 (0.8) | 3.6 (1.0) | 3.6 (1.2) | 8.0 (1.6)a | 10.6 (2.3)a | 0.6 (0.4)d,e | 0.9 (0.5)d,e | 4.9 (2.0)e | 6.9 (2.1)a |
| Pupil diameter | 2.2* | −0.5 (0.2) | −1.2 (0.2) | −1.9 (0.2)a | −0.6 (0.3) | −0.3 (0.2) | −0.5 (0.1)c | −0.2 (0.1)b,c | −0.8 (0.3)c | −0.8 (0.3)c |
p < 0.05, df (8, 56).
p < 0.001, df (8, 56).
p < 0.05 vs. placebo.
p < 0.05 vs. 4 mg HM.
p < 0.05 vs. 8 mg HM.
p < 0.05 vs. 30 mg MPH.
p < 0.05 vs. 60 mg MPH.
Fig. 2.
Volunteers were asked to rate how similar each test drug condition was to each drug, as identified by letter code. Data represent mean peak change from baseline (+S.E.M.) for visual analog scale scores for similar to placebo (top), similar to HM (middle), and similar to MPH (bottom). Closed symbols indicate a significant difference from placebo. *, p < 0.05 versus hydromorphone (4 and 8 mg); #, p < 0.05 versus methylphenidate (30 and 60 mg).
VAS Ratings of Effects.
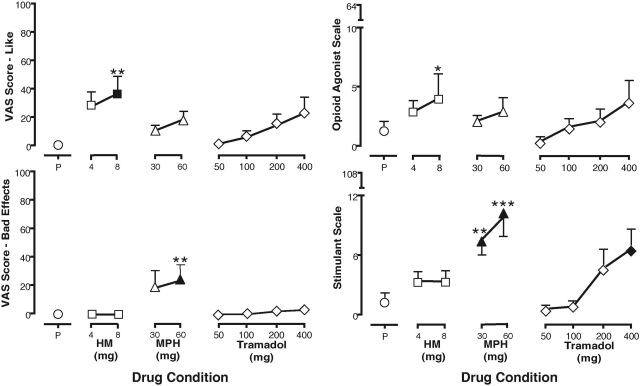
Hydromorphone (8 mg), but not methylphenidate or tramadol, significantly increased ratings of like and good effects. Compared with placebo, hydromorphone (8 mg) and methylphenidate (60 mg) increased ratings of high and drug effect; however, the highest dose of methylphenidate significantly increased ratings of bad effects (Fig. 3; Table 4). Tramadol did not significantly increase ratings of like or good effects compared with placebo.
Fig. 3.
VAS ratings of like and bad effects (left) and subject-rated scores on the opioid agonist scale and stimulant scale (right) during discrimination test sessions. Data represent mean peak (+S.E.M.) scores from eight volunteers; each test drug condition is represented once for each volunteer. A closed symbol indicates a significant difference from placebo. *, p < 0.05 versus tramadol (50 mg); **, p < 0.05 versus tramadol (50 and 100 mg); ***, p < 0.05 versus tramadol (50, 100, and 200 mg).
Adjectives.
Volunteers rated a series of adjectives after each test drug, which constituted an opioid agonist scale and a stimulant scale. Compared with placebo, 8 mg of hydromorphone increased ratings on the opioid agonist scale, whereas both doses of methylphenidate and 400 mg of tramadol significantly increased ratings on the stimulant scale (Fig. 3).
Pupil Diameter.
Hydromorphone (8 mg) significantly decreased pupil diameter compared with placebo and all doses of tramadol (Table 4). A statistically significant increase in pupil diameter was not observed (data not shown).
Discussion
The purpose of the present study was to extend earlier work with tramadol by investigating the discriminative stimulus, subjective, and physiological effects of tramadol in nondependent drug-experienced humans. Given that tramadol is an atypical analgesic that exerts agonist activity at μ-opioid receptors and inhibits monoamine reuptake, subjects were trained to discriminate placebo, an opioid receptor agonist, hydromorphone (8 mg), and a monoamine uptake-inhibiting stimulant, methylphenidate (60 mg; study phase 1). In subsequent discrimination testing, doses of hydromorphone occasioned hydromorphone-appropriate responding during the operant response discrimination task (Fig. 1), whereas doses of methylphenidate occasioned methylphenidate-appropriate responding. Higher doses of tramadol (200 and 400 mg) were associated with hydromorphone-, but not methylphenidate-, appropriate responding. A similar pattern of results was observed across all three discrimination tasks (Table 2).
Examination of subject-rated measures revealed additional effects of the three test drugs. Consistent with previous reports that drug discrimination and drug self-report measures are sensitive to detect the effects of stimulants and opioids (Kelly et al., 2003), effects obtained with these measures were similar across drug test conditions (Bickel et al., 1989). The pattern of results indicates that the 200-mg dose of tramadol engenders effects similar to an opioid agonist, whereas a higher dose of tramadol (400 mg) exerts mixed behavioral effects characteristic of an opioid agonist and stimulant.
To our knowledge, the current study is the first to report the acquisition of a three-choice discrimination using placebo, hydromorphone, and methylphenidate in nondependent drug-experienced humans. The use of methylphenidate and hydromorphone as training drugs in human discrimination procedures can detect stimulant- and opioid-like discriminative effects, respectively. Methylphenidate has been shown to share discriminative stimulus effects with other stimulants such as methamphetamine and d-amphetamine, suggesting that this drug is a useful pharmacological tool to detect stimulant-like effects for novel compounds (Stoops et al., 2005; Sevak et al., 2009). Likewise, hydromorphone has been used as a training drug to detect opioid agonist versus antagonist effects, as well as partial versus full μ-opioid receptor agonist effects (Preston et al., 1987; Jones et al., 1999; Preston and Bigelow, 2000). In the present study, the three-choice discrimination procedure was sensitive to detect opioid agonist effects and stimulant effects, as shown by differential responses to doses of hydromorphone and methylphenidate in discrimination tasks and subject-rated effects.
Doses of tramadol revealed a unique behavioral profile in the present study. Higher doses of tramadol resulted in hydromorphone-appropriate responding, a pattern that was preserved across discrimination tasks (Table 2). Tramadol did not significantly increase ratings of drug liking, good effects, high, or drug effects, but did significantly increase scores on the stimulant scale at the highest dose tested. This unique pattern of results (i.e., hydromorphone-appropriate responding and stimulant scale scores) probably reflects tramadol's activity at both μ-opioid receptors and the monoamine system. However, the lack of subjective effects that are generally associated with increased abuse liability (e.g., significantly increased ratings of drug liking or good effects) is consistent with tramadol's reportedly lower reinforcement efficacy and abuse (Cicero et al., 2005; Adams et al., 2006; Raffa, 2008, O'Connor and Mead, 2010). Although subjects reported stimulant-like effects for tramadol, they may have generally disliked such effects despite their history of stimulant abuse, given the ratings of bad effects associated with methylphenidate.
One explanation for this profile of effects (i.e., mild opioid-like effects with reduced abuse liability) is tramadol's slow onset and lower efficacy at μ-opioid receptors compared with a full μ-opioid receptor agonist. Tramadol's parent compound is approximately 6000 times weaker than morphine; however, the M1 metabolite formed after first-pass metabolism has higher affinity for μ-opioid receptors compared with the parent form and possesses analgesic activity (Hennies et al., 1988). This profile may increase the potential of tramadol to serve as medication for opioid dependence. In addition, the absence of stimulant-like effects is a further advantage if tramadol is used in a drug-abusing population. These results are in line with several laboratory studies that have supported tramadol as a potential treatment for opioid dependence (Carroll et al., 2006; Lofwall et al., 2007; Lanier et al., 2010) and are also consistent with retrospective studies examining tramadol as a treatment for opioid withdrawal (Tamaskar et al., 2003; Threlkeld et al., 2006). Taken together, these converging lines of work suggest tramadol may be a useful medication for the treatment of patients with low levels of opioid dependence or for the treatment of mild to moderate opioid withdrawal.
This mixed profile of discriminative and subjective effects is consistent with previous human studies. Preston et al. (1991) reported no significant ratings of drug liking or decreased pupil size after doses of tramadol in nondependent opioid-using volunteers. In other studies, tramadol has been shown to engender μ-opioid receptor-like effects (Zacny, 2005, Epstein et al., 2006). Preclinical studies have confirmed that effects of tramadol such as analgesia are mediated via both opioid and nonopioid mechanisms (Raffa et al., 1992; Ide et al., 2006). More specifically, Filip et al. (2004) reported that tramadol discrimination in rats was probably mediated by μ-opioid receptors, norepinephrine, and possibly serotonergic activity.
Doses of hydromorphone significantly decreased pupil diameter. In the present study, tramadol failed to change pupil diameter significantly. Examination of the time-dependent changes in pupil dilation and constriction after doses of tramadol revealed a delayed, but not significant, pupillary effect compared with hydromorphone (data not shown). Previous reports of tramadol's pupillary effects have been mixed. Tramadol has been shown to both significantly decrease pupil size (Zacny, 2005; Epstein et al., 2006) and have no effect (Preston et al., 1991) in nondependent opioid volunteers given similar doses as the present study. One possible explanation for the present lack of pupillary effects with tramadol may be differences in metabolism rates related to the polymorphic isoenzyme cytochrome P450 2D6. Individuals who are poor metabolizers of tramadol and express this polymorphism do not show significant miosis after tramadol administration (Fliegert et al., 2005). An alternative explanation may involve the lower efficacy of tramadol compared with full μ-opioid receptor agonists.
The present study expanded knowledge on the effects of tramadol in several ways. First, this study included a large dose range of oral tramadol (for example, compared with Preston et al., 1991; but see Epstein et al., 2006 for a report on oral doses as high as 700 mg). In addition, whereas previous studies have focused on tramadol's opioid effects, the present work examined tramadol's stimulant-like effects along with opioid-like effects. This study also tested tramadol using a human laboratory drug discrimination three-choice procedure. Along with previous reports of tramadol's utility as a potential treatment medication for opioid withdrawal, the present work brings together in one study assessments of the discriminative stimulus and subjective effects for both opioids and stimulants and the abuse liability of oral tramadol in a drug-experienced nondependent population.
To our knowledge, this study is the first to evaluate the discriminative stimulus effects of tramadol in opioid nondependent humans. We used a novel three-choice discrimination procedure with placebo, hydromorphone, and methylphenidate as training drugs. High doses of tramadol shared discriminative stimulus effects with hydromorphone, but not methylphenidate, suggesting a role for μ-opioid receptors in the acute discriminative stimulus effects of tramadol. Consistent with its lower abuse potential compared with full μ-opioid receptor agonists, tramadol did not increase positive subject ratings associated with reinforcement efficacy, such as drug liking and good effects. However, the highest dose of tramadol increased subject rated scores on a stimulant scale. Taken together these data suggest that μ-opioid receptors are involved in the discriminative stimulus effects of tramadol and stimulant-like effects emerge with higher doses of tramadol, but that this profile of effects is still consistent with a modest abuse liability for tramadol.
Acknowledgments
We thank the medical, nursing, and pharmacy staff at The Johns Hopkins University School of Medicine for work on the protocol and the research assistants there for aid in data collection and manuscript preparation.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA18125, T32-DA07209, K24-DA23186].
The drug used in the study described in this article was developed by Gruenenthal. In the past, E.C.S. has been a paid consultant to Gruenenthal. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.181131.
- HM
- hydromorphone
- MPH
- methylphenidate
- VAS
- visual analog scale.
Authorship Contributions
Participated in research design: Bigelow, Lanier, and Strain.
Conducted experiments: Duke, Bigelow, Lanier, and Strain.
Performed data analysis: Duke and Strain.
Wrote or contributed to the writing of the manuscript: Duke, Bigelow, and Strain.
Other: Strain acquired funding for the research.
References
- Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, Senay EC, Woody GE. (2006) A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage 31:465–476 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Bigelow GE, Preston KL, Liebson IA. (1989) Opioid drug discrimination in humans: stability, specificity and relation to self-reported drug effect. J Pharmacol Exp Ther 251:1053–1063 [PubMed] [Google Scholar]
- Cami J, Lama X, Farre M. (1994) Acute effects of tramadol in methadone-maintained volunteers. Drugs 47 (Suppl 1):39–43 [DOI] [PubMed] [Google Scholar]
- Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL. (2006) Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp Clin Psychopharmacol 14:109–120 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Adams EH, Geller A, Inciardi JA, Muñoz A, Schnoll SH, Senay EC, Woody GE. (1999) A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 57:7–22 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Muñoz A. (2005) Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf 14:851–859 [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Piguet V, Collart L, Dayer P. (1996) Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol 41:7–12 [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Preston KL, Bigelow GE. (1997) Discriminative stimulus and subjective effects of opioids with μ and κ activity: data from laboratory animals and human subjects. Psychopharmacology 130:14–27 [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Jasinski DR. (2006) Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol Psychol 73:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegert F, Kurth B, Göhler K. (2005) The effects of tramadol on static and dynamic pupillometry in healthy subjects–the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur J Clin Pharmacol 61:257–266 [DOI] [PubMed] [Google Scholar]
- Filip M, Wydra K, Inan SY, Dziedzicka-Wasylewska M, Przegaliński E. (2004) Opioid and monoamine systems mediate the discriminative stimulus of tramadol in rats. Eur J Pharmacol 498:143–151 [DOI] [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. (2000) Affinity, potency and efficacy of tramadol and its metabolites at the cloned human μ-opioid receptor. Naunyn-Schmiedebergs Arch Pharmacol 362:116–121 [DOI] [PubMed] [Google Scholar]
- Grond S, Sablotzki A. (2004) Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923 [DOI] [PubMed] [Google Scholar]
- Hennies HH, Friderichs E, Schneider J. (1988) Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung 38:877–880 [PubMed] [Google Scholar]
- Herling S, Woods JH. (1981) Discriminative stimulus effects of narcotics: evidence for multiple receptor-mediated actions. Life Sci 28:1571–1584 [DOI] [PubMed] [Google Scholar]
- Ide S, Minami M, Ishihara K, Uhl GR, Sora I, Ikeda K. (2006) Mu opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacology 51:651–658 [DOI] [PubMed] [Google Scholar]
- Inciardi JA, Cicero TJ, Munoz A, Adams EH, Geller A, Senay EC, Woody GE. (2006) The diversion of Ultram, Ultracet, and generic tramadol HCL. J Addict Dis 25:53–58 [DOI] [PubMed] [Google Scholar]
- Jones HE, Bigelow GE, Preston KL. (1999) Assessment of opioid partial agonist activity with a three-choice hydromoprhone dose-discrimination procedure. J Pharmacol Exp Ther 289:1350–1361 [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Hughes JR, Higgins ST, Smith BJ. (1993) Drug discrimination by humans compared to nonhumans: current status and future directions. Psychopharmacology 111:259–270 [DOI] [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. (2003) Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cogn Neurosci Rev 2:227–260 [DOI] [PubMed] [Google Scholar]
- Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC. (2010) Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology 211:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC. (2007) Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology 194:381–393 [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Mead AN. (2010) Tramadol acts as a weak reinforcer in the rat self-administration model, consistent with its low abuse liability in humans. Pharmacol Biochem Behav 96:279–286 [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. (2000) Effects of agonist-antagonist opioids in humans trained in a hydromorphone/not hydromorphone discrimination. J Pharmacol Exp Ther 295:114–124 [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel W, Liebson IA. (1987) Three-choice drug discrimination in opioid-dependent humans: hydromorphone, naloxone and saline. J Pharmacol Exp Ther 243:1002–1009 [PubMed] [Google Scholar]
- Preston KL, Jasinski DR, Testa M. (1991) Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend 27:7–17 [DOI] [PubMed] [Google Scholar]
- Raffa RB. (2008) Basic pharmacology relevant to drug abuse assessment: tramadol as example. J Clin Pharm Ther 33:101–108 [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. (1992) Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260:275–285 [PubMed] [Google Scholar]
- Ren YH, Zheng JW. (2000) Influence of tramadol on morphine discriminative behavior in rats. Acta Pharmacol Sin 21:924–926 [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Hays LR, Rush CR. (2009) Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther 328:1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Rush CR. (2005) Discriminative stimulus and self-reported effects of methylphenidate, d-amphetamine, and triazolam in methylphenidate-trained humans. Exp Clin Psychopharmacol 13:56–64 [DOI] [PubMed] [Google Scholar]
- Tamaskar R, Parran TV, Jr, Heggi A, Brateanu A, Rabb M, Yu J. (2003) Tramadol versus buprenorphine for the treatment of opioid withdrawal: a rretrospective cohort control study. J Addict Dis 22:5–12 [DOI] [PubMed] [Google Scholar]
- Threlkeld M, Parran TV, Adelman CA, Grey SF, Yu J. (2006) Tramadol versus buprenorphine for the management of acute heroin withdrawal: a retrospective matched cohort controlled study. Am J Addict 15:186–191 [DOI] [PubMed] [Google Scholar]
- Woody GE, Senay EC, Geller A, Adams EH, Inciardi JA, Schnoll S, Muñoz A, Cicero TJ. (2003) An independent assessment of MEDWatch reporting for abuse/dependence and withdrawal from Ultram (tramadol hydrochloride). Drug Alcohol Depend 72:163–168 [DOI] [PubMed] [Google Scholar]
- Yanagita T. (1978) Drug dependence potential of 1-(m-methoxyphenyl)-2-dimethylaminomethyl)-cyclohexan-1-ol hydrochloride (tramadol) tested in monkeys. Arzneimittelforschung 28:158–163 [PubMed] [Google Scholar]
- Young AM, Stephens KR, Hein DW, Woods JH. (1984) Reinforcing and discriminative stimulus properties of mixed agonist-antagonist opioids. J Pharmacol Exp Ther 229:118–126 [PubMed] [Google Scholar]
- Zacny JP. (2005) Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend 80:273–278 [DOI] [PubMed] [Google Scholar]