Abstract
Selective endothelin A (ETA) and combined ETA and ETB receptor antagonists are being investigated for use in treating diabetic nephropathy. However, the receptor-specific mechanisms responsible for producing the potential benefits have not been discerned. Thus, we determined the actions of ETA and ETB receptors on measures of glomerular function and renal inflammation in the early stages of diabetic renal injury in rats through the use of selective and combined antagonists. Six weeks after streptozotocin (STZ)-induced hyperglycemia, rats were given 2R-(4-methoxyphenyl)-4S-(1,3-benzodioxol-5-yl)-1-(N,N-di(n-butyl)aminocarbonyl-methyl)-pyrrolidine-3R-carboxylic acid (ABT-627) (5 mg/kg/day), a selective ETA antagonist; (2R,3R,4S)-4-(benzo[d][1,3]dioxol-5-yl)-2-(3-fluoro-4-methoxyphenyl)-1-(2-(N-propylpentylsulfonamido)ethyl)pyrrolidine-3-carboxylic acid hydrochloride (A-182086) (10 mg/kg/day), a combined ETA/B antagonist; or vehicle for 1 week. Sham controls received STZ vehicle (saline). Hyperglycemia led to significant proteinuria, increased glomerular permeability to albumin (Palb), nephrinuria, and an increase in total matrix metalloprotease (MMP) and transforming growth factor-β1 (TGF-β1) activities in glomeruli. Plasma and glomerular soluble intercellular adhesion molecule-1 (sICAM-1) and monocyte chemoattractant protein-1 (MCP-1) were elevated after 7 weeks of hyperglycemia. Daily administration of both ABT-627 and A-182086 for 1 week significantly attenuated proteinuria, the increase in Palb, nephrinuria, and total MMP and TGF-β1 activity. However, glomerular sICAM-1 and MCP-1 expression was attenuated with ABT-627, but not A-182086, treatment. In summary, both selective ETA and combined ETA/B antagonists reduced proteinuria and glomerular permeability and restored glomerular filtration barrier component integrity, but only ETA-selective blockade had anti-inflammatory and antifibrotic effects. We conclude that selective ETA antagonists are more likely to be preferred for the treatment of diabetic kidney disease.
Introduction
Diabetic nephropathy is the most common cause of end-stage renal disease in patients with diabetes mellitus. An early marker for diabetic nephropathy is the occurrence of microalbuminuria (Rosenstock and Raskin, 1986). Defects in the glomerular filtration barrier including the podocytes result in both functional and histopathological changes observed in the glomerulus of diabetic kidneys. Podocytes attach to the glomerular basement membrane (GBM) through adhesion proteins, mainly α3β1 integrins and the dystroglycan complex. The filtration slit between adjacent podocytes includes a number of cell-surface proteins including nephrin, podocalyxin, and P-cadherin, which ensure retaining of large macromolecules, such as serum albumin. In diabetic nephropathy, the etiology of podocyte injury and subsequent proteinuria is via two primary mechanisms: podocyte apoptosis and/or reduced podocyte adhesion to the GBM (Shankland, 2006). Detached cells are shed in the urine as live podocytes (Petermann et al., 2003). Loss of cell anchorage to the GBM may result from down-regulation of the α3β1 integrins (Dessapt et al., 2009) and increased levels of TGF-β1 (Chen et al., 2000).
The endothelin (ET) system plays an important role in the development of diabetic nephropathy. ETA receptors account for the majority of the vasoconstrictor and proliferative effects of ET-1 (Benigni and Remuzzi, 1995) as well as the promotion of mononuclear cell infiltration (Suzuki et al., 2001) and production of matrix proteins (Gómez-Garre et al., 1996). In contrast, ETB receptors mediate endothelial-dependent nitric oxide (NO) release (Tack et al., 1997), contribute to ET-1 clearance from plasma (Fukuroda et al., 1994), and regulate renal sodium (Na+) and water excretion in the collecting duct and other tubular segments (Kohan et al., 1992). Our laboratory recently reported that glomerular and plasma serum levels of interstitial adhesion molecule 1 (ICAM-1) and monocyte chemoattractant protein 1 (MCP-1) were increased after 6 weeks of STZ-induced diabetes (Saleh et al., 2011) with subsequent increase in macrophage infiltration into renal cortices after 10 weeks (Sasser et al., 2007). These effects were shown to be ETA receptor-dependent. Several studies have noted that ETA-selective or combined ETA/B antagonists prevent the development of diabetic nephropathy (Hocher et al., 2001). However, given the contrasting actions of these receptor subtypes in the vasculature and kidney, it remains obscure as to whether blockade of the ETB receptor in conjunction with ETA antagonism would be harmful, beneficial, or neutral for the treatment of patients with diabetic nephropathy.
Previous animal studies have examined treatment with antagonists at the time of STZ administration, thus we wanted to elucidate the effects of ET antagonist treatment after establishing diabetic nephropathy. This study addressed two basic questions. First, does administration of an ETA or combined ETA/B antagonist reverse proteinuria and the glomerular permeability defect in STZ-induced hyperglycemic (HG) rats? And second, do ETA-selective antagonists have an advantage over combined ETA/B antagonists with regard to attenuating glomerular injury and inflammatory mediators?
Materials and Methods
STZ-Induced Hyperglycemia.
Experiments used male Sprague-Dawley rats (250–275 g) from Harlan (Indianapolis, IN). All protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia and followed the American Physiological Society Guidelines for the Care and Use of Laboratory Animals. Rats were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light/dark cycle. Hyperglycemia was induced by injection of STZ (65 mg/kg; Sigma-Aldrich, St. Louis, MO), dissolved in sterile saline in the penile vein (n = 30). Onset of diabetes was confirmed by the presence of glycosuria 48 h after injection. To check the hyperglycemic state of the rats, the glucose concentrations in tail blood samples were measured with an Accu-Chek glucometer (Roche Diagnostics, Indianapolis, IN). All rats with blood glucose levels 400 mg/dl or higher were considered hyperglycemic. A group of control rats (sham) were injected with the sterile saline (n = 18) and kept under identical housing conditions with free access to water and food ad libitum. After induction of hyperglycemia, both groups of rats were kept under observation for 6 weeks before the start of 1-week treatment with endothelin antagonists. During this period, body weight, nonfasting blood glucose, and severity of diabetic-related symptoms were monitored once a week. To prevent hyperglycemic rats from dying during the observation period, those animals were treated with a low level of insulin by using time-release implants (Linplant, Scarborough, ON, Canada), whereas sham rats received the implant excipient, palmitic acid. The implants were sterilized in 2% povidone-iodine solution and inserted by a 16-gauge hypodermic needle under the dorsal skin of the neck. Every implant gradually released the insulin at a dose of approximately 1 unit per day.
Experimental Protocol.
Six weeks after induction of hyperglycemia, rats were randomly allocated to the following six experimental groups: 1) untreated nonhyperglycemic sham rats (S; n = 6), 2) sham rats treated with ETA antagonist, 2R-(4-methoxyphenyl)-4S-(1,3-benzodioxol-5-yl)-1-(N,N-di(n-butyl)aminocarbonyl-methyl)-pyrrolidine-3R-carboxylic acid (ABT-627) (atrasentan); 5 mg/kg/day (S + ABT-627; n = 6), 3) sham rats treated with mixed ETA/B receptor antagonist, A-182086; 10 mg/kg/day (S + A-182086; n = 6), 4) untreated HG rats (n = 10), 5) hyperglycemic rats treated with ABT-627, 5 mg/kg/day (HG + ABT-627; n = 10), and 6) hyperglycemic rats treated with A-182086, 10 mg/kg/d (HG + A-182086; n = 10). ABT-627 and A-182086 were kindly provided by Abbott Laboratories (Abbott Park, IL). Both drugs have been shown to produce near maximum inhibition of the pressor response to ET-1 or the precursor big ET-1 in conscious rats when administered at these doses (Wessale et al., 2002; Wu-Wong et al., 2002). Oral administration of A-182086 at 10 mg/kg completely abolished the vasodilator response to sarafotoxin 6c and significantly inhibited the pressor response as well (Wessale et al., 2002). ABT-627 and A-182086 had very high binding affinity (Ki) in Chinese hamster ovary cell preparations expressing ETA receptors at 0.034 and 0.20 nM, respectively, whereas the Ki values for ETB binding were 63.3 and 1.23 nM, respectively (Wu-Wong et al., 2002). The drugs were administered in the drinking fluid at concentrations calculated to deliver the above-mentioned doses. Because of poor water solubility of the antagonists, dilute solutions of sodium hydroxide were used to prepare a concentrated stock solution (1 g/l of 0.1 M NaOH) before being diluted into the drinking water. Daily food and water consumption were monitored, and the concentrations of drugs in the drinking water were adjusted to maintain appropriate dosing. All rats were kept in metabolic cages during the 1-week treatment for urine collection and proteinuria analysis. On the seventh day after treatment had been started, rats were anesthetized with sodium pentobarbital (50 mg/kg). Plasma samples were collected from arterial blood drawn from the abdominal aorta. The kidneys were removed for further evaluation.
Isolation of Glomeruli.
Kidneys were decapsulated and placed in phosphate-buffered saline (PBS; pH 7.4, 4°C) containing phenylmethylsulfonyl fluoride (PMSF, 1 mM). Glomeruli were isolated as described previously (Saleh et al., 2010, 2011). In brief, cortical tissue was minced and then passed through a 180-μm stainless-steel sieve to separate glomeruli from tubular fragments and vasculature. The material was again filtered through a 200-μm microcellulose filter. The filtrate was passed through a smaller pore size microcellulose filter (70 μm) with glomeruli being retained on the top. Glomeruli were then washed with ice-cold PBS/PMSF, and decapsulated glomeruli were resuspended in ice-cold PBS buffer. Tubular contamination was always confirmed at less than 5% of total tissue under light microscopy. The glomeruli were washed two more times and resuspended in PBS before the final pellet was snap-frozen in liquid nitrogen and stored at −80°C.
For immunoassays and Western blotting, the glomeruli were resuspended in lysis buffer (20 mM HEPES, pH 7.4, 10 mM NaCl, 5 mM EDTA, 0.2% Triton X-100, 10 mM sodium fluoride, 1 mM sodium ortho-vanadate, 1 mM PMSF, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) and homogenized by using an ultrasonic homogenizer (20 s). This suspension was centrifuged at 10,000g for 10 min, and the supernatant was used for immunoassays and Western blotting as well as protein determination by the Bradford method (Bio-Rad Laboratories, Hercules, CA).
For analysis of mRNA expression, quantitative real-time polymerase chain reaction (RT-PCR) was conducted using frozen glomeruli that were first processed for RNA extraction using a QIAGEN RNeasy RNA isolation kit and QIAshredder homogenizer columns (QIAGEN, Valencia, CA).
Measurements and Calculation of Glomerular Permeability.
Palb was determined in isolated glomeruli without freezing. Glomeruli were resuspended at room temperature in 5% bovine serum albumin containing 115 mM NaCl, 5 mM KCl, 10 mM sodium acetate, 1.2 mM dibasic sodium phosphate, 25 mM sodium bicarbonate, 1.2 mM magnesium sulfate, 1 mM calcium chloride, and 3.5 mM glucose, pH 7.4.
The theory and detailed method for determining albumin permeability has been described previously (Saleh et al., 2010, 2011). In brief, images of individual glomeruli (usually 5–10 glomeruli per rat) were taken through an inverted microscope equipped with a digital camera before and after changing the standard media (described above) to one containing 1% bovine serum albumin. The change in media creates an oncotic gradient across the filtration barrier to produce a change in glomerular volume [ΔV = (Vfinal − Vinitial)/Vinitial] that was analyzed off-line (Digimizer; MedCalc Software, Mariakerke, Belgium). The program calculated the average glomerular radius to then calculate glomerular volume (V = 4/3 π r3). The change in volume (ΔV) was used to calculate the albumin reflection coefficient (σalb) by the following equation: (σalb)experimental = (ΔV)experimental/(ΔV)control.
The σalb of the control glomeruli was set at 1.0. Palb is defined as (1 − σalb) and is an index of albumin movement across the filtration barrier relative to water. A σalb of zero indicates albumin movement at the same velocity as water such that Palb is 1.0. When σalb is 1.0, albumin cannot cross the filtration barrier down its concentration gradient, so Palb is zero.
Biochemical Analyses.
Commercially available kits for sICAM-1 (Quantikine, sICAM-1 Immunoassay; R&D Systems, Minneapolis, MN) and MCP-1 (RayBioTech, Inc., Norcross, GA) were used for determining concentrations of these two cytokines in plasma and glomerular homogenates. Nephrin concentration was determined in urine with an enzyme-linked immunosorbent assay kit (Excoell, Philadelphia, PA). Urinary protein concentrations were determined using the Bradford method (Bio-Rad Laboratories). Glomerular active TGF-β1 concentrations were measured with the Quantikine mouse/rat/porcine/canine TGF-β1 kit (R&D Systems). Total MMP activity was determined in the same supernatant as that for collagen assay using the SensoLyteTM 520 Generic MMP assay kit (AnaSpec Inc., San Jose, CA).
Western Blotting.
Glomeruli were homogenized as described above with total protein determined by the Bradford method (Bio-Rad Laboratories). Samples (20 μg) were assessed by standard SDS-polyacrylamide gel electrophoresis followed by blotting to polyvinylidene difluoride membranes as described previously (Foster et al., 2009). All blots were incubated overnight with the following primary antibodies: integrin α3 antibody (goat polyclonal IgG) or integrin β1 antibody (rabbit polyclonal IgG). Both antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Blots were then developed for 1 h using a secondary antibody tagged with infrared dye 680 (AlexaFluor 680 anti-rabbit or anti-mouse IgG; Invitrogen, Carlsbald, CA). To normalize all proteins, blots were then double-labeled by overnight incubation with monoclonal anti-β-actin antibody (Sigma-Aldrich) and redeveloped for 1 h with the secondary antibody tagged with infrared dye 800 (Rockland Immunochemicals, Gilbertsville, PA). Densitometry was performed on the Odyssey Infrared Imaging System version 3.0 (LI-COR Biosciences, Lincoln, NE).
Quantitative RT-PCR.
Total RNA concentration and purity were determined using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) via measuring absorbance at 260 nm (A260) and the ratio of A260 to A280, respectively. RNA (1 μg) was reverse-transcribed using the QuantiTect RT kit (QIAGEN). A dilution of the resulting cDNA was used to quantify the relative content of mRNA by RT-PCR (StepOnePlus Real-Time PCR System; Applied Biosystems, Foster City, CA) using commercially available QuantiTect primer assays (QIAGEN) to detect rat GAPDH, prepro-ET-1, ETA, ETB, nephrin, zonula occludens-1 (ZO-1), and podocin with SYBR green as the fluorescent probe. Fluorescence data were acquired at the end of extension. A melt analysis was run for all products to determine the specificity of the amplification. The cycle threshold (CT) values for each gene were measured and calculated automatically by Applied Biosystems software. Expression of each target gene mRNA relative to GAPDH was calculated on the basis of the change in CT, in which dCT = CT,target − CT,GAPDH, and normalized between the control group and corresponding treatment group and expressed as −(ddCT). With this method, an mRNA that is expressed at a greater level in the experimental than in the control group will have a negative ddCT value and a positive −(ddCT) value. The relative fold expression was calculated as 2−(ddCT).
Statistical Analyses.
All data are presented as mean ± S.E. Differences between data obtained from sham, sham + ABT-627, sham + A-182086, HG, HG + ABT-627, and HG + A-182086 are compared using two-way analysis of variance followed by Bonferroni post hoc tests. p < 0.05 was considered statistically significant. Analyses were performed using Prism version 5.0 software (GraphPad Software Inc., San Diego, CA).
Results
Metabolic Characteristics.
As depicted in Table 1, after 6 weeks of hyperglycemia (referred to as pretreatment), rats had significant lower body weights and elevated nonfasting glucose levels, food consumption, water intake, and urine flow compared with sham groups. One-week treatment with either ABT-627 or A-182086 did not change of any of these characteristics.
TABLE 1.
Characteristics of experimental animals after 6 weeks of STZ-induced diabetes (pretreatment) and after 1 week of treatment with ET receptor antagonists (post-treatment)
Data are means ± S.E.M. (n = 6 in sham and sham-treated groups and n = 10 in HG and HG-treated groups). Urine flow data were derived from 24-h urine collections in metabolic cages.
| Body Weight |
Glycemia |
Food Intake |
Water Intake |
Urine Flow |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Post-Treatment | Pretreatment | Post-Treatment | Pretreatment | Post-Treatment | Pretreatment | Post-Treatment | Pretreatment | Post-Treatment | |
| g | mg/dl | g | ml | |||||||
| Sham | 369 ± 1 | 380 ± 1 | 102 ± 8 | 109 ± 5 | 25 ± 2 | 33 ± 2 | 32 ± 1 | 28 ± 3 | 14 ± 1 | 18 ± 1 |
| Sham + ABT-672 | 369 ± 1 | 382 ± 2 | 95 ± 4 | 106 ± 3 | 27 ± 1 | 30 ± 2 | 34 ± 3 | 32 ± 2 | 15 ± 1 | 20 ± 1 |
| Sham + A-182086 | 370 ± 2 | 386 ± 2 | 98 ± 1 | 108 ± 2 | 25 ± 1 | 28 ± 2 | 31 ± 3 | 29 ± 3 | 14 ± 1 | 20 ± 1 |
| HG | 320 ± 4* | 326 ± 3* | 569 ± 9* | 566 ± 16* | 48 ± 2* | 54 ± 2* | 240 ± 3* | 235 ± 3* | 220 ± 3* | 222 ± 4* |
| HG + ABT-627 | 319 ± 3 | 323 ± 2 | 567 ± 11 | 594 ± 4 | 52 ± 2 | 53 ± 2 | 247 ± 3 | 237 ± 3 | 221 ± 4 | 213 ± 2 |
| HG + A-182086 | 312 ± 2 | 317 ± 2 | 577 ± 6 | 589 ± 3 | 56 ± 2 | 51 ± 2 | 246 ± 4 | 234 ± 3 | 205 ± 4 | 213 ± 4 |
p < 0.05 versus sham group.
Proteinuria.
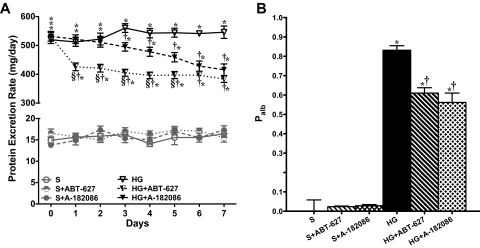
To investigate the pathophysiological relevance of endothelin in incipient diabetic nephropathy, we examined the effects of relatively short-term (1 week) treatment with ET antagonists on urinary protein excretion in rats with established hyperglycemia. As illustrated in Fig. 1A, after 6 weeks of hyperglycemia, rats had very high protein excretion rates (≈ 530 mg/day) compared with sham groups (≈15 mg/day). Figure 1A depicts protein excretion over the course of the 1-week treatment period (weeks 6–7) in untreated/treated sham and hyperglycemic groups. ET antagonists had no effect on proteinuria in sham groups. After only 1 day of treatment, ABT-627 produced a significant decrease in proteinuria compared with the corresponding values in untreated hyperglycemic rats (426 ± 13 versus 512 ± 15 mg/day). In contrast, A-182086 had no effect on proteinuria at day 1 (521 ± 15 versus 512 ± 15 mg/day). During the 1-week treatment, ABT-627 did not produce any additional decrease in proteinuria compared with day 1. However, A-182086 produced a gradual decrease in protein excretion level in hyperglycemic rats. On day 7, both ABT-627- and A-182086-treated hyperglycemic groups had similar decreases in proteinuria compared with the hyperglycemic untreated group (HG + ABT-627, 384 ± 12; HG + A-182086, 416 ± 20 versus HG, 546 ± 22 mg/day).
Fig. 1.
Proteinuria (A) and glomerular Palb (B) increase significantly after 6 weeks of hyperglycemic induction. Both ET receptor antagonists significantly reduced proteinuria and Palb after 1 week of treatment.
Palb Ex Vivo and In Vitro.
Palb was increased to 0.83 after 7 weeks of hyperglycemia (Fig. 1B). One-week treatment with both ABT-627 and A-182086 markedly reduced Palb in hyperglycemic rats. Neither antagonist had any effect on Palb in sham-treated rats. Palb was closely correlated to proteinuria (r = 0.9959, p < 0.0001).
Podocyte Markers.
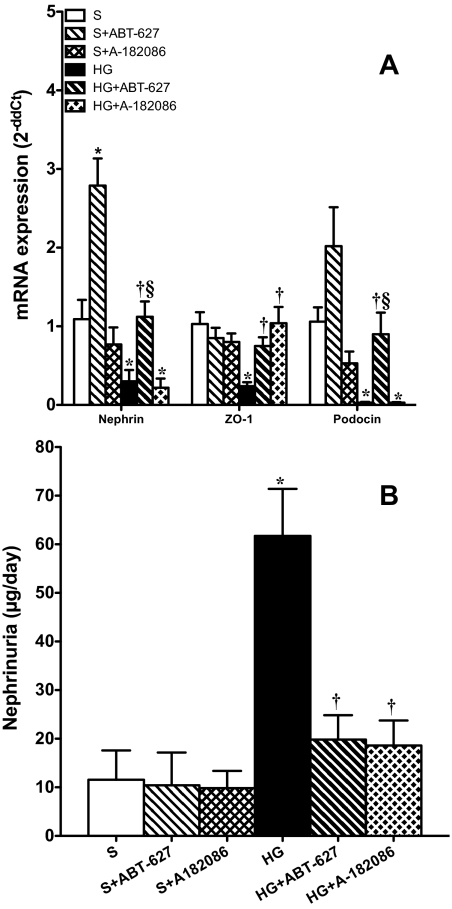
Nephrin, ZO-1, and podocin gene expression in isolated glomeruli was significantly decreased at 7 weeks in hyperglycemic rats compared with sham as quantified by RT-PCR. Treatment of hyperglycemic rats with ABT-627 significantly increased the expression of all of these podocyte-marker genes. We were surprised to find that ABT-627 also increased the gene expression of nephrin in sham rats. A-182086 significantly increased ZO-1 gene in the HG + A-182086 group without any effect on the other two podocyte-marker genes (Fig. 2A). Figure 2B illustrates the 24-h nephrin excretion rate at the 7-week time point, which was significantly increased in hyperglycemic rats compared with shams (61.7 ± 9.7 versus 11.6 ± 6.0 ng/day; p < 0.05). Rats treated for 1 week with either ABT-627 or A-182086 restored urinary nephrin levels to sham levels (19.8 ± 5.0 and 18.6 ± 5.6 ng/day, respectively).
Fig. 2.
A, after 7 weeks of hyperglycemia, gene expression of nephrin, ZO-1, and podocin in glomeruli was significantly attenuated. Both ET-1 antagonists significantly increased the gene expression of these proteins at different levels in the HG-treated groups. *, p < 0.05 versus sham; †, p < 0.05 versus HG; §, p < 0.05 versus HG + A-182086. B, 7-week hyperglycemic rats exhibited significant increases in nephrin excretion compared with sham rats. Both ET receptor antagonists reduced nephrinuria to its control nonhyperglycemic levels. *, p < 0.05 versus sham; †, p < 0.05 versus HG.
α3β1 Integrin Expression.
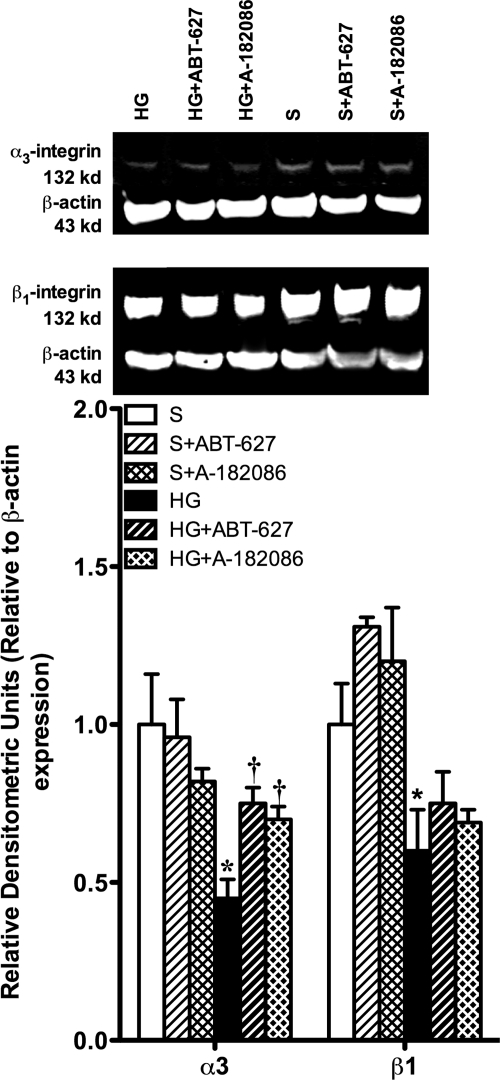
Under baseline conditions, a single band (132 kDa) was detected for both α3 and β1 integrins (Fig. 3). Both α3 and β1 integrins expression was significantly down-regulated as a result of hyperglycemia (p < 0.05). Treatment with ABT-627 or A-182086 resulted in a significant increase in the α3 integrin compared with hyperglycemic controls. However, neither ET antagonist affected the glomerular expression of β1 integrin.
Fig. 3.
Seven-week hyperglycemia decreased the expression of both α3 and β1 integrins, which attach the podocytes to the GBM. Both ET receptor antagonists significantly increased the expression of α3 integrin compared with the control hyperglycemic glomerular levels; however, neither ET receptor antagonist had an effect on the expression of β1 integrin. *, p < 0.05 versus sham; †, p < 0.05 versus HG.
MMP Activity and Active TGF-β1 Content.
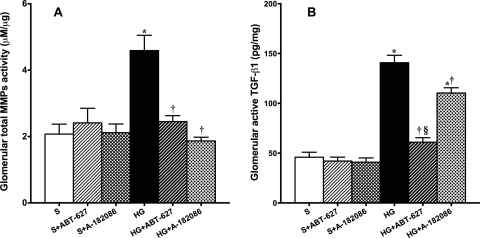
Hyperglycemic rats displayed 122 and 207% increases in total glomerular MMPs (Fig. 4A) and active TGF-β1 (Fig. 4B) content compared with sham rats. Both ABT-627 and A-182086 significantly reduced total MMP activity and restored them to sham levels. ABT-627, but not A-182086, prevented the increase in active TGF-β1 content; however, the levels of active TGF-β1 remained significantly higher in HG + A182086 compared with levels from both sham and HG + ABT-627 rats.
Fig. 4.
Hyperglycemia induction increased both total MMP activity (A) and active TGF-β1 (B) in glomeruli after 7 weeks of established hyperglycemia. Both ET receptor antagonists significantly reduced these changes. However, the attenuation effect of the mixed antagonist on active TGF-β1 remained significantly higher compared with ETA receptor-selective blockade. *, p < 0.05 versus sham; †, p < 0.05 versus HG; §, p < 0.05 versus HG + ABT-627.
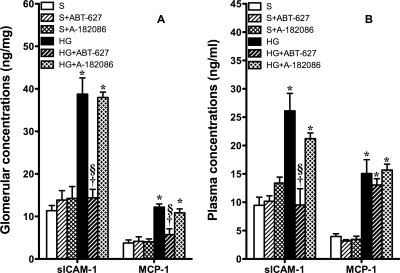
Glomerular and Systemic Inflammation.
ICAM-1 and MCP-1 are an important adhesion molecule and inflammatory chemokine, respectively, that have been documented to participate in the onset and development of diabetic nephropathy (Wu et al., 2006). Glomerular content (Fig. 5A) and plasma concentrations (Fig. 5B) of ICAM-1 and MCP-1 were increased significantly after 7 weeks of established hyperglycemia. Treatment of hyperglycemic rats for 1 week with ABT-627 completely abolished the increase in glomerular sICAM-1 and MCP-1 and plasma sICAM-1 concentration. A-182086 had no effect on the elevated levels of glomerular sICAM-1 and MCP-1 and plasma sICAM-1 concentration in hyperglycemic rats. Neither antagonist produced any change in plasma MCP-1 concentrations.
Fig. 5.
A, glomerular sICAM-1 and MCP-1 were significantly increased after 7 weeks of established hyperglycemia. Selective ETA receptor antagonism significantly reduced the elevated inflammatory protein levels. Nonselective ET receptor antagonists did not have any effect on glomerular sICAM-1 and MCP-1, indicating the counteracting effects of the ETA receptor and ETB receptor in hyperglycemia-induced inflammation. B, plasma concentrations of inflammatory molecules, sICAM-1 and MCP-1, were significantly increased after 7 weeks of established hyperglycemia. Selective ETA receptor antagonism significantly reduced the elevated sICAM-1 but not MCP-1 in HG rats. Nonselective ET receptor antagonists did not have any effect on plasma sICAM-1 (or MCP-1), suggesting a counteracting effect of the ETA receptor and ETB receptor in hyperglycemia-induced inflammation. *, p < 0.05 versus sham; †, p < 0.05 versus HG; §, p < 0.05 versus HG + ABT-627.
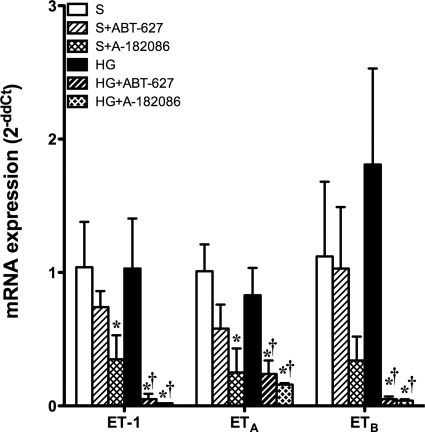
Glomerular Expression of ET Pathway Genes.
Seven weeks of hyperglycemia did not evoke any change in glomerular prepro-ET-1, ETA, or ETB mRNA levels compared with their corresponding shams. However, 1-week treatment with ETA or ETA/B antagonist produced a significant reduction in glomerular expression of all ET pathway genes in hyperglycemic rats. In sham groups, treatment with A-182086, but not ABT-627, resulted in a significant decrease in prepro-ET-1 and ETA gene expression with no effect on ETB expression (Fig. 6).
Fig. 6.
Glomerular gene expression of ET-1, ETA receptor, and ETB receptor did not change after 7 weeks of hyperglycemia. Treatment with either antagonist, ABT-627 or A-182086, significantly suppressed gene expression of all ET-1 system components: ET-1, ETA receptor, and ETB receptor. However, the one exception was that A-182086 significantly decreased ET-1 and ETA receptor gene expression compared with the sham. *, p < 0.05 versus sham; †, p < 0.05 versus HG.
Discussion
This study provides further evidence that the endothelin pathway initiates a functional glomerular defect and injury in a type 1 diabetes animal model. An important new finding is that ETA-selective or mixed ETA/B antagonists can produce rapid reversal of established hyperglycemia-induced changes in the glomerular filtration barrier. Our experimental protocol was designed to provide clinical relevance by determining whether proteinuria and increased glomerular Palb could be reversed even after diabetes was established. Using an ex vivo method that eliminates the influence of glomerular capillary pressure and flow dynamics, we demonstrated that the ability of ETA or ETA/B antagonists to improve glomerular permeability to albumin after hyperglycemia was similar. These data suggest that the ETA receptor contributes to the glomerular defect seen in diabetes, whereas the ETB receptor has little role, if any. These findings are consistent with direct in vitro measurements that ETA, but not ETB, receptor antagonists improve Palb in this model (Saleh et al., 2010). We also observed that ETA-selective antagonism had a profound anti-inflammatory effect on the diabetic kidney in contrast to the combined ETA/B antagonist. Together, these findings support the potential therapeutic advantage of selective ETA versus combined ETA/B antagonists.
We observed a difference in the rate of proteinuria reduction between the two antagonists. In a preclinical study conducted by our group, Sasser et al. (2007) showed that albuminuria increases during the first 10 weeks of STZ-induced hyperglycemia and the ETA antagonist, ABT-627, completely ameliorated albuminuria. These data are consistent with those of Hocher et al. (2001) who investigated both a selective ETA antagonist [(2S)-2-(4,6-dimethoxypyrimidin-2-yl)oxy-3-methoxy-3,3-di(phenyl)propanoic acid (LU 135252)] and a mixed ETA/B (LU 224332) antagonist (Hocher et al., 2001). In contrast, Gross et al. (2003) observed that the ETA-selective antagonist, LU 135252 (darusentan), prevented renal histological alterations in STZ-diabetic rats and significantly decreased urinary ET-1 excretion, but had no effect on albuminuria, although the reason for these different findings is unclear. Nonetheless, our current findings demonstrating a differential effect of the selective versus combined antagonists would suggest that a lack of selectivity could reduce efficacy in terms of reducing proteinuria.
In previous studies using selective ETA antagonists, blood pressure was significantly reduced along with albuminuria (Sasser et al., 2007; Gagliardini et al., 2009); so, it has been impossible to distinguish the benefits of reduced glomerular capillary pressure versus direct effects on permeability. Preclinical studies in subjects with nondiabetic chronic kidney disease showed a blood pressure-independent reduction in proteinuria in response to acute selective ETA antagonist administration (Dhaun et al., 2009). These studies support the hypothesis that ETA antagonism can reverse renal dysfunction independent of blood pressure and renal hemodynamics and suggest a direct effect on the glomerular filtration barrier structure.
The specific independent role of ETB receptors in modulating proteinuria and glomerular permeability cannot be identified using ETB-selective antagonists in vivo because pharmacological blockade of ETB receptors results in hypertension via increased ETA activity by reduced ET-1 clearance (Pollock and Pollock, 2001). The current study demonstrated a more rapid and efficient level of reduction in proteinuria with the ETA-selective blocker compared with the mixed antagonist. This is consistent with the hypothesis that the ETB receptor functions to oppose the direct role of the ETA receptor in terms of influencing the integrity of glomerular permeability and proteinuria.
The significant improvement of proteinuria progression produced by both types of antagonists was accompanied by marked restoration of glomerular filtration barrier components and function. One of the molecular mechanisms that leads to proteinuria in diabetic nephropathy is the down-regulation of podocyte- and filtration-slit molecule expression. We observed that hyperglycemia was associated with reduced expression of podocyte foot-process proteins, namely nephrin, ZO-1, and podocin in isolated glomeruli, with an increase in nephrin urinary excretion rate. We have shown previously that chronic ET-1 infusion in normoglycemic rats resulted in an ETA-dependent increase in nephrin excretion rate (Saleh et al., 2010b). In addition, serum from pre-eclamptic women contains a factor or a group of factors that stimulate the production of ET-1 from glomerular endothelial cells triggering nephrin loss from podocytes (Collino et al., 2008). Loss of podocyte attachment to glomerular basement membrane and subsequent podocyturia may result from down-regulation of the glomerular basement membrane integrin, α3β1 (Korhonen et al., 1990; Adler, 1992). Our data showed that both ABT-627 and A-182086 restore integrin α3 protein but not the β1 subunit.
We observed that both ABT-627 and A-182086 significantly reduced the diabetes-induced increases in active glomerular TGF-β, but the mixed antagonist was less effective. We have reported previously that this model of hyperglycemia does not have any overt fibrosis as detected by histological staining (Sasser et al., 2007); so, we have relied on early markers of fibrosis such as TGF-β. Inhibition of TGF-β prevents fibrosis under experimental diabetic conditions (Sharma et al., 1996; Chen et al., 2003). Several previous studies reported antifibrotic properties of long-term treatment with either selective ETA or nonselective ETA/B antagonists (Nakamura et al., 1995; Hocher et al., 2001). We reported previously that ABT-627 prevents increases in TGF-β in this model (Sasser et al., 2007). Prior evidence also suggests that ETB receptors may possess antifibrotic action via endothelial NO synthase-derived NO signaling after inhibition of TGF-β (Dreieicher et al., 2009); so, the observation that the combined ETA/B antagonist was less effective was perhaps predictable. However, comparison of the two types of antagonists on measures of glomerular fibrosis in our studies was somewhat inconclusive because both ETA and ETA/B antagonists normalized activity of glomerular MMPs. Overexpression of glomerular TGF-β1 in diabetes is associated with increased activity of MMPs, mainly gelatinases (MMP-2 and MMP-9) that contribute to glomerular basement membrane thickening (Krag et al., 2007).
We recently reported that chronic ET-1 infusion in the rat increases glomerular and renal inflammation independent of hypertension (Saleh et al., 2010). Furthermore, we have also reported that selective ETA blockade in the STZ-diabetic rat prevents the increase in early markers of inflammation, ICAM-1 and MCP-1 (Saleh et al., 2011). The current study extends these findings to demonstrate that we could reverse the diabetes-induced increase in inflammation, but only with an ETA-selective antagonist and not the combined blocker. These findings suggest that the ETB receptor functions as an anti-inflammatory factor and that targeting the endothelin pathway for treatment of diabetes should be restricted to ETA-selective compounds.
The general beneficial effects of both ETA-selective and combined receptor antagonism have been demonstrated in a wide variety of studies including our own. However, in terms of systemic and glomerular inflammation, ETB receptor antagonism seems to counteract the benefit of ETA blockade. ETB receptors are involved in the synthesis of the vasodilator NO by endothelial cells, through activation by either ET-1 or ET-3 (Namiki et al., 1992). Inhibition of NO has also been associated with an increase in leukocyte adhesion to mesenteric venules, reflecting that the increases in monocyte/macrophage infiltration are caused by decreases in NO production (Dubey et al., 1996). Another possible explanation for the nonbeneficial effect of ETA/B blockade is the involvement of the ETB receptor in the clearance of ET (Fukuroda et al., 1994). The nonselective NOS inhibitor, Nω-nitro-l-arginine methyl ester, exacerbates liver and kidney injury accompanied with increased leukocyte infiltration in animal models of endotoxemia (Saetre et al., 2001). Part of these effects has been attributed to increased adhesion molecule expression (ICAM-1, P-selectin) after inhibition of endogenous NO production. In addition, mice lacking endothelial NO synthase aggravate anti-GBM glomerulonephritis, indicating a protective role of endogenous NO during renal inflammation (Heeringa et al., 2000).
We did not observe any changes in mRNA expression of glomerular ET-1, ETA, and ETB receptors after 7 weeks of hyperglycemia; however, treatment with an ETA or ETA/B antagonist produced marked decreases in glomerular expression of each of these genes. A previous study from another lab reported that glomerular ET-1 mRNA levels were increased in kidneys from STZ-treated rats, whereas the mRNA levels for ETA and ETB receptors remained unchanged (Fukui et al., 1993). Of course, mRNA levels do not always reflect protein expression and function. On the contrary, Shin et al. (1995) reported that moderate hyperglycemia in diabetic rats is associated with a reduction in renal ET-1 mRNA levels early after the induction of diabetes; however, plasma ET-1 levels were not affected. These data demonstrated that the intrarenal ET-1 system may be affected independently of the systemic ET-1 system.
Factors such as hyperglycemia (Yamauchi et al., 1990), shear stress (Hocher et al., 1997) caused by glomerular hyperfiltration, and urine flow (Hocher et al., 1998) have been shown to stimulate ET-1 synthesis or release. Glucose stimulates also ET-1 synthesis in vitro. Studies examining systemic and intrarenal ET-1 in diabetes have yielded conflicting results. Plasma ET-1 levels have been described as either undetectable (Takahashi et al., 1991), unchanged (Shin et al., 1995), enhanced (Nakamura et al., 1995; Hocher et al., 1998), or suppressed (Hu et al., 1993), and renal ET-1 levels have been shown to be unchanged (Takahashi et al., 1991), enhanced (Fukui et al., 1993), or reduced (Shin et al., 1995). Accordingly, it is suggested that these changes are caused by differences in the diabetic state, and these differences may be caused by the degree of hyperglycemia, renal localization, or varying duration of diabetes. Glomerular ET-1 mRNA levels have been reported to increase with progression of diabetic nephropathy in STZ-diabetic rats, whereas the mRNA levels for ETA and ETB do not change in diabetes (Fukui et al., 1993). Of course, mRNA levels do not always reflect protein expression and function. On the contrary, early after the induction of diabetes, renal ET-1 mRNA and protein expression have been reported to be reduced and plasma ET-1 levels were unchanged, implying that the intrarenal ET-1 system may be affected independently of the systemic ET-1 system (Shin et al., 1995).
From a clinical perspective, both ETA and ETA/B antagonists would seem to have beneficial effects for reducing proteinuria in the long term. However, the ETA-selective antagonist was significantly more effective than the combined antagonist at reducing TGF-β and inflammatory mediators in the model of type 1 diabetes. Our data suggest an antifibrotic and anti-inflammatory role for the ETB receptor, thus providing rationale for use of ETA-selective rather than mixed ETA/B antagonists for treatment of diabetic nephropathy and perhaps other forms of proteinuric renal disease and inflammation.
Acknowledgments
We thank Dr. Kelly Hyndman and Wararat Kittikulsuth for help in teaching analytical methods.
This work was supported by the National Institutes of Health National Heart Lung and Blood Institute [Grants HL95499, HL64776, HL60653]; the American Heart Association [Predoctoral Fellowship 09PRE2050253]; and a grant from the government of Egypt.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.178988.
- GBM
- glomerular basement membrane
- ET
- endothelin
- HG
- hyperglycemic
- MCP-1
- monocyte chemoattractant protein-1
- MMP
- matrix metalloprotease
- NO
- nitric oxide
- Palb
- permeability to albumin
- ICAM-1
- intercellular adhesion molecule-1
- sICAM-1
- soluble ICAM-1
- STZ
- streptozotocin
- TGF-β
- transforming growth factor β
- ZO-1
- zonula occludens-1
- S
- sham
- PBS
- phosphate-buffered saline
- PMSF
- phenylmethylsulfonyl fluoride
- RT-PCR
- real-time polymerase chain reaction
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ABT-627
- 2R-(4-methoxyphenyl)-4S-(1,3-benzodioxol-5-yl)-1-(N,N-di(n-butyl)aminocarbonyl-methyl)-pyrrolidine-3R-carboxylic acid
- LU 135252
- (2S)-2-(4,6-dimethoxypyrimidin-2-yl)oxy-3-methoxy-3,3-di(phenyl)propanoic acid
- A-182086
- (2R,3R,4S)-4-(benzo[d][1,3]dioxol-5-yl)-2-(3-fluoro-4-methoxyphenyl)-1-(2-(N-propylpentylsulfonamido)ethyl)pyrrolidine-3-carboxylic acid hydrochloride.
Authorship Contributions
Participated in research design: Saleh, J. S. Pollock, and D. M. Pollock.
Conducted experiments: Saleh.
Performed data analysis: Saleh and D. M. Pollock.
Wrote or contributed to the writing of the manuscript: Saleh, J. S. Pollock, and D. M. Pollock.
Other: J. S. Pollock and D. M. Pollock acquired funding for the research.
References
- Adler S. (1992) Characterization of glomerular epithelial cell matrix receptors. Am J Pathol 141:571–578 [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Remuzzi G. (1995) Endothelin in the progressive renal disease of glomerulopathies. Miner Electrolyte Metab 21:283–291 [PubMed] [Google Scholar]
- Chen HC, Chen CA, Guh JY, Chang JM, Shin SJ, Lai YH. (2000) Altering expression of α3ß1 integrin on podocytes of human and rats with diabetes. Life Sci 67:2345–2353 [DOI] [PubMed] [Google Scholar]
- Chen S, Jim B, Ziyadeh FN. (2003) Diabetic nephropathy and transforming growth factor-β: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 23:532–543 [DOI] [PubMed] [Google Scholar]
- Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G. (2008) Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294:F1185–F1194 [DOI] [PubMed] [Google Scholar]
- Dessapt C, Baradez MO, Hayward A, Dei Cas A, Thomas SM, Viberti G, Gnudi L. (2009) Mechanical forces and TGFβ1 reduce podocyte adhesion through α3β1 integrin downregulation. Nephrol Dial Transplant 24:2645–2655 [DOI] [PubMed] [Google Scholar]
- Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, Webb DJ. (2009) Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension 54:113–119 [DOI] [PubMed] [Google Scholar]
- Dreieicher E, Beck KF, Lazaroski S, Boosen M, Tsalastra-Greul W, Beck M, Fleming I, Schaefer L, Pfeilschifter J. (2009) Nitric oxide inhibits glomerular TGF-β signaling via SMOC-1. J Am Soc Nephrol 20:1963–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Boegehold MA, Gillespie DG, Rosselli M. (1996) Increased nitric oxide activity in early renovascular hypertension. Am J Physiol Regul Integr Comp Physiol 270:R118–R124 [DOI] [PubMed] [Google Scholar]
- Foster JM, Carmines PK, Pollock JS. (2009) PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol 297:F471–F480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Nakamura T, Ebihara I, Osada S, Tomino Y, Masaki T, Goto K, Furuichi Y, Koide H. (1993) Gene expression for endothelins and their receptors in glomeruli of diabetic rats. J Lab Clin Med 122:149–156 [PubMed] [Google Scholar]
- Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. (1994) Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun 199:1461–1465 [DOI] [PubMed] [Google Scholar]
- Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, Conti S, Rottoli D, Longaretti L, Remuzzi A, et al. (2009) Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol 297:F1448–F1456 [DOI] [PubMed] [Google Scholar]
- Gómez-Garre D, Ruiz-Ortega M, Ortego M, Largo R, López-Armada MJ, Plaza JJ, González E, Egido J. (1996) Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension 27:885–892 [DOI] [PubMed] [Google Scholar]
- Gross ML, El-Shakmak A, Szábó A, Koch A, Kuhlmann A, Münter K, Ritz E, Amann K. (2003) ACE-inhibitors but not endothelin receptor blockers prevent podocyte loss in early diabetic nephropathy. Diabetologia 46:856–868 [DOI] [PubMed] [Google Scholar]
- Heeringa P, van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, Kallenberg CG, Jennette JC. (2000) Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am J Pathol 156:879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocher B, Lun A, Priem F, Neumayer HH, Raschack M. (1998) Renal endothelin system in diabetes: comparison of angiotensin-converting enzyme inhibition and endothelin-A antagonism. J Cardiovasc Pharmacol 31:S492–S495 [DOI] [PubMed] [Google Scholar]
- Hocher B, Schwarz A, Reinbacher D, Jacobi J, Lun A, Priem F, Bauer C, Neumayer HH, Raschack M. (2001) Effects of endothelin receptor antagonists on the progression of diabetic nephropathy. Nephron 87:161–169 [DOI] [PubMed] [Google Scholar]
- Hocher B, Thöne-Reineke C, Bauer C, Raschack M, Neumayer HH. (1997) The paracrine endothelin system: pathophysiology and implications in clinical medicine. Eur J Clin Chem Clin Biochem 35:175–189 [PubMed] [Google Scholar]
- Hu RM, Levin ER, Pedram A, Frank HJ. (1993) Insulin stimulates production and secretion of endothelin from bovine endothelial cells. Diabetes 42:351–358 [DOI] [PubMed] [Google Scholar]
- Kohan DE, Hughes AK, Perkins SL. (1992) Characterization of endothelin receptors in the inner medullary collecting duct of the rat. J Biol Chem 267:12336–12340 [PubMed] [Google Scholar]
- Korhonen M, Ylänne J, Laitinen L, Virtanen I. (1990) Distribution of β1 and β3 integrins in human fetal and adult kidney. Lab Invest 62:616–625 [PubMed] [Google Scholar]
- Krag S, Nyengaard JR, Wogensen L. (2007) Combined effects of moderately elevated blood glucose and locally produced TGF-β1 on glomerular morphology and renal collagen production. Nephrol Dial Transplant 22:2485–2496 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ebihara I, Fukui M, Tomino Y, Koide H. (1995) Effect of a specific endothelin receptor A antagonist on mRNA levels for extracellular matrix components and growth factors in diabetic glomeruli. Diabetes 44:895–899 [DOI] [PubMed] [Google Scholar]
- Namiki A, Hirata Y, Ishikawa M, Moroi M, Aikawa J, Machii K. (1992) Endothelin-1- and endothelin-3-induced vasorelaxation via common generation of endothelium-derived nitric oxide. Life Sci 50:677–682 [DOI] [PubMed] [Google Scholar]
- Petermann AT, Krofft R, Blonski M, Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J, Durvasula R, et al. (2003) Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64:1222–1231 [DOI] [PubMed] [Google Scholar]
- Pollock DM, Pollock JS. (2001) Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281:F144–F150 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Raskin P. (1986) Early diabetic nephropathy: assessment and potential therapeutic interventions. Diabetes Care 9:529–545 [DOI] [PubMed] [Google Scholar]
- Saetre T, Hovig T, Røger M, Gundersen Y, Aasen AO. (2001) Hepatocellular damage in porcine endotoxemia: beneficial effects of selective versus non-selective nitric oxide synthase inhibition? Scand J Clin Lab Invest 61:503–512 [DOI] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. (2010) Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56:942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. (2011) Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. (2007) Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ. (2006) The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69:2131–2147 [DOI] [PubMed] [Google Scholar]
- Sharma K, Jin Y, Guo J, Ziyadeh FN. (1996) Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45:522–530 [DOI] [PubMed] [Google Scholar]
- Shin SJ, Lee YJ, Lin SR, Tan MS, Lai YH, Tsai JH. (1995) Decrease of renal endothelin 1 content and gene expression in diabetic rats with moderate hyperglycemia. Nephron 70:486–493 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Lopez-Franco O, Gomez-Garre D, Tejera N, Gomez-Guerrero C, Sugaya T, Bernal R, Blanco J, Ortega L, Egido J. (2001) Renal tubulointerstitial damage caused by persistent proteinuria is attenuated in AT1-deficient mice: role of endothelin-1. Am J Pathol 159:1895–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack I, Marin Castano E, Pêcher C, Praddaude F, Bascands JL, Bompart G, Ader JL, Girolami JP. (1997) Endothelin increases NO-dependent cGMP production in isolated glomeruli but not in mesangial cells. Am J Physiol Renal Physiol 272:F31–F39 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Suda K, Lam HC, Ghatei MA, Bloom SR. (1991) Endothelin-like immunoreactivity in rat models of diabetes mellitus. J Endocrinol 130:123–127 [DOI] [PubMed] [Google Scholar]
- Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, Winn M, Jae HS, von Geldern TW, Opgenorth TJ, et al. (2002) Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci (Lond) 103 (Suppl 48):112S–117S [DOI] [PubMed] [Google Scholar]
- Wu Y, Wu G, Qi X, Lin H, Qian H, Shen J, Lin S. (2006) Protein kinase Cβ inhibitor LY333531 attenuates intercellular adhesion molecule-1 and monocyte chemotactic protein-1 expression in the kidney in diabetic rats. J Pharmacol Sci 101:335–343 [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Dixon DB, Chiou WJ, Sorensen BK, Liu G, Jae HS, Tasker A, von Geldern TW, Winn M, Opgenorth TJ. (2002) Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Clin Sci (Lond) 103 (Suppl 48):107S–111S [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Ohnaka K, Takayanagi R, Umeda F, Nawata H. (1990) Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett 267:16–18 [DOI] [PubMed] [Google Scholar]