Abstract
Qualitative urinalysis can verify abstinence of drug misuse but cannot detect changes in drug intake. For drugs with slow elimination, such as methamphetamine (MA), a single episode of abuse can result in up to 5 days of positive urine drug screens. Thus, interventions that produce substantial decreases in drug use but do not achieve almost complete abstinence are classified as ineffective. Using nonpharmacologic doses of deuterium-labeled l-methamphetamine (l-MA-d3) we have developed a simple, robust method that reliably estimates changes in MA intake. Twelve subjects were dosed with 5 mg of l-MA-d3 daily and challenged with 15, 30, and 45 mg of nonlabeled d-MA (d-MA-d0) after reaching plasma steady status of l-MA-d3. Urinary concentration ratios of d-MA-d0 to l-MA-d3 provided clear separation of the administered doses with as little as 15-mg dose increments. Administered doses could not be resolved using d-MA-d0 concentrations alone. In conclusion, the urinary [d-MA-d0]:[l-MA-d3] provides a quantitative, continuous measure of illicit MA exposure. The method reliably detects small, clinically relevant changes in illicit MA intake from random urine specimens, is amenable to deployment in clinical trials, and can be used to quantify patterns of MA abuse.
Introduction
Epidemics of methamphetamine (MA) abuse and addiction are occurring throughout the world (Schifano et al., 2007; Degenhardt et al., 2008; McKetin et al., 2008), fueled by the illicit synthesis of 197 to 624 metric tons of illicit amphetamine-like drugs per year, enough for more than 10 billion 30-mg MA doses (http://www.unodc.org/documents/wdr/WDR_2010/World_Drug_Report_2010_lo-res.pdf). Some of these abusers become the addicts who create social, health, and crime consequences that affect all levels of society (Watanabe-Galloway et al., 2009). Thus, there is a pressing need to develop treatments for MA addiction. Unfortunately, despite an intense effort over the last 20 years, no medications have been proven effective for the treatment of MA addiction (Karila et al., 2010).
Results of qualitative urine toxicology tests are the primary objective outcome measures for most antiaddiction trials, including trials for MA addiction. Urine immunoassays that are sensitive (but not specific) and inexpensive and can be deployed in the clinic are commonly used in these trials. To eliminate false-positive results drug identity is confirmed and a urine drug concentration measured using sensitive and specific assay methods that always include mass spectrometry (MS). Although these methods yield precise and accurate urine concentrations, several factors, including age, hydration status, urine pH, and urine flow, all make back-extrapolation from urine concentration to the quantity of drug abused difficult, if not impossible. As a consequence, the results of urine drug tests are only scored as a time series of binary outcomes of “positive” or “negative.”
Abstinence is the goal of addiction treatments, and qualitative urine toxicology is exceedingly sensitive for detecting drug use in usually abstinent individuals. However, it is not sensitive in detecting either reductions or brief periods (up to 2–3 days) of abstinence in individuals. Thus, extremely large reductions in abuse (perhaps up to 90%) are needed before even a modest reduction in urinalysis-positive results will be evident and the treatment will be accepted as effective (National Institute on Drug Abuse/College on Problems of Drug Dependence, 1999). This degree of stringency may be the reason for failure of all treatments for MA addiction tested to date. If new treatments for stimulant abuse are unlikely to yield sudden, total abstinence, then qualitative methods that are unable to measure less than total abstinence are not likely to be useful in selecting drug or other treatment candidates that may decrease but not eliminate illicit intake. Considering the growing list of failed trials for MA dependence, developing methods that allow nonbinary continuous estimation of drug intake has become essential.
To determine illicit intake we have been testing the utility of giving small, pharmacologically inactive oral doses of deuterium-labeled drugs or metabolites that have a pharmacokinetic profiles similar to the abused drug of interest. We then determine urinary concentration ratios of unlabeled (illicit and self-administered) to deuterium-labeled drug (or metabolite) to arrive at an estimate of intake and exposure to the addictive drug. The method is analogous to using an internal standard in analytic chemistry.
In this article we present laboratory validation of a method for quantitatively estimating exposure to MA. When used in a clinical trial this method changes a binary to a continuous measure and will allow evaluation of partial efficacy of a putative treatment. To assess MA intake we used trideuterated l-MA with deuterium labeling on the methyl group. In prior work we have shown that this level of deuteration does not alter the pharmacology of MA in humans (Harris et al., 2003). l-MA [also notated R-(−)-MA] is the less pharmacologically active isomer compared with d-MA [also notated S-(+)-MA]. In work leading to this study we established that 5-mg oral doses of l-MA are completely absorbed, have no measurable subjective or cardiovascular effects, and are easily detected in urine (Li et al., 2010).
Materials and Methods
Subjects.
Twelve healthy, nondependent, MA-using subjects (eight men, four women; mean age 31 ± 10 years; mean weight 72 ± 13 kg; 83% white) participated in this study. To be included subjects had to have used MA for at least 1 year with more than 20 lifetime exposures but not be MA-dependent by criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Participants were in good health as judged by medical examination, laboratory tests (including hematologic, hepatic, and renal serum chemistries), urinalysis, and ECG. The study was approved by the California Pacific Medical Center and University of California, San Francisco institutional review boards. The study was carried out in accordance with the Declaration of Helsinki.
Study Design.
A fixed-sequence, open-label design with sequential outpatient-inpatient phases was used. Oral doses of 5 mg of l-MA-d3 were administered for 14 days. On days 1 to 7 subjects were outpatients. During this period, a single oral dose of 5-mg l-MA-d3 was administered every morning under direct supervision. Subjects were admitted to the research ward on day 7. On study days 8, 10, and 12, a series of ascending intravenous d-MA-d0 doses of 15, 30, and 45 mg were given. Each d-MA-d0 dose was administered over 1 min under infusion pump control (Harvard Apparatus Inc., Holliston, MA). The 15-mg dose was given as a single infusion. The 30-mg dose was given as two 15-mg infusions with doses separated by 1 h. The 45-mg dose was given as five 9-mg infusions each separated by 1 h. This pattern was designed to simulate a d-MA binge.
Before each outpatient l-MA-d3 dose pharmacodynamic effects were assessed. Subjects were monitored for 1 h after dosing and had Visual Analog Scale measures and vital signs measured before discharge. During the inpatient phase, vital signs and subjective-effect measures were obtained frequently. During infusions subjective and cardiovascular measures were obtained before and 15 min after each infusion and at 0.5, 1, 1.5, 2, 4 8, 24, and 48 h after the last infusion.
Blood Collection.
Venous blood samples (approximately 7 ml) were obtained using sterile techniques from an indwelling intravenous catheter. During the outpatient phase trough plasma levels were obtained before dosing. On infusion days plasma samples for d-MA-d0 and l-MA-d3 levels were obtained before and at 0.25, 0.5, 1, 2, 4, 8, 24, and 48 h after dosing. For the 30- and 45-mg doses, additional plasma samples were obtained immediately before and 15 min after each infusion and at 0.5, 1, 2, 4, 8, 24, and 48 h after the last dose.
Urine Collection.
During the inpatient phase subjects voided as needed. All voided urine was collected with time, volume, and urine pH of each individual sample recorded.
Bioassay.
d-MA-d0 and l-MA-d3 in plasma and urine were measured by combined gas chromatography (GC)-MS, using dl-MA-d9 as the internal standard. The analytes were extracted from the respective biofluids, converted to the trifluoroacetyl amide derivatives, separated by gas chromatography on a Restek Rtx-200 MS analytical column (Restek, Bellefonte, PA), and detected by mass spectrometry operated in the chemical ionization mode, using isobutane as the reagent gas. The molecular ion species (M + H)+, m/z 246, 249, and 255, were monitored for the trifluoroacetyl amides of MA-d0, MA-d3, and MA-d9, respectively. Interday accuracy for the measurement of MA-d0 and MA-d3 in urine ranged from 108 to 109%, respectively, at the 5 ng/ml limit of quantitation, and from 100 and 105%, respectively, at 2500 ng/ml. The respective coefficients of variation were 12 and 7.5% at the limit of quantitation and 4.5 and 5.3% at 2500 ng/ml. In all cases MA-d3 could easily be quantified against a background of MA-d0.
Pharmacokinetic Analysis.
Pharmacokinetic data for d-MA-d0 and l-MA-d3 were analyzed using nonlinear mixed-effect models implemented using the program NONMEM (version 7; NONMEM Project Group, University of California, San Francisco). A population pharmacokinetic model (based on complete data from 12 subjects) of oral repeated l-MA-d3 dosing indicates that pharmacokinetic steady state reached within 5 days of daily oral doses of 5 mg of l-MA-d3. The full model will be presented in a separate article.
Urinary Data Analysis.
The unlabeled-to-labeled MA urine concentration ratio, which we formally notate as [d-MA-d0]:[l-MA-d3], was determined for each collected urinary sample. Linear discriminant analysis was used to test whether [d-MA-d0]:[l-MA-d3]differentiated between administered doses of d-MA-d0. Classifier accuracy was evaluated by subject-based leave-one-out cross-validation. Each subject's data were classified based on a training set consisting of the other subject's data. Because of the incomplete systemic distribution of MA, urine specimens collected within the first 5 h of d-MA-d0 dosing were not used to train the classifier but were used as test data. A separate analysis was conducted for urine specimens collected more than 24 h after dosing, because the concentration ratio is affected by continued daily oral l-MA-d3 administration at 24 h. McNemar's test was used to compare accuracy between classification methods. A linear regression model was used to describe the relationship between urinary [d-MA-d0]:[l-MA-d3] and the corresponding MA dose. Prediction bands of 95% were calculated to reflect the uncertainty about future observations and indicate the distribution within which 95% of future observations are expected to fall (Dalgaard, 2008). All computations were performed using R.
Results
Safety and Tolerability.
All MA doses tested were well tolerated, and no serious adverse events occurred. There were no measurable pharmacodynamic effects after any of the l-MA-d3 doses; d-MA produced expected increases in heart rate, blood pressure, and subjective effects.
Urinary Concentration Ratio, [d-MA-d0]:[l-MA-d3].
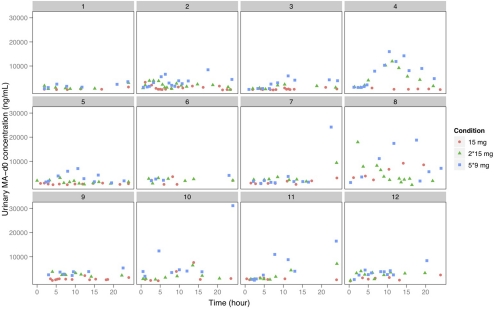
A total of 589 urine samples were collected; 331 between 0 and 24 h and 238 between 24 and 48 h after doses of d-MA-d0. In Fig. 1 we show urine d-MA-d0 concentrations plotted against time. Here, the urine concentrations after the three doses of d-MA-d0 (analogous to increasing amounts of illicit intake) overlap substantially and cannot be separated by dose. This finding is consistent with a previous study in MA addicts presenting for treatment where MA urine concentrations varied from undetectable to 300,000 ng/ml. Despite a concentration range spanning six orders of magnitude, MA urine concentrations did not allow prediction of the amount of illicit intake (Batki et al., 2000).
Fig. 1.
Urine d-MA-d0 concentrations after 15, 30, and 45 mg of d-MA-d0 by individual subject.
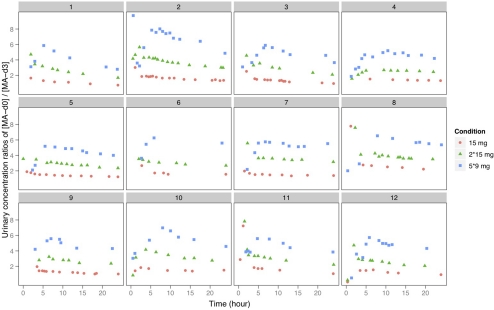
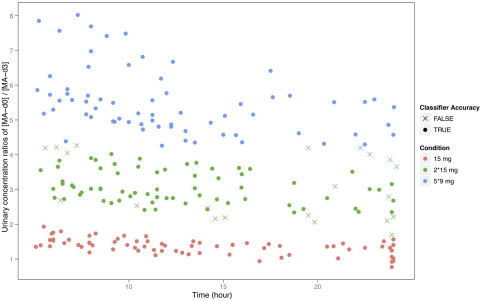
In Fig. 2 we present the urinary [d-MA-d0]:[l-MA-d3] plotted against time. Now dose-dependent increases can easily be visually discriminated. Visual (and statistical) discrimination is particularly evident at times more than 5 h after the first d-MA-d0 dose. As described above, classification methods based on the dependent variables of time, urine [d-MA-d0], or urine [d-MA-d0]:[l-MA-d3] as predictors were developed. The overall accuracy and sensitivity/specificity for each dose condition derived from each classification method are summarized in Table 1. For urine samples collected from 5 h after d-MA-d0 dosing through the next l-MA-d3 dose, the classification accuracy was 91% using urine [d-MA-d0]:[l-MA-d3], which was a significant (p < 0.001) improvement over the 54% accuracy using urine [d-MA-d0] alone. Classification based on both urine [d-MA-d0]:[l-MA-d3] and time since dosing further improved accuracy to 96% (p < 0.001), and this is displayed in Fig. 3. From 24 to 48 h classifier accuracy using the urine [d-MA-d0]:[l-MA-d3] fell to 60.0% for 15-mg dose differences, but if the analysis was restricted to 30-mg dose increments accuracy remained robust at 84.6% and was a significant improvement over the 72.8% accuracy obtained using urine [d-MA-d0] alone (p < 0.01). From 24 to 48 h, including time as a predictor did not significantly improve classification accuracy (83.4%; p = 0.77).
Fig. 2.
Urine concentration ratio, [d-MA-d0]:[l-MA-d3], after 5 mg of l-MA-d3 and 15, 30, and 45 mg of d-MA-d0 by individual subject.
TABLE 1.
Performance of different classification methods
| Time Period | Predictors | Overall Accuracy | Sensitivity |
Specificity |
||||
|---|---|---|---|---|---|---|---|---|
| 15 mg | 30 mg | 45 mg | 15 mg | 30 mg | 45 mg | |||
| h | % | % | % | % | % | % | % | |
| 5–24 | [d-MA-d0] | 54 | 76 | 50 | 36 | 75 | 66 | 90 |
| 5–24 | [d-MA-d0]:[l-MA-d3] | 91 | 95 | 87 | 91 | 96 | 93 | 97 |
| 5–24 | [d-MA-d0]:[l-MA-d3], time | 96 | 95 | 97 | 95 | 99 | 95 | 100 |
| 24–48 | [d-MA-d0]:[l-MA-d3] | 60 | 78 | 39 | 63 | 73 | 74 | 92 |
| 24–48 (15 vs. 45 mg) | [d-MA-d0] | 73 | 96 | 46 | 46 | 96 | ||
| 24–48 (15 vs. 45 mg) | [d-MA-d0]:[l-MA-d3] | 85 | 98 | 69 | 69 | 98 | ||
| 24–48 (15 vs. 45 mg) | [d-MA-d0]:[l-MA-d3], time | 83 | 96 | 69 | 69 | 96 | ||
Fig. 3.
Classification of urine samples into different dosage conditions based on the method using [d-MA-d0]:[l-MA-d3] and time since dosing as predictors.
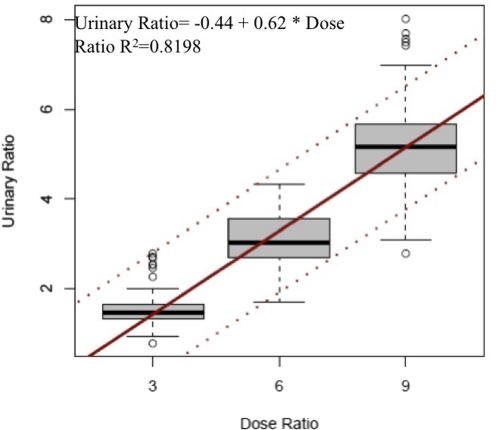
The ratio of d-MA-d0 to l-MA-d3 doses in this study were 3 (15 mg of MA-d0:5 mg of MA-d3), 6 (30 mg of MA-d0:5 mg of MA-d3), and 9 (45 mg of MA-d0:5 mg of MA-d3). When the dose ratios were treated as continuous variables instead of categorical variables, the ratios of doses were linearly related to urinary [d-MA-d0]:[l-MA-d3] (Fig. 4; urinary ratio = − 0.44 + 0.62 × dose ratio; R2 = 0.8198).
Fig. 4.
Relationships between urine [d-MA-d0]:[l-MA-d3] and d-MA-d0/l-MA-d3 dose ratio. The line within the box is the median, the area within the box contains the second and third quartiles, and whiskers include data points that fall within 1.5 times the interquartile range. The solid black line is the regression line. Dashed lines are 95% prediction bands.
Discussion
We present a simple, robust method of using pharmacologically inactive oral doses of l-MA-d3 to estimate the illicit MA amount consumed. With this method, useful estimates of MA exposure can be made from spontaneously voided urine specimens within a relatively wide time window.
For both detection and confirmation, urine toxicology tests classify a sample as positive if it contains an amount equal to or greater than the lowest concentration of the drug that can be reliably detected in the urine after a single dose. This degree of sensitivity minimizes the possibility of missing an episode of drug use (Dolan et al., 2004). However, it also minimizes the sensitivity of these tests for detecting decreases in drug taking. For example, because of its slow elimination, low concentrations of MA can be detected in urine for up to 7 days after a single oral dose of 30 mg (Valentine et al., 1995) or up to 60 h after a single 15-mg smoked or intravenous dose (Cook et al., 1993). These data suggest that the highly sensitive urine toxicology methods used in addiction trials may overdetect MA abuse, probably decreasing the ability of trials to identify effective treatments. Overdetection of abuse also exists for other abused drugs such as cocaine, amphetamine, and marijuana. For example, based on urine benzyolecgonine concentrations, Preston et al. (1997) found that a cyclical pattern of cocaine abuse was not detected using binary outcome assignments of urine results. In a study of recently incarcerated drug abusers, amphetamine remained detectable in urine for more than 48 h in all subjects; one subject had positive urine results for 9 days (Smith-Kielland et al., 1997).
Self-reports of drug use are commonly used to assess the quantity of illicit intake. These measures can be inaccurate because illicit drug abusers often consume impure, diluted drugs and use dosing methods with incomplete bioavailability (oral and nasal) or where variable amounts of drug are destroyed (i.e., pyrolysis with smoked drugs). Our method estimates the bioavailable fraction of the illicit dose, the amount associated with pharmacologic activity and toxicity.
The detection window (the length of time in days after the last use of a drug) that sequentially collected urine samples continue to produce positive drug test results can be affected by many variables. Pharmacological factors include dose, route of administration, duration of use (acute or chronic), and rate of elimination. Several factors affect elimination including age, organ function, urine pH, hydration status, and polymorphisms of drug-metabolizing enzymes (http://www.ndci.org/sites/default/files/ndci/DCR.VI__2.pdf). For example, urine acidification dramatically increases MA elimination. Because of the accumulation of drug in deep compartments, longer detection windows are more likely in chronic abusers, a group often targeted in clinical trials. Analytical factors such as the sensitivity of the test (cutoff concentration) and the method's specificity (the actual drug and/or metabolite that is being detected) can also affect the detection window (Jaffee et al., 2008). Labeled and unlabeled MA have identical absorption, distribution, metabolism, and elimination; thus our method controls for most of these intraindividual and interindividual factors.
There are two clear advantages of using urine concentration ratios of non-labeled to deuterium labeled drugs or metabolites as a quantitative endpoint in clinical trials. First, modest reductions in drug misuse can be tracked, allowing better estimates of therapeutic drug efficacy or rational selection of combination therapies. In contrast to currently available qualitative technologies urine concentration ratios yield a continuous outcome measure. Our data suggest than the urinary [d-MA-d0]:[l-MA-d3] can differentiate as little as 15-mg increases in exposure to d-MA-d0 in a wide detection window. The ratio is directly related to the total d-MA-d0 exposure without being affected by administration regimen (single or multiple) at least for samples obtained from 1 h after the last unlabeled dose. These properties make the urinary [d-MA-d0]:[l-MA-d3] an attractive biomarker of disease severity and therapeutic response that can be easily adapted for MA treatment trials. It is noteworthy that the analytic technology needed to quantify the isotopes of MA (GC-MS or liquid chromatography-MS) is already widely available and currently used to confirm qualitative results. The only change required in current analytic technologies will be use of a differently deuterated internal standard; both MA-d3 and MA-d9 are commercially available as internal standards for MA assays. Second, quantitative estimates of drug exposure will allow better stratification of the severity of illness. Other instruments that grade the severity of addiction, such as the Addiction Severity Index, primarily reflect slowly changing factors, such as employment, relationships, and legal status. Our method offers a finer temporal resolution. Logically, the severity of an addictive disorder is related to the amount of drug exposure; the ability to quantify exposure to illicit MA will permit a better assessment of the relationship between drug misuse and disease.
There are limits to our method. First, subjects need to take l-MA-d3 but may not do so. In upcoming trials we plan to coadminister l-MA-d3 with the treatment medication. Subjects with no l-MA-d3 in urine can be assumed not to be adherent to the treatment medication; thus our method allows evaluation of adherence as well as outcome. Second, we only tested intravenous MA administration, and urinary ratios may be slightly different if MA is abused by routes with slower absorption (oral and nasal). Samples collected immediately after abuse of MA, while drug continues to be absorbed and distributed, may lead to inaccurate estimates of use. However, for samples obtained in the elimination phase urine concentration ratios should remain robust in estimating the absorbed abused dose. Many participants in drug treatment attend group or individual counseling; obtaining urine samples after therapy visits may attenuate this limitation. Finally, our method may increase the cost of conducting trials. We estimate the costs of synthesizing deuterated MA and preparing individual dose units containing l-MA-d3 are $5 to 10 per dose. There should be no additional costs if confirmatory assays using mass spectrometry are used. Thus, for an 8-week trial where subjects are dosed daily with deuterated drug the additional cost would be $280 to 560 per subject. This cost is balanced by the increased power from use of a continuous primary outcome variable, probably decreasing the number of subjects needed and ultimately the trial cost. In addition, the cost of rejecting potentially efficacious therapies caused by inadequate endpoints is unaffordable.
In conclusion, administration of pharmacologically inactive doses of oral l-MA-d3 followed by quantifying the urine [d-MA-d0]:[l-MA-d3] permits estimation of the amount of MA abuse from a single random urine specimen. Quantification of drug exposure from easily obtained biological specimens will be useful in developing new treatments for MA addiction, understanding the patterns of abuse, and determining compliance with pharmacotherapies during clinical trials. Introduction of a continuous outcome measure may be a substantial improvement from the current qualitative binary outcome measures used to assess MA abuse. Finally, development of similar methods for other addictive drugs is possible.
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grants P50-DA018179, DA012521, P30-DA12393]; the National Institutes of Health National Institute of Allergy and Infectious Diseases Extramural Activities [Grant R01-AI50587]; and the National Institutes of Health National Institute of General Medical Sciences [Grant GM26696].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179176.
- MA
- methamphetamine
- l-MA-d3
- deuterium-labeled l-methamphetamine
- d-MA-d0
- nonlabeled d-MA
- GC
- gas chromatography
- MS
- mass spectrometry.
Authorship Contributions
Participated in research design: Galloway and Mendelson.
Conducted experiments: Galloway, Baggott, Lopez, and Mendelson.
Contributed new reagents or analytic tools: Everhart.
Performed data analysis: Li and Coyle.
Wrote or contributed to the writing of the manuscript: Li, Verotta, Baggott, and Mendelson.
References
- Batki SL, Moon J, Delucchi K, Bradley M, Hersh D, Smolar S, Mengis M, Lefkowitz E, Sexe D, Morello L, et al. (2000) Methamphetamine quantitative urine concentrations during a controlled trial of fluoxetine treatment. Preliminary analysis. Ann NY Acad Sci 909:260–263 [DOI] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. (1993) Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos 21:717–723 [PubMed] [Google Scholar]
- Dalgaard P. (2008) Introductory Statistics with R. Springer, New York [Google Scholar]
- Degenhardt L, Roxburgh A, Black E, Bruno R, Campbell G, Kinner S, Fetherston J. (2008) The epidemiology of methamphetamine use and harm in Australia. Drug Alcohol Rev 27:243–252 [DOI] [PubMed] [Google Scholar]
- Dolan K, Rouen D, Kimber J. (2004) An overview of the use of urine, hair, sweat and saliva to detect drug use. Drug Alcohol Rev 23:213–217 [DOI] [PubMed] [Google Scholar]
- Harris DS, Boxenbaum H, Everhart ET, Sequeira G, Mendelson JE, Jones RT. (2003) The bioavailability of intranasal and smoked methamphetamine. Clin Pharmacol Ther 74:475–486 [DOI] [PubMed] [Google Scholar]
- Jaffee WB, Trucco E, Teter C, Levy S, Weiss RD. (2008) Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv 59:140–142 [DOI] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. (2010) Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol 69:578–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lopez JC, Galloway GP, Baggott MJ, Everhart T, Mendelson J. (2010) Estimating the intake of abused methamphetamines using experimenter-administered deuterium labeled R-methamphetamine: selection of the R-methamphetamine dose. Ther Drug Monit 32:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Kozel N, Douglas J, Ali R, Vicknasingam B, Lund J, Li JH. (2008) The rise of methamphetamine in Southeast and East Asia. Drug Alcohol Rev 27:220–228 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse/College on Problems of Drug Dependence (1999) Consensus statement on evaluation of outcome of pharmacotherapy for substance abuse/dependence report. National Institute on Drug Abuse/College on Problems of Drug Dependence Meeting; 1999 April 23–24; Washington, DC [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. (1997) Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction 92:717–727 [PubMed] [Google Scholar]
- Schifano F, Corkery JM, Cuffolo G. (2007) Smokable (“ice”, “crystal meth”) and non smokable amphetamine-type stimulants: clinical pharmacological and epidemiological issues, with special reference to the UK. Ann Ist Super Sanita 43:110–115 [PubMed] [Google Scholar]
- Smith-Kielland A, Skuterud B, Mørland J. (1997) Urinary excretion of amphetamine after termination of drug abuse. J Anal Toxicol 21:325–329 [DOI] [PubMed] [Google Scholar]
- Valentine JL, Kearns GL, Sparks C, Letzig LG, Valentine CR, Shappell SA, Neri DF, DeJohn CA. (1995) GC-MS determination of amphetamine and methamphetamine in human urine for 12 hours following oral administration of dextro-methamphetamine: lack of evidence supporting the established forensic guidelines for methamphetamine confirmation. J Anal Toxicol 19:581–590 [DOI] [PubMed] [Google Scholar]
- Watanabe-Galloway S, Ryan S, Hansen K, Hullsiek B, Muli V, Malone AC. (2009) Effects of methamphetamine abuse beyond individual users. J Psychoactive Drugs 41:241–248 [DOI] [PubMed] [Google Scholar]