Abstract
Reactive oxygen species (ROS) superoxide anion (O2⨪) and hydrogen peroxide (H2O2) produced by activated leukocytes and endothelial cells in sites of inflammation or ischemia cause endothelial barrier dysfunction that may lead to tissue edema. Antioxidant enzymes (AOEs) catalase and superoxide dismutase (SOD) conjugated with antibodies to platelet-endothelial cell adhesion molecule-1 (PECAM-1) specifically bind to endothelium, quench the corresponding ROS, and alleviate vascular oxidative stress and inflammation. In the present work, we studied the effects of anti-PECAM/catalase and anti-PECAM/SOD conjugates on the abnormal permeability manifested by transendothelial electrical resistance decline, increased fluorescein isothiocyanate-dextran influx, and redistribution of vascular endothelial-cadherin in human umbilical vein endothelial cell (HUVEC) monolayers. Anti-PECAM/catalase protected HUVEC monolayers against H2O2-induced endothelial barrier dysfunction. Polyethylene glycol-conjugated catalase exerted orders of magnitude lower endothelial uptake and no protective effect, similarly to IgG/catalase. Anti-PECAM/catalase, but not anti-PECAM/SOD, alleviated endothelial hyperpermeability caused by exposure to hypoxanthine/xanthine oxidase, implicating primarily H2O2 in the disruption of the endothelial barrier in this model. Thrombin-induced endothelial permeability was not affected by treatment with anti-PECAM/AOEs or the NADPH oxidase inhibitor apocynin or overexpression of AOEs, indicating that the endogenous ROS play no key role in thrombin-mediated endothelial barrier dysfunction. In contrast, anti-PECAM/SOD, but not anti-PECAM/catalase, inhibited a vascular endothelial growth factor (VEGF)-induced increase in endothelial permeability, identifying a key role of endogenous O2⨪ in the VEGF-mediated regulation of endothelial barrier function. Therefore, AOEs targeted to endothelial cells provide versatile molecular tools for testing the roles of specific ROS in vascular pathology and may be translated into remedies for these ROS-induced abnormalities.
Introduction
The integrity and barrier function of the endothelial cell monolayer lining the vascular lumen are critical for the maintenance of cardiovascular homeostasis. Agents including thrombin, bradykinin, and vascular endothelial growth factor (VEGF) cause endothelial activation and structural rearrangements manifested by actin remodeling, cell shape changes, contraction, and barrier disruption leading to the leakage of blood components across the endothelial monolayer (Stevens et al., 2000). An abnormal increase in endothelial permeability may lead to edema, a process involved in the pathogenesis of inflammation, allergic reactions, ischemia/reperfusion injury, sepsis, acute lung injury, vasculopathy, and stroke (Lucas et al., 2009).
Reactive oxygen species (ROS), including superoxide anion O and hydrogen peroxide H2O2, are excessively generated in sites of inflammation, ischemia, and other vascular disorders. The vascular endothelium constitutes a primary target for oxidants released during these inflammatory events (Birukov, 2009). ROS produced by activated leukocytes and endothelial cells have been implicated in endothelial contraction and loss of barrier integrity (Boueiz and Hassoun, 2009). The initial extracellular ROS to which endothelial cells are exposed is O2⨪ released by activated leukocytes. O2⨪ poorly diffuses through membranes and spontaneously dismutates into H2O2 at a high rate and may act only in the close microenvironment of its generation. H2O2 is more stable and capable to pass through cell membranes, resulting in higher cytotoxicity compared with that of superoxide. Catalase, a potent antioxidant enzyme decomposing H2O2 into water and oxygen, was found to be protective against endothelial damage caused by oxidative stress induced by either activated polymorphonuclear leukocytes or extracellular xanthine/xanthine oxidase (XO) (Boueiz and Hassoun, 2009). Endothelial cells produce ROS at a markedly lower level than leukocytes (Thomas et al., 2008). However, an increasing body of evidence indicates an important role for endogenous endothelial ROS as signaling molecules (Alom-Ruiz et al., 2008), including proinflammatory cell activation through the nuclear factor-κB transcription factor-mediated inflammatory cascade (Forman et al., 2010; Shuvaev et al., 2011). The major sources of ROS in endothelial cells include NADPH oxidases, the respiratory chain of mitochondria, and several cytosolic enzymatic systems such as XO or uncoupled endothelial nitric-oxide (NO) synthase (Thomas et al., 2008). NADPH oxidase-produced ROS are of particular importance in the regulation of endothelial functions (Alom-Ruiz et al., 2008). In this study, we focus on signaling functions of both H2O2 and superoxide that are produced by NADPH oxidase (NOX), particularly NOX2, because human umbilical vein endothelial cells (HUVECs) express only this member of the NOX family on the cell membrane (Thomas et al., 2008). Endothelial cells produce ROS by NADPH oxidase in response to vasoactive and proinflammatory agents including thrombin and VEGF (Birukov, 2009). To date, it has not been known whether thrombin-induced ROS are involved in the regulation of endothelial barrier function. A recent study reported that VEGF-induced endothelial ROS production is implicated in barrier disruption (Monaghan-Benson and Burridge, 2009). Further elucidation of these pathophysiological mechanisms and devising their remedies represent important biomedical goals.
Protection against increased ROS generation can be achieved using antioxidant enzymes superoxide dismutase (SOD), which dismutates O2⨪ into oxygen and H2O2, and catalase, which decomposes H2O2 into water and oxygen (Freeman and Crapo, 1982). In this context, we have devised formulations of SOD and catalase conjugated with antibodies providing specific delivery to endothelial cells (i.e., “vascular immunotargeting”) (Muzykantov et al., 1996; Atochina et al., 1998; Shuvaev et al., 2004, 2007). In particular, conjugation with antibodies to the endothelial surface molecule platelet-endothelial cell adhesion molecule-1 (PECAM-1) provides specific endothelial delivery of anti-PECAM/SOD and anti-PECAM/catalase in vitro and in vivo, quenching of the corresponding ROS, and antioxidant effects unattainable by nontargeted formulations including polyethylene glycol (PEG)/catalase and PEG/SOD (Shuvaev et al., 2007, 2011). In the present work, we have used anti-PECAM/catalase and anti-PECAM/SOD to study the roles of exogenous and endogenous (e.g., produced in response to thrombin and VEGF) superoxide anion and H2O2 in the disruption of the endothelial barrier and evaluate specific interventions in this abnormality.
Materials and Methods
Antibodies and Reagents.
Monoclonal antibody (mAb 62) against PECAM-1 used for conjugation was provided by Dr. Marian Nakada (Centocor, Malvern, PA). Polyclonal antibodies against the N or C terminus of vascular endothelial (VE)-cadherin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Alexa Fluor 594 phalloidin and Alexa Fluor 488-conjugated secondary antibody (Ab) were from Invitrogen (Carlsbad, CA). Catalase and Cu,Zn-SOD from bovine liver were purchased from Calbiochem (San Diego, CA). PEG/catalase and PEG/SOD, XO, hypoxanthine (HX), apocynin [1-(4-hydroxy-3-methoxyphenyl)ethanone], diphenylene iodonium (DPI), and fetal bovine serum were purchased from Sigma-Aldrich (St. Louis, MO). Radioisotope-containing sodium iodide ([125I]Na) was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Succinimidyl-6-(biotinamido)hexanoate, succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate, N-succinimidyl-S-acetylthioacetate, and iodination tubes were from Thermo Fisher Scientific (Waltham, MA). Recombinant human VEGF was purchased from R&D Systems (Minneapolis, MN). Human α thrombin was from Enzyme Research Laboratories (South Bend, IN). Adenovirus expressing luciferase, catalase, and SOD1 were purchased from Vector Biolabs (Philadelphia, PA).
Conjugate Preparation.
Anti-PECAM-1/enzyme conjugates were prepared via amino chemistry as described previously (Shuvaev et al., 2011). Maleimide reactive groups were introduced into the antioxidant enzyme (AOE) molecule using a 20- to 100-fold molar excess of succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate, a maleimide-containing hetero-bifunctional cross-linker that allows covalent attachment of the maleimide group through amino groups of the protein. N-Succinimidyl-S-acetylthioacetate at a 20-fold molar excess was added to IgG (anti-PECAM-1 Ab or control mouse IgG) and incubated at room temperature for 30 min, introducing sulfhydryl groups into the Ab molecules. Thereafter, the introduced sulfhydryl groups were deprotected using 50 mM hydroxylamine for 2 h. Ab molecule was conjugated with catalase or SOD at a 1:1 or 1:2 molar ratio, respectively. The effective diameter of the prepared conjugates was approximately 300 nm, measured by a dynamic light scattering apparatus (90Plus Particle Size Analyzer; Brookhaven Instruments Corp., Holtsville, NY). The conjugates at this size have been shown to optimally bind to endothelial cells and enter cells via cell adhesion molecule-mediated endocytosis (Muzykantov et al., 1999; Shuvaev et al., 2011). Conjugated catalase and SOD retained approximately 80 and 70% of their initial catalytic activities, respectively, as we described elsewhere (Shuvaev et al., 2004, 2007).
Cell Culture and Treatment.
HUVECs at the first passage were purchased from Lonza Walkersville (Walkersville, MD) and were grown in Falcon tissue culture flasks (BD Biosciences, San Jose, CA) coated with 1% gelatin (Sigma-Aldrich) in EGM-2 BulletKit (Lonza Walkersville) containing 10% fetal bovine serum. Passages from 4 to 5 were used throughout the study. Confluent HUVECs (at a densities of 4 × 104 cells/cm2 for fluorescent immunostaining and enzyme-linked immunosorbent assay (ELISA) assays and 3 × 105 cells/cm2 for permeability assays) were preincubated with EBM-2 medium supplemented with 0.5% fetal bovine serum for overnight and incubated with conjugates for 30 min before stimulation with H2O2 (400 μM), HX/XO (200 μM/20 mU), thrombin (20 nM), or VEGF (200 ng/ml).
Adenoviral Infection of HUVECs.
HUVECs grown on Transwell inserts were infected with adenovirus expressing luciferase, catalase, or SOD1 for 18 h. The medium was replaced with fresh culture medium, and the cells were cultured for another 24 h to allow protein expression (Sweitzer et al., 2003). Expression of catalase and SOD1 in HUVECs was determined by Western blot analysis.
SOD and Catalase Radiolabeling.
Catalase, SOD, PEG/catalase, and PEG/SOD were radiolabeled with [125I]Na using iodination tubes as recommended by the manufacturer. An excess of free label was removed by gel-filtration chromatography using Bio-Spin 6 chromatography columns (Bio-Rad Laboratories, Hercules, CA), while iodinated enzymes were transferred to phosphate-buffered saline.
Binding of Antioxidant Enzymes to Endothelial Cells.
HUVECs were plated in 24-well culture dishes and grown to confluent culture. Radiolabeled conjugates were added to the cells at sequential dilutions. Cells were incubated at 37°C for 1 h, unbound label was washed out, and cells were lysed with lysis buffer (1% Triton X-100 and 1.0 M NaOH). Bound radioactivity was measured using a Wizard 1470 gamma counter (PerkinElmer Life and Analytical Sciences). Bound material was expressed as nanograms of enzyme per well. Each point was performed in quadruplicate, and results are expressed as mean ± S.E.M. (Shuvaev et al., 2011).
Endothelial Permeability to Fluorescein Isothiocyanate-Dextran Assay.
HUVECs were seeded onto gelatin-coated, 24-well Transwell 3.0-μm pore-size culture inserts (Corning Life Sciences, Lowell, MA) at a density of 0.1 million cells/well and cultured for 72 h to form restrictive endothelial monolayers. Cells then were starved overnight and exposed to conjugates and/or stimulus for the indicated period of time. After treatment, fluorescein isothiocyanate (FITC)-dextran (30 kDa) dissolved in starvation medium was placed in the apical compartment at a final concentration of 500 μg/ml and allowed to equilibrate for 2 h. Samples were taken from both apical and basolateral chambers for fluorescence measurement. The results were expressed as the percentage of FITC-dextran influx across HUVEC monolayers.
Transendothelial Electrical Resistance Measurements.
The transendothelial electrical resistance (TEER) across HUVEC monolayers prepared as describe above, reflecting the endothelial barrier integrity, was measured using an EVOM resistance meter (World Precision Instruments, Sarasota, FL). The TEER value was first measured at 72 h after seeding (0.1 million cells per well), and this time was defined as the start point. The data are presented as the change in the resistive (in-phase) portion of the impedance normalized to its initial value at time zero.
Immunostaining and Fluorescent Microscopy.
HUVECs were grown on gelatin-coated μ slides (8 wells; ibidi, Martinsried, Germany), treated with conjugates and/or H2O2 for the indicated period of time, washed with Hanks' balanced salt solution (HBSS), fixed with 1% paraformaldehyde, and permeabilized with 0.1% Triton X-100. Nonspecific binding was blocked with blocking solution containing 1% bovine serum albumin and 0.05% Tween 20 in HBSS for 1 h followed by incubation with Alexa Fluor 488-conjugated Ab to VE-cadherin and Alexa Fluor 594-conjugated phalloidin for F-actin staining. The slides then were mounted using a ProLong antifade kit (Invitrogen). Images were taken by fluorescence microscopy (Eclipse TE2000-U; Nikon, Melville, NY) using filters optimized for Alexa Fluor 488 and 594.
Measurement of HUVEC Surface Expression of VE-Cadherin by Whole-Cell ELISA.
The amount of VE-cadherin expressed on the surface of the HUVECs was assessed using a whole-cell ELISA as described previously (Bae et al., 2007) with some modifications. The HUVEC monolayers, grown on gelatin-coated, 96-well plates, were starved overnight before conjugate and H2O2 treatment. Thereafter, cells were washed, fixed with 1% paraformaldehyde for 10 min, and then blocked with 1% bovine serum albumin for 1 h at room temperature. The cells were incubated with polyclonal Ab against human VE-cadherin (N terminus) for 1 h, followed by washing three times with 0.05% Tween 20 in HBSS. Cells were further incubated for 1 h with horseradish peroxidase-conjugated anti-goat IgG. Unbound secondary antibodies were removed by extensive washing. The ortho-phenylenediamine substrate (0.5 mg/ml ortho-phenylenediamine and 0.015% H2O2 in phosphate-buffered saline) was added and incubated at room temperature for 15 min. The reaction was stopped by the addition of 15% H2SO4, and the absorbance was determined at 491 nm.
Results
Anti-PECAM/AOE Conjugates Do Not Impair Basal Endothelial Barrier Function.
First, we tested the effects of anti-PECAM/AOE conjugates (indicated as Ab conjugates) on endothelial monolayer integrity. To delineate the possible effects of basal ROS quenching, we have compared the effects of Ab/catalase and Ab/SOD with that of drug-free, or “empty,” Ab conjugate of a similar size lacking AOE cargo. To inspect the cytoskeleton and the integrity of intercellular junctions, a HUVEC monolayer treated with Ab conjugates was stained for F-actin and VE-cadherin, a molecular marker of adhesion junctions. Supplemental Fig. S1 shows that phalloidin staining was accentuated in Ab conjugate-treated versus control cells, revealing increased actin polymerization into filamentous actin. The Ab conjugate-induced actin filaments were arranged into stress fibers and a cortical network with dense peripheral bands at the cell boundaries. However, similar to control cells, Ab conjugate-treated cells maintained the cobblestone morphology and continuous zipperlike staining of VE-cadherin in the intercellular junctions, indicating preserved endothelial barrier integrity. In support of this notion, a direct analysis of endothelial permeability (FITC-dextran influx assay) showed that diverse Ab conjugates at doses ranging from 12.5 to 100 μg/ml did not change endothelial permeability, regardless whether the conjugate carried AOE or not (Supplemental Fig. S2).
Anti-PECAM/Catalase, but Not IgG/Catalase or PEG-Modified Enzymes, Protects against Endothelial Barrier Dysfunction Caused by Exposure to H2O2.
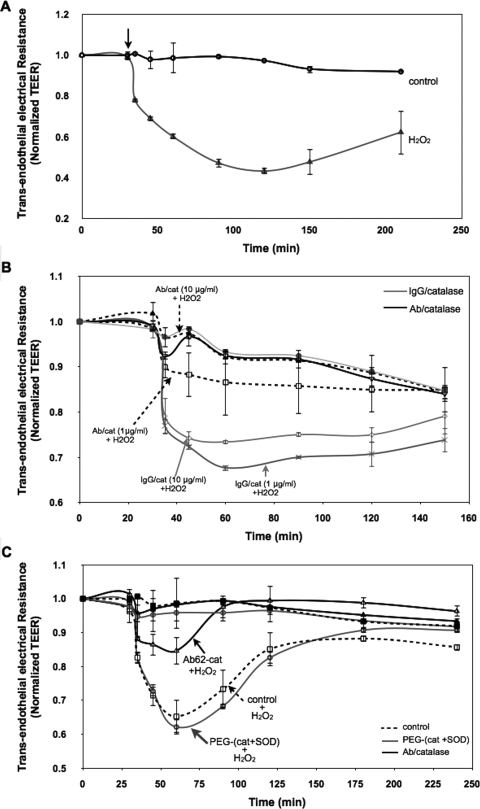
We have used three approaches to test endothelial permeability: 1) real-time measurement of TEER, 2) endpoint measurement of FITC-dextran transfer, and 3) analysis of VE-cadherin distribution. Exposure of HUVEC monolayers to H2O2 (400 μM) caused an immediate increase in endothelial permeability evident as TEER decline (Fig. 1A). Pretreatment with Ab/catalase, but not IgG/catalase, markedly diminished H2O2-induced TEER decline in a dose-dependent manner (Fig. 1B). In contrast to Ab/catalase, pretreatment with a mixture of PEG/AOEs had no effect on the subsequent H2O2-induced TEER index of endothelial permeability (Fig. 1C).
Fig. 1.
Anti-PECAM/catalase conjugates protect against endothelial barrier dysfunction induced by H2O2 insult. A, TEER across HUVEC monolayers was used to evaluate endothelial permeability. HUVEC monolayers grown onto Transwell inserts (3.0-μm pore size) were transferred to Endohm (World Precision Instruments) chambers for resistance measurement at different time points. At the arrow-indicated time point, the cells were stimulated with H2O2 (400 μM). The recorded values were normalized to the initial resistances of the monolayers before H2O2 treatment. The normalized TEER values are represented as means ± S.D. (n = 3). B, Ab/catalase treatment (1 and 10 μg/ml) abolished H2O2-induced endothelial hyperpermeability. HUVEC monolayers were preincubated with conjugates for 30 min and washed three times to remove unbound conjugates. Cells then were stimulated with H2O2 (400 μM) and subjected to TEER measurement. C, mixture of PEGylated SOD and catalase (10 μg/ml of each) failed to attenuate H2O2-induced TEER decline.
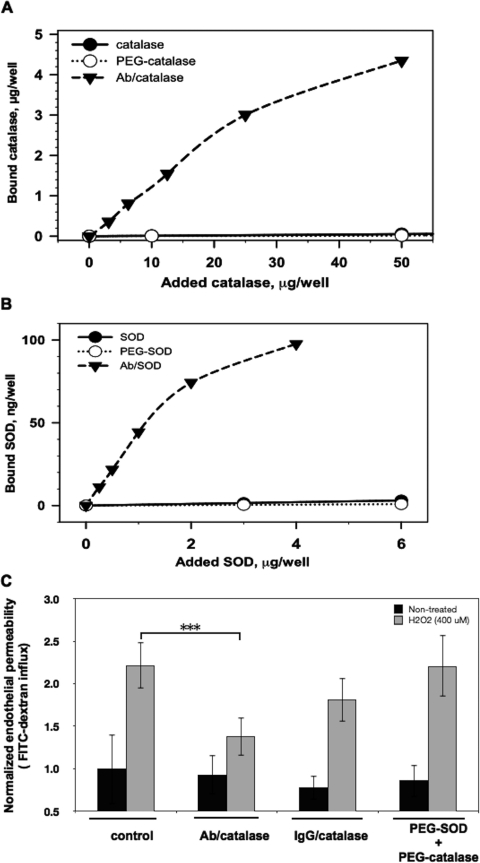
This result can be explained by the fact that Ab/AOEs bind to endothelial cells with a high affinity and are taken up by the cells, in contrast to other AOE formulations including PEG/AOEs. Endothelial uptake of 125I-labeled PEG/AOEs did not differ from that of naked AOEs, trivial in a wide range of doses, and was exceeded by the Ab/AOE uptake by orders of magnitude (Fig. 2, A and B). A separate study using FITC-dextran tracing affirmed that Ab/catalase, but not IgG/catalase or PEG/catalase, attenuates H2O2-induced endothelial permeability (Fig. 2C).
Fig. 2.
PEGylated SOD and catalase do not bind to endothelial cells and do not inhibit H2O2-stimulated endothelial permeability for macromolecules. A, Ab/catalase, but not PEGylated catalase, specifically bound to HUVECs. B, Ab/SOD, but not PEGylated SOD, specifically targeted HUVECs. C, Ab/catalase, but not PEGylated SOD and catalase, alleviated H2O2-induced endothelial permeability for macromolecules as measured by the FITC-dextran influx assay. Values are the normalized percentages of total FITC-dextran passing across cells relative that of the control group. Error bars represent the S.D. (n = 3; ***, p < 0.001).
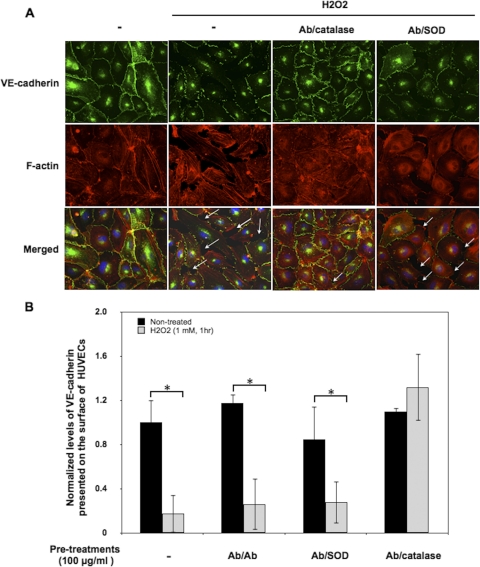
Anti-PECAM/Catalase Inhibits H2O2-Induced Redistribution of VE-Cadherin.
We used immunostaining to examine the effects of Ab/catalase on H2O2-induced redistribution of VE-cadherin, an endothelial adhesion molecule. Figure 3A shows that H2O2 caused the formation of gaps between cells and the loss of VE-cadherin from the adhesion junctions in the gaps. Ab/catalase, but not drug-free Ab conjugate or Ab/SOD conjugate, attenuated this effect. In agreement with qualitative microscopy data, quantitative whole-cell ELISA showed that 1) H2O2 caused a dramatic reduction in surface VE-cadherin in HUVECs and 2) pretreatment with Ab/catalase eliminated this abnormality (Fig. 3B). Taken together, data shown in Figs. 1 to 3 indicate that Ab/catalase, but not other AOE formulations, alleviates H2O2-induced endothelial barrier abnormalities.
Fig. 3.
Ab/catalase inhibits H2O2-induced redistribution of VE-cadherin. A, immunocytochemical staining of VE-cadherin and F-actin in HUVECs. Confluent HUVECs treated with Ab/Ab, Ab/catalase, and Ab/SOD conjugate (100 μg/ml, 30 min) were washed and stimulated with H2O2 (1 mM) for 1 h. Cells then were washed, fixed, and immunostained for VE-cadherin (green fluorescence) and F-actin (red fluorescence). Arrows point to the intercellular gaps. B, whole-cell ELISA was used to quantify VE-cadherin presented on the cell surface. The data are normalized to control cells without H2O2 stimulation and are means from three experiments ± S.D. (*, p < 0.05 in a Student's t test).
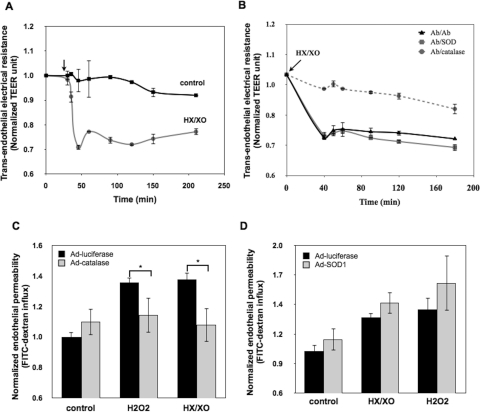
Ab/Catalase, but Not Ab/SOD, Inhibits Barrier Dysfunction Caused by Extracellular ROS Influx.
Next, we examined the effects of Ab/AOEs on endothelial barrier disruption caused by extracellular generation of O2⨪ and H2O2. The exposure of HUVEC monolayers to the xanthine (200 μM) and XO (20 mU) system generating superoxide that transforms into H2O2 caused a rapid decline in TEER (Fig. 4A). Pretreatment with Ab/catalase, but not Ab/SOD, inhibited this effect (Fig. 4B), suggesting that H2O2, but not superoxide anion, causes monolayer disruption. This notion was affirmed by testing the endothelial permeability of HUVEC monolayers infected with adenovirus expressing luciferase (control), catalase, or SOD1. Overexpression of catalase, but not SOD, alleviated endothelial permeability caused by either H2O2 or XO (compare Fig. 4, C and D).
Fig. 4.
Ab/catalase, but not Ab/SOD, specifically inhibited superoxide-induced endothelial permeability. A, superoxide anion generated by the HX/XO system increased endothelial permeability. HUVEC monolayers were exposed to HX (200 μM) and XO (20 mU), and the TEER values at indicated time points were recorded. The data shown are normalized TEER values relative to the initial TEER values of the monolayers before stimulation. Means ± S.D. are shown. (n = 3). B, Ab/catalase, but not Ab/SOD, rescued superoxide-induced endothelial permeability. HUVECs were treated with drug-free conjugates Ab/Ab (100 μg/ml), Ab/catalase (10 μg/ml), and Ab/SOD (100 μg/ml). Cells then were washed and stimulated with HX/XO. Only Ab/catalase treatment reversed HX/XO-induced TEER decline. C, overexpression of cytosolic catalase significantly attenuated endothelial permeability induced by HX/XO. HUVECs overexpressing luciferase (negative control) or catalase were stimulated with H2O2 (600 μM) or HX/XO (400 μM/40 mU) for 90 min followed by FITC-dextran influx measurement. Data shown are normalized dextran influx (fold of basal) and are means ± S.D. (n = 6; *, p < 0.05). D, overexpression of cytosolic SOD1 did not show protective effects on superoxide anion- or H2O2-induced hyperpermeability. HUVECs overexpressing SOD1 were treated with HX/XO (400 μM/40 mU) or H2O2 (600 μM) for 90 min. The endothelial permeability to FITC-dextran was measured.
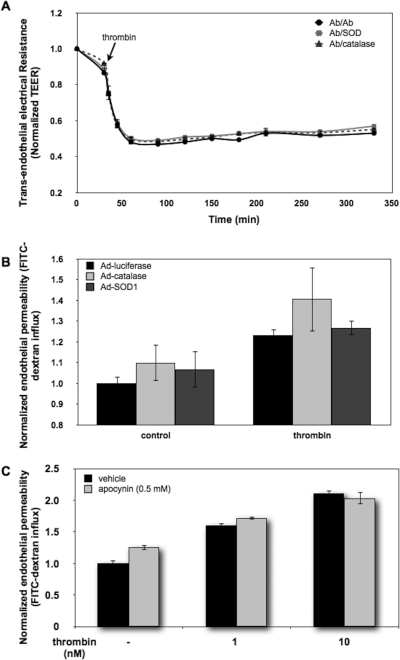
Antioxidant Enzyme Interventions Do Not Attenuate Thrombin-Induced Endothelial Permeability.
A recent finding that thrombin, a potent endothelial permeability enhancer, also induces the generation of endothelial ROS through the activation of NADPH oxidase (Djordjevic et al., 2005) prompted us to test the effects of Ab/AOEs on thrombin-induced endothelial permeability. As shown in Fig. 5A, neither Ab/catalase nor Ab/SOD affected thrombin-induced TEER decline. In accord with this result, overexpression of catalase or SOD in HUVECs had no effect (Fig. 5B). Furthermore, the NADPH oxidase inhibitor apocynin had no effect on thrombin-induced endothelial permeability (Fig. 5C). Taken together, these data imply that endogenous endothelial ROS play rather minor roles in this effect of thrombin.
Fig. 5.
Thrombin-induced intracellular ROS is not involved in the regulation of endothelial permeability. A, pretreatment of Ab/catalase and Ab/SOD (100 μg/ml, 30 min) did not attenuate thrombin-induced endothelial permeability. Conjugate-treated HUVEC monolayers were exposed to thrombin (20 nM), and the TEER values of the monolayers were measured at the indicated time points. Data shown are means ± S.D. (n = 3). B, overexpression of cytosolic catalase and SOD1 did not modulate thrombin-induced endothelial permeability. HUVECs expressing cytosolic luciferase serving as a control, catalase, and SOD1 by adenoviral infection were stimulated with thrombin (40 nM, 1 h) followed by the FITC-dextran influx assay. C, blockage of thrombin-induced ROS generation did not affect endothelial permeability. HUVEC monolayers were preincubated with dimethyl sulfoxide (vehicle) or apocynin (0.5 mM) for 1 h and then exposed to thrombin (1 and 10 nM) for 1 h. Endothelial permeability to macromolecules was assessed by the FITC-dextran influx assay. Thrombin stimulation increased permeability in a dose-dependent manner, and no significant difference was detected between control and apocynin-treated cells.
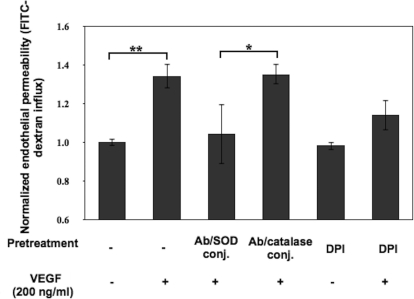
Ab/SOD, but Not Ab/Catalase, Inhibits Barrier Dysfunction Caused by VEGF.
Finally, we have tested whether Ab/AOEs affect endothelial barrier disruption caused by VEGF, a potent endogenous peptide that elevates vascular permeability and endothelial ROS as well (Monaghan-Benson and Burridge, 2009; Bates, 2010). HUVEC monolayers preincubated with Ab/SOD or Ab/catalase or the NADPH oxidase inhibitor DPI were exposed to VEGF. The result in Fig. 6 showed that Ab/SOD, but not Ab/catalase, inhibited VEGF-induced endothelial permeability. Taken together with the inhibitory effect of DPI, this result implicates superoxide anion, not H2O2, in VEGF-induced barrier dysfunction.
Fig. 6.
Ab/SOD conjugate specifically protects the endothelial barrier from VEGF insult. Ab/SOD, but not Ab/catalase, inhibited VEGF-induced endothelial permeability. HUVEC monolayers were preincubated with conjugates of Ab/catalase and Ab/SOD (100 μg/ml, 30 min) or DPI (10 μM, 1 h). Cells then were stimulated with VEGF (200 ng/ml) for 30 min followed by the FITC-dextran influx assay. Data shown are means ± S.D. (n = 4; *, p < 0.05; **, p < 0.01).
Discussion
The elevated level of ROS influx is implicated in abnormal endothelial activation, redox signaling, and cellular dysfunction (Birukov, 2009), all involved in the pathogenesis of vascular disorders including inflammation and ischemia (Boueiz and Hassoun, 2009). Both endogenous and exogenous ROS may impair endothelial barrier function via mechanisms involving rearrangements of the endothelial cytoskeleton and intercellular junctions. For example, exposure of endothelial cells to H2O2 or xanthine/XO causes remodeling of the actin filament networks, leading to increased intracellular tension and intercellular gap formation (Boueiz and Hassoun, 2009) and redistribution of cell junction components including VE-cadherin (Alexander et al., 2000).
The toxicity of ROS to endothelial cells depends on their source, concentration, and main species produced. Thus, the major mechanism of superoxide toxicity is depletion of the NO endothelial pool and as a result the decrease in NO bioavailability that may cause endothelial dysfunction (Thomas et al., 2008; Boueiz and Hassoun, 2009). The increased level of H2O2 may cause damage to cell proteins, DNA, and lipids mostly mediated by the formation of the highly reactive hydroxyl radical. Depending on the ROS level, oxidative stress may cause cell damage, apoptosis, or necrosis (Thomas et al., 2008).
Mechanisms of abnormal endothelial permeability caused by exogenous and endogenous ROS remain to be more fully understood, and means for specific therapeutic interventions in these mechanisms remain to be devised. Antioxidant therapies scavenging the ROS surplus have long been proposed to protect endothelial barrier function. Prolonged use of large doses of nonenzymatic antioxidants may confer some degree of protection in relatively subtle forms of chronic oxidative stress, whereas the use of potent antioxidant enzymes, AOEs targeted to the endothelium, may be more appropriate in the treatment of severe and acute forms of the pathology, such as inflammation and ischemia (Muzykantov et al., 1996; Atochina et al., 1998; Shuvaev et al., 2004). Immunotargeting of catalase and SOD to PECAM-1 stably expressed on endothelial lumen allows adequate and specific delivery of these AOEs to endothelial cells (Muzykantov et al., 1999; Shuvaev et al., 2007). Anti-PECAM/catalase attenuates lung ischemia/reperfusion injury in rats and mice (Kozower et al., 2003; Shuvaev et al., 2009), whereas anti-PECAM/SOD inhibits angiotensin II-induced vasoconstriction in mice (Shuvaev et al., 2009) and endothelial proinflammatory activation in response to tumor necrosis factor, interleukin-1β, and lipopolysaccharide (Shuvaev et al., 2011).
Taking advantage of the abilities of anti-PECAM/SOD and anti-PECAM/catalase to specifically quench endothelial superoxide and H2O2, respectively, in this study, we assessed their effects on endothelial permeability induced by exogenous ROS and by endogenous ROS generated in response to thrombin and VEGF. We elected to do this in a model of endothelial monolayers cultured on porous membranes that allow direct quantitative measurement of endothelial permeability. Analysis of TEER to ion flow relies on a simplified equivalent circuit model viewing the endothelium as a parallel circuit consisting of both paracellular and transcellular pathways. Because the intercellular junction is often rate limiting to paracellular solute movement, an alternation in TEER is commonly used as an index of intercellular junction permeability. The TEER assay allows monitoring of the change in endothelial permeability in real time. Loss of barrier function also leads to increased passage of inert macromolecules such as fluorescently labeled dextran and albumin via the endothelial monolayer, thereby providing an alternative assay for the quantitative assessment of endothelial permeability to macromolecules (Draijer et al., 1995). Adhesion junctions and the adhesion junction component VE-cadherin in particular play an important role in the control of vascular permeability and integrity. Therefore, we examined the effects of anti-PECAM/AOEs on the structural integrity of endothelial monolayers through immunostaining VE-cadherin to visualize the endothelial intercellular junctions.
Anti-PECAM conjugates and anti-PECAM-carrying nanocarriers cross-link PECAM-1 molecules and thereby activate endothelial cells (e.g., inducing endocytosis) (Muro et al., 2003; Shuvaev et al., 2011). Anti-PECAM-carrying nanocarriers cause Ras homolog gene family, member A activation and rearrangement of the actin cytoskeleton (Garnacho et al., 2008). In theory, these changes as well as quenching the basal ROS level by anti-PECAM/AOEs can affect endothelial barrier integrity. However, anti-PECAM/AOEs caused no changes in endothelial integrity and permeability in HUVEC monolayers (Supplemental Figs. S1 and S2). This result is in agreement with the lack of proedematous changes in the lungs of animals treated with anti-PECAM conjugates including anti-PECAM/AOEs (Scherpereel et al., 2002; Kozower et al., 2003; Shuvaev et al., 2009) and thus further supports the safety and potential translational potential of this targeted antioxidant intervention. Of note, PECAM-1 is involved in the maintenance of endothelial monolayer integrity and anti-inflammatory signaling (Ilan and Madri, 2003; Privratsky et al., 2010). Changes in PECAM signaling activities or its localization by anti-PECAM conjugates could potentially affect these functions. However, our results show that 1) neither anti-PECAM/AOEs nor unloaded drug-free anti-PECAM conjugates affect basal endothelial permeability (Supplemental Figs. S1 and S2) and 2) in each case of ROS-mediated endothelial permeability, only one type of anti-PECAM/AOE altered the outcome (carrying either catalase or SOD), whereas another type had no discernible effect. Presumably, effects of PECAM engagement and/or blocking would be similar in all of these cases. The fact that no effects unrelated to the delivery of the given AOEs have been observed argues that the anti-PECAM/AOE conjugates described in this study did not affect PECAM anti-inflammatory functions.
Anti-PECAM/catalase, but not other AOE formulations, protected against endothelial barrier disruption caused by exogenous H2O2 (Figs. 1–3). The lack of binding explains the failure of PEG/catalase (Fig. 2). PEG/AOEs and AOE formulations modified with PEG-based Pluronic have prolonged half-lives in circulation and showed protective effects in models of oxidative stress (Liu et al., 1989; Machtay et al., 2006; Yi et al., 2010), including a radiation injury model (Machtay et al., 2006). Antioxidants (e.g., N-acetyl-l-cysteine), XO inhibitors (allopurinol), and AOEs (catalase or SOD) attenuate vascular leakage in some animal models (Horgan et al., 1989; Barnard and Matalon, 1992). However, it is plausible that the specific endothelial delivery of AOEs may be needed for effective intervention in ROS acting in the endothelium. For example, a mutant recombinant SOD fusion protein that binds to negatively charged glycocalyx showed promising protective effects in models of oxidative stress and inflammation (Gao et al., 2003; Clarke et al., 2010). Furthermore, a protective effect of anti-PECAM/SOD but not PEG/SOD has been observed in cell and animal models of endothelial cytokine activation (Shuvaev et al., 2011). Data presented in this article showing the alleviation of abnormal endothelial permeability by anti-PECAM/AOEs but not untargeted AOE formulations further affirm the importance of targeted AOE delivery in the management of endothelial oxidative stress.
H2O2 and superoxide are among the extracellular ROS attacking the endothelium at the sites of inflammation or ischemia. Exposure to H2O2 or xanthine/XO-produced ROS disrupts the endothelial barrier (Barnard and Matalon, 1992). In the present study, endothelial targeting or overexpression of catalase, but not SOD, inhibited endothelial permeability in response to xanthine/XO-generated ROS, indicating the key role of H2O2 in endothelial barrier dysfunction, likely due to the rapid transformation of extracellular superoxide into H2O2, a more stable, diffusible, and arguably more toxic ROS. In endothelium, extracellular SOD (ecSOD or SOD3) bound to glycocalyx further accelerates this reaction (Gongora et al., 2006). This observation helps to explain previous findings showing that the injection of anti-PECAM/catalase, but not anti-PECAM/SOD, alleviated alveolar edema in a model of in situ lung ischemia/reperfusion (Shuvaev et al., 2009).
Thrombin disrupts endothelial barrier function by activating protease-activated receptor-1, which initiates intracellular signals leading to the disassembly of the intercellular adhesion junctions and cytoskeleton reorganization, with resultant barrier disruption (Minshall et al., 2010). Thrombin also induces ROS generation via the activation of NADPH oxidase (Djordjevic et al., 2005). However, we found that the thrombin-induced increase in endothelial permeability could not be interfered by 1) anti-PECAM/catalase and anti-PECAM/SOD treatment, 2) overexpression of cytosolic catalase and SOD that decomposed intracellular ROS, and 3) treatment with apocynin, which blocked NADPH oxidase activity. These data suggest that ROS play rather a minor role in thrombin-induced endothelial permeability.
In contrast, anti-PECAM/SOD, but not anti-PECAM/catalase, attenuated VEGF-induced endothelial barrier disruption, implicating endogenous superoxide in this type of pathological redox signaling. VEGF is a potent inducer of angiogenesis and vascular permeability (Bates, 2010). Recent studies implicated ROS in barrier dysfunction induced by VEGF and other mediators (Monaghan-Benson and Burridge, 2009); hence, direct identification of superoxide, not H2O2, as a redox mediator of the VEGF effect is of specific importance. VEGF is a multifunctional mediator, however. For example, conditional deletion of VEGF in type II pneumocytes attenuated pulmonary permeability yet was associated with enhanced apoptosis of alveolar epithelial cells during ischemia/reperfusion-induced acute lung injury (Mura et al., 2010). However, recombinant VEGF administration enhanced alveolar and vascular regeneration in rat pups subjected to hyperoxia, albeit at the expense of increasing permeability (Kunig et al., 2006). The present finding of attenuation of VEGF-induced endothelial barrier dysfunction by anti-PECAM/SOD therefore may have important translational implications. It is tempting to speculate, for example, that the endothelial delivery of anti-PECAM/SOD may inhibit VEGF-induced endothelial permeability without influencing the protective role of VEGF in epithelial repair after lung injury. Specific scavenging of the VEGF-induced ROS offered by anti-PECAM/SOD may protect the endothelial barrier without compromising the beneficial effects of VEGF on the recovery process of impaired vascular endothelial cells during ischemia/reperfusion or inflammatory injury.
Supplementary Material
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL073940] (to V.R.M.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.180620.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- VEGF
- vascular endothelial growth factor
- ROS
- reactive oxygen species
- AOE
- antioxidant enzyme
- SOD
- superoxide dismutase
- PECAM-1
- platelet-endothelial cell adhesion molecule-1
- PEG
- polyethylene glycol
- NOX
- NADPH oxidase
- HUVEC
- human umbilical vein endothelial cell
- VE
- vascular endothelial
- HX
- hypoxanthine
- XO
- xanthine oxidase
- NO
- nitric oxide
- Ab
- antibody
- ELISA
- enzyme-linked immunosorbent assay
- DPI
- diphenylene iodonium
- FITC
- fluorescein isothiocyanate
- TEER
- transendothelial electrical resistance
- HBSS
- Hanks' balanced salt solution.
Authorship Contributions
Participated in research design: Han, Shuvaev, and Muzykantov.
Conducted experiments: Han and Shuvaev.
Performed data analysis: Han and Shuvaev.
Wrote or contributed to the writing of the manuscript: Han, Shuvaev, and Muzykantov.
Other: Muzykantov acquired funding for the research.
References
- Alexander JS, Alexander BC, Eppihimer LA, Goodyear N, Haque R, Davis CP, Kalogeris TJ, Carden DL, Zhu YN, Kevil CG. (2000) Inflammatory mediators induce sequestration of VE-cadherin in cultured human endothelial cells. Inflammation 24:99–113 [DOI] [PubMed] [Google Scholar]
- Alom-Ruiz SP, Anilkumar N, Shah AM. (2008) Reactive oxygen species and endothelial activation. Antioxid Redox Signal 10:1089–1100 [DOI] [PubMed] [Google Scholar]
- Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR. (1998) Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am J Physiol 275:L806–L817 [DOI] [PubMed] [Google Scholar]
- Bae JS, Yang L, Manithody C, Rezaie AR. (2007) Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem 282:9251–9259 [DOI] [PubMed] [Google Scholar]
- Barnard ML, Matalon S. (1992) Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J Appl Physiol 72:1724–1729 [DOI] [PubMed] [Google Scholar]
- Bates DO. (2010) Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 87:262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov KG. (2009) Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal 11:1651–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boueiz A, Hassoun PM. (2009) Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res 77:26–34 [DOI] [PubMed] [Google Scholar]
- Clarke MB, Wright R, Irwin D, Bose S, Van Rheen Z, Birari R, Stenmark KR, McCord JM, Nozik-Grayck E. (2010) Sustained lung activity of a novel chimeric protein, SOD2/3, after intratracheal administration. Free Radic Biol Med 49:2032–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Görlach A. (2005) The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med 38:616–630 [DOI] [PubMed] [Google Scholar]
- Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VW. (1995) cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res 76:199–208 [DOI] [PubMed] [Google Scholar]
- Forman HJ, Maiorino M, Ursini F. (2010) Signaling functions of reactive oxygen species. Biochemistry 49:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. (1982) Biology of disease: free radicals and tissue injury. Lab Invest 47:412–426 [PubMed] [Google Scholar]
- Gao B, Flores SC, Leff JA, Bose SK, McCord JM. (2003) Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am J Physiol Lung Cell Mol Physiol 284:L917–L925 [DOI] [PubMed] [Google Scholar]
- Garnacho C, Shuvaev V, Thomas A, McKenna L, Sun J, Koval M, Albelda S, Muzykantov V, Muro S. (2008) RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood 111:3024–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. (2006) Role of extracellular superoxide dismutase in hypertension. Hypertension 48:473–481 [DOI] [PubMed] [Google Scholar]
- Horgan MJ, Lum H, Malik AB. (1989) Pulmonary edema after pulmonary artery occlusion and reperfusion. Am Rev Respir Dis 140:1421–1428 [DOI] [PubMed] [Google Scholar]
- Ilan N, Madri JA. (2003) PECAM-1: old friend, new partners. Curr Opin Cell Biol 15:515–524 [DOI] [PubMed] [Google Scholar]
- Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. (2003) Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol 21:392–398 [DOI] [PubMed] [Google Scholar]
- Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. (2006) Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 291:L1068–L1078 [DOI] [PubMed] [Google Scholar]
- Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. (1989) Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol 256:H589–H593 [DOI] [PubMed] [Google Scholar]
- Lucas R, Verin AD, Black SM, Catravas JD. (2009) Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol 77:1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, Arguiri E, Shuvaev VV, Sun J, Cengel K, et al. (2006) Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol 81:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall RD, Vandenbroucke EE, Holinstat M, Place AT, Tiruppathi C, Vogel SM, van Nieuw Amerongen GP, Mehta D, Malik AB. (2010) Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res 80:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan-Benson E, Burridge K. (2009) The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 284:25602–25611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura M, Binnie M, Han B, Li C, Andrade CF, Shiozaki A, Zhang Y, Ferrara N, Hwang D, Waddell TK, et al. (2010) Functions of type II pneumocyte-derived vascular endothelial growth factor in alveolar structure, acute inflammation, and vascular permeability. Am J Pathol 176:1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. (2003) A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci 116:1599–1609 [DOI] [PubMed] [Google Scholar]
- Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. (1996) Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci USA 93:5213–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. (1999) Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA 96:2379–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privratsky JR, Newman DK, Newman PJ. (2010) PECAM-1: conflicts of interest in inflammation. Life Sci 87:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, et al. (2002) Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther 300:777–786 [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, et al. (2009) Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther 331:404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Dziubla T, Wiewrodt R, Muzykantov VR. (2004) Streptavidin-biotin crosslinking of therapeutic enzymes with carrier antibodies: nanoconjugates for protection against endothelial oxidative stress. Methods Mol Biol 283:3–19 [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. (2011) PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J 25:348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. (2007) Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther 323:450–457 [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, Albelda SM, Muzykantov VR. (2011) Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release 149:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. (2000) Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol 279:L419–L422 [DOI] [PubMed] [Google Scholar]
- Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR. (2003) PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic Biol Med 34:1035–1046 [DOI] [PubMed] [Google Scholar]
- Thomas SR, Witting PK, Drummond GR. (2008) Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10:1713–1765 [DOI] [PubMed] [Google Scholar]
- Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. (2010) Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic Biol Med 49:548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.