Abstract
Background
Severe bleeding after injury requires transfusion of blood products, including fresh frozen plasma (FFP). Many centers are keeping thawed plasma (TP) ready for massively transfused patients. According to the American Association of Blood Banks Standards, TP is approved for transfusion up to 5 days after thawing, when stored at 1°C to 6°C. However, there are no clinical data analyzing the effects of the approved 5-day storage on plasma. We hypothesize that the hemostatic potential (HP) of freshly thawed (FFP-0) was superior to plasma stored for 5 days (FFP-5).
Methods
FFP from 30 single donors were thawed at 37°C and kept at 1°C to 6°C for 5 days. HP was evaluated at day 0 and 5 by measuring kinetics of thrombin generation (TG), kinetics of clot formation by thromboelastography, clotting factors and inhibitors, and cell-derived microparticles (MPs) by flow cytometry.
Results
When comparing FFP-5 to FFP-0, FFP-5 exhibited only 40% of the potential of FFP-0 for TG (6.2 nM/min vs. 14.3 nM/min, p < 0.0001), a slower clotting response via thromboelastography (reaction time: 4.3 minutes vs. 3.2 minutes, p < 0.0001) and a longer delay in reaching maximum thrombus generation (5.7 minutes vs. 4.6 minutes, p < 0.01). Diminished HP was accompanied by a significant decline in multiple coagulation proteins, including FV, VII, VIII, von Willebrand factor, and free Protein S, by up to 30%, and a decrease of 50% in MP counts.
Conclusion
The HP and clot forming ability of TP significantly declined with storage. Hence, freshly TP may have a greater ability to restore hemostasis and correct coagulopathy compared with FFP-5. The clinical consequences for transfused patients deserve further exploration.
Keywords: FFP storage, Thrombin generation, TEG, Microparticles, Trauma
Hemorrhage remains a major cause of potentially preventable death after both civilian and military severe traumatic injury, and transfused trauma patients are frequently coagulopathic. Several comprehensive reviews of traumatic coagulopathy indicate that the mechanism of acute coagulopathy of trauma with hemorrhagic shock (ACoTS) is not fully understood and is likely multifactorial.1,2 Cohen and coworkers have recently described the relationship between acute traumatic coagulopathy in mice and generation of elevated activated protein C (APC), suggesting APC may play a major role in development of ACoTS.3,4 Patients with severe bleeding after major traumatic injury often require massive transfusion (MT) of blood products including fresh frozen plasma (FFP), the early use of which has been associated with improved survival.5 The improvement in survival with FFP is conventionally assumed to be because of its hemostatic potential (HP) by delivery of components necessary for hemostasis, which becomes depleted with hemorrhage, MT, and crystalloid fluid resuscitation. Although this plasma HP is important, we have shown that plasma also has profound effects on repairing endothelial permeability.6
The HP of FFP, as it relates to the ability to correct the hypocoagulable state of bleeding patients, is likely the result of several parameters including coagulation factor activity, presence or absence of inhibitors, and the procoagulant activity of cellular microparticles (MPs). Recent data support transfusing earlier and greater amounts of FFP in MT patients. To accommodate this change in practice, and improve the rapid availability of plasma in emergency situations, many centers are using thawed plasma (TP) as an alternative for these patients. Although TP has been shown to contain the same coagulation factors as FFP with the exception of lower levels of FV and FVIII, there are no clinical data suggesting that the efficacy of TP is equal to that of FFP. Thus, quantitative or qualitative declines of these plasma components may result in reduced HP of TP.
FFP is prepared by centrifugation of whole blood with separation of plasma from cellular components or by apheresis collection. If the separated plasma is frozen within 8 hours of blood collection, it is labeled “FFP,” and if it is frozen within 24 hours, it is labeled “FP24.” According to the American Association of Blood Banks Standards, FFP is thawed at 37°C for 30 minutes to 40 minutes, after which it can be used in clinical situations within a 24-hour period; if not used within the 24 hours, the thawed FFP can be relabeled and designated as TP.7,8 Both FFP and FP24 are approved for transfusion up to 5 days after thawing, when stored at 1°C to 6°C. We, and others, have shown that levels of coagulation factors in plasma are variable and change with storage. However, there are no published studies correlating the age of plasma and clinical outcomes. Furthermore, there are no studies conclusively documenting what constitutes adequate levels of hemostatic components for patients with ACoTS.
In this study, we applied a comprehensive set of in vitro measures to (a) further characterize the HP and clot forming kinetics of FFP-0, FFP-5, and FP24-0, FP24-5; and (b) relate changes in HP to the functional assays of clotting factors, physiologic inhibitors, and cellular MPs.
METHODS
Plasma
Frozen plasma units from 30 single donors were obtained from the Gulf Coast Regional Blood Center (Houston, TX). All the FFP units used in the study were donated and frozen at the blood donation center <2 months before our experiment. The mean donor age was 42.5 years (range, 17–66 years), 47% were from male donors. Plasma samples were divided into four groups according to plasma separation time from whole blood before freezing (FFP vs. FP24) and a storage time after thawing (day 0 vs. day 5; Table 1). Fourteen plasma units were FFP and the rest were FP24. Plasma units included different blood groups: 13O, 11A, 4B, and 2AB. The blood group O to non-O ratio was 1:1.8 in FFPs and 1:1 in FP24s. Frozen plasma was thawed in a water bath at 37°C according to standard operating procedures8 and stored at 1°C to 6°C for 5 days. Aliquots were taken from each of the 30 TP units on day 0 (FFP-0 or FP24-0) and day 5 (FFP-5 or FP24-5), centrifuged, frozen, and stored at −80°C until use for parallel testing. All plasma assays were measured concurrently to minimize differences attributable to variable assay conditions.
TABLE 1.
Plasma Groups Definitions
| Group | Label | Assay Conditions | Criteria |
|---|---|---|---|
| I | FFP-0 (n = 14) | Freshly thawed plasma, day 0 | Plasma separated and frozen within 8 h of blood collection (<8 h) |
| II | FFP-5 (n = 14) | Refrigerated thawed plasma, day 5 | Plasma separated and frozen within 8 h of blood collection (<8 h) |
| III | FP24-0 (n = 16) | Freshly thawed plasma, day 0 | Plasma separated and frozen within 24 h of blood collection (>8 h and <24 h) |
| IV | FP24-5 (n = 16) | Refrigerated thawed plasma, day 5 | Plasma separated and frozen within 24 h of blood collection (>8 h and <24 h) |
Plasma samples were characterized for the following functional and quantitative measures: (a) thrombin generation (TG), using the Calibrated Automated Thrombogram (CAT; Thrombinoscope, Maastricht, Netherlands), using the platelet poor plasma-low (PPP-low), platelet rich plasma, and MP reagents, providing reaction concentrations of 1 pM tissue factor (TF)/4 μmol/L phospholipid (PL), 1 pM TF/no PL, and no TF/4 μmol/L PL, respectively; (b) Thromboelastography (TEG 5000 Thromboelastograph Analyzer; Hemoscope, Niles, IL), which measures the kinetics of clot formation and clot strength and stability; and (c) residual cell and MP phenotypes, and MP procoagulant activity, using flow cytometry and a functional prothrombinase assay (Zymuphen MP-activity; Hyphen BioMed, Neuville-sur-Oise, France).
TG Assay
In this study, the HP of FFP and FP24 samples were analyzed by TG assay,9 using the CAT (Thrombinoscope), with PPP and PPP-low reagents, providing reaction concentrations of 5 pM and 1 pM TF, and 4 μmol/L PL. The CAT assay renders a TG curve that provides information on several parameters including the lag time (representing the time until initial thrombin had formed [min], the thrombin peak height [nM thrombin], the time to peak [ttPeak, min], the endogenous thrombin potential [reflects the area under the curve], and the rate of TG [mean slope = peak height/{time to peak − lag time}]). When assayed in a donor plasma unit, TG estimates its HP for correcting the impaired hemostasis in a patient when transfused, whereas when assayed in a patient plasma sample, TG reflects current intravascular functionality and hemostatic capacity of the plasma.
Thromboelastography
The kinetics of plasma clot formation and clot strength and stability were assayed by TEG (TEG 5000 Thromboelastograph Analyzer).10 Clot formation was triggered with a recombinant TF (Recombiplastin; Instrumentation Laboratory, Lexington, MA) final concentration 3 pM. The analyzed TEG parameters included split point (SP, representing the time to initial fibrin formation), reaction time to clot initiation (R), the speed of clot propagation (α-angle), final clot strength as maximum amplitude (MA), and time to MA (TMA). The analysis also included a set of parameters from the mathematical first derivative of the standard TEG: maximum rate of thrombus generation (MRTG), time to MRTG (TMRTG), and total thrombus generation, as potentially more sensitive measures of clot growth.
Coagulation Factors and Inhibitors
Plasma samples were analyzed for a panel of coagulation factors and physiologic inhibitors on the ACL TOP coagulation analyzer (Instrumentation Laboratory). Measurements included prothrombin time, activated partial thromboplastin time, fibrinogen, coagulation factors II, V, VII, VIII, IX, X, XI, XII, XIII, protein C (PC), free Protein S antigen and activity, von Willebrand factor (vWF) antigen and activity, antithrombin, plasminogen, plasmin inhibitor, and D-dimer. Thrombin-activatable fibrinolysis inhibitor (total) was analyzed using an enzyme-linked immunosorbent assay (ELISA; American Diagnostica), and protein C inhibitor (PCI) activity was measured by an in-house immunoassay.11
Cellular MPs
Microparticle counts and phenotypes were analyzed by multicolor flow cytometry.12 Briefly, MPs were identified and defined as particles less than 1 μm in size. Phenotypes include platelet (PMP), red blood cell, leukocyte, monocyte, endothelial cell, TF-positive, and total Annexin-V-positive MPs, which were assayed using the following antibodies: CD41-PE, CD235a-PE, CD45-PC5, CD14-FITC, CD144-PE, CD142-PE, and Annexin-V FITC, respectively. Thirty microliters of plasma was mixed with 10 μL of lineage-specific monoclonal antibodies or with Annexin-V FITC. After 30-minute incubation at room temperature in the dark, samples were diluted in 1 mL of a 0.22 μm-filtered sheath fluid (Isoflow, Beckman Coulter, Miami, FL) or Annexin-V binding buffer. The flowcount beads (Flow-Count, Beckman Coulter) with known concentration were added to samples before reading and used to calculate the absolute MP number per μL of plasma.
Statistical Analysis
The results are expressed as mean with SE of means (mean ± SEM) or median (interquartile range) levels. Statistical differences between FFP-0 and FFP-5 and between FFP and FP24 were evaluated with a paired Student’s t test. Statistical comparisons of mean activities in blood group O versus non-O, and male versus female plasma donors were accomplished using an unpaired Student’s t test assuming either equal or unequal variance, as appropriate. Pearson’s correlation coefficient (r) was calculated and tested for statistical significance to determine correlation between two continuous variables. A p value of less than 0.05 was considered statistically significant. Contributions of clotting factor changes between FFP-0 and FFP-5 to changes in CAT and TEG parameters were evaluated by multivariate regression analysis, after adjusting for age, gender, and ABO blood group.
RESULTS
TG Assay
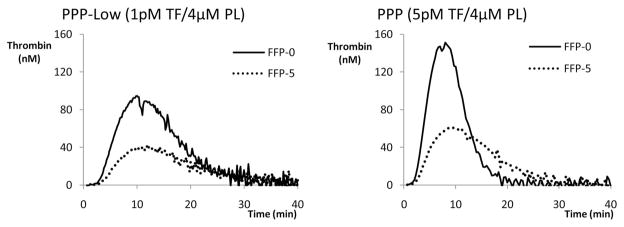
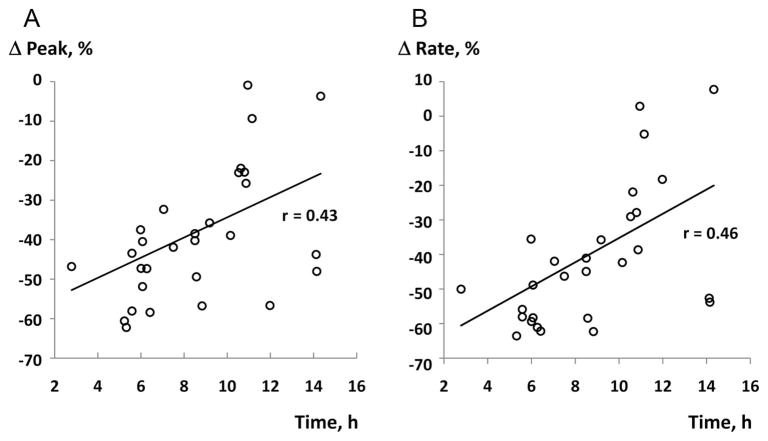
Analysis of thrombograms demonstrated significant changes in all parameters between day 0 and day 5. The largest decline was observed for the rate and the peak thrombin in FFP-5 (58.1% and 47.3%, both p < 0.0001) with PPP-low reagent, as shown in Table 2. Similarly, comparison of FP24-0 and FP24-5 thrombograms demonstrated a statistically significant decline in all parameters. When TG parameters were compared between FFP-0 and FP24-0, higher values of thrombin peak and rate, and a shorter time to peak, were noticed in FP24 (Table 2). Representative tracings of single plasma day 0 and day 5 thrombograms with PPP-low and PPP reagents are presented at Figure 1. When thrombogram parameters were analyzed according to the time (duration) of whole blood storage before plasma separation, there was a significant inverse correlation between the time of plasma separation and changes in the rate of TG, and thrombin peak (Fig. 2). This association indicates the largest decline in HP of plasma separated earlier. None of the CAT parameters were influenced by donor’s age, gender, or ABO blood group (data not shown).
TABLE 2.
Thrombin Generation Results in FFP-0 vs. FFP-5 and FP24-0 vs. FP24-5
| FFP-0 | FFP-5 | Δ, % | FP24-0 | FP24-5 | Δ, % | p* | |
|---|---|---|---|---|---|---|---|
| PPP-low reagent | |||||||
| Lag time (min) | 3.5 (3.4–4.2) | 4.2 (4–4.8) | 21.2† | 3.6 (3.3–3.9) | 4.5 (4.2–4.8) | 23.7† | NS |
| Endogenous thrombin potential (nM thrombin · min) | 1,449 (1,268–1,515) | 1,016 (841–1,156) | 24.5‡ | 1,293 (1,243–1,470) | 1,131 (854–1,255) | 14.7‡ | NS |
| Peak thrombin (nM) | 93 (88–139) | 54 (46–83) | 47.3† | 141 (116–161) | 87 (60–130) | 32.1† | 0.04 |
| Time to peak (min) | 10.6 (9.8–13) | 12.6 (10.5–14.2) | 17.9‡ | 9.3 (8.1–10.2) | 10.9 (8.5–11.8) | 14.6‡ | 0.03 |
| Rate (nM/min) | 14.3 (10.6–22.8) | 6.2 (5.1–12.6) | 58.1† | 22.1 (19.1–33) | 13.3 (10.5–27.9) | 33.8‡ | 0.04 |
| PPP reagent | |||||||
| Lag time (min) | 1.8 (1.7–1.9) | 2.3 (2.3–2.4) | 27.3† | 2.1 (2.0–2.3) | 2.7 (2.5–3.0) | 25.0† | 0.03 |
| Endogenous thrombin potential (nM thrombin · min) | 1,196 (1,090–1,382) | 1,145 (1,032–1,214) | 6.0§ | 1,171 (1,089–1,356) | 1,004 (936–1,278) | 10.0§ | NS |
| Peak thrombin (nM) | 216 (194–241) | 189 (152–204) | 16.2‡ | 207 (194–225) | 173 (146–202) | 20.4§ | NS |
| Time to peak (min) | 4.8 (4.5–5.0) | 5.6 (5.4–5.8) | 18.1‡ | 5.4 (4.9–6.1) | 6.5 (5.7–6.7) | 17.6‡ | NS |
| Rate (nM/min) | 80.9 (53.2–91.1) | 60.4 (43.5–63.9) | 27.2† | 58.0 (49.8–84.4) | 47.2 (39.0–56.3) | 31.4§ | NS |
Values are represented as median (interquartile range).
Statistical significance between FFP-0 and FP24-0.
p < 0.0001.
p < 0.001.
p < 0.05.
Figure 1.
Representative thrombogram tracings of FFP-0 and FFP-5 with PPP-low and PPP reagents. HP of plasma significantly decreased on day 5.
Figure 2.
Correlations of the plasma separation time with changes (Δ, %) in CAT parameters: (A) peak thrombin, and (B) rate of TG.
Thromboelastography
We have shown that TEG parameters (SP, R, TMA, and TMRTG) significantly change during 5 days of refrigerated plasma storage (manuscript submitted), and that the removal of MPs by filtration further reduces plasma clotting properties, significantly affecting MA and G. In this study, we evaluated the effects of plasma preparation times on its clot forming properties, and the results are presented in the Table 3. Noticeably, the majority of parameters declined between day 0 and day 5 in FFP. This was also true for the FP24 with the exception of MRTG, which was significantly higher in FP24-5 compared with FP24-0 (6.7 vs. 7.0, p < 0.02). However, comparison of FFP and FP24 revealed shorter SP, R, TMA, TMRTG, and higher MRTG in FP24, indicating its greater ability to form a clot compared with FFP. There was no difference in TEG values when analyzed by donor’s age, gender, or ABO blood group (data not shown).
TABLE 3.
Thromboelastography Values for FFP-0 vs. FFP-5 and FP24-0 vs. FP24-5
| Variable | FFP-0 | FFP-5 | Δ (%) | FP24–0 | FP24–5 | Δ (%) | p* |
|---|---|---|---|---|---|---|---|
| Split point, SP (min) | 2.3 (2.2–2.4) | 3.5 (3.1–3.6) | 53.4† | 1.9 (1.8–2.2) | 3 (2.5–3.3) | 44.7‡ | <0.02 |
| Reaction time, R (min) | 3.2 (3.0–3.3) | 4.3 (3.9–4.7) | 40.7† | 2.7 (2.2–3.0) | 3.7 (2.8–4.0) | 31.5‡ | <0.01 |
| Alpha angle (degrees) | 47.9 (41.5–51.2) | 42.9 (33.7–53.1) | 5.8 | 51.9 (45.2–55.8) | 53.6 (48.7–60.6) | 3.5 | NS |
| Maximum amplitude, MA (mm) | 29 (25–30) | 28.1 (21.9–30.20) | 0.9 | 24.1 (22.7–28.2) | 26 (21.7–29.8) | 6.4 | NS |
| Time to MA (min) | 15.1 (13.6–16.2) | 18.3 (16.4–20.1) | 19.9§ | 14.3 (12.7–15.4) | 14.8 (14.2–16.3) | 6.3 | <0.03 |
| Maximum rate of thrombus generation, MRTG (mm/min) | 5.9 (4.4–6.6) | 4.9 (3.3–6.8) | 13.6|| | 6.7 (4.8–7.4) | 7.0 (5.7–8.3) | 6.5 | 0.02 |
| Time to MRTG (min) | 4.6 (4.2–4.8) | 5.7 (5.1–6.2) | 23.7‡ | 3.6 (3.2–4.1) | 4.8 (3.7–5.2) | 20.7§ | 0.002 |
| Total thrombus generated, TTG (mm/min) | 374 (330–386) | 373 (301–401) | 1.4 | 322 (300–367) | 342 (297–386) | 5.6¶ | NS |
Values are represented as median (interquartile range). * Difference between FFP-0 and FP24-0.
p < 0.0001.
p < 0.002.
p < 0.01.
p < 0.05.
p = 0.05.
Coagulation Factors and Inhibitors
The results for mean activities of coagulation factors and physiologic inhibitors in FFP-0 and FFP-5 for all ABO blood groups, expressed as a percentages of calibration plasma which was referenced against WHO International Standards, and the percentages of change in factors between FFP-0 and FFP-5 are presented in Table 4. During storage, multiple factors decreased. We observed significant changes in 15 of 24 analytes in FFP-5 compared with FFP-0. The largest decreases were noted for FV (30%), FVIII (24.5%), free Protein S activity (23.4%), FVII (18.9%), and vWF activity (15.7%). There were no significant changes in fibrinogen, FXIII, antithrombin, protein C, plasminogen, plasmin inhibitor, D-dimer, and thrombin-activatable fibrinolysis inhibitor.
TABLE 4.
Coagulation Factors and Inhibitors in Thawed Plasma Day 0 and Day 5 (All ABO Groups)
| Analyte | Day 0 | Day 5 | % Change | p* |
|---|---|---|---|---|
| Prothrombin time (sec) | 11.2 (10.7–11.6) | 13.3 (12.7–13.8) | 18.5 | <0.0001 |
| International normalized ratio | 1.02 (0.97–1.05) | 1.2 (1.15–1.25) | 18.8 | <0.0001 |
| Activated partial thromboplastin time (sec) | 36.4 (33.7–37.7) | 39 (36.8–42.7) | 9.5 | <0.0001 |
| Fibrinogen (mg/dL) | 299 (272–323) | 297 (267–331) | 1.0 | NS |
| Factor II (%) | 95.5 (90.5–105.4) | 90.5 (85.8–96.9) | 5.5 | <0.0001 |
| Factor V (%) | 73.2 (69.3–86.7) | 53.1 (41.8–61.5) | 30.0 | <0.0001 |
| Factor VII (%) | 97.1 (85.2–116.3) | 77.2 (69.8–94.1) | 18.9 | <0.0001 |
| Factor VIII (%) | 83.6 (68.5–99.3) | 59.9 (47.8–73.9) | 24.5 | <0.0001 |
| von Willebrand factor antigen (%) | 117.9 (85.6–155.6) | 100.5 (74.8–145.1, 55.3) | 8.3 | <0.01 |
| von Willebrand factor activity (%) | 102.4 (79.1–122.3) | 77.6 (47.4–103.5) | 15.7 | <0.0001 |
| Factor IX (%) | 112.3 (100.9–115.9) | 104.5 (97.8–106.7) | 6.0 | <0.0001 |
| Factor X (%) | 94.2 (85.2–102.8) | 93.1 (83.7–97.8) | 2.4 | <0.0001 |
| Factor XI (%) | 94.6 (85–102) | 91.9 (82.2–100.6) | 2.8 | <0.001 |
| Factor XII (%) | 104.6 (87–114.4) | 99.3 (84.4–116.2) | 1.1 | <0.05 |
| Factor XIII (%) | 110 (90.9–126) | 102.8 (89.8–116.2) | 2.6 | NS |
| Antithrombin (%) | 96 (93–104) | 96 (91.5–102.5) | 0 | NS |
| Protein C (%), chromogenic | 99 (91–114.5) | 99 (93–114.5) | 0 | NS |
| Protein C (%), clotting | 103.6 (93.7–123.1) | 106.2 (96–126) | −0.6 | NS |
| Free Protein S antigen (%) | 93.7 (82.2–99.3) | 90.8 (79.9–98.1) | 2.1 | <0.001 |
| Free Protein S activity (%) | 87.5 (71.8–100.4) | 64.4 (53.3–77.9) | 23.4 | <0.0001 |
| Plasminogen (%) | 92.2 (84.9–100.3) | 92.1 (85.4–99.7) | 0.2 | NS |
| Plasmin inhibitor (%) | 99.2 (92.3–105.4) | 99.4 (92.7–105.1) | 1.0 | NS |
| D-dimer (ng/mL) | 148 (119–255) | 167 (113–261) | 3.5 | NS |
| Protein C inhibitor (%) | 97 (88–108.3) | 94 (84.3–106) | 3.5 | 0.02 |
| Thrombin-activatable fibrinolysis inhibitor (%) | 82 (71.5–91.8) | 80.5 (74.8–91.5) | 1.2 | NS |
Values are represented as median (interquartile range).
Statistical significance of the difference between day 0 and day 5.
As expected, when factors were analyzed by blood group, the ABO blood group-dependent variations in levels of FVIII and vWF were noted. Blood group O had significantly lower activities of FVIII (67% [55–82%] vs. 99% [81–104%], p < 0.001) and VWF (81% [65–93%] vs. 110% [95–128%], p < 0.01), and a longer activated partial thromboplastin time (37.2 seconds [36.4–39.9 seconds] vs. 34.7s [33.2–36.6 seconds], p < 0.02) when compared with non-O blood groups. The comparison of coagulation proteins by gender showed significantly higher activity of plasmin inhibitor (105% [100–107%] vs. 93% [91–96%], p < 0.001), and lower activity of protein C inhibitor (90% [80–99%] vs. 103% [97–115%], p < 0.02), in females. There was no difference in factor levels by donor age.
When analyzed by the time of plasma separation, only FV declined significantly more in FFP24 compared with FFP (37% vs. 27%, p < 0.01), whereas FVIII showed a trend toward lower levels but did not reach statistical significance (data not shown).
Cellular MPs
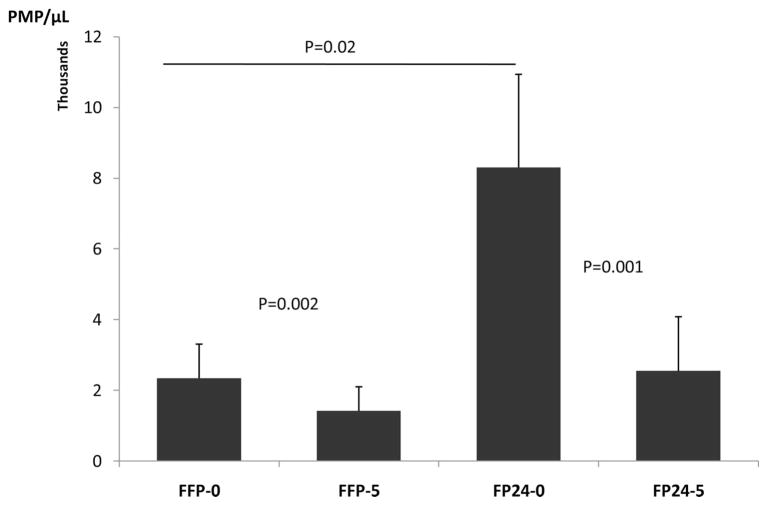
The majority (95%) of MPs in plasma originated from platelets, and the rest from other blood and vascular cells. When the PMP counts were analyzed in relation to duration of whole blood storage (<8 hours vs. >8 hours and <24 hours) before plasma separation, a significantly higher PMP count was observed in FP24-0 compared with FFP-0 (8,302/μL vs. 2,344/μL, p = 0.02). However, PMP levels declined both in FFP-5 and FP24-5 during storage by 40% (p = 0.002) and 69% (p = 0.001), respectively, (Fig. 3). Furthermore, there was a correlation between PMP count and time of plasma separation in FP24 but not in FFP. This indicates longer blood storage, before plasma separation, may contribute to PMP generation. The trend toward increased MPs of other cell types was also noted but did not reach statistical significance (data not shown).
Figure 3.
PMP levels in FFP-0, FFP-5, FP24-0, and FP24-5. PMP levels were significantly higher in FP24-0 compared with FFP-0. However, PMP levels declined both in FFP-5 and FP24-5 during storage by 40% and 69%, respectively.
Multivariate Analysis
The results of a multivariate analysis identifying the association of changes in individual variables with changes in plasma’s HP and ability to form a clot are presented in Table 5.
TABLE 5.
Individual Coagulation Factor Changes Associated With Changes in Plasma’s Hemostatic Potential (CAT) and Ability to Form a Clot (TEG)
| FII | FV | FVII | FVIII | FIX | FX | FXI | FXII | vWFag | vWFact | Free PS Act | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAT | |||||||||||
| Lag | x | x | x | x | |||||||
| ETP | x | x | x | x | x | x | x | ||||
| ttPeak | x | x | |||||||||
| Rate | x | x | |||||||||
| TEG | |||||||||||
| SP | x | x | x | x | x | x | |||||
| R | x | x | x | x | x | ||||||
| MA | x | ||||||||||
| TMA | x | x | x | x | x | ||||||
| MRTG | x | x | x | ||||||||
| TMRTG | x | x | x | x | x | x | |||||
Lag, lag time; ETP, endogenous thrombin potential; ttPeak, the time to peak; Rate, the rate of thrombin generation; SP, split point; R, reaction time; MA, maximum amplitude; TMA, time to MA; MRTG, maximum rate of thrombus generation (MRTG); TMRTG, time to MRTG.
DISCUSSION
This study demonstrates that the standard 5-day storage of TP decreases the capacity to generate thrombin, the central mediator of fibrin generation, and the ability to form a fibrin clot. Both processes were significantly reduced in FFP-5 versus FFP-0. Multiple clotting factors and PMPs also significantly declined. Interestingly, plasma prepared after extended whole blood storage (>8 hours and <24 hours) retained better HP compared with plasma separated within 8 hours. These findings are particularly relevant at trauma centers whose current protocols use TP so that they can infuse plasma sooner to massively bleeding trauma patients, where ≈30% of severely injured patients are coagulopathic on admission.
By definition, each mL of fresh normal plasma should contain 1 IU of each coagulation factor (=100%). However, there is significant variation in levels and activity of coagulation factors in an individual blood donor, resulting in a wide “normal range.” The Council of Europe requires testing of factor VIII levels in 10 randomly selected plasma units in the first month of storage and indicates that plasma containing at least 0.7 IU/mL of FVIII is satisfactory for transfusion.13 There are no requirements for evaluation of other clotting factors. Regulatory agencies in the United States do not have similar guidelines.
FFP also contains cellular MPs, which interact with clot formation. Information about the cellular MPs and their contribution to hemostatic capacity of stored blood products are limited.14,15 Based on FDA Guidance for Industry and transfusion guidelines from other regulatory agencies, it is assumed that TP transfused at day 5 will provide sufficient clotting proteins to bleeding patients; however, there are no clinical data supporting this widely held opinion. There are several reports describing the stability of common clotting factors in TP under different storage conditions and/or preparation and preservation methods.16–18 However, functional measures presented in this study indicate that despite acceptable levels of coagulation factor activity, the overall HP of TP is significantly reduced. In addition, the decline in HP seems to be inversely correlated with the time of plasma separation, such that the HP of FP24 decreases less than that of FFP.
TG is a key process that determines the extent of a hemostatic plug or a thrombotic process. The thrombin burst is crucial for the formation of a stable fibrin clot. Thrombogram analysis has been increasingly used in evaluating hemostatic capacity in various inherited or acquired bleeding or thrombophilic conditions and is often correlated with the risk prediction for bleeding or thrombosis.19,20 Dysregulated thrombogram results have been reported in trauma patients with acute coagulopathy at admission; however, the association of an abnormal laboratory measurement and clinical outcomes was not explored.21
As mentioned previously, ACoTS is the acquired hypocoagulable state that occurs in the most severely injured patients who often require MTs. The mechanism of the ACoTS seems to be multifactorial and is not fully understood. It is the sum of the effects of blood loss and dilution, coagulation factor and platelet consumption, hypothermic platelet dysfunction, acidosis-induced decreases in coagulation factor activity, and fibrinolysis. Recent research showed that the combination of trauma and shock in patients and in mouse model was associated with an activation of PC, early coagulopathy, and poor outcomes.3,4 Once generated, APC exerts its anticoagulant effects by irreversibly inactivating factors Va and VIIIa and through derepression of fibrinolysis by directly inhibiting plasminogen activator inhibitor. However, the mechanism of this PC activation is, as yet, unknown. Importantly, if elevated APC is a major player in the ACoTS, not only is the HP of transfused plasma important but also the ability of that plasma to generate thrombin in the presence of elevated APC.
The present data show significant decreases in multiple coagulation factors, which has been widely reported.16–18 Similarly, the inherent variability in coagulation factors between individual donors is well described. The activities of the factors V, VII, and VIII, free Protein S, and vWF decreased with storage of TP. With the exception of decreased levels of factor V, there was no major difference between FFP and FP24. According to current opinion, TP contains hemostatic levels of coagulation factors necessary for bleeding patients. Restoring in vivo clotting function toward normal is widely considered important in patients with massive hemorrhage and severe hemorrhagic shock. In cases of massive blood loss requiring infusion of large amounts of asanguineous fluids and blood products, coagulation factors are reduced to 30% of normal levels after the loss of one blood volume (10 units of blood components) and to 15% after the loss of two blood volumes. Although 30% to 40% is considered to be a minimum individual factor activity level required for safe surgical hemostasis in euvolemic and normothermic surgical patients,22 there are no data regarding factor activity levels required for hemostasis in severely injured patients suffering from hypothermia, acidosis, hemodilution, and multiple coexisting factor depletions. The potential clinical significance of transfusing a plasma product with preserved HP, especially in MT patients, is clear. At the very least, clinicians should be knowledgeable about the products they are ordering and receiving from the blood bank.
Our results show that although individual factor levels remained above 50% of normal levels in TP stored for 5 days, the overall HP significantly diminished by as much as 58%. When multiple in vivo factors are depleted, which is often the case in major trauma with bleeding, it is difficult to estimate the necessary factor levels for adequate hemostasis, and it is likely that replacement with a plasma product (FFP-5 or FP24-5) that has decreased factor activity levels will be inadequate to restore in vivo hemostatic capacity. The results further indicate that global functional tests of hemostasis, TG and TEG, are likely a better reflection of the HP of plasma to support adequate hemostasis than the simple retention of clotting factor levels.
Interestingly, a recent report indicated that low plasma thrombin peak (<100 nM) was associated with ongoing bleeding in patients during major surgery. Furthermore, either impaired TG or insufficient fibrin clot formation was present in 88% to 93% of patients with acquired dilutional coagulopathy during major surgery, whereas both tests were low in 50% to 59% of cases with bleeding.23 Hence, transfusing plasma or other hemostatic products with preserved and persistent HP for the TG and clot formation, especially in MT patients, is likely of great significance. Importantly, studies are still required to evaluate their clinical efficacy.24
Our studies demonstrated that platelet-derived MPs decreased significantly during storage, contributing to a lower HP of TP. MPs are small cellular membrane fragments (<1 μm) shed from activated or apoptotic blood and EC.25 They express PS, adhesion receptors, and TF, rendering them highly procoagulant. MPs are considered essential for hemostasis, as they expose PLs on their surface, providing binding sites for activated clotting factors. Comparison of MP levels in FFP and FP24 revealed increased levels of PMPs in FP24, suggesting cell-derived MPs, generated during extended blood storage, contribute to functional hemostasis in stored plasma.26 Lawrie et al.14 reported elevated red blood cell-derived MPs in overnight stored whole blood before separation of plasma. This finding is in line with reports of increased PMPs in platelet concentrates during storage. The clinical relevance of PMPs in supporting coagulation was documented in leukemic and thrombocytopenic patients, showing that patients with high levels of circulating PMPs did not bleed despite having low platelet counts. In addition, the presence of high levels of PMPs in cryoprecipitate is considered to contribute to its therapeutic effects in bleeding patients,15,27 and it has been reported that PMPs have 100-fold higher procoagulant activity than platelets. The combined activities and interactions of clotting factors, inhibitors, MPs, and other plasma components contribute to plasma’s overall hemostatic phenotype as a determinant of its hemostatic capacity. However, the physiologic and pathophysiological roles of circulating MPs generated intravascularly from various blood and vascular cells are largely unknown and can be beneficial or deleterious depending on the endogenous disease state. They may have potent prothrombotic and proinflammatory effects and potentially modify vascular functions.28
This study has several potential limitations. First, FFP and FP24 were collected from different donors, and we might have either under- or overestimated the differences between the two. Ideally, one should compare pairs of FFP and FP24 prepared from same donor. Second, studies were performed only at day 0 and day 5, not at other periods in between; therefore, we do not know if there is a point where HP decreases abruptly or if it is gradual. Third, to minimize differences attributable to variability in assay conditions, reagent lot numbers, or substrates, plasma aliquots were refrozen and assayed later concurrently under the same assay conditions. Keeping in mind the large number of different assays performed on plasma samples, this would not be feasible otherwise. Importantly, since the storage at ultra low temperatures minimizes analyte degradation and all the samples in our study were treated in the same manner, we believe that refreezing process likely did not have any significant impact on analyte activity. Furthermore, we did not evaluate the different hemostatic capacity of TP at the various temperatures common to severely injured trauma patients. Finally, we did not evaluate the effects of the length of FFP storage before thawing and testing. As all the FFP units used in the study were donated and frozen at the blood donation center <2 months before our experiment, we believe this short storage time (compared with 1 year of storage limit allowed by the American Association of Blood Banks Standards) did not have any significant effects on measured analytes. The greatest limitation is the lack of data regarding correlation of these in vitro tests with clinical outcomes. The separation of blood banking storage practice from clinical results is an area of clinical practice and research that must be reconciled.
Trauma and hemorrhagic shock result in major perturbations of the coagulation system and significant and systemic damage to endothelium. Our recent study showed freshly TP had beneficial effects on endothelial permeability, vascular stability, and resuscitation in an animal model of HS, whereas aged plasma exacerbated the permeability and inflammation of endothelial cells in vitro.6 The clinical consequences for patients transfused with TP possessing decreased HP, insufficient TG capacity, and delayed clot propagation warrant further in vivo investigation. The clinical benefits versus risks associated with transfusion of TP need to be evaluated.
Multiple studies have already shown an association between poor outcome and the age of transfused blood.29,30 On the basis of the multiple levels of degradation affecting HP of stored TP, we hypothesize a similar correlation between TP and adverse clinical outcomes, especially in MT trauma patients. In summary, we have demonstrated that the HP and clot forming ability of TP declines over 5 days of refrigerated storage. The clinical consequences for transfused patients are uncertain and deserve further in vivo exploration.
Acknowledgments
Supported by the US Department of Defense via a grant W81XWH-08-C-0712, titled PROMMTT (Prospective, Observational, Multicenter Massive Transfusion sTudy), and by the P50 GM38529 grant to Department of Surgery at the University of Texas Health Science Center-Houston from the National Institutes of Health.
We thank Ms. Robin Fuller from the Gulf Coast Regional Blood Center for providing assistance in procurement of plasma products and Dr. R. Michelle Sauer for her editorial support.
Footnotes
Presented in parts as posters at the Shock Society meetings in San Antonio, TX, June 6–9, 2009, and Portland, OR, June 12–15, 2010.
Presented at the 69th Annual Meeting of the American Association for the Surgery of Trauma, September 22–25, 2010, Boston, Massachusetts.
References
- 1.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23:231–240. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesebro BB, Rahn P, Carles M, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32:659–665. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 6.Pati S, Matijevic N, Doursout MF, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;69 (Suppl 1):S55–S63. doi: 10.1097/TA.0b013e3181e453d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein HG, editor. Standards for Blood Banks and Transfusion Services. 26. Bethesda, MD: AABB; 2009. [Google Scholar]
- 8.Roback JD, editor. Technical Manual. 16. Bethesda, MD: AABB; 2008. [Google Scholar]
- 9.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 10.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 11.Espana F, Hendl S, Gilabert J, Estellés A, Aznar J. Evaluation of two functional assays for protein C inhibitor/plasminogen activator inhibitor-3 activity. Thromb Res. 1993;70:375–384. doi: 10.1016/0049-3848(93)90079-4. [DOI] [PubMed] [Google Scholar]
- 12.Robert S, Poncelet P, Lacroix R, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–197. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 13.Council of Europe. Guide to Preparation, Use and Quality Assurance of Blood Components: Recommendation No. R (95) 15. 13. Strasbourg: Council of Europe Publishing; 2007. [Google Scholar]
- 14.Lawrie AS, Harrison P, Cardigan RA, Mackie IJ. The characterization and impact of microparticles on haemostasis within fresh-frozen plasma. Vox Sang. 2008;95:197–204. doi: 10.1111/j.1423-0410.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 15.Seghatchian MJ. The quality of clinical FFP, cryoprecipitate and cryosupernatant derived from single donor apheresis procedures and random donations collected in a BAT system: assessment of the activity states of FVIII, contact activation, microvesicle content and cytokines. Transfus Sci. 1997;18:115–118. doi: 10.1016/s0955-3886(96)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.Lawrie AS, Cardigan RA, Williamson LM, Machin SJ, Mackie IJ. The dynamics of clot formation in fresh-frozen plasma. Vox Sang. 2008;94:306–314. doi: 10.1111/j.1423-0410.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 17.Lamboo M, Poland DC, Eikenboom JC, et al. Coagulation parameters of thawed fresh-frozen plasma during storage at different temperatures. Transfus Med. 2007;17:182–186. doi: 10.1111/j.1365-3148.2007.00729.x. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu RS, Le T, Brimhall B, Thompson H. Study of coagulation factor activities in apheresed thawed fresh frozen plasma at 1–6 degrees C for five days. J Clin Apher. 2006;21:224–226. doi: 10.1002/jca.20095. [DOI] [PubMed] [Google Scholar]
- 19.Brummel-Ziedins K, Undas A, Orfeo T, et al. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. 2008;6:104–110. doi: 10.1111/j.1538-7836.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- 20.Gatt A, van Veen JJ, Woolley AM, Kitchen S, Cooper P, Makris M. Thrombin generation assays are superior to traditional tests in assessing anticoagulation reversal in vitro. Thromb Haemost. 2008;100:350–355. [PubMed] [Google Scholar]
- 21.Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009;49:2652–2660. doi: 10.1111/j.1537-2995.2009.02335.x. [DOI] [PubMed] [Google Scholar]
- 22.Edmunds L, Salzman E. Hemostatic problems, transfusion therapy, and cardiopulmonary bypass in surgical patients. In: Colman R, Hirsh J, Marder V, et al., editors. Hemostatis and Thrombosis: Basic Principles and Clinical Practice. 4. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 1031–1043. [Google Scholar]
- 23.Schols SE, Lance MD, Feijge MA, et al. Impaired thrombin generation and fibrin clot formation in patients with dilutional coagulopathy during major surgery. Thromb Haemost. 2010;103:318–328. doi: 10.1160/TH09-06-0396. [DOI] [PubMed] [Google Scholar]
- 24.Nascimento B, Callum J, Rubenfeld G, Neto JB, Lin Y, Rizoli S. Clinical review: fresh frozen plasma in massive bleedings—more questions than answers. Crit Care. 2010;14:202. doi: 10.1186/cc8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. [PubMed] [Google Scholar]
- 26.Keuren JF, Magdeleyns EJ, Govers-Riemslag JW, Lindhout T, Curvers J. Effects of storage-induced platelet microparticles on the initiation and propagation phase of blood coagulation. Br J Haematol. 2006;134:307–313. doi: 10.1111/j.1365-2141.2006.06167.x. [DOI] [PubMed] [Google Scholar]
- 27.George JN, Pickett EB, Heinz R. Platelet membrane microparticles in blood bank fresh frozen plasma and cryoprecipitate. Blood. 1986;68:307–309. [PubMed] [Google Scholar]
- 28.Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 29.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]