Abstract
Purpose of review
The purpose of the study was to review recent research on Rift Valley fever virus (RVFV) infection, encompassing four main areas: epidemiology and outbreak prediction, viral pathogenesis, human diagnostics and therapeutics, and vaccine and therapeutic candidates.
Recent findings
RVFV continues to extend its range in Africa and the Middle East. Better definition of RVFV-related clinical syndromes and human risk factors for severe disease, combined with early-warning systems based on remote-sensing, simplified rapid diagnostics, and tele-epidemiology, hold promise for earlier deployment of effective outbreak control measures. Advances in understanding of viral replication pathways and host cell-related pathogenesis suggest means for antiviral therapeutics and for more effective vaccination strategies based on genetically engineered virus strains or subunit vaccines.
Summary
RVFV is a significant health and economic burden in many areas of Africa, and remains a serious threat to other parts of the world. Development of more effective methods for RVFV outbreak prevention and control remains a global health priority.
Keywords: Rift Valley fever; Rift Valley fever virus; arbovirus infections; hemorrhagic fevers, viral; vaccines
Introduction
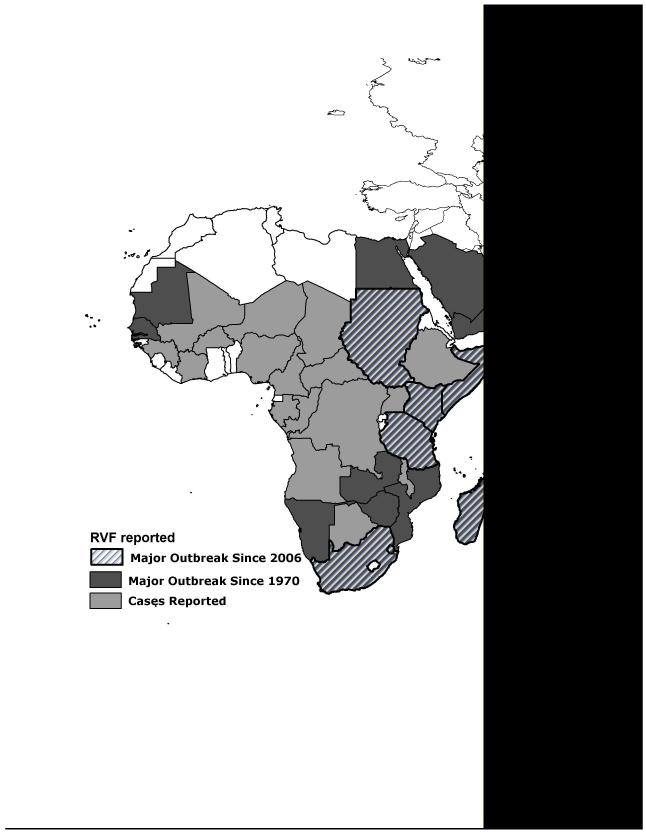
Rift Valley fever virus (RVFV), the cause of Rift Valley Fever (RVF), is a zoonosis endemic to Africa and the Arabian peninsula that now represents a looming health threat to Europe, Asia, and the New World [1,2]. Areas with the most recent RVF outbreaks include Somalia (2006-7), Kenya (2006-7), Tanzania (2007), Sudan (2007-8), Mayotte (2007-8), Madagascar (2008), Swaziland (2008) and South Africa (2008, 2009, and 2010) (see Figure 1). In affected areas, RVFV epizootics cause catastrophic livestock losses, while concurrent epidemics result in a significant number of severe human cases, including RVFV-associated encephalitis, retinitis (leading to blindness) and, in ~ 1% of cases, a highly lethal hemorrhagic fever [3-4, 5••, 6-8]. New data describing human RVFV disease are helping to inform clinical and public health practice, and should enable earlier recognition of RVFV outbreaks. Because no disease-specific treatments are currently available, rapid deployment of measures to prevent RVFV transmission are essential for disease control. New research programs that focus on prediction of outbreaks, transmission factors, human disease determinants, rapid diagnosis of RVFV infection, and vaccine development, are all top priorities in RVFV research. Efforts to prevent RVFV transmission in endemic areas may halt the emergence of RVFV globally, as new evidence supports the idea that once introduced, RVFV may easily embed itself in North American ecosystems [9].
Figure 1. Map of current world distribution of Rift Valley fever virus.
Diagonal lines indicate countries reporting major RVF outbreaks since 2005, solid dark gray indicates countries reporting major RVF outbreaks since 1970, light gray areas indicate countries that have previously reported human or animal cases of RVFV infection or evidence of RVFV transmission. Sources: U.S. Centers for Disease Control [3], and OIE-World Organization for Animal Health.
Outbreak Prediction
One way that RVFV is maintained in nature is via transovarial transmission in flood-water Aedes mosquitoes [10] (and perhaps other species, such as Anopheles arabiensis [7]). After periods of prolonged or heavy rainfall, depressions in the terrain fill with standing water, and dormant infected eggs hatch into feeding mosquitoes that reintroduce RVFV locally (Figure 2). Persisting rainfall results in large blooms of other competent mosquito ‘bridge vectors’ (e.g., Culex spp.) that magnify an early outbreak by transmitting RVFV among amplifying vertebrate hosts, including cattle, goats, and sheep. Because RVFV outbreaks generally follow anomalous heavy rainfall in endemic areas [8,11,12], meteorological forecasting of extreme weather events has been shown to be a useful tool to predict RVFV activity [13]. The most recent outbreak of RVFV in East Africa was forecast using climate prediction tools, which provided a modest early warning [14]. The 2-6 week alert enabled deployment of vector surveillance, but was not early enough to enable widespread livestock vaccination or implementation of vector control to prevent spread of the disease. Future research will need to focus on providing even earlier warnings for all stakeholders, so that prediction can have greater impact on mobilization of preventive interventions. Integrated tele-epidemiologic approaches are being developed that link remotely-sensed data with real-time vector and host data to enable fine-scale RVF prediction in areas with varied landscapes and changing weather patterns [15]. Such research, combined with proactive monitoring, will advance our understanding of the factors involved in the initiation and maintenance of RVFV outbreaks.
Figure 2. Pathways of Rift Valley fever virus transmission and development of human RVF disease.
Arrows indicate the progression of virus transmission from the local reservoir maintained by vertical transmission of virus among floodwater mosquitoes to local amplifying livestock, and onward to human populations in the same locales. The time from initial flooding to the first reports of severe human cases may be 3 months or more.
New Knowledge on Viral Pathogenesis
Rift Valley fever virus is a negative sense single stranded RNA virus with a tripartite genome consisting of small (S), medium (M), and large (L) segments [16]. It is a member of the genus Phlebovirus in the family Bunyaviridae. Cryoelectron tomography has recently revealed that RVFV, once thought to be pleomorphic, has, an icosahedral structure, with a T=12 triangulation number. Among viruses, this type of structure is only known to occur in Uukuniemi virus, a related phlebovirus [17]. Analysis of RVFV strains collected during the recent Kenyan outbreak in 2006-2007 has demonstrated the concurrent circulation of multiple virus lineages that manifest gene segment reassortments but share common ancestry from the 1997-1998 East African outbreak of RVF [18•]. This analysis also indicated continuing RVF virus circulation and evolution during the 1998-2006 interepizootic/epidemic period. Of note, wildlife surveys in Kenya have further demonstrated the continued circulation of RVFV among many different types of wildlife during inter-epizootic periods [19]. Additional research is needed to evaluate the significance of these wild vertebrate species in maintaining RVFV transmission in high-risk ecosystems.
Many bunyaviruses, including RVFV, produce a non-structural protein encoded by the S gene segment, known as NSs protein, which serves as an important virulence factor [20-22, 23•]. NSs protein is known to interfere with host transcription and antagonize beta- interferon (IFN-β) production, thereby limiting early phases of host innate immunity and likely increasing pathogenesis [24-26]. A new role for NSs protein was recently defined; beyond IFN-β suppression, NSs protein was also found to downregulate dsRNA-dependent protein kinase (PKR), a ubiquitous protein that suppresses viral translation in response to viral replication [27]. By downregulating PKR, RVFV is able to effectively translate its viral proteins and replicate despite deficient cellular transcription. This downregulation of PKR was shown to be due to the degradation of PKR via the proteasome [21]. Finally, an additional virulence factor, the NSm protein (a non-structural protein encoded on the M segment of RVFV) has been shown to suppress viral-induced apoptosis of infected cells [28].
Although RVFV replicates in host cell cytoplasm, it has been known that NSs protein forms filamentous structures in the nuclei of infected cells [29,30]. A recent study has elucidated the importance of Sin3A-associated protein 30 (SAP30) and NSs interactions in host cell nuclei [26]. After RVFV infection a multi-protein complex containing NSs protein and host transcription factors, SAP30 and YY1, the activator/repressor of interferon transcription, along with other cofactors allows for repression of the IFN-β promoter and evasion of host antiviral response. Another recent study has demonstrated that NSs interacts with constitutive heterochromatin clusters of pericentromeric DNA sequences in host cells [23•]. Formation of NSs filaments (phosphorylated multimers of NSs protein) enhances interaction with heterochromatin and leads to chromosome cohesion and segregation defects, which is dependent on SAP30 interaction. This finding reinforces previous observations concerning the main role of NSs-SAP30 interaction on RVFV pathogenicity, and may help to explain the mechanism by which RVFV causes abortions and fetal deformities in infected animals.
Risk Factors for Human Infection and Disease
Although RVF garners the most attention during its intermittent, large-scale epidemics and epizootics [8,31], in high-risk areas, interepidemic transmission to humans has been well documented [6]. Older age, male gender, and rural location continue to be risk factors for RVFV exposure [6,7]. Animal contacts, particularly the handling of animal abortus, have been shown to be important risk factors for human infection during both interepidemic [6] and epidemic [4] periods. A formal outbreak investigation, conducted during the recent 2006-7 outbreak of RVF in Kenya, has determined relative patient risk for developing severe vs. mild disease associated with RVFV infection [4]. Patients who had touched an aborted animal fetus had nearly 4 times the risk of subsequently developing severe RVF disease, and consuming or handling sick animals was found to be the only personal exposure that was associated with RVF-related deaths in this study [4]. This suggests that animal exposures (Figure 2) are the most significant mode of transmission of RVFV to humans during epidemics, and that the route of infection (aerosol vs. insect bite) may be of particular importance in determining the subsequent progress of infection and the course of clinical disease.
Human RVF Disease
RVFV causes a wide range of disease in humans, varying from a week-long undifferentiated febrile illness to hemorrhagic diathesis and death [5••]. Ophthalmologic complications remain important sequelae of human RVF disease and result in long-lasting visual disturbance in affected individuals [6]. Based on recent case series, we now know that, in its most severe forms, RVF can also lead to acute renal dysfunction. During the recent 2007-2008 outbreak in Sudan, a majority (60%) of patients hospitalized with RVF had renal impairment and 90% of those required dialysis [32]. Mortality was high (40%) among these patients and occurred early during hospitalization. However, survivors did not show evidence of persisting chronic renal failure.
During the most recent RVFV outbreak in Kenya, a characteristic clinical syndrome emerged for RVF and its features and time-course were documented [5••,12]. As described, the fever, malaise, and headaches seen in RVF were non-specific, but combined with large joint arthralgias (elbows, knees, and shoulders), nausea, vomiting, and mid-epigastric pain, followed by tender hepatomegaly, jaundice, and delirium, a characteristic RVF syndrome emerged. This more thorough documentation of the clinical RVF syndrome is important, in that RVFV outbreaks are typically identified only late in the course of an epidemic, because detection is nominally based on the reporting of cases of hemorrhagic fever, which is the rarest complication of RVFV infection. Identification of an RVF clinical syndrome should lead to earlier recognition of epidemics in remote, resource-poor areas where transmission would otherwise easily evade detection.
A previous case study from 2006 had suggested that RVFV can spread by vertical transmission from infected mothers to their unborn children [33]. During the recent 2007-2008 Sudanese outbreak, preterm labor due to acute RVF disease was also found to result in delivery of an anti-RVFV IgM positive baby with skin rash and hepatosplenomegaly, again suggesting that vertical transmission of RVFV probably does occur among humans [34].
Rapid Diagnosis
In endemic areas, RVFV-infection is most often diagnosed using a combination of clinical acumen and available diagnostic testing. As mentioned above, it is usually the recognition of acute hemorrhagic fever cases that triggers an outbreak investigation. Newer, multiplexed PCR and RT-PCR enzyme hybridization assays are being developed that can simultaneously detect multiple pathogens, including many hemorrhagic fever viruses [35]. During the recent RVF outbreak in Kenya, field RT-PCR testing was rapidly able to identify those individuals with high viremia who were more likely to progress to severe disease [36]. Because RVF outbreaks occur most commonly in very remote locations, field deployable techniques are essential for effective detection and control. Of note, a molecular technique for practical field deployment, the real-time reverse transcription-loop-mediated isothermal amplification assay (RT-LAMP), which does not rely on thermocycler equipment, has been designed for field diagnosis of RVFV [37•,38•]. The advantages of RT-LAMP are that it has a similar sensitivity and specificity as real-time PCR, but, in contrast, LAMP assays are one-step, single tube reactions that are less expensive and faster than traditional PCR, and can be assessed with the unaided eye. Continued focus on field deployable techniques for RVFV diagnosis is warranted in order to enable more rapid and accurate detection of both RVF outbreaks of all sizes.
Promising Vaccines and Therapeutics
Public health and animal health agencies agree that it is now a priority to develop RVFV vaccines (whether for humans, animals, or both) that will yield highly effective, long-term protective immunity. Past experience with killed inactivated RVFV vaccines has shown them to be protective but expensive to produce and inconvenient to administer, often requiring multiple booster immunizations to achieve and maintain protection [39]. In the past, live-attenuated strains of RVFV have been used during outbreaks as vaccines for susceptible livestock, but these appear to have caused miscarriage and teratogenicity among pregnant animals of some species [39,40]. The ideal RVF vaccine would confer protection after a single dose, be completely non-pathogenic with no potential for reversion to wild-type virus, be safe to produce in standard vaccine facilities, and maintain its stability at ambient temperatures for long periods of time. In addition, because of the animal-trade embargoes imposed during RVF epizootics, it is considered highly desirable that commercial livestock vaccines have the ability to ‘differentiate between naturally infected and vaccinated animals’ (DIVA) before we implement them as part of routine RVF prevention programs in livestock [41,42].
Vaccine development
Currently, different strategies are being employed to approach these vaccine goals, including development of safer, live-attenuated and genetically-engineered RVFV strains [43••], testing of chimeric alphavirus-RVFV replicon constructs [40], viral DNA-based vaccines [44], and approaches based on production of recombinant viral proteins within virus-like particles [45]. Each approach has its advantages and disadvantages. Inactivated vaccines are safe, but need repeat doses and boosting [44,45]. Replicon and viral-like particle approaches (VLPs) cannot revert to virulent forms because they are devoid of the essential genes for RVFV replication and can be differentiated between wild type infection and vaccination, but these are expensive to produce. Live attenuated vaccines are highly immunogenic and don’t require boosting, but have the safety concern of reversion to virulence [39,41]. However, a recently published reverse genetics method [46•] has been shown to produce RVFV from cDNA constructs containing specific mutations and/or viral gene deletions, which enables the manipulation of the RVFV genome to elucidate specific viral protein functions and determine the contribution(s) of their antigen loci to vaccine strain efficacy and stability [41].
For virus-free constructs, gene-gun immunizations with cDNA encoding RVFV structural proteins (G1 and G2) have been shown to induce neutralizing antibody titers in experimental animals, but some immunized mice still developed clinical signs of infection after sub-lethal challenge [44]. Another approach under development is the use of Sindbis virus replicon vectors that express RVFV Gn and Gc glycoproteins and NSm protein [40]. These replicon vectors induce protective antibody responses in immunized mice and sheep, and provided 100% protection against lethal RVFV challenge. In other research, RVFV-like VLPs have been produced efficiently by reverse genetics [47] and have been shown to be highly immunogenic and protective against lethal challenge in mice [45]. A new reverse-engineered live attenuated vaccine candidate, rZH501-ΔNSs:GFP-ΔNSm, created by replacing viral virulence genes, NSs and NSm, with a labeling gene, green fluorescent protein, is highly attenuated in rat models and results in the production of high neutralizing antibody titers [43]. This RVFV vaccine-candidate has very limited potential for reversion to wild-type RVFV virulence, and allows for DIVA with the use of simplified side-by-side ELISAs [42].
Progress on chemotherapy for enveloped viruses
For treatment of symptomatic Rift Valley Fever, no highly effective RVFV-specific therapeutics currently exist [41]. However, beyond supportive care, there is hope that viable antiviral therapeutic options will emerge. The aryl-methyldiene rhodanine derivative, LJ001, is a small molecule that intercalates in viral membranes and prevents virus-cell fusion. It has recently been shown to have broad spectrum activity against enveloped viruses such as RVFV, and may prove to be an important therapeutic adjunct in the future [48••]. Another broad antiviral strategy, bavituximab, targets exposed phosphatidylserine on the cell membranes of enveloped viruses and virus-infected cells, and this may also prove to be an important broad spectrum anti-viral therapeutic [49••].
Conclusion
Rift Valley fever virus continues to circulate among animals and humans in sub-Saharan Africa and parts of the Middle East, both during high profile, intermittent epidemics and epizootics and during interepidemic periods. New insights into the maintenance, transmission, and pathogenesis of RVFV have enabled new strides in our quest for more effective control and prevention of RVF in high-risk areas. These advances should also help us to limit the spread of this high-risk pathogen to other parts of the world. As human risk factors and RVFV-related disease syndromes become better defined, promising vaccine candidates and therapeutics may help to prevent morbidity and mortality from this emerging arboviral pathogen.
Acknowledgements
The authors have no conflicts of interest to report. Drs. LaBeaud, Kazura, and King are supported by the Midwest Research Center of Excellence (RCE) in Biodefense (funded by the US National Institute of Allergy and Infectious Diseases (NIAID) through grant U54 AI057160), and by the National Center for Foreign Animal and Zoonotic Disease Defense (FAZD Center) of the US Department of Homeland Security, which facilitated work on this manuscript. The content of this publication does not necessarily reflect the views or policies of these agencies, nor does mention of trade names, commercial projects, or organizations imply endorsement by the US Government.
This work was supported in part by funding from the NIH and the US Department of Homeland Security.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1. [accessed March 29, 2010];W.H.O. information sheet: Haemorrhagic fevers, Viral. on World Wide Web URL: http://www.who.int/topics/haemorrhagic_fevers_viral/en/
- 2.Peters CJ. Emergence of Rift Valley fever. In: Saluzzo JF, Dodet B, editors. Factors in the Emergence of Arbovirusesedition. Elsevier; Paris: 1997. pp. 253–264. [Google Scholar]
- 3. [accessed March 29, 2010];CDC Viral Hemorrhagic Fevers Fact Sheet. on World Wide Web URL: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/vhf.htm.
- 4.Anyangu AS, Gould LH, Sharif SK, et al. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am J Trop Med Hyg. 2010 doi: 10.4269/ajtmh.2010.09-0293. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Kahlon SS, Peters CJ, Leduc J, et al. Severe Rift Valley fever may present with a characteristic clinical syndrome. Am J Trop Med Hyg. 2010;82:371–375. doi: 10.4269/ajtmh.2010.09-0669. Presents a small case series of RVF human hemorrhagic fever during the last Kenyan outbreak and clarifies the clinical phenotype of disease yielding a succinct clinical syndrome that will allow for earlier recognition and classification of severe human RVF disease.
- 6.LaBeaud AD, Muchiri EM, Ndzovu M, et al. Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya. Emerg Infect Dis. 2008;14:1240–1246. doi: 10.3201/eid1408.080082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seufi AM, Galal FH. Role of Culex and Anopheles mosquito species as potential vectors of Rift Valley fever virus in Sudan outbreak, 2007. BMC Infect Dis. 2010;10:65. doi: 10.1186/1471-2334-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods CW, Karpati AM, Grein T, et al. An outbreak of Rift Valley fever in Northeastern Kenya, 1997-98. Emerg Infect Dis. 2002;8:138–144. doi: 10.3201/eid0802.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turell MJ, Dohm DJ, Mores CN, et al. Potential for North American mosquitoes to transmit Rift Valley fever virus. J Am Mosq Control Assoc. 2008;24:502–507. doi: 10.2987/08-5791.1. [DOI] [PubMed] [Google Scholar]
- 10.Linthicum KJ, Davies FG, Kairo A, Bailey CL. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic period in Kenya. J Hyg (Lond) 1985;95:197–209. doi: 10.1017/s0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies FG, Linthicum KJ, James AD. Rainfall and epizootic Rift Valley fever. Bull World Health Organ. 1985;63:941–943. [PMC free article] [PubMed] [Google Scholar]
- 12.King CH, Kahlon SS, Muiruri S, Labeaud AD. Facets of the Rift Valley fever outbreak in Northeastern Province, Kenya, 2006-2007. Am J Trop Med Hyg. 2010;82:363. doi: 10.4269/ajtmh.2010.09-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linthicum KJ, Anyamba A, Tucker CJ, et al. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 14.Anyamba A, Chretien JP, Small J, et al. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci U S A. 2009;106:955–959. doi: 10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tourre YM, Lacaux JP, Vignolles C, Lafaye M. Climate impacts on environmental risks evaluated from space: a conceptual approach to the case of Rift Valley fever in Senegal. Glob Health Action. 2009:2. doi: 10.3402/gha.v2i0.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmaljohn C, Hooper JW. Bunyaviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edition Lippincott-Raven; Philadelphia PA: 2001. pp. 1581–1602. [Google Scholar]
- 17.Freiberg AN, Sherman MB, Morais MC, et al. Three-dimensional organization of Rift Valley fever virus revealed by cryoelectron tomography. J Virol. 2008;82:10341–10348. doi: 10.1128/JVI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Bird BH, Githinji JW, Macharia JM, et al. Multiple virus lineages sharing recent common ancestry were associated with a large Rift Valley fever outbreak among livestock in Kenya during 2006-2007. J Virol. 2008;82:11152–11166. doi: 10.1128/JVI.01519-08. Demonstrated the widespread circulation of RVFV during the last Kenyan epidemic and showed the continued evolution of RVFV strains during interepidemic periods, highlighting the ongoing under-recognized regional transmission of RVFV.
- 19.Evans A, Gakuya F, Paweska JT, et al. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect. 2008;136:1261–1269. doi: 10.1017/S0950268807009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billecocq A, Spiegel M, Vialat P, et al. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habjan M, Pichlmair A, Elliott RM, et al. NSs protein of Rift Valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J Virol. 2009;83:4365–4375. doi: 10.1128/JVI.02148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikegami T, Narayanan K, Won S, et al. Dual functions of Rift Valley fever virus NSs protein: inhibition of host mRNA transcription and post-transcriptional downregulation of protein kinase PKR. Ann N Y Acad Sci. 2009;1171(Suppl 1):E75–85. doi: 10.1111/j.1749-6632.2009.05054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Mansuroglu Z, Josse T, Gilleron J, et al. Nonstructural NSs protein of Rift Valley fever virus interacts with pericentromeric DNA sequences of the host cell, inducing chromosome cohesion and segregation defects. J Virol. 2010;84:928–939. doi: 10.1128/JVI.01165-09. Demonstrated the interaction of NSs with host DNA yielding a possible mechanism for the teratogenicity and abortafacient properties of RVFV infection.
- 24.Bouloy M, Janzen C, Vialat P, et al. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J Virology. 2001;75:1371–1377. doi: 10.1128/JVI.75.3.1371-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le May N, Dubaele S, Proietti De Santis L, et al. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell. 2004;116:541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- 26.Le May N, Mansuroglu Z, Leger P, et al. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 2008;4:e13. doi: 10.1371/journal.ppat.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikegami T, Narayanan K, Won S, et al. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 2009;5:e1000287. doi: 10.1371/journal.ppat.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won S, Ikegami T, Peters CJ, Makino S. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J Virol. 2007;81:13335–13345. doi: 10.1128/JVI.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanepoel R, Blackburn NK. Demonstration of nuclear immunofluorescence in Rift Valley fever infected cells. J Gen Virol. 1977;34:557–561. doi: 10.1099/0022-1317-34-3-557. [DOI] [PubMed] [Google Scholar]
- 30.Yadani FZ, Kohl A, Prehaud C, et al. The carboxy-terminal acidic domain of Rift Valley fever virus NSs protein is essential for the formation of filamentous structures but not for the nuclear localization of the protein. J Virol. 1999;73:5018–5025. doi: 10.1128/jvi.73.6.5018-5025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Outbreaks of Rift Valley fever in Kenya, Somalia and United Republic of Tanzania, December 2006-April 2007. Wkly Epidemiol Rec. 2007;82:169–178. [PubMed] [Google Scholar]
- 32.El Imam M, El Sabiq M, Omran M, et al. Acute renal failure associated with the Rift Valley fever: a single center study. Saudi J Kidney Dis Transpl. 2009;20:1047–1052. [PubMed] [Google Scholar]
- 33.Arishi HM, Aqeel AY, Al Hazmi MM. Vertical transmission of fatal Rift Valley fever in a newborn. Ann Trop Paediatr. 2006;26:251–253. doi: 10.1179/146532806X120363. [DOI] [PubMed] [Google Scholar]
- 34.Adam I, Karsany MS. Case report: Rift Valley fever with vertical transmission in a pregnant Sudanese woman. J Med Virol. 2008;80:929. doi: 10.1002/jmv.21132. [DOI] [PubMed] [Google Scholar]
- 35.He J, Kraft AJ, Fan J, et al. Simultaneous Detection of CDC Category “A” DNA and RNA Bioterrorism Agents by Use of Multiplex PCR & RT-PCR Enzyme Hybridization Assays. Viruses. 2009;1:441–459. doi: 10.3390/v1030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Njenga MK, Paweska J, Wanjala R, et al. Using a field quantitative real-time PCR test to rapidly identify highly viremic Rift Valley fever cases. J Clin Microbiol. 2009;47:1166–1171. doi: 10.1128/JCM.01905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Le Roux CA, Kubo T, Grobbelaar AA, et al. Development and evaluation of a real-time reverse transcription-loop-mediated isothermal amplification assay for rapid detection of Rift Valley fever virus in clinical specimens. J Clin Microbiol. 2009;47:645–651. doi: 10.1128/JCM.01412-08. One of two papers that demonstrated that RVFV could be detected in clinical specimens using a LAMP assay and that results were comparable to real time PCR.
- 38•.Peyrefitte CN, Boubis L, Coudrier D, et al. Real-time reverse-transcription loop-mediated isothermal amplification for rapid detection of Rift Valley fever virus. J Clin Microbiol. 2008;46:3653–3659. doi: 10.1128/JCM.01188-08. One of two papers that demonstrated that RVFV could be detected in clinical specimens using a LAMP assay and that results were comparable to real time PCR.
- 39.Ikegami T, Makino S. Rift Valley fever vaccines. Vaccine. 2009;27(Suppl 4):D69–72. doi: 10.1016/j.vaccine.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heise MT, Whitmore A, Thompson J, et al. An alphavirus replicon-derived candidate vaccine against Rift Valley fever virus. Epidemiol Infect. 2009;137:1309–1318. doi: 10.1017/S0950268808001696. [DOI] [PubMed] [Google Scholar]
- 41.Bouloy M, Flick R. Reverse genetics technology for Rift Valley fever virus: current and future applications for the development of therapeutics and vaccines. Antiviral Res. 2009;84:101–118. doi: 10.1016/j.antiviral.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McElroy AK, Albarino CG, Nichol ST. Development of a RVFV ELISA that can distinguish infected from vaccinated animals. Virol J. 2009;6:125. doi: 10.1186/1743-422X-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Bird BH, Albarino CG, Hartman AL, et al. Rift Valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol. 2008;82:2681–2691. doi: 10.1128/JVI.02501-07. Reverse genetics technology used to create vaccine candidates that have precisely defined deletions of virulence factors (NSs and NSm), contain markers (GFP) that allow for DIVA, eliminate concerns about reversion to virulence, and provide protective immunity after one injection.
- 44.Lagerqvist N, Naslund J, Lundkvist A, et al. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley Fever virus cDNA constructs. Virol J. 2009;6:6. doi: 10.1186/1743-422X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naslund J, Lagerqvist N, Habjan M, et al. Vaccination with virus-like particles protects mice from lethal infection of Rift Valley fever virus. Virology. 2009;385:409–415. doi: 10.1016/j.virol.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 46•.Billecocq A, Gauliard N, Le May N, et al. RNA polymerase I-mediated expression of viral RNA for the rescue of infectious virulent and avirulent Rift Valley fever viruses. Virology. 2008;378:377–384. doi: 10.1016/j.virol.2008.05.033. Used RNA polymerase-based system to create RVFV strains with specific mutations to allow for a greater understanding of the specific functionality of mutations in vaccine candidate strains.
- 47.Habjan M, Penski N, Wagner V, et al. Efficient production of Rift Valley fever virus-like particles: The antiviral protein MxA can inhibit primary transcription of bunyaviruses. Virology. 2009;385:400–408. doi: 10.1016/j.virol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 48••.Wolf MC, Freiberg AN, Zhang T, et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci U S A. 2010;107:3157–3162. doi: 10.1073/pnas.0909587107. Discusses, LJ001, a potential broad spectrum anti-enveloped virus therapeutic that inhibits virus-cell fusion.
- 49••.Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. Discusses, bavituximab, a potential broad spectrum anti-enveloped virus therapeutic that binds to phosphatidylserine on virions and virus-infected cells allowing for improved antibody- dependent cellular cytotoxicity and opsonization.