Abstract
Background
Clinical studies have shown that resuscitation with fresh frozen plasma (FFP) is associated with improved outcome after severe hemorrhagic shock (HS). We hypothesized that in addition to its effects on hemostasis, FFP has protective and stabilizing effects on the endothelium that translate into diminished endothelial cell (EC) permeability and improved resuscitation in vivo after HS. We further hypothesized that the beneficial effects of FFP would diminish over 5 days of routine storage at 4°C.
Methods
EC permeability was induced by hypoxia and assessed by the passage of 70-kDa Dextran between monolayers. Thrombin generation time and coagulation factor levels or activity were assessed in FFP. An in vivo rat model of HS and resuscitation was used to determine the effects of FFP on hemodynamic stability.
Results
Thawed FFP inhibits EC permeability in vitro by 10.2-fold. Protective effects diminish (to 2.5-fold) by day 5. Thrombin generation time is increased in plasma that has been stored between days 0 and 5. In vivo data show that day 0 FFP is superior to day 5 FFP in maintaining mean arterial pressure in rats undergoing HS with resuscitation.
Conclusion
Both in vitro and in vivo studies show that FFP has beneficial effects on endothelial permeability, vascular stability, and resuscitation in rats after HS. The benefits are independent of hemostasis and diminish between days 0 and 5 of storage.
Keywords: FFP, FFP storage lesion, Hemorrhagic shock, Vascular stability, Endothelial permeability
After major traumatic injury and hemorrhage, patients often require multiple transfusions of blood products, including fresh frozen plasma (FFP). Traditional use of FFP in trauma and hemorrhagic shock (HS) has been to provide a source of clotting factors that commonly become depleted with hemorrhage, massive transfusion (MT), and fluid resuscitation. Recent clinical studies have shown that resuscitation with FFP is associated with improved outcome after severe HS.1–4 Data emerging from casualty care in the war in Iraq revealed that there is an inverse correlation between the ratio of units of plasma to units of red blood cells (RBCs) transfused and mortality.1 Civilian studies have shown mixed results but predominantly support the use of FFP in massively transfused patients.5–12 Concerns over the use of FFP involve its rare association with transfusion-related acute lung injury and, the even more uncommon, transmission of viral and bacterial infections. Although some trauma centers have advocated the sparing use of FFP to prevent side effects, such as transfusion-related acute lung injury,13 recent data on FFP and its use in MT patients have initiated patterns of change in blood product administration in many trauma centers around the country. Unfortunately, little is known about the mechanism of action of FFP, and it is widely believed that most of FFP's clinical benefit in HS treatment is due to its hemostatic properties.14
According to American Association of Blood Banks guidelines, thawed FFP is stored between 1°C and 6°C and can be used within a 24-hour period. If not used within the 24 hours, the FFP is relabeled and designated as thawed plasma (TP). TP is available for clinical use if stored between 1°C and 6°C for up to 5 days.15–17 Storage of FFP for 5 days has obvious advantages from a standpoint of reducing waste and allowing for rapid administration when needed in an emergency situation. As with other blood components, the question of storage stability has been addressed for FFP. Most studies have focused on whether postthaw storage of FFP maintains adequate coagulation factor levels, specifically factors V and VIII. Both factors V and VIII have been shown to decrease after 5 days of storage, but remain within acceptable levels for hemostasis.18–22 Unfortunately, there are no studies conclusively documenting what constitutes adequate or acceptable levels of these labile factors in patients with HS. Furthermore, no studies are available to explain the changes that occur in the more than 1,000 proteins found in a unit of plasma.23
Importantly, there are no published studies correlating the age of plasma and clinical outcomes. Plasma proteins comprise a highly heterogeneous class of biological macromolecules. Many are unstable when not in their native environments, which can vary considerably among organ compartments and extracellular fluids. If certain buffer conditions are not maintained, extracted proteins may not function properly or remain soluble. Although optimal conditions for storage are distinctive to each protein, it is well known that proteins generally lose structural integrity and activity as a result of storage at 4°C because of proteolysis, aggregation, and suboptimal buffer conditions.24 Consequently, to prevent rapid degradation of proteins, preserve function, and prevent variable results, standard techniques in the basic science laboratories mandates –80°C storage of proteins used in experiments.
Studies on the effects of storage on other blood components have revealed that a myriad of biochemical, metabolic, and molecular alterations can occur over time. The “storage lesion” for RBCs has been defined as a spectrum of changes that can potentially affect the quality of the product for clinical use.1,25,26 Routine storage of blood has been shown to prime the neutrophil NADPH oxidase, suggesting a proinflammatory change in aged RBCs.27 Recent work by other groups shows that storage of platelets increases growth factors including soluble CD40 ligand, transforming growth factor-β, vascular endothelial growth factor-A, platelet derived growth factor, and bfibroblast growth factor. All these proteins have potential to destabilize the vasculature and adversely affect clinical outcome.27,28 Furthermore, plasma from stored platelets and RBCs has been shown to activate human pulmonary endothelial cells (PECs).29 In a multivariate analysis, Silliman and coworkers demonstrate that the age of transfused blood was an independent predictor of multiple organ failure after injury.30–32 As mentioned previously, it is universally recognized that most proteins lack stability at 4°C. Proteins need to be stored appropriately to inhibit degradation and preserve consistent function, which would hypothetically vary depending on the age of the blood product used. Taken together, these findings suggest that the age of the stored blood products (RBCs, plasma, and platelets) has the potential to alter the consistency of the clinical outcome, an undesirable effect in critically injured patients.
Although resuscitation with FFP has been associated with improved outcome after HS,33 the mechanism of action of FFP is wholly unknown. Traditionally, clinicians have been taught to use plasma to replace consumed, depleted, or diluted coagulation proteins and to reverse an assumed or documented coagulopathy. However, we question whether the effects of FFP are solely related to replacing coagulation proteins because clinical observations suggest that there are other effects. With the increasing use of plasma and decreasing use of crystalloid, most clinicians have observed a decrease in the edema that was common in resuscitated trauma patients. Preclinical data in animals suggest that plasma is less inflammatory than artificial colloid, albumin, or lactated ringer's (LR) solution when transfused into swine and rats undergoing HS.34,35 We think that it is simplistic to assume that the beneficial effects of plasma are solely related to replenishment of coagulation factors. Thus, we hypothesized that the beneficial effects of FFP are due, in part, to its ability to globally promote systemic vascular stability, as defined by decreased endothelial permeability and improved hemodynamic stabilization after HS. Our studies are based around the central hypothesis that FFP has the capacity to “normalize” injured endothelium by inhibiting and repairing the damage from a number of detrimental processes induced by HS. These processes include increased and prolonged vascular permeability, endothelial basement membrane breakdown, exposure of subendothelium, nonspecific initiation of coagulation, endothelial contraction and death, interstitial edema, leukocyte infiltration, inflammation, and tissue hypoxia.36 Normalization of these damaging effects should result in restoration of the normal structure and function of the vessel, improved vascular endothelial function, decreased permeability, targeted hemostasis, and overall better clinical outcome. Integrated into our central hypothesis is our secondary hypothesis that the beneficial effects of FFP on the vascular endothelium, hemostasis, and resuscitation will diminish with increased storage time from day 0 through day 5 at 4°C. In this article, we attempt to begin to address these questions by studying the effects of FFP both in vitro and in vivo in a rat model of HS and resuscitation.
METHODS
In Vitro Studies
Primary Cells, Media, and Other Reagents
First passage human pulmonary endothelial cells (PECs) were purchased from PromoCell (Suttgart, Germany). PECs were cultured in pulmonary microvascular growth media (endothelial growth media-MV). PECs were used at passage 3 to 6 for all experiments and were grown in 5% CO2 with hypoxia (2% O2) or without (19% O2) hypoxia. FFP (ABO blood types) was obtained from the Gulf Coast Blood Center in Houston, TX. The anticoagulant used was 63 mL citrate-phosphate-dextrose per 450 mL blood. The FFPs used were from the same donor at days 0 and 5. Day 0 FFP was aliquoted after thaw and stored at 4°C. Aliquots were aged and used at days 5 and 10 (endothelial cell [EC] permeability only) for the sake of comparison. For both in vitro studies of permeability and in vivo studies, thawed days 0 and 5 FFP were pooled from three donors.
Endothelial Cell Permeability
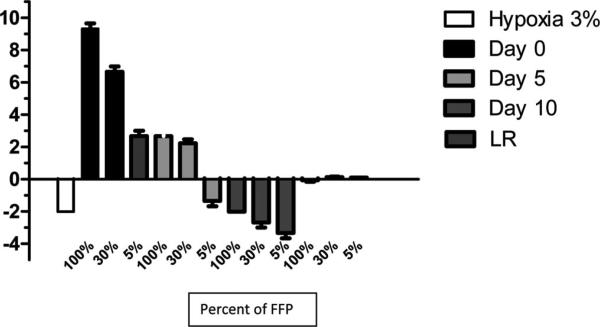
Collagen IV-coated 0.4-μm pore site inserts were obtained from BD Biosciences (San Jose, CA). PECs were seeded at 40,000 cells per insert well (24-well format) and cultured for 48 hours allowing cell attachment, adhesion, and monolayer formation. Cells were treated with hypoxia (2% O2) for 18 hours. Hypoxia was induced by incubating the cells in a hypoxia incubator. Further studies (data not shown), confirmed the induction of hypoxia by showing induction of hypoxia inducible factor-1α and vascular endothelial growth factor-A. PEC monolayers were then treated with FFP diluted in media for 1 hour. Permeability was tested by adding fluorescein isothiocyanate-conjugated 10 mg/mL Dextran (70 kDa; Sigma-Aldrich, St. Louis, MO) to the upper chamber of each well. Readings were taken over 1 hour, and the measurements were determined using a flourimeter (excitation/emission wavelengths of 485 nm/530 nm). The largest differences were noted at 30 minutes, which is depicted in Figure 1. Data represent the mean and standard deviation from three independent experiments. Figure 1 is representative of one set of experiments conducted in triplicate wells with FFP pooled from three individual donors. Permeability is plotted as fold decrease (FFP-treated ECs) above control (media)-treated cells. This experiment was repeated three times total (with FFP from nine donors total), all with similar results (data not shown).
Figure 1.
Day 0 FFP inhibits PECs permeability in vitro, which diminishes after 5 days of routine storage at 4°C. PECs were seeded in transwell chambers (0.4-μM pore size) and allowed to adhere for 48 hours. PECs were cultured with different concentrations (100%, 30%, and 5%) of FFP in media. FFP was stored and aged at 4°C between days 0, 5, and 10. Permeability was induced by 3% hypoxia in hypoxia incubators for 18 hours. Permeability was determined by the passage of 70-kDa fluorescein isothiocyanate-conjugated dextran to the bottom chamber over 1 hour. The 30-minute time point is presented here. All decreases in permeability were analyzed according to the mean and standard deviation of three experiments, and they were statistically significant (p < 0.05).
Clotting Factor Assays
Clotting factors assays were performed on the ACL TOP automated coagulation analyzer (Beckman Coulter, Fullerton, CA) in nine different single-donor FFPs, which were thawed (day 0), split, and then kept for 5 days at 4°C. Measurements were performed in days 0 and 5 plasma aliquots from nine donors using standard reagents and manufacturers’ protocols. von Willebrand factor (vWF) antigen and activity, factor XIII antigen, free protein S, and D-dimer were measured by immunoturbidimetric assays, and the antithrombin, protein C, plasminogen, and plasmin inhibitor were measured by chromogenic assays on the ACL TOP analyzer.
Calibrated Automated Thrombogram Assay
Hemostatic potential of freshly thawed (day 0) and day 5 FFPs from seven individual donors was presented as thrombin-generating capacity (TGC) measured by Calibrated Automated Thrombogram (CAT; Thrombinoscope, Maastricht, The Netherlands), a global functional test of hemostasis.37 The CAT assay was performed on seven of the nine bags of FFP that were used to measure clotting factors in days 0 and 5 aliquots of TP. The CAT assay measures thrombin generation time (TGT) in plasma. The TGT test was performed with two reagents: (1) platelet poor plasma (PPP)-low reagent that contains 1 pmol/L tissue factor/4 μmol/L phospholipid and has an increased sensitivity to factors VIII, IX, and XI, and (2) microparticle (MP)-reagent (0 pmol/L tissue factor/4 μmol/L phospholipid) to detect tissue factor-bearing microparticles. TGT provides information on the lag time before thrombin generation, the time to peak, the peak of thrombin generation, the area under the thrombin generation curve (the endogenous thrombin potential), and time to tail.
In Vivo Rat Model of HS and Resuscitation
The animal model used a Sprague-Dawley rat model of HS, chronically instrumented for continuous monitoring of blood pressure, heart rate, and body temperature. Briefly, rats were anesthetized with isoflurane, intubated, and ventilated under isothermic conditions. Tygon catheters were placed into the abdominal aorta through the femoral artery to record arterial blood pressure and heart rate and into the femoral vein for drug administration. HS was induced by withdrawing blood from the femoral vein until mean arterial blood pressure (MAP) stabilized at 25 mm Hg. One hour after HS, animals were randomly assigned to one of the following resuscitation groups: FFP at day 0 (group 1) or FFP at day 5 (group 2). Resuscitation fluids were infused over 45 minutes after HS. FFP at days 0 and 5 were infused at a volume equal to the blood lost (range of 2 mL/100 g body weight). Hemodynamic parameters were recorded before HS, at the end of HS, and for 6 hours after fluid resuscitation, at which time surviving animals were killed. Rats were 335 ± 15 g. Hemorrhage volume of all rats was similar (approximately 6 mL/rat) to achieve a MAP of 30 mm Hg.
Statistical Analysis
For EC permeability, data were analyzed using a Student's t test for two group comparisons. One-way analysis of variance was used for dose response studies in permeability. Comparisons of hemostatic parameters between days 0 and 5 and the hemodynamic changes between HS and baseline measurements were analyzed using paired t-tests. To compare the hemodynamic changes induced by FFP days 0 and 5, data were analyzed by analysis of variance to assess overall significance. When significant, an appropriate multiple comparison method (Dunnett's t test) was applied.38 The magnitude of changes produced by resuscitation fluids was compared using an unpaired t test. Data are presented as mean ± standard error of the mean. A p value of <0.05 was considered significant.
RESULTS
In Vitro Studies
Day 0 FFP Inhibits PEC Permeability In Vitro, Which Diminishes After 5 Days of Routine Storage at 4°C
EC permeability is a hallmark characteristic of injured vasculature. To determine whether FFP has effects on EC permeability, we induced permeability in PEC monolayers with 2% hypoxia for 18 hours followed by treatment with 100%, 30%, and 5% FPP diluted into culture media. The FFP used was pooled from three independent donors. Indeed, we found that PECs treated with FFP do demonstrate a dose-dependent decrease in permeability to 70-kDa fluorescein isothiocyanate–conjugated Dextran (Fig. 1) compared with PECs cultured with media. Day 5 FFP demonstrated a diminished capacity to inhibit PEC permeability compared with day 0 FFP. Although day 10 FFP is not used clinically in the United States, it is used in Europe. We showed a time-dependent effect of FFP storage on EC permeability. Treatment of PECs with day 10 plasma has no protective effect and in fact exacerbates the endothelial permeability. These studies were repeated three times (total of nine donors), all with similar results (data not shown).
Thrombin-Generating Capacity of FFP Is Diminished in Between Days 0 and 5 of Storage
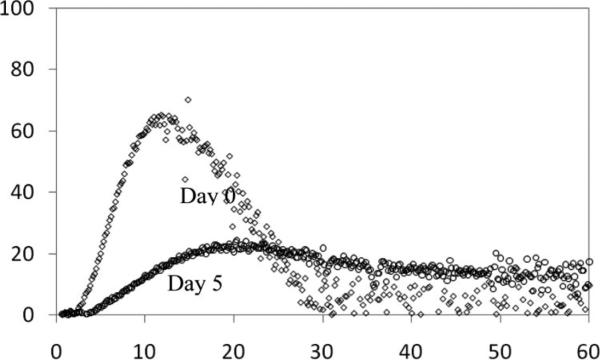
As a global measure of the ability to form a clot, we studied the effect of age (day 0 vs. day 5) on the capacity of the FFP to generate thrombin using a CAT Assay.37 TGC of fresh FFP showed high variability between single donors with either PPP-low or MP reagent. The majority of the TGT parameters significantly changed during storage, indicating a decrease in hemostatic capacity of the aged plasma (Table 1). Figure 2 shows representative TGT curves from a single-donor FFP on days 0 and 5.
TABLE 1.
Thrombin Generation Test With PPP-Low and MP Reagents
| Day 0 | Day 5 | p | |
|---|---|---|---|
| PPP-low | |||
| Lag time (min) | 3.6 ± 0.2 (2.8–4.1) | 5.1 ± 0.3 (3.2–6.2) | 0.0003 |
| ETP (nmol/L thrombin · min) | 1421 ± 89 (107–1715) | 1096 ± 176 (433–1525) | 0.03 |
| Peak thrombin (nmol/L) | 124 ± 20 (63–230) | 94 ± 21 (17–167) | 0.04 |
| Time to peak (min) | 10.4 ± 0.7 (6.9–12.1) | 12.9 ± 1.5 (8.6–21) | NS |
| Start tail (min) | 35.6 ± 3 (25–48) | 41.3 ± 4.5 (27–58) | 0.05 |
| MP | |||
| Lag time (min) | 18.3 ± 0.7 (15.7–21.5) | 33.3 ± 3.7 (17–53) | 0.008 |
| ETP (nmol/L thrombin · min) | 409.5 ± 59 (127–838) | 154.8 ± 61 (86–412) | NS |
| Peak thrombin (nmol/L) | 44 ± 5.8 (16–78.2) | 29.1 ± 6.4 (9–61) | 0.04 |
| Time to peak (min) | 26.4 ± 1.2 (21.5–31) | 40 ± 3.8 (25–57) | 0.02 |
| Start tail (min) | 60.9 ± 3 (50.7–70) | 68 ± 4.7 (55–77) | NS |
ETP, endogenous thrombin potential; NS, not significant.
TGC of FFP is diminished in between days 0 and 5 of storage. Hemostatic potential of freshly thawed and stored FFPs from seven individual donors was presented as TGC measured by CAT, a global functional test of hemostasis. The CAT assay measures TGT in plasma. TGT shows increased lag time, increased time to peak, decreased peak of thrombin generation, decreased area under the thrombin generation curve (ETP), and increased time to tail for day 5 FFP. Data are presented as mean ± standard error of mean (range).
Figure 2.
Representative curve of thrombin-generating capacity of FFP between days 0 and 5 of storage. As a global measure of the ability to form a clot, we studied the effect of storage on the capacity of the FFP to generate thrombin using a CAT Assay. The plot shows representative thrombin generation time curves from a single-donor FFP on days 0 and 5.
Coagulation Proteins and Inhibitors Decrease in FFP Between Days 0 and 5 of Storage
Others have reported that some coagulation factors and clotting cascade intermediates do change between days 0 and 5 storage of FFP.18–22 We sought to confirm this and found that this is indeed a reproducible finding, extending beyond just factors V and VIII. Levels of the coagulation factors and inhibitors measured in days 0 and 5 FFPs showed variable degrees of stability. Multiple factors significantly decreased after 5 days of storage (Table 2), including factors II, V, VII, VIII, IX, X, XI, XIII, vWF, and protein S activity. Conversely, fibrinogen, antithrombin, protein C, free protein S antigen, plasminogen, antiplasmin, vWF antigen, and factor XII seem to be stable during plasma storage (Table 2).
TABLE 2.
Levels of Coagulation Factors and Inhibitors in Thawed Plasma Between Days 0 and 5
| Analyte | Day 0 (n = 9) | Day 5 (n = 9) | p |
|---|---|---|---|
| PT (s) | 11.0 (0.6) | 13.4 (0.8) | <0.001 |
| APTT (s) | 36.7 (3.7) | 41.3 (6.0) | 0.001 |
| Fibrinogen (mg/dL) | 279 (51) | 273 (51) | NS |
| Factor II (%) | 95.6 (6.8) | 89.2 (6.0) | 0.004 |
| Factor V (%) | 80.3 (20.3) | 51.0 (19.4) | <0.001 |
| Factor VII (%) | 108.7 (25.1) | 89.1 (16.6) | 0.003 |
| Factor VIII (%) | 74.7 (15.8) | 57.8 (12.4) | 0.002 |
| vWF antigen (%) | 122.9 (57.1) | 107.7 (54.7) | NS |
| vWF activity (%) | 91.5 (26.1) | 70.5 (28.8) | 0.02 |
| Factor IX (%) | 111.8 (11.1) | 103.5 (7.9) | 0.003 |
| Factor X (%) | 98.7 (9.6) | 95.2 (8.0) | 0.001 |
| Factor XI (%) | 101.5 (17.3) | 98.9 (17.1) | <0.001 |
| Factor XII (%) | 108.4 (25.2) | 106.9 (25.9) | NS |
| Factor XIII (%) | 127.6 (31.2) | 120.8 (29.1) | 0.009 |
| Antithrombin (%) | 93.1 (3.8) | 93.2 (4.1) | NS |
| Protein C (%) | 110.0 (21.5) | 110.3 (20.9) | NS |
| APCR V+/V– ratio | 2.8 (0.2) | 2.6 (0.2) | <0.001 |
| Free Protein S activity (%) | 84.5 (14.1) | 63.2 (13.8) | 0.001 |
| Free Protein S antigen (%) | 82.8 (10.6) | 82.0 (11.1) | NS |
| Plasminogen (%) | 95.6 (9.8) | 95.7 (10.0) | NS |
| Plasmin inhibitor (%) | 105.8 (6.4) | 105.0 (6.3) | NS |
| DD (ng/mL) | 214.7 (115.5) | 219.8 (105.1) | NS |
PT, prothrombin time; APTT, activated partial thromboplastin time; APCR, activated protein C resistance; NS, not significant at the 5% level.
Coagulation proteins and inhibitors decrease in FFP between days 0 and 5. Coagulation factors measured in days 0 and 5 FFPs showed variable degrees of stability. Multiple factors significantly decreased after 5 d of storage, including factors II, V, VII, VIII, IX, X, XI, XIII, vWF activity, and protein S. Fibrinogen, antithrombin, protein C, free protein S, plasminogen, antiplasmin, vWF antigen, and factor XII seem to be stable during plasma storage. Data are presented as mean (standard deviation).
In Vivo Studies
Preliminary Data: Day 5 FFP Has Decreased Capacity to Restore MAP in a Rat Model of HS and Resuscitation
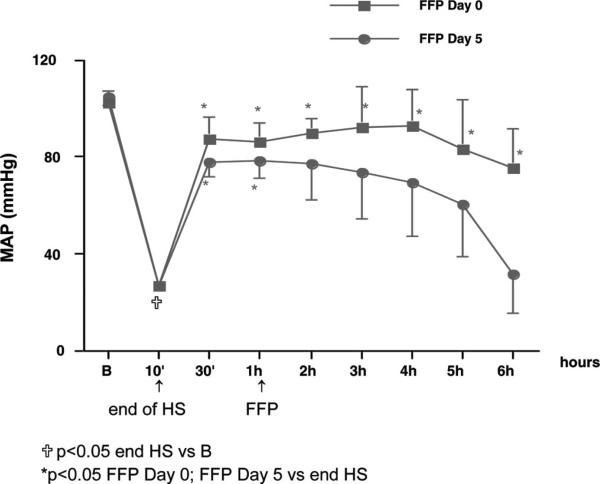
To address the question of whether day 0 or 5 FFP has an altered capacity to resuscitate rats subjected to HS, animals were subjected to HS as described previously.39 Rats were hemorrhaged over 10 minutes (2 mL/100 g body weight) and resuscitated with a fixed volume of plasma after 60 minutes. One hour after HS, animals were randomly assigned to one of the following resuscitation groups: FFP at day 0 (group 1, n = 5) and FFP at day 5 (group 2, n = 5). FFP was pooled from three individual donors and infused at a volume equal to the blood lost. All rats in group 1 (FFP day 0) survived the entire duration of the study. In contrast, two rats in group 2 (FFP day 5) did not survive the full 6 hours and died 3 hours to 4 hours after FFP infusion. As depicted in Figure 3, hemorrhage alone induced an immediate and significant decrease in MAP (70%), reaching a nadir at the end of hemorrhage (10 minutes). After 60 minutes of HS, FFP was administered. In HS rats resuscitated with FFP at day 0 (group 1), blood pressure was fully restored to baseline and remained increased throughout the study. In contrast, for HS rats resuscitated with FFP at day 5 (group 2), MAP was decreased compared with day 0 rats (group 1). In conclusion, these data indicate that day 0 FFP, but not at day 5 FFP, restores blood pressure to baseline in hemorrhaged rats. These preliminary studies suggest that resuscitation with day 0 FFP is superior to day 5 FFP in maintaining hemodynamic stability.
Figure 3.
Effects of FFP at days 0 and 5 on MAP in rats subjected to hemorrhagic shock. Rats were hemorrhaged over 10 minutes and resuscitated with a fixed volume of plasma after 60 minutes. One hour after HS, FFP was administered: day 0 (group 1, n = 5) and day 5 (group 2, n = 5). FFP was infused at a volume equal to the blood lost. Data indicate that FFP infused at day 0, but not at day 5, restores blood pressure to baseline in hemorrhaged rats. These studies suggest that resuscitation with day 0 FFP is superior to day 5 FFP in maintaining hemodynamic stability. B, baseline.
DISCUSSION
In this article, we show that day 0 FFP has potent effects on endothelial function and hemodynamic stability in vitro and in vivo, respectively; these effects diminish after 5 days of routine storage at 4°C. In our studies with FFP and EC function, we found that day 0 FFP has the capacity to repair EC permeability induced by hypoxia in vitro. In addition, we show that this reparative capacity is diminished between days 0 and 5 of storage at 4°C. Our findings in vitro also suggest that day 5 FFP is superior to LR, which has no effect on EC barrier repair. There are significant differences in coagulation proteins between days 0 and 5 FFP, and there are changes in TGC, a global measure of coagulation potential. The American Association of Blood Bank does not provide guidelines for quality control of FFP in regard to clotting factors. However, the Council of Europe requires testing of factor VIII level in 10 randomly selected units in their first month of storage (FVIII level should be >70% of the freshly collected plasma unit). There are no guidelines for other clotting factors. Regarding bleeding tendencies, approximately 40% of a single-factor activity is considered to be enough to support hemostasis in a single-factor deficiency. However, when multiple factors are depleted, which is often the case in major trauma with bleeding, it is difficult to estimate the necessary factor levels for adequate hemostasis. Interestingly, a recent report indicated that low plasma thrombin peak (<100 nmol/L) was associated with ongoing bleeding in patients during major surgery.40 Our in vivo studies support and extend our in vitro results and show that day 0 FFP is highly effective at restoring hemodynamic stability in HS rats, whereas day 5 FFP demonstrates a diminished ability to effectively maintain MAP after HS. These characteristics of FFP have not been reported previously and support our central hypothesis that some of the beneficial effects of increased FFP in MT patients may be, in part, due to the ability of plasma to normalize injured endothelium by inhibiting endothelial permeability and restoring hemodynamic stability. Furthermore, our data also support our secondary hypothesis that the beneficial capacity of FFP to normalize endothelial function and hemodynamic stability in HS diminishes with storage at 4°C.
As mentioned previously, it is generally thought that administration of FFP helps to reverse or prevent coagulopathy by providing a source of coagulation factors that replenish endogenous factors depleted by hemorrhage, hemodilution, and nonspecific consumptive clotting. However, a recent comprehensive review of traumatic coagulopathy concluded that because the underlying mechanism for traumatic coagulopathy is still unknown, our current treatment modalities lack rationale.41 Work on the composition of plasma recently documented that there are literally hundreds of bioactive proteins in FFP.23 Our data suggest that the therapeutic role of FFP is far greater than its effects on simply increasing the amount of classic coagulation proteins. Therefore, we propose a new concept and hypothesize that FFP preserves, repairs, and normalizes the vascular endothelium to a steady state, which results in effects such as the inhibition of EC permeability. This function of FFP could be through the action of soluble factors present in FFP and the induction of stabilizing factors by ECs or other cells exposed to FFP. It is also possible that platelets may be producing some of the effects we find in vivo. These results are even more thought provoking when one considers that ECs are the first cell type that FFP comes into contact with after intravenous administration in HS. ECs are considered by some to make up the largest organ in the body, as opposed to being inert liners of the blood vessel tube. ECs are the central platform on which a number of critical processes involved in hemostasis, inflammation, and edema occur.42
In terms of hemostasis, day 5 FFP demonstrated a lower TGC, which was also in line with statistically significant decrease in levels of multiple coagulation factors. The effect of plasma storage affected mostly prothrombin time, activated partial thromboplastin time, factors V and VIII, and protein S, which all fell below their normal ranges. These results suggest that fresh TP may have greater ability to restore hemostasis. The effect of these changes in FFP on transfused patients is unknown and is of worthy of future investigation.
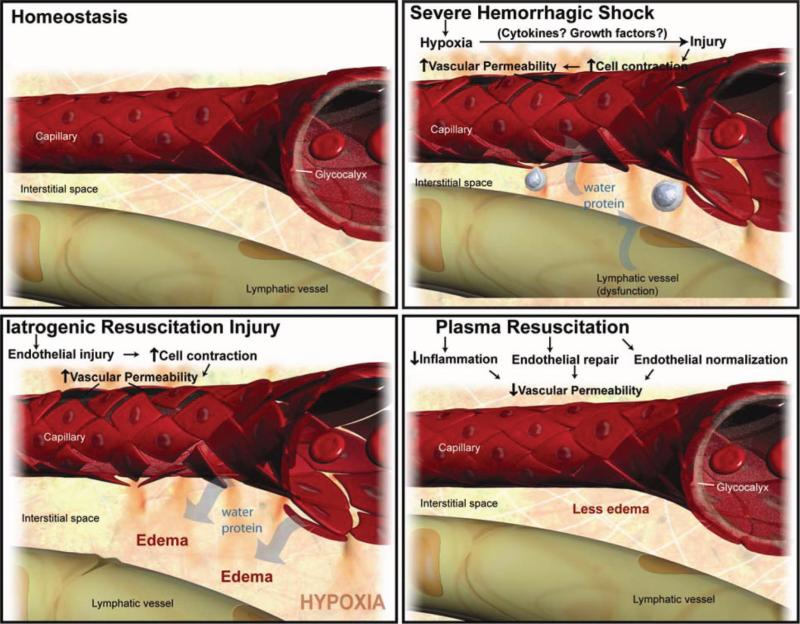
Vascular integrity and permeability are compromised by a number of factors induced by HS including hypoxia, coagulation cascade intermediates (i.e., thrombin) and inflammatory factors such as tumor necrosis factor-α. Systemic vascular stability is most likely maintained by the complex interplay and balance of these groups of factors and others that oppose their action. It is possible that the hypoxia and thrombin, induced early endothelial permeability and seen after HS, is beneficial and leads to translocation of extravascular fluid into the intravascular space, However, we believe that iatrogenic resuscitation injury with fluids that perpetuate EC permeability may lead to vascular instability, pathologic edema, and the adverse outcomes commonly associated with resuscitation. One key component in regulating vascular integrity is the inhibition of EC permeability. A main consequence of EC permeability and compromise of the blood-organ barrier is the development of interstitial edema, a condition found in the gut and lungs of HS patients with multiple organ failure.43 At the molecular level, EC permeability and vascular stability are regulated by molecular “zippers” that are composed of tight junctions and adherens junctions, structures that are crucial in holding together EC and prevent the passage of water and molecules into the interstitial space.44 When platelet counts become low enough, EC junctional stability is compromised and manifests in endothelial fragility and spontaneous bleeding.44 One can speculate that these effects are present in the coagulopathy of trauma. It is possible that the factors present in FFP, or induced by FFP, promote vascular stability through regulation of these critical junction proteins. Compromise of EC junctions by factors present or absent in day 5 plasma could lead to a number of deleterious effects including barrier dysfunction, interstitial edema, tissue hypoxia, inflammatory cell infiltration, detached pericytes, extracellular matrix breakdown, and exposed subendothelium. One can further speculate that the exposure of the subendothelium could lead to nonspecific, untargeted activation of the coagulation cascade and may theoretically worsen and propagate a coagulopathic state in HS patients. Our finding would suggest a possible beneficial indirect effect of FFP on hemostasis at the EC level, as opposed to the traditional view of FFP as a source of clotting factors. Figure 4 depicts our working biological model of the mechanism of action of FFP. In our model, FFP repairs and normalizes the vascular endothelium after HS by restoring tight junctions, rebuilding the glycocalyx, and inhibiting inflammation and edema, all detrimental processes that can be caused by iatrogenic fluid resuscitation with LR.
Figure 4.
Working biological model of the mechanism of action of FFP. This figure depicts our working biological model of the mechanism of action of FFP. HS leads to a deviation of the vasculature from homeostasis. HS induces hypoxia, endothelial cell tight junction breakdown, inflammation, and leukocyte diapedesis. FFP repairs and “normalizes” the vascular endothelium by restoring tight junctions, building the glycocalyx, and inhibiting inflammation and edema, all detrimental processes that are caused by iatrogenic injury with fluids such as lactated ringer's solution.
Cohen and coworkers45 have recently described the relationship between acute traumatic coagulpathy in mice with increased activated protein C. They and others aim to understand how HS directly causes increased bleeding, although the release of activated protein C, a protein that could be described as having cytoprotective and anticoagulant properties. Previously undefined effects of HS and novel applications of existing products can be tied together to optimize current therapy and elucidate a better mechanistic understanding of underlying pathologic processes, which enables us to optimize treatment. Our data in vitro and in vivo suggest that FFP has beneficial effects on vascular stability and resuscitation that are independent of its effects on hemostasis and dependent on the age of the FFP.
Future studies will aim to better characterize the mechanism of action of FFP both in vitro and in vivo in our rodent model of HS. Appropriate controls using no treatment and other resuscitation arms including fresh whole blood and various crystalloid solutions (i.e., LR) will be evaluated. Differences found between days 0 and 5 FFP both in vitro and in vivo will be used as a discovery platform to identify what factors are responsible for the noted beneficial effects of FFP on endothelial permeability and hemodynamic stability after HS. Regarding the age of plasma and its potential for hemostasis, we show that day 5 FFP has a lower TGC than day 0 FFP. Future studies will also aim to determine whether there is an in vivo difference in the hemostatic potential of day 0 versus day 5 plasma. Although therapeutic significance of changes observed in stored versus fresh FFP are unclear without further investigation, we believe that these data have clinical significance in MT trauma patients with potential to directly impact patient care. The larger issue is that most current clinical use of blood products has very little clinical outcome data associated with their use and reiterates the need for further investigation.
ACKNOWLEDGMENTS
We thank Dr. R. Michelle Boggs for her editorial support. We also thank Willa Wang, Michael Gerber, and Yangyan Liang for their technical expertise.
Supported by an NIH grant (NIGMS 5 P50 GM038529).
DISCUSSION
Dr. Stephen M. Cohn, MD, FACS (US Army Institute of Surgical Research, Fort Sam Houston, TX): Prolonged storage of red blood cells has been associated with poorer outcomes in the clinical setting, but the optimal “age of blood” for infusion is not known. Dr. Pati et al. focused their investigations on the effects of storage on thawed plasma. They hypothesized that fresh frozen plasma (FFP) had the capacity to “normalize” injured endothelium by inhibiting or repairing the damage from detrimental processes induced by hemorrhagic shock. The authors performed in vitro and in vivo studies in rats, which demonstrated the salutary impact of plasma on endothelial permeability, vascular stability, and resuscitation after hemorrhagic shock. The beneficial effects noted with day 0 FFP were diminished when thawed plasma was stored for 5 days at 4°C.
Review of this interesting study generated a number of questions: In each comparison, was the donors’ plasma at day 0 compared with the same individuals’ plasma at day 5? This would minimize the variability in plasma composition inherent in a population of subjects. How does the increase in endothelial permeability seen with older plasma reflect on the clinical scenario? Are these changes similar to those seen in capillary leak syndrome with acute respiratory distress syndrome? It seems that 5% diluted day 0 FFP has the same effect on permeability as 100% concentrated day 5 thawed plasma. If someone infused multiple units of thawed 5-day-old plasma, do you expect to see the same impact on endothelial permeability as a few units of FFP? Can this information be applied to a rat model of ARDS to determine the in vivo effects of various doses of plasma of varying ages?
In interpreting the calibrated automated thrombogram assay, how do these findings relate to likelihood of clot formation? Is the plasma stored at day 5 incapable of inducing clot or do the values remain within the range necessary to permit thrombus generation? Are the differences in clotting factors found between days 0 and 5 clinically relevant? Many of the levels of coagulation factors found were statistically significantly diminished on day 5 when compared with day 0 but seem to unimportant (e.g., prothrombin time rising from 11 to 13.4 or partial thromboplastin time changing from 36.7 to 41.3 seconds). Which, if any of these changes in factors, is germane to patient care?
The replacement of blood lost with plasma exclusively, and no crystalloid, led to a surprising benefits both in maintenance of blood pressure and improved survival. However, the small number of rats used (five per group) is less than is normally used for resuscitation experiments. The Wigger's model of maintaining hypotension in rats by continual blood withdrawal over an hour may not be applicable to our typical patient scenario. As your findings are quite provocative, have the authors considered expanding their work, using more animals, and subjecting them to an alternative hemorrhage or resuscitation model to confirm their findings?
Investigating the impact of the age of plasma is certainly a novel approach to understand the utility of this commonly used fluid. As with most unique experiments, this work has generated as many questions as it has answered.
Dr. Shibani Pati (University of Texas Health Science Center, Houston, TX): We are very grateful for Dr. Cohn's thoughtful comments and suggestions for this article. Our responses to his comments follow.
Dr. Cohn questioned whether the donor's plasma at day 0 was compared with the same individuals plasma at day 5. This is a valid question, and we proceeded to clarify this point in the methods and article. The FFP used was from the same donor at days 0 and 5. Day 0 FFP was aliquoted after thaw and stored at 4°C. Aliquots were aged and used at days 5 and 10 for the sake of comparison. He also raises the question as to how the changes in permeability reflect on the clinical scenario and whether they are similar to ARDS. As far as we know, there have been no studies to date to answer this question. It is a clinical study that we would like to embark on after we have shown clear preclinical differences and characterized them further. We speculate that there will be differences in ARDS. Some of our work currently suggests increased leukocyte infiltrates and thickening in the lung parenchyma of animals undergoing HS that are resuscitated with day 5 FFP. However, we feel that this is beyond the scope of this article and hence have not included this information in the text.
Footnotes
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the US Department of the Air Force, Army, Navy, Defense, or the Government. This work was prepared as part of their official duties; and, as such, there is no copyright to be transferred.
Presented at the Conference on Shock, San Antonio, TX, June 2009.
REFERENCES
- 1.Spinella PC, Perkins JG, Grathwohl KW, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64(2 Suppl):S69–S77. doi: 10.1097/TA.0b013e318160ba2f. discussion S77–S78. [DOI] [PubMed] [Google Scholar]
- 2.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B. Working Group on Polytrauma of the German Society of Trauma Surgery (DGU). Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 3.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 5.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–271. [DOI] [PubMed] [Google Scholar]
- 6.Scalea TM, Bochicchio KM, Lumpkins K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248:578–584. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 7.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–570. doi: 10.1016/j.amjsurg.2008.12.014. discussion 570. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira PG, Inaba K, Shulman I, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66:693–697. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- 9.Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276. doi: 10.1097/TA.0b013e31817e5166. discussion 276–278. [DOI] [PubMed] [Google Scholar]
- 10.Moore FA, Nelson T, McKinley BA, et al. StO2 Study Group. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients? Am J Surg. 2008;196:948–958. doi: 10.1016/j.amjsurg.2008.07.043. discussion 958–960. [DOI] [PubMed] [Google Scholar]
- 11.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 12.Dente CJ, Shaz BH, Nicholas JM, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–1624. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 13.Eder AF, Sebok MA. Plasma components: FFP, FP24, and thawed plasma. Immunohematology. 2007;23:150–157. [PubMed] [Google Scholar]
- 14.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–139. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 15.Murthi SB, Stansbury LG, Hess JR. Blood and coagulation support in trauma. Blood Rev. 2009;23:149–155. doi: 10.1016/j.blre.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Hess JR, Dutton RB, Holcomb JB, Scalea TM. Giving plasma at a 1:1 ratio with red cells in resuscitation: who might benefit? Transfusion. 2008;48:1763–1765. doi: 10.1111/j.1537-2995.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 17.Hess JR. Blood and coagulation support in trauma care. Hematology Am Soc Hematol Educ Program. 2007:187–191. doi: 10.1182/asheducation-2007.1.187. [DOI] [PubMed] [Google Scholar]
- 18.Boström F, Sjödahl M, Wehlin L, Egberg N, Lundahl J. Coagulation parameters in apheresis and leukodepleted whole-blood plasma during storage. Transfusion. 2007;47:460–463. doi: 10.1111/j.1537-2995.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 19.Downes KA, Wilson E, Yomtovian R, Sarode R. Serial measurement of clotting factors in thawed plasma stored for 5 days. Transfusion. 2001;41:570. doi: 10.1046/j.1537-2995.2001.41040570.x. [DOI] [PubMed] [Google Scholar]
- 20.Lamboo M, Poland DC, Eikenboom JC, et al. Coagulation parameters of thawed fresh-frozen plasma during storage at different temperatures. Transfus Med. 2007;17:182–186. doi: 10.1111/j.1365-3148.2007.00729.x. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson L, Hedner U, Nilsson IM, Robertson B. Shelf-life of bank blood and stored plasma with special reference to coagulation factors. Trans-fusion. 1983;23:377–381. doi: 10.1046/j.1537-2995.1983.23584018713.x. [DOI] [PubMed] [Google Scholar]
- 22.Scott EA, Puca KE, Pietz BC, Duchateau BK, Friedman KD. Comparison and stability of ADAMTS13 activity in therapeutic plasma products. Transfusion. 2007;47:120–125. doi: 10.1111/j.1537-2995.2007.01074.x. [DOI] [PubMed] [Google Scholar]
- 23.Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter JF. Long term storage of proteins. Curr Protoc Protein Sci. 2002 doi: 10.1002/0471140864.ps0406s27. chapter 4:unit 4.6. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner JM, Nydam TL, Clarke JH, Banerjee A, Silliman CC, McCarter MD. Red blood cell supernatant potentiates LPS-induced proinflammatory cytokine response from peripheral blood mononuclear cells. J Interferon Cytokine Res. 2009;29:333–338. doi: 10.1089/jir.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin-Yee I, Keeney M, Krueger L, Dietz G, Moses G. Supernatant from stored red cells activates neutrophils. Transfus Med. 1998;8:49–56. doi: 10.1046/j.1365-3148.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 27.Silliman CC. The transfusion of pre storage leukoreduced packed red blood cells to injured patients. Crit Care Med. 2008;36:1661–1662. doi: 10.1097/CCM.0b013e3181704602. [DOI] [PubMed] [Google Scholar]
- 28.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 31.Frenzel T, Westphal-Varghese B, Westphal M. Role of storage time of red blood cells on microcirculation and tissue oxygenation in critically ill patients. Curr Opin Anaesthesiol. 2009;22:275–280. doi: 10.1097/ACO.0b013e328323f7c4. [DOI] [PubMed] [Google Scholar]
- 32.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624. discussion 624–625. [PubMed] [Google Scholar]
- 33.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 34.Alam HB, Stanton K, Koustova E, Burris D, Rich N, Rhee P. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60:91–99. doi: 10.1016/j.resuscitation.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Deb S, Sun L, Martin B, et al. Lactated ringer's solution and hetastarch but not plasma resuscitation after rat hemorrhagic shock is associated with immediate lung apoptosis by the up-regulation of the Bax protein. J Trauma. 2000;49:47–53. doi: 10.1097/00005373-200007000-00007. discussion 53–55. [DOI] [PubMed] [Google Scholar]
- 36.Jain RK. A new target for tumor therapy. N Engl J Med. 2009;360:2669–2671. doi: 10.1056/NEJMcibr0902054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemker HC, Giesen P, AlDieri R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 38.Keppel B. Multihospital affiliation in hand, Beverly aims to double its size. Mod Healthc. 1982;12:70–72. [PubMed] [Google Scholar]
- 39.Tharakan B, Holder-Haynes JG, Hunter FA, Smythe WR, Childs EW. Cyclosporine A prevents vascular hyperpermeability after hemorrhagic shock by inhibiting apoptotic signaling. J Trauma. 2009;66:1033–1039. doi: 10.1097/TA.0b013e31816c905f. [DOI] [PubMed] [Google Scholar]
- 40.Schols SEM, van der Meijden PEJ, van Oerle R, Curvers J, Heemskerk JW, van Pampus EC. Increased thrombin generation and fibrinogen level after therapeutic plasma transfusuion: relation to bleeding. Thromb Haemost. 2008;99:64–70. doi: 10.1160/TH07-07-0438. [DOI] [PubMed] [Google Scholar]
- 41.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 42.Vandendries ER, Furie BC, Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb Haemost. 2004;92:459–466. doi: 10.1160/TH04-05-0306. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez EA, Kozar RA, Suliburk JW, Weisbrodt NW, Mercer DW, Moore FA. Conventional dose hypertonic saline provides optimal gut protection and limits remote organ injury after gut ischemia reperfusion. J Trauma. 2006;61:66–73. doi: 10.1097/01.ta.0000224190.65542.e2. discussion 73–74. [DOI] [PubMed] [Google Scholar]
- 44.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359:1261–1270. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chesebro BB, Rahn P, Carles M, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32:659–665. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]