Abstract
Objective
To evaluate the impact of hepatitis C virus (HCV) on the immune system before receipt of highly active antiretroviral therapy (HAART) and on immune recovery after receipt of HAART among human immunodeficiency virus (HIV)/HCV–coinfected women enrolled in the Women’s Interagency HIV Study.
Methods
The study included 294 HIV-infected women who initiated HAART and attended 2 follow-up visits. The women were grouped on the basis of positive HCV antibody and HCV RNA tests. There were 148 women who were HCV antibody negative, 34 who were HCV antibody positive but RNA negative, and 112 who were HCV antibody and RNA positive. Immune recovery was measured by flow-cytometric assessment for markers of activation and maturation on CD4+ and CD8+ T cells. Data analysis used repeated measures of variance.
Results
HIV/HCV coinfection is associated with an increased number of CD4+ and CD8+ primed/memory T cells. HIV/HCV coinfection, however, did not affect any further decreases in CD4+ or CD4+ and CD8+ naive/memory T cell counts or enhanced T cell activation. HIV/HCV coinfection also did not affect HAART responses in the CD4+ and CD8+ T cell compartment.
Conclusions
HCV does not affect immune responses to HAART in HIV/HCV–coinfected individuals but is associated with an expansion of CD4+ and CD8+ memory T cell subsets. Functional impairment in the CD4+ and CD8+ T cell compartments still needs to be assessed in coinfected patients.
Between 30% and 100% of HIV-infected patients are coinfected with hepatitis C virus (HCV) [1–4]. A number of studies have negatively associated HIV with the progression of HCV disease [5–7]. HIV accelerates HCV-associated complications, such as liver cirrhosis, end-stage liver disease, and hepatocellular carcinoma. The impact of HCV on the progression of HIV disease is less clear—there have been a number of discordant findings. Some studies have reported that HCV accelerates the progression of HIV disease [8–11], whereas others found no impact [12, 13]. Variation in the definition of the progression of HIV disease—based on incidences of opportunistic infections or decreases in CD4+ T cell count, sample size, duration of follow-up, and other cofounding variables—may have contributed to these conflicting findings.
Few studies have evaluated the effect of HCV co-infection on immune recovery after the initiation of highly active antiretroviral therapy (HAART). In those studies, immune recovery was defined by either the magnitude or rate of increases in CD4+ T cell counts over time, with controversial findings. Some studies have reported that the level and rate of increases in CD4+ T cell counts are lessened and slower in coinfected than in monoinfected patients [10, 14, 15], whereas others did not find a negative association between HCV coinfection and CD4+ T cell responses after the initiation of HAART [16–18]. None of these studies, however, evaluated a larger breadth of immunophenotypic markers relevant to immune function.
Through the Women’s Interagency HIV Study (WIHS), a multicenter cohort established in 1993 in the United States, where ~39% of HIV-infected women are coinfected with HCV, we retrospectively evaluated the impact of HIV/HCV coinfection on immune recovery after the initiation of HAART between HIV-monoinfected women and women coinfected with HCV who have either cleared or did not clear HCV. Immune recovery was evaluated by examining alterations in CD4+ and CD8+ T cell counts and alterations in the dynamics of activation and naive/memory T cell status within the CD4+ and CD8+ T cell compartments.
MATERIALS AND METHODS
Study design and classifications
The present study included adult HIV-positive women enrolled in the WIHS cohort. Characteristics of the WIHS cohort have been described elsewhere [19]. WIHS used a standard definition of antiretroviral therapy: (1) untreated, (2) monotherapy (any single antiretroviral therapy within the preceding 6 months), (3) combination therapy (all combination therapies except HAART within the preceding 6 months), and (4) HAART within the preceding 6 months. HAART was defined as (1) ≥2 nucleoside reverse-transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor (PI) or 1 nonnucleoside reverse-transcriptase inhibitor (NNRTI; 82% of regimens classified as HAART); (2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI (14% of regimens); (3) a regimen that contained ritonavir and saquinavir in combination with 1 NRTI and no NNRTIs (2% of regimens); and (4) a regimen of ≥3 NRTIs including abacavir or tenofovir, in the absence of both PIs and NNRTIs (2% of regimens). Combinations of zidovudine and stavudine with either a PI or NNRTI were not considered to constitute HAART. HIV treatment was initiated from 1996 to 2001. None of the participants received treatment for HCV for the duration of the study.
Women in WIHS are monitored at 6-month intervals; clinical information (including self-reported treatment history) and blood are collected at each visit. Women included in the study initiated HAART and had a pre-HAART visit (defined here as the baseline) and 2 consecutive post-HAART follow-up visits, defined as post-HAART visit 1 (at 0–6 months) and post-HAART visit 2 (at 6–12 months). Details of patients by HCV status and visit are shown in table 1. On the basis of HCV serostatus and RNA status at the pre-HAART visit, 148 women were HCV antibody negative (HCV−), 34 were HCV antibody positive but RNA negative (HCV+RNA−), and 112 were HCV antibody and RNA positive (HCV+RNA+). A total of 893 samples were analyzed in this retrospective study. Clinical information at each time point, plasma, and peripheral blood mononuclear cells (PBMCs) were available for these patients. The study was conducted in accordance with institutional review board guidelines for human research at each of the participating sites.
Table 1.
Demographic characteristics of the cohort, by hepatitis C virus (HCV) infection status.
| Characteristic | No. of patients (N = 294) | HCV status, no. (%)
|
P | ||
|---|---|---|---|---|---|
| HCV− (n = 148) | HCV+ RNA− (n = 34) | HCV+ RNA+ (n = 112) | |||
| Age group | |||||
| <35 years | 90 | 70 (47.3) | 7 (20.6) | 13 (11.6) | <.01 |
| ≥35 years | 204 | 78 (52.7) | 27 (79.4) | 99 (88.4) | |
| Ethnicity | |||||
| White | 41 | 18 (12.2) | 3 (8.8) | 20 (17.9) | .03 |
| Black | 165 | 80 (54.1) | 23 (67.6) | 62 (55.4) | |
| Hispanic | 84 | 50 (33.8) | 6 (17.6) | 28 (25.0) | |
| Other | 4 | 2 (5.9) | 2 (1.8) | ||
| Employed | |||||
| Yes | 84 | 53 (35.8) | 7 (20.6) | 24 (21.4) | .02 |
| No | 210 | 95 (64.2) | 27 (79.4) | 88 (78.6) | |
| No. of sex partners | |||||
| 0–4 | 153 | 90 (60.8) | 12 (35.3) | 51 (45.5) | <.01 |
| 5–10 | 51 | 23 (15.5) | 3 (8.8) | 25 (22.3) | |
| 11–100 | 67 | 27 (18.2) | 13 (38.2) | 27 (24.1) | |
| >100 | 23 | 8 (5.4) | 6 (17.6) | 9 (8.0) | |
| Current smoking | |||||
| Yes | 147 | 51 (34.5) | 18 (52.9) | 78 (69.6) | <.01 |
| No | 146 | 96 (64.9) | 16 (47.1) | 34 (30.4) | |
| Missing | 1 | 1 (0.7) | … | … | |
| Ever IDU | |||||
| Yes | 128 | 6 (4.1) | 27 (79.4) | 95 (84.8) | <.01 |
| No | 166 | 142 (95.9) | 7 (20.6) | 17 (15.2) | |
| Current IDU | |||||
| Yes | 11 | 1 (0.7) | 0 | 10 (8.9) | <.01 |
| No | 282 | 146 (98.6) | 34 (100) | 102 (91.1) | |
| Missing | 1 | 1 (0.7) | … | … | |
| Current CD4+ T cell count | |||||
| >500 cells/mm3 | 68 | 36 (24.3) | 7 (20.6) | 25 (22.3) | .65 |
| 351–500 cells/mm3 | 67 | 38 (25.7) | 9 (26.5) | 20 (17.9) | |
| 201–350 cells/mm3 | 87 | 42 (28.4) | 11 (32.4) | 34 (30.4) | |
| <200 cells/mm3 | 72 | 32 (21.6) | 7 (20.6) | 33 (29.5) | |
| Current plasma HIV RNA load | |||||
| ≤4000 copies/mL | 104 | 46 (31.1) | 12 (35.3) | 46 (41.1) | .26 |
| 4001–20,000 copies/mL | 73 | 45 (30.4) | 8 (23.5) | 20 (17.9) | |
| 20,001–55,000 copies/mL | 37 | 18 (12.2) | 6 (17.6) | 13 (11.6) | |
| >55,000 copies/mL | 78 | 39 (26.4) | 8 (23.5) | 31 (27.7) | |
| Missing | 2 | … | … | 2 (1.8) | |
NOTE. Current injection drug use (IDU) reflects injection drug use during the 6 months preceding the pre-HAART visit. HCV+, HCV antibody positive; HCV−, HCV antibody negative; RNA+, HCV RNA positive; RNA−, HCV RNA negative.
Viral load measurements and HCV serologic results
Blood was collected for viral load measurements in sodium citrate cell-preparation tubes (Vacutainer brand tubes; Becton-Dickinson), which were either processed within 6 h or centrifuged at 1500 g and then processed and stored at −80°C as directed by the manufacturer. Plasma HIV RNA levels were measured using the NASBA/NucliSens HIV RNA assay (bioMérieux), in accordance with the manufacturer’s recommendations. Plasma HIV RNA measurements were conducted by personnel in laboratories that participate in and are certified by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases, Virology Quality Assurance certification program [20]. HCV RNA was measured using COBAS Amplicor HCV Monitor 2.0 (Roche Diagnostics) with a linear range of 600–700,000 IU/mL. Because we anticipated that coinfected women would have HCV RNA levels higher than the upper limit of the assay, all samples were initially diluted 1:10; if they were found to be negative for HCV RNA, they were retested in undiluted form using a qualitative Amplicor HCV assay, which has a lower detection limit of 50 IU/mL (Roche Diagnostics); if these were positive, they were retested in undiluted form with the quantitative assay. All specimens that were nonreactive in both HCV quantitative and qualitative polymerase chain reaction assays were considered to be HCV RNA negative. HCV serologic results were determined at entry by the contemporary HCV commercial EIAs. Additionally, all women with undetectable HCV RNA were retested by HCV 3.0 EIA (Ortho Diagnostic), and all results with a signal-to-cutoff ratio <3.8 were confirmed by RIBA 3.0 (available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5203a1.htm#fig4).
Immunofluorescence staining and flow-cytometric analysis
The expression of naive/memory, activation, and functional markers on CD4+ and CD8+ T cells were analyzed using frozen PBMCs and 3- or 4-color flow cytometry. All antibodies (anti-CD3, -CD4, -CD8, –HLA-DR, -CD38, -CD27, -CD45RO, -CD95, -CD45RA, and -IgG) were purchased from either Becton Dickinson or PharMingen. Flow-cytometric analyses were performed using a FACSCalibur flow cytometer with Cell Quest software (Becton Dickinson). Activated cells were defined as HLA−DR+CD38+, naive T cells were defined as CD45RO−CD27+CD95−, and memory T cells were defined as CD45RO+CD27−CD95+.
Statistical analysis
χ2 square tests were used for the comparison of demographic and clinical characteristics among HCV−, HCV+RNA−, and HCV+RNA+ women. To evaluate the level of markers of activation and maturation on CD4+ and CD8+ T cells among the 3 HCV groups at the pre-HAART visit, a general linear model was used for each marker. The change in marker levels after HAART, in comparison with pre-HAART levels, was also assessed within each HCV group for each marker, using a general linear model. To compare overall marker levels and patterns of change over the pre-HAART, and post-HAART 1, and post-HAART 2 visits among the 3 HCV groups, repeated-measures analysis of variance was used. To investigate the relationship between immune markers and demographic and clinical factors, multivariate analysis of data from the pre-HAART to post-HAART 1 and post-HAART 2 visits was performed, using repeated-measures analysis of variance adjusting for age, ethnicity, current smoking, current injection drug use, pre-HAART CD4+ cell counts (>500, 351–500, 201–350, or <200 cells/mm3), pre-HAART antiretroviral treatment (no treatment, monotherapy, or combination therapy), HCV status (HCV−, HCV+RNA−, or HCV+RNA+), and HIV response (not applicable [pre-HAART HIV load of ≤1000 copies/mL or at the limit of assay detection], complete response [HIV load at post-HAART visit 1 undetectable, <80 copies/mL], no response [decrease in HIV load at post-HAART visit 1 from pre-HAART visit of <0.5 log10], and partial response [decrease in HIV load at post-HAART visit 1 from pre-HAART visit of >0.5 log10 but not <80 copies/mL]). These definitions were based on NIH standards (available at: http://aidsinfo.nih.gov/guidelines/adult/AA_100605.pdf). For all the statistical tests applied, 2-tailed P < .05 was considered to be statistically significant. SAS statistical software (version 9; SAS Institute) was used to conduct the analyses.
RESULTS
Demographic characteristics of the participants
All of the participants in the study were infected with HIV. A detailed demographic description of the cohort is listed in table 1. HCV-negative women, as indicated by serologic and virologic testing, constituted ~50% of the participants (HCV−); ~38% of the women were HCV positive by both serologic and virologic testing (HCV+RNA+); and ~12% of the women showed cleared/suppressed HCV replication, as indicated by a positive serologic and a negative virologic response (HCV+RNA−). HCV-positive women were, in general, older and more likely to have had >10 sex partners, and women with HCV viremia were more likely to be active injection drug users. CD4+ T cell counts and HIV RNA levels were similar among the 3 groups.
Impact of HCV coinfection on alterations in the CD4+ T cell compartment at the pre-HAART and post-HAART visits
To examine the impact of HCV coinfection on the status of the immune system and on its possible influence on immune recovery after the initiation of HAART, we evaluated absolute CD4+ T cell counts and a number of immunophenotypic markers on CD4+ T cells reflective of immune activation (CD4+HLA-DR+CD38+), naive (CD4+CD45RO−CD27+CD95−), and memory (CD4+CD45RO+CD27−CD95+) T cell status at baseline and at the post-HAART 1 and 2 visits in the 3 HIV-infected groups (HCV−, HCV+RNA−, and HCV+RNA+). All analyses consisted of a comparison between the unadjusted analysis for the mean percentage expression of each marker among the 3 groups and multivariate repeated-measures analyses of variance, adjusting for age, ethnicity, current smoking, current injection drug use, pre-HAART CD4+ T cell counts (>500, 351–500, 201–350, or <200 cells/mm3), pre-HAART antiretroviral treatment (no treatment, monotherapy, or combination therapy), HCV status (HCV−, HCV+RNA−, or HCV+RNA+), and HIV response.
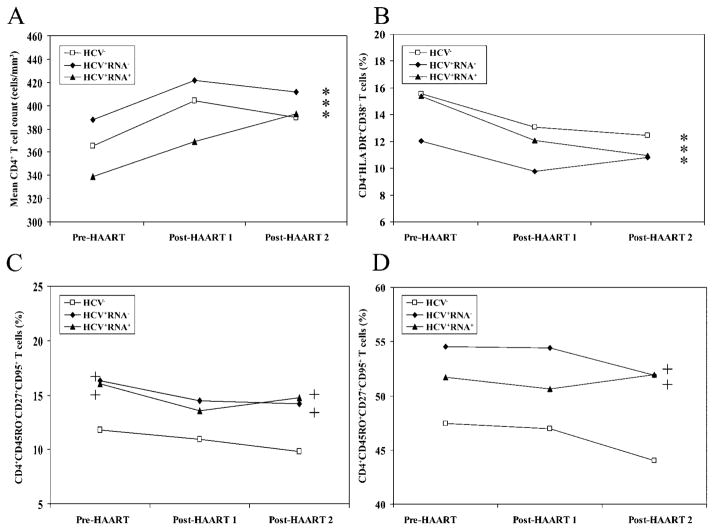
The absolute number of CD4+ T cells was similar among the 3 HCV groups at baseline (figure 1A). Specifically, mean CD4+ T cell expression in the HCV− group was 365 cells/μL; in the HCV+RNA− group, it was 388 cells/μL, and in the HCV+RNA+ group, it was 339 cells/μL (P > .2; figure 1A). After the initiation of HAART, absolute CD4+ T cell counts increased within each group, and the pattern of change, as indicated by the slope of the line, was similar among the 3 groups (figure 1A). The percentage of activated CD4+ T cells were also similar among the 3 groups (P > .1; figure 1B) at baseline. After the initiation of HAART, the percentage of activated CD4+ T cells was reduced among the 3 groups (P < .001, figure 1B), independent of HCV serostatus.
Figure 1.
Comparative analysis of alterations in the CD4+ T cell compartment within and between the 3 HIV-infected groups. A, Mean no. of CD4+ T cells/mm3. B, Mean percentage of CD4+ activated T cells. C, Percentage of CD4+CD45RO−CD27+CD95+ T cells. D, Percentage of CD4+CD45RO+CD27+CD95+ T cells. Asterisks in the far right of each graph indicate statistical significance (P < .05) after the initiation of highly active antiretroviral therapy (HAART; combining post-HAART visits 1 and 2), compared with pre-HAART values, within each group. The plus sign to either the left or right within each panel designates a significant value (P < .05) between the group and the hepatitis C virus–negative (HCV−) group at baseline or after the initiation of HAART, respectively. No plus sign indicates that the difference between groups was not significant (P > .05 ). HCV+, HCV antibody positive; RNA+, HCV RNA positive; RNA−, HCV RNA negative.
Absolute numbers of naive and memory CD4+ T cells were also similar among the 3 groups at baseline, and the pattern of change was similar among the 3 groups (data not shown). Of interest are markers demonstrating the coexpression of CD27 and CD95, with or without CD45RO expression. These markers were higher in HCV+RNA+ and HCV+RNA− than in HCV− women. Specifically, CD4+CD45RO−CD27+CD95+ cell counts were increased in the HCV+ groups, independent of RNA status, in comparison to the HCV− group (P < .05; figure 1C). After the initiation of HAART, the percentage of these cells remained unchanged within each group but was still higher in the HCV+RNA+ and HCV+RNA− groups than in the HCV− group. Similarly, CD4+CD45RO+CD27+CD95+ cells exhibited a trend toward higher expression in the HCV+RNA+ and HCV+RNA− groups (HCV+RNA−, P =.06; HCV+RNA+, P =.08), but this trend did not reach statistical significance until after the initiation of HAART (HCV+RNA−, P =.006; HCV+RNA−, P =.004) (figure 1D). On the basis of current knowledge of T cell differentiation markers, it is likely that CD4+CD45RO−CD27+CD95+ and CD4+CD45RO+CD27+CD95+ cells represent central memory (CM) and effector memory (EM) T cells, respectively. Collectively, within the CD4+ T cell compartment, our data indicate that HCV coinfection does not affect pre-HAART values of absolute CD4+ T cell counts, the percentage of activated cells, and absolute naive and memory T cells but that it leads to enhanced expression of primed/memory T cells. HCV coinfection also did not alter HAART-mediated responses in the immunophenotypic profile of CD4+ T cells.
Impact of HCV coinfection on alterations in the CD8+ T cell compartment at the pre-HAART and post-HAART visits
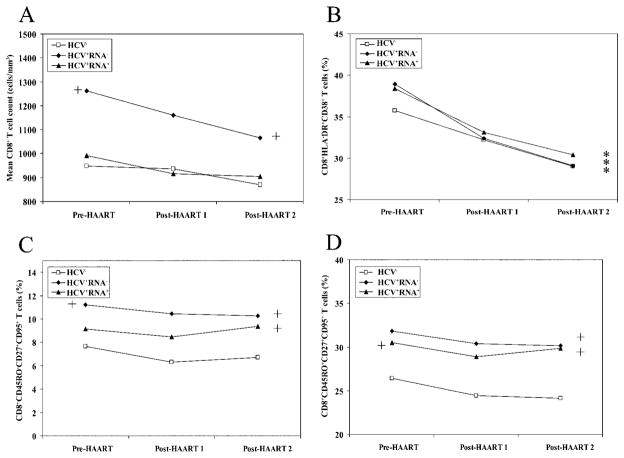
At baseline, although absolute numbers of CD8+ T cells were similar between the HCV− and HCV+RNA+ groups, they were significantly higher in the HCV+RNA− group (P =.003), compared with the HCV− group (figure 2A). After the initiation of HAART, numbers of CD8+ T cells did not significantly change within each group from pre-HAART values, with CD8+ T cell counts being still higher in the HCV+RNA− group (P < .001; figure 2A). The percentage of activated CD8+ T cells was similar at baseline among the 3 HCV groups (P > .2), and the magnitude of decline among the 3 groups after the initiation of HAART was similar (figure 2B). The percentage of CD8+ naive T cells was equivalent before and after the initiation of HAART among the 3 groups (data not shown). The percentage of CD8+ memory T cells was higher in the HCV+RNA+ groups than in the HCV− group, whereas the HCV+RNA− group demonstrated a trend toward higher baseline values of CD8+ naive T cells (P =.08, data not shown). It is likely that this trend did not reach statistically significant values because of the smaller number of HCV+RNA− women in the study (n =34), which is inherent to the observation that only 10%–15% of HCV-infected individuals will clear HCV. The overall pattern of change after the initiation of HAART within the percentage of CD8+ memory T cells was similar among the 3 groups.
Figure 2.
Comparative analysis of alterations in the CD8+ T cell compartment within and between the 3 HIV-infected groups. A, Mean no. of CD8+ T cells/mm3. B, Mean percentage of CD8+ activated T cells. C, Percentage of CD8+CD45RO−CD27+CD95+ T cells. D, Percentage of CD8+CD45RO+CD27+CD95+ T cells. Asterisks in the far right of each graph indicate statistical significance (P < .05) after the initiation of highly active antiretroviral therapy (HAART; combining post-HAART visits 1 and 2), compared with pre-HAART values, within each group. The plus sign to either the left or right within each panel designates a significant value (P < .05) between the group and the hepatitis C virus–negative (HCV−) group at baseline or after the initiation of HAART, respectively. No plus sign indicates that the difference between the groups was not significant (P > .05). HCV+, HCV antibody positive; RNA+, HCV RNA positive; RNA−, HCV RNA negative.
As in the CD4+ T cell compartment, alterations in CD27 and CD95 on CD45RO+ or CD45RO− cells were also observed in CD8+ T cells. Specifically, CD8+CD45RO−CD27+CD95+ cells were higher in HCV+RNA− group than in the HCV− group at baseline (P =.02; figure 2C). After the initiation of HAART, they remained higher in the HCV+RNA− group and reached statistical significance in the HCV+RNA+ group (figure 2C). The pattern of change within each group after the initiation of HAART was similar in the percentage expression of this population. The other effector population (CD8+CD45RO+CD27+CD95+) within the CD8+ T cell compartment was also higher in the HCV+RNA+ group at baseline than in the HCV− group (P =.04) while exhibiting a trend toward higher levels in HCV+RNA− group (P =.07) (figure 2D). After the initiation of HAART, the percentage expression of CD8+CD45RO+CD27+CD95+ T cells remained increased in both HCV+ (RNA+ or RNA−) groups, compared with the HCV− group (P < .0001; figure 2D). HAART per se, however, did not alter the percentage of these cells, and the pattern of change was similar among the 3 groups after the initiation of HAART. Current smoking did not affect any of the immunologic markers examined in the CD4+ and CD8+ T cell compartments.
Multivariate analysis of CD4+ T cell count and percentage expression of CD45RO−CD27+CD95+ and CD45RO+ CD27+CD95+ T cells in the CD4+ and CD8+ T cell compartments
Given that CD4+ and CD8+ CD45RO−CD27+ CD95+ and CD45RO+CD27+CD95+ T cells were higher in the HCV+RNA+ and HCV+RNA− groups at baseline, we investigated the relationship with clinical (pre-HAART CD4+ T cell count, HIV response, HCV status, and HAART visit) and demographic (age, ethnicity, current smoking, and current injection drug use) parameters. Viral responses were classified as no response, partial response, complete response, or not applicable as defined in the statistical analysis subsection. As shown in table 2, the percentage of CD4+CD45RO+CD27+CD95+ T cells was higher if the pre-HAART CD4+ T cell count was between 201 and 350 cells/mm3 (P =.04). As was reported above, the CD4+CD45RO+CD27+CD95+ T cell population was higher in the HCV+ groups, independent of RNA status, compared with the HCV− group (table 2). The percentage expression of CD4+CD45RO−CD27+CD95+ T cells was inversely correlated with the pre-HAART CD4+ T cell count. Specifically, as the pre-HAART CD4+ T cell counts decreased from 201–350 to <200 cells/mm3, the percentage difference in these cells increased from 4.2% to 8.0%, compared with women who had pre-HAART CD4+ T cell counts >500 cells/mm3 (P < .01 for both) (table 2). The CD4+CD45RO−CD27+CD95+ T cell population was lower in HIV responders and partial responders (P < .01 for both), and the decrease was evident at post-HAART visits both 1 and 2 (table 2). Finally, as expected, CD4+ T cell counts were higher for patients with the highest pre-HAART CD4+ T cell counts, were higher for both virologic responders and partial responders, and were higher after the initiation of HAART (table 2). None of these changes, as reported above, were affected by HCV serostatus (table 2).
Table 2.
Adjusted analysis of CD4+ and CD8+ T cell counts and immune markers by demographic and clinical characteristics.
| Characteristic | No. of patientsa | Difference from reference group
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean total CD4+ T cell count | P | CD4+CD45RO+ CD27+CD95+ % | P | CD4+CD45RO− CD27+CD95+ % | P | Mean total CD8+ T cell count | P | CD8+CD45RO+ CD27+CD95+ % | P | CD8+CD45RO− CD27+CD95+ % | P | ||
| Age group | |||||||||||||
| <35 years | 90 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| ≥35 years | 204 | 11 | .37 | −1.1 | .57 | 2.1 | .06 | 26 | .63 | −1.2 | .47 | 0.3 | .66 |
| Ethnicity | |||||||||||||
| White | 41 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| Black | 165 | 7 | .68 | −4.2 | .10 | −0.5 | .72 | 126 | .11 | −0.3 | .90 | 0.1 | .94 |
| Hispanic | 84 | 6 | .74 | −5.4 | .05 | −0.1 | .96 | 37 | .66 | −1.8 | .44 | −1.0 | .34 |
| Other | 4 | 2 | .96 | −11.8 | .13 | −8.2 | .06 | 32 | .89 | −0.1 | .98 | −2.0 | .47 |
| Current smoking | |||||||||||||
| Yes | 147 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| No | 146 | 3 | .81 | −1.3 | .43 | −1.7 | .09 | −34 | .41 | −1.9 | .18 | −0.6 | .37 |
| Missing | 1 | … | … | … | … | … | … | ||||||
| Current IDU | |||||||||||||
| Yes | 11 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| No | 282 | −25 | .33 | 0.7 | .85 | 0.4 | .85 | −141 | .10 | 1.6 | .59 | 1.2 | .39 |
| Missing | 1 | ||||||||||||
| Pre-HAART CD4+ T cell count | |||||||||||||
| >500 cells/mm3 | 68 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| 351–500 cells/mm3 | 67 | −238 | <.01 | 1.9 | .45 | 0.9 | .53 | −118 | .12 | −2.8 | .18 | −0.9 | .33 |
| 201–350 cells/mm3 | 87 | −396 | <.01 | 4.9 | .04 | 4.2 | <.01 | −216 | <.01 | 0.3 | .87 | 1.6 | .07 |
| <200 cells/mm3 | 72 | −536 | <.01 | 1.3 | .60 | 8.0 | <.01 | −349 | <.01 | −2.0 | .35 | 2.0 | .03 |
| Pre-HAART treatment | |||||||||||||
| No treatment | 89 | 0 | 0.0 | 0 | 0.0 | 0.0 | |||||||
| Monotherapy | 31 | 9 | .67 | −3.4 | .29 | −1.2 | .52 | −37 | .71 | −1.9 | .47 | −2.0 | .09 |
| Combination therapy | 174 | 11 | .43 | −1.0 | .61 | −1.6 | .18 | −15 | .80 | −0.9 | .61 | −1.7 | .02 |
| HIV response | |||||||||||||
| Nonresponder | 84 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| Partial responder | 94 | 21 | .16 | −4.3 | .06 | −3.3 | .01 | −41 | .54 | −1.2 | .52 | −1.7 | .03 |
| Responder | 62 | 32 | .06 | −1.4 | .57 | −4.4 | <.01 | −70 | .37 | 0.3 | .90 | −1.1 | .24 |
| Not applicable | 51 | 28 | .12 | −3.1 | .24 | −4.7 | <.01 | −160 | .05 | −1.0 | .64 | −0.3 | .78 |
| Missing | 3 | … | … | … | … | … | … | ||||||
| HCV status | |||||||||||||
| Antibody negative | 148 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| Antibody positive, RNA negative | 34 | 27 | .14 | 7.4 | <.01 | 4.1 | <.01 | 196 | .01 | 5.4 | .02 | 3.7 | <.01 |
| Antibody and RNA positive | 112 | 2 | .91 | 4.6 | .03 | 2.0 | .09 | 43 | .48 | 4.3 | .01 | 1.4 | .06 |
| HAART visit | |||||||||||||
| Pre-HAART | 294 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | ||||||
| Post-HAART 1 | 294 | 35 | <.01 | −0.9 | .49 | −1.6 | .03 | −36 | .13 | −1.7 | .12 | −1.0 | .03 |
| Post-HAART 2 | 290 | 39 | <.01 | −1.6 | .25 | −2.0 | .03 | −91 | <.01 | −1.3 | .25 | −0.5 | .38 |
NOTE. Bold type indicates statistical significance. Complete response, HIV load at post–highly antiretroviral therapy (HAART) visit 1 (0–6 months) undetectable (<80 copies/mL); no response, decrease in HIV load at post-HAART visit 1 from pre-HAART visit of <0.5 log10; not applicable, pre-HAART HIV load ≤ 1000 copies/mL or less than the lower limit of detection; partial response, decrease in HIV load at post-HAART visit 1 from pre-HAART visit of >0.5 log10, but detectable.
At the pre-HAART visit.
In the CD8+ T cell compartment, the percentage of CD8+CD45RO+CD27+CD95+ T cells was higher in the HCV+ groups, independent of HCV RNA status (table 2). This population did not correlate with any other clinical or demographic parameter evaluated (table 2). The percentage of CD8+CD45RO−CD27+CD95+ T cells was higher in the HCV+RNA− group (P =.0002), and there was a trend toward higher levels in the HCV+RNA+ group (P =.06). This population was lowest in partial responders (P =.03), was lower in women who received combination therapy (P =.02), and was highest in women with pre-HAART CD4+ T cell counts <200 cells/mm3 (P =.03) (table 2).
DISCUSSION
We evaluated the impact in HIV-infected individuals of HCV coinfection on immunophenotypic markers of the immune system and on clinical responses to HAART. HCV coinfection does not appear to alter the extent of the CD4+ T cell decline or immune responses to HAART in coinfected women. This observation indicates that the lower in CD4+ T cell count in coinfected patients is driven by HIV and not HCV, because CD4+ T cell counts were similar at baseline between the HIV-monoinfected and HIV/HCV-coinfected women and increased at the same rate after the initiation of HAART. Additionally, these data suggest that HCV in coinfected patients does not add to the HIV-mediated destruction of CD4+ T cells. This finding is in contrast to an observation reported in a Swiss cohort by Greub et al. [10], which pointed to an impairment in CD4+ T cell recovery in HCV-coinfected patients, but our study is in agreement with other results [16–18]. Given that both our study and that of Greub et al. had a large number of patients, it is unlikely that the opposing conclusion regarding CD4+ T cell recovery is driven by a lack of statistical power, but it could be driven by variations in the demographic characteristics of the patients included in both studies. In particular, in the Greub et al. study, HCV-infected and -uninfected patients were at opposite end of the spectrum in terms of reported injection drug use; ~5% of HCV-uninfected patients reported injection drug use, compared with 89% of HCV-infected patients. In the present study, the HCV-infected and -uninfected patients were similar in their active use of injection drugs, with>86% in each group reporting no current injection drug use. Additionally, our data were exclusively based on women, whereas, in the Swiss cohort, women constituted only ~30% of the cohort [10]. Conflicting results may also have been driven by sex differences.
There are several populations of memory T cells: EM, CM, and terminally differentiated EM [21–25]. Although all memory cells can mount a recall immune response, CM cells are more long-lived and have higher proliferative capacity [26]. CD62L and CCR7 coexpression is widely used to distinguish between memory subsets [27], but they are not ideal for retrospective study, because CD62L can be shed from the cell surface and is not stable on frozen samples. These markers also seem to be more indicative of homing location rather than differentiation status [25, 28]. We evaluated the frequency of CD45RO+CD27−CD95+ and CD45RO−CD27+CD95− T cells, to distinguish between memory and naive T cells, respectively. Similar patterns emerged in the CD4+ and CD8+ T cell compartments; naive T cells were not affected by HCV serostatus at baseline or in response to HAART. However, although memory CD4+ T cells were not affected by HCV coinfection, memory CD8+ T cell counts were higher in the HCV+RNA+ group. This finding suggests a role of this population in HCV and warrants further investigation.
Certain subsets of CD4+ and CD8+ memory T cells were also increased in HCV-infected women, and they remained higher after the initiation of HAART. These populations were CD45RO−CD27+CD95+ and CD45RO+CD27+CD95+ T cells; on the basis of CD45RO and CD27 expression, it is plausible that they represent CM and EM T cells, respectively. Further phenotypic markers need to be assessed to determine the nature of these effector populations. The CM population is believed to be a more potent responder to recall antigen, and it may have been responsible for driving HCV clearance in the HCV+ RNA− group. These data also suggest that HCV coinfection may induce the expansion and/or preservation of these memory T cell populations. These cells may be maintained as a response to continuous antigen stimulation by HIV and HCV. Evidence for extrahepatic HCV replication in PBMCs, especially in HIV/HCV-coinfected patients, is well documented [29–33], and it may be responsible for the antigen load in the periphery, leading to the expansion of these populations. It would be of interest to determine whether these cells are indeed HCV specific, as can be evaluated by tetramer technology. Because these cells are CD95+ (Fas receptor), they may be more susceptible to Fas-and Fas ligand–mediated apoptosis. In vitro studies, however, have indicated otherwise. Cells coinfected with HCV and HIV are protected against apoptosis through a factor or factors secreted by HCV [34–36]. However, ex vivo data may be different from those in vivo studies, and CD95 expression on cells alone does not necessarily indicate that those cells are programmed to undergo apoptosis; they may be susceptible to apoptosis under the appropriate signals.
Although we did not find that HCV coinfection leads to further reductions in the number of CD4+ T cells or altered HAART-mediated immune responses, immunophenotypic analysis alone is not a comprehensive indicator of immune integrity. A recent report indicated that T helper responses are impaired in coinfected patients to a greater extent than monoinfected patients, as measured by effector function responses [37]. Therefore, HCV infection may lead to additional functional impairments without altering the number of such cells.
Acknowledgments
We thank the study participants for their cooperation.
Data were collected by the Women’s Interagency HIV Study Collaborative Study Group, with centers (Principal Investigators) at New York City/Bronx Consortium, Bronx, NY (Kathryn Anastos); Brooklyn, NY (Howard Mink-off); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California, San Francisco (Ruth Green-blatt); Los Angeles County/Southern California Consortium, Los Angeles (Alexandra Levine); Chicago Consortium, Chicago, IL (Mardge Cohen); and Data Coordinating Center, Boston, MA (Stephen J. Gange).
Financial support. The Women’s Interagency HIV Study was supported by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute and the National Institute on Drug Abuse (grants U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-HD-32632, U01-AI-34993, and U01-AI-42590). Funding was also provided by the National Institute of Child Health and Human Development (grant U01-HD-3-2632-11) and the National Center for Research Resources (M01-RR-00071, M01-RR-00079, and M01-RR-00083).
Financial support: National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant R01 052065 to A.K.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77–84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 2.Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–7. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 4.Beld M, Penning M, Lukashov V, et al. Evidence that both HIV and HIV-induced immunodeficiency enhance HCV replication among HCV seroconverters. Virology. 1998;244:504–12. doi: 10.1006/viro.1998.9130. [DOI] [PubMed] [Google Scholar]
- 5.Poles MA, Dieterich DT. Infections of the liver in HIV-infected patients. Infect Dis Clin North Am. 2000;14:741–59. doi: 10.1016/s0891-5520(05)70129-x. [DOI] [PubMed] [Google Scholar]
- 6.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 7.Valdez H, Chowdhry TK, Asaad R, et al. Changing spectrum of mortality due to human immunodeficiency virus: analysis of 260 deaths during 1995–1999. Clin Infect Dis. 2001;32:1487–93. doi: 10.1086/320164. [DOI] [PubMed] [Google Scholar]
- 8.Piroth L, Duong M, Quantin C, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–8. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lesens O, Deschênes M, Steben M, Bélanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus–positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179:1254–8. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 10.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 11.Sabin CA, Telfer P, Phillips AN, Bhagani S, Lee CA. The association between hepatitis C virus genotype and human immunodeficiency virus disease progression in a cohort of hemophilic men. J Infect Dis. 1997;175:164–8. doi: 10.1093/infdis/175.1.164. [DOI] [PubMed] [Google Scholar]
- 12.Dorrucci M, Pezzotti P, Phillips AN, Lepri AC, Rezza G. Coinfection of hepatitis C virus with human immunodeficiency virus and progression to AIDS. Italian Seroconversion Study. J Infect Dis. 1995;172:1503–8. doi: 10.1093/infdis/172.6.1503. [DOI] [PubMed] [Google Scholar]
- 13.Macias J, Pineda JA, Leal M, et al. Influence of hepatitis C virus infection on the mortality of antiretroviral-treated patients with HIV disease. Eur J Clin Microbiol Infect Dis. 1998;17:167–70. doi: 10.1007/BF01691112. [DOI] [PubMed] [Google Scholar]
- 14.De Luca A, Bugarini R, Lepri AC, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162:2125–32. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 15.Macias J, Pineda JA, Lozano F, et al. Impaired recovery of CD4+ cell counts following highly active antiretroviral therapy in drug-naive patients coinfected with human immunodeficiency virus and hepatitis C virus. Eur J Clin Microbiol Infect Dis. 2003;22:675–80. doi: 10.1007/s10096-003-1015-2. [DOI] [PubMed] [Google Scholar]
- 16.Chung RT, Evans SR, Yang Y, et al. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS. 2002;16:1915–23. doi: 10.1097/00002030-200209270-00008. [DOI] [PubMed] [Google Scholar]
- 17.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Carbonero L, Nunez M, Rios P, Perez-Olmeda M, Gonzalez-Lahoz J, Soriano V. Liver injury after beginning antiretroviral therapy in HIV/hepatitis C virus co-infected patients is not related to immune reconstitution. AIDS. 2002;16:1423–5. doi: 10.1097/00002030-200207050-00016. [DOI] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Inter-agency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 20.Nowicki MJ, Benning L, Bremer JW, et al. Longitudinal variability of human immunodeficiency virus type 1 RNA viral load measurements by nucleic acid sequence-based amplification and NucliSens assays in a large multicenter study. J Clin Microbiol. 2001;39:3760–3. doi: 10.1128/JCM.39.10.3760-3763.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 23.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–9. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 25.Wherry EJ, Ahmed R, Crotty S. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maus MV, Kovacs B, Kwok WW, et al. Extensive replicative capacity of human central memory T cells. J Immunol. 2004;172:6675–83. doi: 10.4049/jimmunol.172.11.6675. [DOI] [PubMed] [Google Scholar]
- 27.Managlia EZ, Landay A, Al-Harthi L. Interleukin-7 signalling is sufficient to phenotypically and functionally prime human CD4 naive T cells. Immunology. 2005;114:322–35. doi: 10.1111/j.1365-2567.2004.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antia R, Ganusov VV, Ahmed R, Crotty S. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005;5:101–11. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 29.Laskus T, Radkowski M, Jablonska J, et al. Human immunodeficiency virus facilitates infection/replication of hepatitis C virus in native human macrophages. Blood. 2004;103:3854–9. doi: 10.1182/blood-2003-08-2923. [DOI] [PubMed] [Google Scholar]
- 30.Kaminska A, Bednarska A, Radkowski M. Extrahepatic replication of the hepatitis C virus (HCV) Przegl Epidemiol. 2003;57:317–20. [PubMed] [Google Scholar]
- 31.Goutagny N, Fatmi A, De Ledinghen V, et al. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951–8. doi: 10.1086/375350. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Shahidi A, Park S, Guilfoyle D, Hirshfield I. Detection of extrahepatic hepatitis C virus replication by a novel, highly sensitive, single-tube nested polymerase chain reaction. Am J Clin Pathol. 2003;119:95–100. doi: 10.1309/33TA-JLB7-48KL-MXVG. [DOI] [PubMed] [Google Scholar]
- 33.Yan FM, Chen AS, Hao F, et al. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805–811. doi: 10.3748/wjg.v6.i6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Street A, Macdonald A, Crowder K, Harris M. The hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependentsurvival signaling cascade. J Biol Chem. 2004;279:12232–41. doi: 10.1074/jbc.M312245200. [DOI] [PubMed] [Google Scholar]
- 35.Gale M, Jr, Kwieciszewski B, Dossett M, Nakao H, Katze MG. Anti-apoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J Virol. 1999;73:6506–16. doi: 10.1128/jvi.73.8.6506-6516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung YL, Sheu ML, Yen SH. Hepatitis C virus NS5A as a potential viral Bcl-2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int J Cancer. 2003;107:65–73. doi: 10.1002/ijc.11303. [DOI] [PubMed] [Google Scholar]
- 37.Villacres MC, Literat O, Degiacomo M, et al. Reduced type 1 and type 2 cytokines in antiviral memory T helper function among women coinfected with HIV and HCV. J Clin Immunol. 2005;25:134–41. doi: 10.1007/s10875-005-2819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]