Abstract
Virologic response to highly active antiretroviral therapy (HAART) typically results in a substantial rise in CD4 cell counts. We investigated factors associated with poor CD4 response among HIV-infected women followed at 6-monthly intervals in the Women’s Interagency HIV Study. Women with nadir CD4 counts <350 cells/mm3 who achieved at least 6 months of plasma HIV RNA < 400 copies/ml were studied. Demographic, clinical, and treatment factors were compared between immunologic nonresponders, defined as the lower quartile of CD4 count change after two visits with virologic suppression (<56 cell/mm3; n = 38), and the remaining group of responders (n = 115). Immunologic nonresponders had lower baseline HIV RNA levels and higher CD4 counts, more frequently used HAART 6 months prior to achieving consistent viral suppression, and more commonly had HIV RNA levels >80 but <400 copies/mL at both suppressive visits (21 vs. 7.8%, p = 0.024). In multivariate analysis, higher CD4 count and lower HIV RNA level at the last presuppressive visit were associated with immune nonresponse. We conclude that higher baseline CD4 count and lower HIV RNA level were associated with poor immunologic response to HAART in women with virologic suppression for at least 6 months. Persistent low level viremia may also contribute.
INTRODUCTION
Highly active antiretroviral therapy (HAART) has dramatically modified the morbidity and mortality of HIV disease.1,2 Virologic response to HAART, specifically the suppression of plasma HIV RNA to below the level of quantification, typically results in a substantial rise in CD4 cell counts. This rise, sometimes termed the immunologic response to HAART, is central to restoration of integrity of the immune system. A summary analysis of 23 clinical trials of triple combination therapy in HAART-naive subjects found that the mean CD4 cell increase 12 months after HAART initiation was 160 cells/mm3 (weighted 95% confidence interval, 146 to 175 cells/mm3).3 However, immunological and virologic response to HAART is neither universal nor homogeneous.4 Moreover, discordant immunologic and virologic responses, where either CD4 counts rise without complete viral suppression or HIV RNA levels are suppressed to below detection but CD4 counts do not rise, have been documented.5 “Complete” responses, where patients experience both virologic and immunologic responses, are associated with improved clinical outcomes when compared with patients with discordant responses or compared with patients who experience neither a virologic nor immunologic response.6,7
Several studies have investigated risk factors for poor immune responses to HAART. To date only increasing age, poorer virologic response,8,9 injection drug use as an HIV acquisition risk factor,10 hepatitis C virus coinfection,11 and lower baseline CD4 cell count and HIV RNA viral load12 have been associated with diminished immunologic response to HAART.
If factors associated with poor immunologic response could be identified, strategies could perhaps be developed to address these risk factors, or otherwise augment immune response, in poor immunologic responders. Examples include discontinuation of implicated medications, treatment of hepatitis C infection, intensification of antiretroviral therapy, or perhaps use of immune-modulating therapy such as interleukin-2.13,14 In addition, with iterations of clinical guidelines advocating deferral of HAART initiation until lower CD4 cell counts,15,16 identification of risk factors for poor immunologic response would allow subjects with those factors to become candidates for earlier initiation of HAART. Therefore, we investigated clinical and laboratory factors associated with poor immunologic response among HIV-infected women monitored in the Women’s Interagency HIV Study (WIHS) with nadir pre-HAART suppression CD4 cell counts of <350 cells/mm3 who achieved at least 6 months of plasma HIV RNA suppression to below 400 copies/ml on HAART.
MATERIALS AND METHODS
Study population
The WIHS is a prospective, multicenter cohort study of the natural history of HIV infection in women conducted at six centers across the United States. The methods of this cohort study have been described previously.17 The study originally enrolled 2059 HIV-seropositive women and 568 HIV-seronegative women from October 1994 through November 1995. These women were seen at study visits every 6 months, interviewed using a standardized questionnaire, received a physical examination, and had blood collected for HIV RNA level, CD4/CD8 lymphocyte subset analysis, complete blood cell count, and creatinine determination. The standardized questionnaire specifically sought all new diagnoses of HIV-associated conditions (including AIDS-defining illnesses), opportunistic infections, hospitalizations, and discharge diagnoses since the previous WIHS visit. Also, all current medication use and any medication use since the last WIHS visit were elicited.
This analysis included all HIV-infected WIHS subjects with nadir presuppression CD4 cell counts of <350 cells/mm3 who experienced at least two consecutive visits with complete HIV RNA suppression while reporting continuous HAART use. Complete viral suppression was considered a plasma HIV RNA of <400 copies/ml. Patients were included if they had reported prior antiretroviral therapy, including HAART, as long as they never achieved transient HIV RNA suppression (a single isolated HIV RNA count of <400 copies/ml) at any time prior to the two consecutive visits with HIV RNA suppression. HAART was defined as one of the following combinations18: two or more nucleoside reverse transcriptase inhibitors (NRTIs), in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitor (NNRTI); one NRTI in combination with at least one PI and at least one NNRTI; one NRTI in combination with ritonavir plus saquinavir; or, an abacavir-containing regimen of three or more NRTIs in the absence of both PIs and NNRTIs. Because of their antagonism, the combination of stavudine and zidovudine with either an NNRTI or PI was not considered to be a HAART regimen.
Statistical analysis
For analysis, each subject’s WIHS visits were reordered based on the first of their two consecutive WIHS visits with HIV RNA suppression to <400 copies/ml. The first visit with suppression of HIV RNA to <400 copies/mL while on HAART was classified as visit 0. All other visits were classified based on the number of months between visit 0 and the visit being considered. WIHS cohort study visits occurred every 6 months, such that the visit immediately preceding visit 0 was classified the −6M visit, the visit scheduled for 18 months after visit 0, the +18M visit, and so forth. In addition, each subject’s nadir presuppressive CD4 cell count, and the visit at which this test was drawn, were determined. All laboratory and medication use variables were identified at each of the above study time points.
Change in absolute CD4 cell count after at least 6 months of virologic suppression on HAART was determined for each subject by calculating the difference between the absolute CD4 cell count at the second suppressive HAART visit (the +6M visit), and the visit immediately preceding the first suppressive HAART visit (i.e., the −6M visit). A subject was considered an immunologic responder if her CD4 cell count change on at least 6 months of suppressive HAART was equal to or greater than the magnitude of the increase of the 25th percentile of the CD4 cell count change of this study cohort. A subject with an increase of less than the 25th percentile was declared an immunologic nonresponder.
Variables of interest
Host-related factors tested for association with immunologic nonresponse to HAART included age at visit 0, ethnicity, HIV acquisition risk factors, clinical AIDS diagnosis at visit 0, hepatitis C virus (HCV) antibody seroreactivity, self-reported alcohol use, self-reported illicit drug use (heroin, cocaine, or crack), and WIHS study center.
The following laboratory values were compared between responders and nonresponders at two time points: (1) the pre-suppressive nadir CD4 cell count visit and (2) the −6M pre-suppression visit: plasma HIV RNA level, absolute CD4 cell count, CD4 percentage, absolute CD8 cell count, CD8 percentage, absolute lymphocyte count, lymphocyte percentage, absolute neutrophil count, neutrophil percentage, white blood cell (WBC) count, hemoglobin concentration, platelet count, serum creatinine concentration, and serum albumin concentration.
The effect of low-level HIV viremia on immune nonresponse was assessed by comparing the number of responders and non-responders with HIV RNA levels between 80 and 400 copies/ml at both visit 0 and the +6M visit.
HIV-1 RNA in plasma was originally quantified for all participants using the isothermal nucleic acid sequence based amplification (NASBA) method, with a lower limit of quantification of 4000 copies/ml (Organon Teknika, Durham, NC), in laboratories that were certified by the National Institutes of Health, Virology Quality Assurance Laboratory proficiency testing program. Starting with WIHS visit 7 (October 1997), plasma HIV-1 RNA was quantified using the more sensitive Nuclisens method of NASBA quantification (lower limit of detection, 80 copies/ml). The NASBA assay correlates well with both branched DNA (Quantiplex; Bayer Diagnostics) and reverse transcriptase-polymerase chain reaction (Cobas Amplicor; Roche Diagnostics) assays, with between assay correlations coefficients of 0.94 for both tests.19 For most women who initiated HAART, HIV-1 RNA determinations were repeated using the more sensitive Nuclisens assay, if needed, at the visit just prior to that at which HAART use was first reported, and for all visits after HAART initiation. Ten women who initiated HAART prior to WIHS visit 7 had their HIV-1 RNA determinations repeated by the early Nuclisens assay with a lower-limit of detection of 400 copies/ml. For the analysis of low-level viremia while taking suppressive HAART, women with plasma HIV-1 RNA levels below the lower limit of detection were considered to have an HIV RNA viral load equal to the cutoff value of the RNA quantification assay utilized at that time point. Thus, the women with viral load determinations performed by the assay with a lower limit of detection of less than 400 copies/ml were considered to have HIV RNA viral loads of 400 copies/ml. CD4 and CD8 lymphocyte subsets were quantified using standard flow cytometric methods in laboratories participating in the NIH/NIAID Flow Cytometry Quality Assessment Program.20
Antiretroviral therapy related factors were tested for association with immunologic nonresponse to HAART. General class of HAART (PI-based, NNRTI-based, or combination PI- and NNRTI-based used in the suppressive HAART regimen), and use of the potentially bone marrow-suppressive medications zidovudine and trimethoprim–sulfamethoxasole, were compared.
Presuppressive antiretroviral medication history was also considered in a variety of ways. The intensity of prior antiretroviral use was hierarchically classified as use of no therapy, monotherapy, dual therapy, or HAART at and prior to the −6M presuppression visit. Also, the number of presuppressive WIHS visits where the subject reported any antiretroviral or HAART use was determined.
Comparisons between immunologic responders and nonresponders were done using Student’s t test for continuous variables (rank tests were also used but not reported as they gave similar results) and by Fisher’s exact test for categorical variables. Individual variables significantly associated with nonresponse were then used in multivariate stepwise logistic regression models to find the set(s) of variables that adequately predicted immunologic nonresponse, using SAS default criteria of p = 0.05 to enter and stay in the model. All statistical procedures were performed with SAS version 8.2 (SAS Institute, Cary, NC).
RESULTS
Figure 1 details the stepwise exclusion of subjects from the WIHS cohort for this study. Most of the women monitored in WIHS who did not qualify for this study were excluded due to lack of consistent continuous HAART use. Of 2059 HIV-sero-prevalent women, 799 were excluded for never reporting HAART use and 291 for initiating HAART but not reporting its use over two consecutive visits. The bulk of the remainder of exclusions were due to either lack of continuous RNA suppression over two consecutive visits (356 women) or transient HIV RNA suppression at some point prior to continuous suppression (166 women). Of the 153 women included in this study, none reported use of chemotherapy or radiation therapy, treatments known to affect bone marrow function and CD4 cell levels. The age, ethnicity, and HIV transmission risk factor of the women included in this study were comparable to those of an analysis of the 1132 women in the WIHS cohort initiating HAART after 1995 (data not shown).21 All women had their first suppressive visit (visit 0) between 1996 and 2002.
FIG. 1.
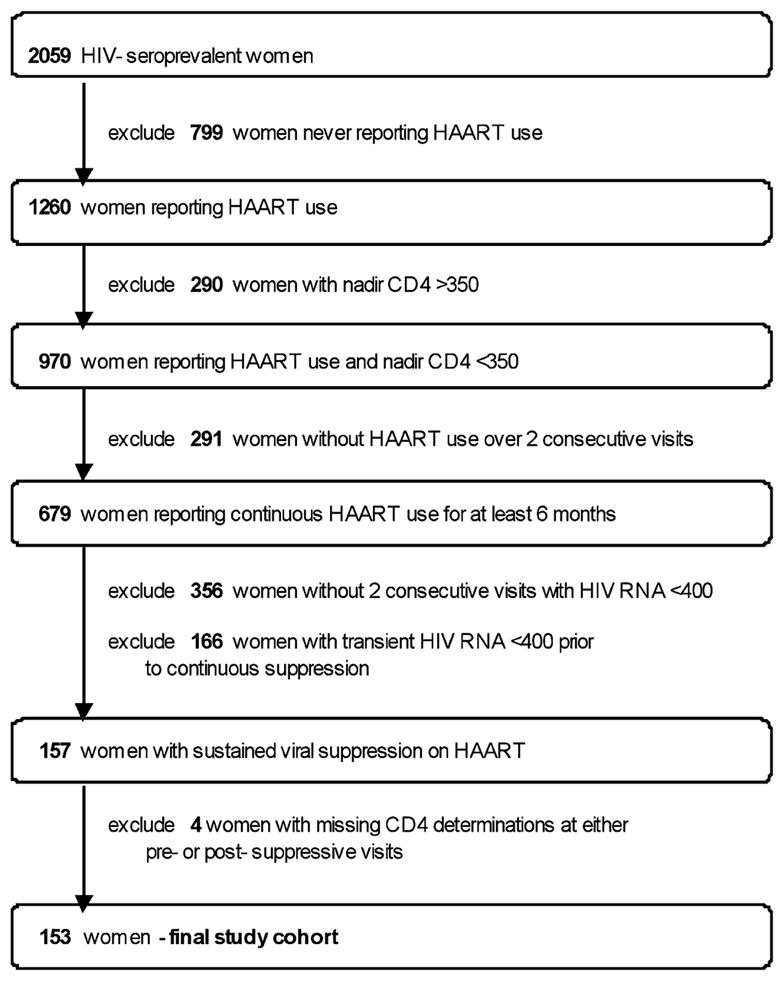
Selection of women enrolled in the WIHS cohort reporting HAART use with sustained virologic suppression for at least 6 months and with a nadir CD4 cell count of <350 cells/mm3.
This study cohort’s mean change in CD4 cell counts from the -6M presuppression visit to the +6M visit under at least 6 months of virologic suppression on HAART was +142 cells/mm3 (standard deviation [SD], 168.6; range, −282 to +839 cells/mm3). The median value and interquartile ranges (IQR) for this pre- to postsuppression change were +116 cells/mm3, and +56 to +199 cells/mm3, respectively. The 25th percentile was used as the cutoff for a successful immune response, such that a 56-cell/mm3 or greater increase on suppressive HAART was required to classify a woman as an immunologic responder. One hundred and fifteen women experienced a 56-cell/mm3 or greater increase and were classified as immunologic responders (range of CD4 change, +56 to +839 cells/mm3). Thirty-eight women had less than a 56-cell/mm3 increase and were classified as immunologic nonresponders (range of CD4 change, −282 to +51 cells/mm3). Twenty-nine of these 38 (74%) immunologic nonresponders had less than a 10-cell/mm3 increase and 22 of 38 (58%) had a net decrease in CD4 cell counts on suppressive HAART. Overall, only eight women (three nonresponders and five responders) did not have a CD4 value of >200 cells/mm3 at either the −6 month, 0, or +6 month visit.
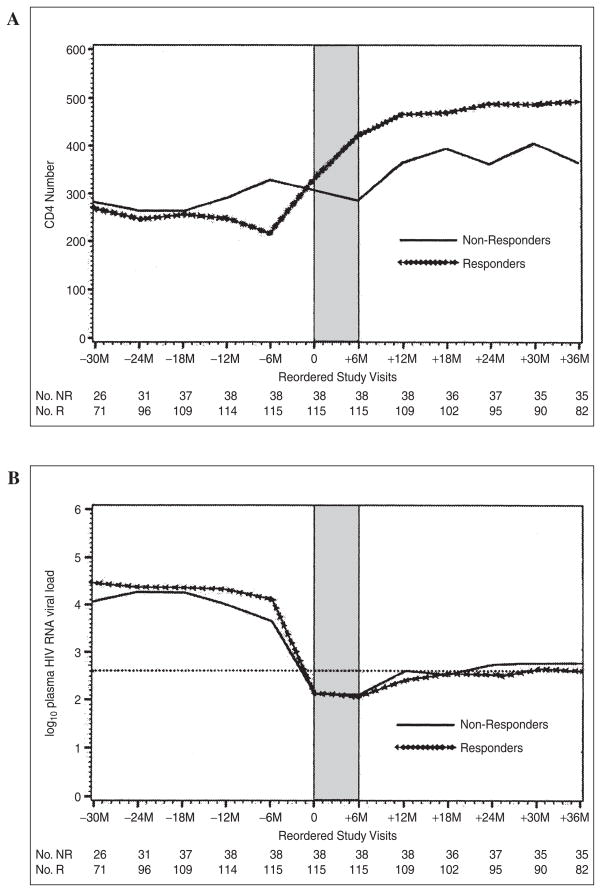
Figure 2 is composed of plots spanning all reordered WIHS visits between the −24M visit (the visit 24 months prior to visit 0, the first suppressive HAART visit) and the +36M visit (the visit 36 months after visit 0). Figure 2A separately plots mean absolute CD4 cell counts whereas Fig. 2B separately plots mean log10 plasma HIV RNA for immunologic nonresponders and responders. As can be seen in Fig. 2A, the difference between immunologic nonresponders and responders in mean CD4 cell count was about 100 cells and persisted for 36 months beyond the initial complete suppression of HIV RNA. In Fig. 2B, the mean plasma log10 HIV RNA viral load at the −6M visit (the presuppressive HAART visit) for immunologic nonresponders was lower than that for responders (3.64 versus 4.12, a difference of 0.48 logs, p = 0.002). This difference did not persist to visits beyond the second suppressive HAART visit (+6M visit).
FIG. 2.
Plot of mean absolute CD4 number (A) and mean log10 HIV RNA level (B) in immunologic responders (R) and nonresponders (NR) at all WIHS visits between the visit 30 months prior to (−30M) and 36 months after (+36M) the first suppressive HAART visit (visit 0). Shaded area denotes required time period of HIV viral suppression below 400 copies/ml.
Table 1 summarizes the clinical characteristics of immunologic responders and nonresponders. The mean age of immunologic nonresponders was 2 years older than responders, although this was not statistically significant (p = 0.13). Race, HIV acquisition risk factor, clinical AIDS diagnosis, hepatitis C serostatus, and substance use (coded as alcohol alone, illicit drug use alone, or either alcohol or illicit drug use) were not significantly associated with immunologic response status. The WIHS center where the subject was enrolled and monitored was also not found to be associated with immunologic response (p = 0.27, data not shown).
Table 1.
Clinical Characteristics of Immunologic Nonresponders versus Immunologic Responders
| Clinical characteristic | Nonresponders (n =38)
|
Responders (n = 115)
|
p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age years: mean (SD) | 38.3 | (8.19) | 36.3 | (6.62) | 0.13a |
| Race | |||||
| White | 10 | 6.3 | 27 | 23.5 | |
| African American | 19 | 50.0 | 57 | 49.6 | |
| Other | 9 | 23.7 | 31 | 26.9 | 0.91b |
| HIV risk factor | |||||
| Injection drug use | 10 | 26.3 | 30 | 26.3 | |
| Heterosexual risk | 17 | 44.7 | 55 | 48.2 | |
| Transfusion risk | 2 | 5.3 | 4 | 3.5 | |
| No identified risk | 9 | 23.7 | 25 | 21.9 | 0.93b |
| Clinical AIDS diagnosis | 26 | 68.4 | 65 | 56.5 | 0.25b |
| Nadir CD4 cell count (cells/mm3): mean (SD) | 190.8 | (94.3) | 151.8 | (107.1) | 0.05a |
| Hepatitis C seropositive | 15 | 41.7 | 36 | 32.1 | 0.32b |
| Substance use | |||||
| Alcohol alone | 18 | 47.4 | 50 | 43.5 | 0.71b |
| Illicit drug use alone | 5 | 13.2 | 12 | 10.4 | 0.77b |
| Alcohol or illicit drug use | 21 | 55.3 | 53 | 46.1 | 0.35b |
t test.
Exact test.
Table 2 contains characteristics of both the suppressive HAART regimen and the intensity and duration of presuppressive antiretroviral use by immunologic responders versus non-responders. At least three-quarters of all subjects reported taking protease inhibitors (PIs) at the second suppressive HAART visit (+6M visit), with no significant differences between immunologic responders and nonresponders. Three women, all classified as immunologic responders, reported use of three or more nucleosides including abacavir without a PI or NNRTI as HAART at the +6M visit. There were also no significant differences in reported zidovudine or trimethoprim–sulfamethoxasole use at the second suppressive HAART visit between immunologic responders and nonresponders. There were no significant differences in the intensity (classified as none, monotherapy, dual therapy, or HAART) of overall past antiretroviral exposure between immunologic responders and non-responders (trend test p value across the four antiretroviral use categories, 0.32, data not shown). A trend existed toward more immunologic nonresponders than responders reporting more intense presuppression antiretroviral therapy in the 12 months prior to visit 0 (trend test, p = 0.16). This difference became statistically significant when subjects were dichotomized to HAART use versus no HAART use, with 21 of 38 immunologic nonresponders (55.3%) versus 40 of 115 immunologic responders (34.8%) reporting HAART use at these presuppression visits (p = 0.025). When considering duration of presuppression antiretroviral use by number of visits a woman reported being on therapy, nonresponders reported a median of 1 visit (IQR, 0 to 2) of HAART use prior to the first suppressive HAART visit (visit 0), compared with immunologic responders, who reported a median of zero visits (IQR, 0 to 1) of HAART use prior to visit 0 (p = 0.39).
Table 2.
HIV-Related Medication Use in Immunologic Nonresponders and Responders
| Medication characteristic | Nonresponders (n = 38)
|
Responders (n = 115)
|
p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Suppressive HAART at the +6M visit | |||||
| PI-based | 29 | 76.3 | 72 | 62.6 | 0.35a |
| NNRTI-based | 5 | 13.2 | 21 | 18.3 | 0.23a |
| PI and NNRTI | 4 | 10.5 | 19 | 16.5 | 0.44a |
| Specific medications at the +6M visit | |||||
| Zidovudine use | 12 | 31.6 | 39 | 33.9 | 0.84a |
| Trimethoprim–sulfamethoxazole use | 7 | 18.4 | 25 | 21.7 | 0.82a |
| Most intense presuppressive ART reported at either the −12M or −6M visit | |||||
| No therapy | 6 | 15.8 | 20 | 17.4 | |
| Monotherapy | 2 | 5.3 | 10 | 8.7 | |
| Dual therapy | 9 | 23.7 | 45 | 39.1 | |
| HAART | 21 | 55.3 | 40 | 34.8 | 0.16b |
Abbreviations: ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Exact test.
Trend test.
The laboratory values of interest were compared between responders and nonresponders at two time points, the presuppressive nadir CD4 count visit and the −6M presuppression visit. The largest difference in presuppression mean absolute CD4 cell counts between nonresponders and responders at the evaluated study points was found at the −6M visit, with immunologic nonresponders having 110 cells/mm3 more than responders (p < 0.0001). Compared with immunologic responders, nonresponders also had higher mean absolute CD4 cell counts at the presuppressive nadir CD4 count visit (191 versus 152 cells/mm3, p = 0.047). In addition, all other laboratory values of interest were also found to have the most statistically significant differences between responders and nonresponders at the −6M presuppression visit. Table 3 contains mean values for plasma HIV RNA (log10), absolute CD4, CD8, and lymphocyte cell counts, and CD4, CD8, and lymphocyte percentages at the −6M visit by immunologic response status. The mean plasma HIV RNA (log10) at the −6M visit was 0.48 log units lower in the nonresponders compared with the immunologic responders (p = 0.0021). Absolute CD4 counts, absolute CD8 and lymphocyte cell counts, and CD4, CD8, and lymphocyte percentages were also higher at −6M visit in immunologic nonresponders than in responders, with all values statistically significantly higher except for the lymphocyte percentage (p = 0.06) and the CD8 percentage (p = 0.98).
Table 3.
Laboratory Characteristics of Immunologic Nonresponders and Responders at the −6 Month Visit
| Characteristic | Nonresponders (n = 38)
|
Responders (n = 115)
|
p Valuea | ||
|---|---|---|---|---|---|
| Mean | SD | n | SD | ||
| Plasma HIV RNA, log10 copies/ml | 3.64 | 0.78 | 4.12 | 0.84 | 0.002 |
| CD4 cell count (cells/mm3) | 327.1 | 173.5 | 216.5 | 134.7 | <0.001 |
| CD4 percentage | 19.5 | 9.18 | 15.4 | 8.77 | 0.016 |
| CD8 cell count (cells/mm3) | 1048 | 663.3 | 772.4 | 419.3 | 0.003 |
| CD8 percentage | 54.8 | 13.47 | 54.7 | 12.46 | 0.976 |
| Lymphocyte count (cells/mm3) | 1785 | 913.2 | 1351 | 602.7 | 0.001 |
| Lymphocyte percentage | 37.7 | 13.18 | 33.4 | 11.92 | 0.060 |
All p values by t test.
There were no significant differences found by immunologic response status in hemoglobin concentration, platelet counts, serum creatinine, and serum albumin concentrations at all study time points (data not shown).
The effect of active low-level HIV viremia on immune non-response was assessed by comparing the number of immunologic nonresponders versus responders with detectable HIV RNA viral loads between 80 and 400 copies/ml at both visit 0 and the +6M visit. Eight of 38 nonresponders (21.0%) versus 9 of 115 responders (7.8%) had HIV RNA levels >80 but <400 copies/ml at both visits 0 and +6M (OR, 2.69; 95% CI, 1.12–6.48; p = 0.024). Of these 17 women considered to have low-level HIV viremia, 4 (2 nonresponders and 2 responders) had one but not both of the suppressive HAART plasma HIV RNA levels determined by the assay with a lower limit of detection of 400 copies/ml, and not the more sensitive <80-copies/ml Nuclisens assay. An additional two nonresponders and four responders had one plasma RNA viral load <80 copies/ml and the other determined by the less sensitive <400-copies/ml assay.
Logistic regression analysis was conducted using the factors significantly associated with immunologic nonresponse in univariate analysis: the −6M visit CD4 cell count, the −6M visit plasma log10 HIV RNA level, HAART use at visits −12M through −6M and HIV RNA levels between 80 and 400 copies/ml at both visits 0 and +6M. In addition, age in years at the first suppressive HAART visit and HCV seropositivity were included in the modeling, as these factors have been found to be associated with the magnitude of immunologic response to HAART in other studies. In stepwise selection logistic regression models that included the above factors, only higher last presuppressive CD4 cell count (OR estimate for each 50 CD4 cell increase, 0.78; 95% CI, 0.67 to 0.90; p = 0.001) and lower last presuppressive HIV RNA level (OR estimate for each 1.0 log10 increase, 1.75; 95% CI, 1.04 to 2.93; p = 0.03) were found to be independently associated with immune nonresponse. A trend for more immune nonresponders than responders with low-level HIV RNA viremia (HIV RNA between 80 and 400 copies/ml at visits 0 and +6M) was noted, but did not reach statistical significance (p = 0.098, OR, 2.63; 95% CI, 0.84 to 8.26).
DISCUSSION
This analysis of HIV-infected women monitored in the WIHS who experienced at least 6 months of HIV RNA suppression while reporting continuous HAART use found several factors associated with immunologic nonresponse. In univariate analysis, higher CD4 cell count, CD4 percentage, CD8 cell count, total lymphocyte count, total white blood cell count, and lower plasma HIV RNA levels during the 6 months prior to suppression were all significantly associated with nonresponse, as was use of HAART during the 6 months prior to the achievement of consistent viral suppression (HAART use at −12M through −6M visits). Also, the degree of viral suppression was greater for more responders, with significantly more nonresponders having low-level HIV viremia between 80 and 400 copies/ml at both visit 0 and visit +6M. Only higher presuppressive CD4 cell count and lower presuppressive HIV RNA viral load remained statistically significant in multivariate logistic regression analysis. Factors not statistically associated with immunologic nonresponse included age at initial viral suppression, ethnicity, HIV acquisition risk factors, clinical AIDS diagnosis, hepatitis C seroreactivity, alcohol and/or illicit drug use, use of PI-based versus NNRTI-based HAART, and use of specific medications such as zidovudine and trimethoprim–sulfamethoxazole.
The two most significant factors associated with immunologic nonresponse in this study were lower presuppression HIV RNA viral load and higher presuppression CD4 cell counts. These findings can have a number of interpretations. First, these associations may represent biologic phenomena. If the magnitude of reduction of plasma viral load under HAART is positively correlated to CD4 cell recovery, then even fully suppressive HAART in an individual with a lower presuppression viral load could have less impact on CD4 cell recovery than in an individual with a high presuppression viral load. Similarly, if the presuppression CD4 cell count level is the result of HIV viral activity, then a lower presuppression CD4 cell count could represent more HIV viral activity and thus greater potential impact of viral suppression with HAART.
Alternatively, as this study included women who were not naive to antriretroviral therapy including HAART, a lower HIV RNA viral load and higher CD4 cell count prior to suppression to below 400 copies/ml could have resulted from longer and/or more effective presuppression antiretroviral use in nonresponders compared with responders. Indeed, this hypothesis is suggested by the observations that nonresponders more commonly reported HAART use at the −12M and −6M presuppression visits and experienced larger increases in CD4 cell counts between the presuppression nadir visit and the presuppression −6M visit.
Finally, regression to the mean is another potential mechanism that may partially explain the association between higher presuppression CD4 cell counts in immunologic nonresponders compared with responders, as the presuppressive −6M visit CD4 cell count was used in the calculation of the immune response to suppressive HAART. Thus, a random, spurious higher presuppressive CD4 cell count may lead to the appearance of less response and a random spurious lower value to greater response. The fact that not more than a 20-cell difference exists in the CD4 cell count mean plots (Fig. 2A) at and prior to the −18M visit suggests that there is not a large difference between responders and nonresponders until response to HAART is examined. To further address this, we assessed for regression to the mean by examining changes in CD4 counts between the −12M and +12M visits, which overlapped the −6M presuppression and the −6M visits, the visits used to calculate immune response. A difference in mean CD4 cell count changes between nonresponders and responders from the −12M visit to the +12M visit of 126 cells/mm3 was of the same magnitude as the mean change of 111 cells/mm3 seen from the −6M presuppression to the +6M visit (data not shown). This suggests a true difference between responders and nonresponders, and not a random effect such as regression to the mean.
Other investigators have evaluated CD4 cell responses to HAART in other cohorts,8,9 but few have restricted analyses to virologic responders. In a Spanish study, investigators assessed factors associated with lack of at least a 100-cell/mm3 increase in CD4 cell count after virologic response for 24 or more months.10 Similar to our findings, lower baseline HIV RNA level and higher baseline CD4 cell count were associated with poor immunologic response in univariate analysis, along with prior injection drug use and receipt of an NNRTI-based regimen. In multivariate analysis, however, only prior injection drug use was associated with poor immunologic response, whereas use of a PI-based regimen was associated with reduced risk of poor immunologic response.
Hepatitis C infection and older age at the time of HAART initiation have been associated with poorer CD4 cell responses in other studies of immunologic outcomes.8–11 In our study, neither the HIV acquisition risk factor of injection drug use nor hepatitis C seropositivity was statistically associated with immune nonresponse. There was a nonsignificant trend toward older age for immune nonresponse, with nonresponders being 2 years older than immune responders at HIV suppression. When included in multivariate models, both differences in age at HIV suppression and hepatitis C serostatus were found not to be significantly associated with immune nonresponse.
Only women with virologic suppression to at least <400 copies/ml were included in this study, as the goal of the analysis was to investigate factors associated with immune response to suppressive antiretroviral therapy. Continued viral replication in the face of antiretroviral therapy has been associated with limited immune responses to antiretroviral therapy, with studies finding CD4 cell increases negatively correlated with plasma HIV RNA levels while on HAART.9,22 In our study, active low-level viral replication with plasma HIV RNA levels between 80 and 400 copies/ml at both visits 0 and +6M while on HAART was found in significantly more immune nonresponders (21.0%) than responders (7.8%) (OR, 2.69; 95% CI, 1.12–6.48; p = 0.036). However, this difference did not remain statistically significant in multivariate modeling, although a trend for this was noted (OR, 2.63; 95% CI, 0.84–8.27; p = 0.098). Four of the women with HIV RNA levels between 80 and 400 copies/ml had one but not both of the two suppressive HAART plasma HIV RNA viral load determinations performed by the less sensitive <400-copies/ml assay and were thus considered to have a viral load of 400 copies/ml in this analysis. If HIV viral loads of these subjects were in fact <80 copies/ml at the visit where they could only be determined to be <400 copies/mL, this would represent a conservative bias. Further study of immunologic nonresponders with more sensitive HIV RNA assays with lower limits of quantification could shed light on the potential role of low levels of viremia in the pathogenesis of poor CD4 count responses.
As others did, we selected an arbitrary CD4 cell count increase as the definition of immune response, namely falling within the lowest quartile of CD4 changes on 6 months of suppressive HAART. We believe that this level well represents women with poor immunologic responses to HAART (relative to their peers) that are of potential clinical significance, since three-quarters of the nonresponders had an increase of 10 or fewer CD4 cells/mm3 and 58% had no increase at all. In addition, we believe that our cutoff for immune nonresponse of fewer than a 52-cell increase under 6 months of suppressive HAART well represents individuals with poor CD4 cell increases on suppressive HAART compared with the reported experience of subjects in clinical trials. Indeed, this cutoff is less than half the mean increase in CD4 cells on 6 months of suppressive HAART reported in an analysis of large numbers of HAART-naive subjects in clinical trials (estimated mean CD4 cell increase of 123 cells/mm3 after 6 months of HAART, based on data on 3204 patients; 95% CI, 111 to 135 cells/mm3).3
It is possible that with further antiretroviral treatment that some of these women would experience meaningful increases in CD4 cell counts. For example, Dronda and colleagues found that 42 of 288 subjects with viral suppression through 24 months were poor immunologic responders at 1 year but had increases from baseline of greater than 100 cells/mm3 between months 12 and 24.10 Because of sample size limitations related to frequent intermittent use of HAART in the WIHS, we were unable to extend the required period of continuous HAART use and virologic suppression in this analysis. By following the plot of mean CD4 cell counts for nonresponders versus responders in Fig. 2A from visit 0 through visit +36M, however, one can see that the mean CD4 cell count for the nonresponders never catches up to the responders and generally remains about 100 cells/mm3 lower. In addition, at visit +36M the mean CD4 increase in nonresponders is less than 50 cells/mm3, compared with a mean increase of more than 250 cells/mm3 in the responders. This sustained difference in CD4 cell counts between the two groups of women suggests that our classification of subjects is robust and biologically relevant.
There are several limitations to this study. Interestingly, few women achieved consecutive visits with viral suppression on HAART, thus limiting both the power of the study to find predictors of immune nonresponse and perhaps the ability to generalize to all HAART users. Nonstatistically significant trends, such as age at initiation of HAART, and factors that were statistically significant in univariate analysis but not in multivariate modeling, such as low-level HIV RNA viremia and presuppression HAART use, were noted. It is possible that a larger sample size would have had the power to determine whether these differences independently predict nonresponse. Women were enrolled in the WIHS cohort in late 1994 through late 1995. This was during the era of combination antiretroviral therapy and at the beginning of first-generation PI-based HAART. Given the generally poor overall results in real world clinical experience with these early PI-based regimens, especially in antiretroviral drug-experienced individuals,23 it is not surprising that so few subjects qualified for this study.
We chose to include only women with nadir presuppression CD4 cell counts of <350 cells/mm3 as this reflects the current threshold for initiating antiretroviral therapy found in several of the guidelines for treatment of HIV infection that are generated by expert panels.15,16 Thus, we could not assess differences in immune response rates in women who start HAART with nadir counts >350 cells/mm3.
Knowing the exact date of HAART initiation in antiretroviral drug-naive individuals and having baseline data points just before this date, as in clinical trials, is the ideal way to analyze immune response to HAART. The lack of exact antiretroviral start and stop dates, as well as the 6-month intervals between WIHS study visits, limited the ability to assess the effect of HAART on CD4 responses in this study, especially as a large portion of the CD4 cell increase seen in subjects initiating HAART has been found to occur in the first months of HAART use. Furthermore, we relied on self-report of HAART use, and, because of the visit frequency, we could only determine that women were suppressed at the beginning and end of a 6-month interval but not whether they were suppressed throughout the interval.
In summary, we found that higher presuppressive CD4 cell count and lower HIV viral load were associated with poor immunologic response to HAART in women with suppressed viral loads for at least 6 months. Our finding of a trend toward a greater prevalence of low-level viremia (between 80 and 400 copies/ml) among immunologic nonresponders warrants further study of similar populations with more sensitive viral load assays, since intensification of antiretroviral therapy could be a useful strategy to study if ongoing viral replication accounts for part of the decreased CD4 cell response to HAART.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, D.C. Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, Phyllis Tien); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange, Alvaro Munoz).
Informed consent was obtained from all subjects and human experimentation guidelines of the U.S. Department of Health and Human Services and those of the authors’ institutions were followed in the conduct of this research.
Financial support: The WIHS is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute of Dental and Craniofacial Research (grants U01-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (grant UO1-CH-32632) and the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, MO1-RR-00083).
Footnotes
The authors have no commercial or other associations that might pose a conflict of interest.
References
- 1.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, d’Arminio MA, Yust I, Bruun JN, Phillips AN, Lundgren JD. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators [see comments] N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 2001;15:1369–1377. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- 4.DeHovitz JA, Kovacs A, Feldman JG, Anastos K, Young M, Cohen M, Gange SJ, Melnick S, Greenblatt RM. The relationship between virus load response to highly active antiretroviral therapy and change in CD4 cell counts: A report from the Women’s Interagency HIV Study. J Infect Dis. 2000;182:1527–1530. doi: 10.1086/315875. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann D, Pantaleo G, Sudre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study. Lancet. 1998;351:723–724. doi: 10.1016/s0140-6736(98)24010-4. [DOI] [PubMed] [Google Scholar]
- 6.Grabar S, Le Moing V, Goujard C, Leport C, Kazatchkine MD, Costagliola D, Weiss L. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 7.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis. 2001;183:1328–1335. doi: 10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 8.Viard JP, Mocroft A, Chiesi A, Kirk O, Roge B, Panos G, Vetter N, Bruun JN, Johnson M, Lundgren JD. Influence of age on CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: Evidence from the EuroSIDA Study. J Infect Dis. 2001;183:1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 9.Le Moing V, Thiebaut R, Chene G, Leport C, Cailleton V, Michelet C, Fleury H, Herson S, Raffi F. Predictors of long-term increase in CD4+ cell counts in human immunodeficiency virus–infected patients receiving a protease inhibitor–containing antiretroviral regimen. J Infect Dis. 2002;185:471–480. doi: 10.1086/338929. [DOI] [PubMed] [Google Scholar]
- 10.Dronda F, Moreno S, Moreno A, Casado JL, Perez-Elias MJ, Antela A. Long-term outcomes among antiretroviral-naive human immunodeficiency virus-infected patients with small increases in CD4+ cell counts after successful virologic suppression. Clin Infect Dis. 2002;35:1005–1009. doi: 10.1086/342695. [DOI] [PubMed] [Google Scholar]
- 11.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti JC, Hirschel B, Janin P, Francioli P, Flepp M, Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: The Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 12.Florence E, Lundgren J, Dreezen C, Fisher M, Kirk O, Blaxhult A, Panos G, Katlama C, Vella S, Phillips A. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA Study. HIV Med. 2003;4:255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti G, Meroni L, Varchetta S, Terzieva V, Bandera A, Manganaro D, Molteni C, Trabattoni D, Fossati S, Clerici M, Galli M, Moroni M, Franzetti F, Gori A. Low-dose prolonged intermittent interleukin-2 adjuvant therapy: Results of a randomized trial among human immunodeficiency virus-positive patients with advanced immune impairment. J Infect Dis. 2002;186:606–616. doi: 10.1086/342479. [DOI] [PubMed] [Google Scholar]
- 14.Katlama C, Carcelain G, Duvivier C, Chouquet C, Tubiana R, De Sa M, Zagury L, Calvez V, Autran B, Costagliola D. Interleukin-2 accelerates CD4 cell reconstitution in HIV-infected patients with severe immunosuppression despite highly active antiretroviral therapy: The ILSTIM Study—ANRS 082. AIDS. 2002;16:2027–2034. doi: 10.1097/00002030-200210180-00007. [DOI] [PubMed] [Google Scholar]
- 15.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the Use of Antiretrovirals in HIV-Infected Adults and Adolescents. U.S. Department of Health and Human Services; Publication no. 10-29-2004. [Google Scholar]
- 16.Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CC, Fischl MA, Gatell JM, Gazzard BG, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Schooley RT, Thompson MA, Vella S, Volberding PA. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–265. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 17.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. The Women’s Interagency HIV Study: WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 18.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137:381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 19.Murphy DG, Cote L, Fauvel M, Rene P, Vincelette J. Multi-center comparison of Roche COBAS AMPLICOR MONITOR version 1.5, Organon Teknika NucliSens QT with Extractor, and Bayer Quantiplex version 3.0 for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2000;38:4034–4041. doi: 10.1128/jcm.38.11.4034-4041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: A report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 21.Anastos K, Barron Y, Cohen MH, Greenblatt RM, Minkoff H, Levine A, Young M, Gange SJ. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140:256–264. doi: 10.7326/0003-4819-140-4-200402170-00007. [DOI] [PubMed] [Google Scholar]
- 22.Connick E, Lederman MM, Kotzin BL, Spritzler J, Kuritzkes DR, St Clair M, Sevin AD, Fox L, Chiozzi MH, Leonard JM, Rousseau F, D’Arc RJ, Martinez A, Kessler H, Landay A. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–363. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 23.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: Risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]