Abstract
Phosphoinositides constitute only a small fraction of cellular phospholipids yet their importance in the regulation of cellular functions can hardly be overstated. The rapid metabolic response of phosphoinositides after stimulation of certain cell surface receptors was the first indication that these lipids could serve as regulatory molecules. These early observations opened research areas that ultimately clarified the plasma membrane role of phosphoinositides in Ca2+ signaling. However, research of the last 10 years has revealed a much broader range of processes dependent on phosphoinositides. These lipids control organelle biology by regulating vesicular trafficking and they modulate lipid distribution and metabolism more generally via their close relationship with lipid transfer proteins. Phosphoinositides also regulate ion channels, pumps and transporters as well as both endocytic and exocytic processes. The significance of phosphoinositides found within the nucleus is still poorly understood and a whole new research concerns the highly phosphorylated inositols that also appear to control multiple nuclear processes. The expansion of research and interest in phosphoinositides naturally created a demand for new approaches to determine where within the cell these lipids exert their effects. Imaging of phosphoinositide dynamics within live cells has become a standard cell biological method. These new tools not only helped us localize phosphoinositides within the cell but also taught us how tightly phosphoinositide control can be linked with distinct effector protein complexes. The recent progress allows us to understand the underlying causes of certain human diseases and design new strategies for therapeutic interventions.
INTRODUCTION
Phospholipids are very important structural elements of all eukaryotic cellular membranes that undergo constant metabolic changes according to the need of the cell to maintain its structural integrity. Each membrane compartment has its unique lipid composition: for example, the plasma membrane (PM) has high phosphatidylserine content showing asymmetric distribution being enriched in the inner leaflet. The PM also has the highest cholesterol content and contains sphingomyelin and complex glycosphingolipids in the outer leaflet of the membrane. Since almost all of the structural lipids or their precursors are synthesized in the endoplasmic reticulum (ER), these lipids have to reach their steady-state destination either with vesicular transport or with the help of lipid transfer proteins. Cellular lipid gradients are a direct consequence of the compartmentalization of the enzymes that generate and metabolize these lipids, and directional lipid transport becomes a key part of their regulation. In addition to their important structural roles, cells also use lipids as signaling molecules. The well-known metabolites of arachidonic acid as pro-inflammatory and hemostatic mediators together with the endogenous cannabinoids are good examples of how cells utilize lipid compounds for intercellular communication, although, the same lipids can also have signaling roles within the cell. Phosphoinositides are the best examples of how phospholipids, namely phosphatidylinositol (PtdIns) can be utilized as a scaffold to generate by phosphorylation a variety of compounds that control a whole range of cellular functions. It is important to distinguish the small amount of regulatory lipids that show high turnover rates from the structural lipids that have a slower turnover. PtdIns is one of only a few lipids that clearly serves as a structural lipid as well as a precursor of multiple signaling molecules. This dual role often makes it more difficult to analyze the importance of PtdIns in cell regulation.
Increased turnover of PtdIns and phosphatidic acid (PtdA) in response to stimulation of some cell surface receptors was the fundamental observation that drew attention to these lipids (76). However, not until 1975 was it recognized that increased turnover of PtdIns is an early signaling event linked to Ca2+ signaling (109). Polyphosphorylated inositides were isolated and structurally characterized in the early 60’s (62), but the function of receptor-regulated phosphoinositide-specific phospholipase C (PLC) enzyme(s) was only discovered in 1983 (19, 30). How increased turnover of PtdIns led to increased Ca2+ uptake and activation of downstream regulatory processes was also highly debated, Given the high Ca2+ sensitivity of the PLC enzymes, it was questioned for some time whether PLC activation was indeed a primary receptor-controlled event or rather only a secondary event in response to the Ca2+ increase (27, 110). These debates were settled with the discovery of the Ca2+ mobilizing effect of Ins(1,4,5)P3, the soluble product of PLC mediated hydrolysis of PtdIns(4,5)P2 (154). In a related line of research it was recognized more than 20 years ago that releasing Ca2+ from the endoplasmic reticulum (ER) alone is a sufficient signal to activate a Ca2+ influx pathway (129), yet the molecular mechanism of ER luminal Ca2+ sensing and its coupling to a PM Ca2+ influx pathways were discovered only very recently (96). The tremendous expansion of the inositol lipid research field has inevitably led to its fragmentation and now a review could be written about each of the special aspects of regulation by phosphoinositides. This review will not attempt to cover all of these areas in great detail. Instead it will try to highlight new developments in the respective areas and find common principles that govern regulatory processes. Finally it will discuss some of the methodological advances that allowed the gathering of new information on these important lipid regulators.
1. CELLULAR PROCESSES REGULATED BY INOSITIDES
It might be easier at this time to list the processes that are not regulated by inositides in a eukaryotic cell, than those that are clearly inositide-dependent (Figure 1). The following sections will outline some research areas where phosphoinositides have gained high profile and discuss some current questions that the author feels are important and unresolved. This selection is not exhaustive and it reflects the author’s views and interests. The various phosphoinositides are formed by a large number of kinases and phosphatase enzymes. Although these enzyme groups have been extensively researched and hence could be the basis of any review article covering this field, no attempt will be made in this review to systematically discuss the phosphoinositide converting enzymes. Such summaries can be found in several other recent reviews (5, 8, 39, 103, 147).
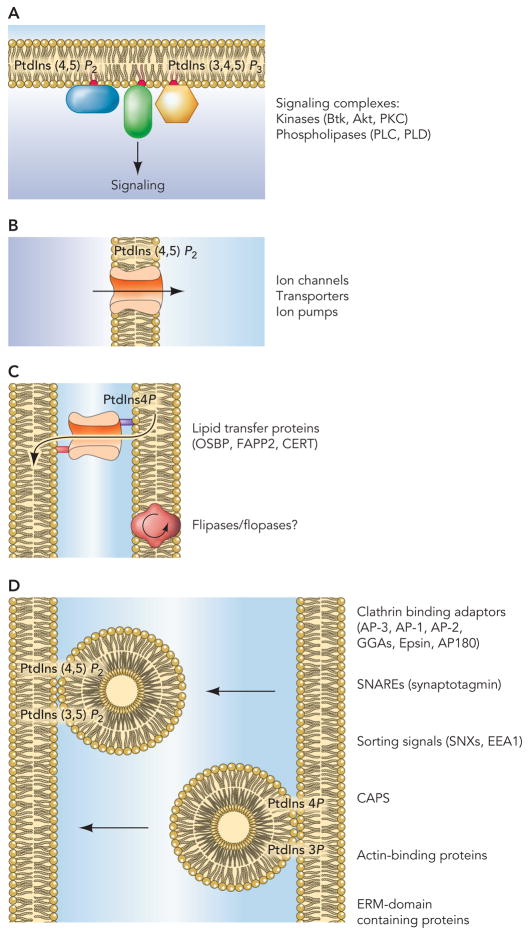
Figure 1.
Phosphoinositides regulate multiple membrane-associated molecular events. (A) Signaling from the inner leaflet of the plasma membrane is the best documented and known function of PtdIns(4,5)P2 and PtdIns(3,4,5)P3. PtdIns(4,5)P2 serves both as a precursor for PLC-generated messengers such as Ins(1,4,5)P3 and diacylglycerol, and an activator of other phospholiases, such as PLD. PtdIns(4,5)P2 is also converted to PtdIns(3,4,5)P3 by PI 3-kinases. This lipid recruits and activates several important protein kinases such as Akt/PKB, Btk and some PKC isoforms. (B) Phosphoinositides also regulate the activity of a number of ion channels and transporters thereby controlling ion distribution and gradients. (C) Phosphoinositides not only help hydrophilic and charged molecules (such as ions) to cross the hydrophobic membranes, but as increasingly being recognized, they also help hydrophobic molecules to traverse the aqueous phase separating the membranes. The lipid transfer proteins may carry their cargo to farther distances within the cell, but more likely they work within functional contact zones formed by adjacent membranes without moving too far from these membranes. Such functional zones exist between the ER and the Golgi, the ER and the mitochondria and the ER and the PM. Phosphoinositides probably also regulate the flipase and flopase proteins that help move certain lipids between the inner and the outer leaflet of membranes. (D) Phosphoinositides control the budding and fission process between membranes and hence are critical regulators of vesicular trafficking. Intriguingly the steady state phosphorylation state of phosphoinositides increases as one moves from the nuclear envelope/ER (very little if any phsophorylated PtdIns) toward the PM which has most of the PtdIns(4,5)P2 and PtdIns(3,4,5)P3. The role of phosphoinositides in these locations is to recruit adaptor proteins and to work together with the small GTP binding proteins often regulating their nucleotide exchange factors or GAP proteins.
1.1. Questions still unanswered about the classical canonical signaling roles of phosphoinositides
The general concept of signal transduction utilizing PM phosphoinositides is clearly established for Ca2+ -mobilizing hormones when they activate their cell surface receptors. These receptors activate phosphoinositide-specific phospholipase C (PLC) enzymes to hydrolyze phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) of the PM. The PLC enzymes that come in a variety of forms (133) can be activated by heterodimeric G protein subunits (PLCβ enzymes, in the case of G protein coupled receptors), or via tyrosine phosphorylation (PLCγ enzymes in case of receptor tyrosine kinases) as well as by small GTP binding proteins (PLCe enzyme, in case of a variety of mostly G protein coupled receptors). There are still some classes of PLC enzymes whose pathways of activations are poorly understood. For example, PLCδ is not activated by any known mechanism other than the cytoplasmic Ca2+ increase that in fact is a regulator of all PLC isoforms. PLC activation has, at least, two components: the enzyme is recruited from the cytosol to the membrane by unique means characteristic to the various isoforms. The membrane attachment is then associated with activation of the enzymes by allosteric mechanisms involving molecular rearrangements. While many details of the isoform-specific PLC regulation have been clarified (132, 133), a recent discovery shed new lights to the PLC activation process. The most conserved parts of phosphoinositide-specific PLCs are the so called X and Y domains that are connected with intervening sequences of various lengths. Interestingly, these parts of the molecule are not resolved in any of the available crystal structures suggesting their greater mobility (44, 82). Intriguingly, Hicks et al. has recently found that these linker segments serve as autoinhibitory loops that need to be rearranged during PLC activation. These authors showed that PLC enzymes with deleted inter-X-Y segments displayed much larger constitutive activities than their wild-type counterparts (73). The mechanism by which these loops are moved during activation is still not clear, but the high content of negatively charged residues found within these loops suggests that electrostatic interactions have an important role in the process.
Although PLC enzymes are capable of hydrolyzing PtdIns, PtdIns4P and PtdIns(4,5)P2 in vitro, the general consensus is that these enzymes primarily hydrolyze PtdIns(4,5)P2 in the PM. However, the limited pool of PtdIns(4,5)P2 needs constant replenishment from a larger PtdIns pool via sequential phosphorylations by PI 4-kinase and PIP 5-kinase enzymes. As straightforward as it seems, this process is very poorly understood. Firstly, the replenishment of PM PtdIns4P in intact cells appears to be mediated by one of two wortmannin-sensitive type III PI 4-kinases, PI4KIIIα (9, 11). The yeast orthologue of this enzyme, Stt4p is localized to the PM and is responsible for the synthesis of the PM pool of PtdIns4P and hence, PtdIns(4,5)P2 (6, 7). However, in mammalian cells, this enzyme is mostly found in the ER and the Golgi (116, 183), and it is not all that clear how it can supply PtdIns4P for the PM since no lipid transfer protein is known to transfer PtdIns4P between membranes. It is possible that a small amount of active PI4KIIIα enzyme is located at the PM that is below the detection limit of immunocytochemical methods. Alternatively, a fraction of the peripheral ER makes contacts with the PM and in this junctional compartment the enzyme is able to directly phosphorylate PM-localized PtdIns. The existence of such a compartment in yeast is also supported by several observations. In S. cerevisiae, a reciprocal situations exists: the PM-localized Stt4p is also responsible for the generation of a PtdIns4P pool that is dephosphorylated by the Sac1 phosphatase, a clearly ER-localized enzyme (48). Moreover, Stt4p is concentrated in specific domains in the PM where it co-localizes with the Ypp1p protein, a molecular chaperone that is required for its activity (7, 188). Ypp1p is presumably a peripherally ER-localized chaperone-like protein that alleviates α-synuclein (a protein that accumulates in familial Parkinson’s disease) toxicity in yeast (46). It is not unreasonable to assume that Stt4p and Ypp1p are part of the ER-PM contact sites in the yeast where they form a signaling complex (7). Such ER-PM contact zones do exist in mammalian cells (170) and they are the sites of the store-operated Ca2+ entry (SOCE) pathway that is activated by the depletion of the ER luminal Ca2+ pools. Here, the contacts are formed by the recently discovered ER-resident Ca2+ sensor molecule STIM1 and the PM Ca2+ channel Orai1 (130). Whether the mammalian PI4KIIIα is located and functions in this compartment is yet to be demonstrated.
Given the high PtdIns4P content of the Golgi, it is also plausible that PtdIns4P reaches the PM in the form of vesicular transport between the Golgi and the PM. Indeed, there are several findings that suggest that the PI4KIIIα mechanism is not the only way cells can get their PM PtdIns4P. Patch clamp studies have shown that many ion channels require PtdIsn(4,5)P2 for proper function (55)(also see below). In one of these studies, the restoration of the channel activity – presumably by resynthesis of PtdIns(4,5)P2 – is insensitive to inhibition of type III PI4Ks, suggesting an alternative mechanism of PtdIns4P resynthesis (184). Yet, another study using a similar experimental paradigm, but a different PtdIns(4,5)P2-sensitive channel, reported that the calcium-binding regulatory protein, NCS-1 can regulate the restoration of the PtdIns(4,5)P2 pools that regulate the channel (54). NCS-1, on the other hand has been shown to regulate the Golgi-localized PI4KIIIbeta enzyme (71, 189) as first demonstrated in yeast (72). These data would be compatible with a role of Golgi PtdIns4P being the ultimate source of PM PtdIns4P. What makes some of these experiments difficult to evaluate is the long time period required for either overexpression or downregulation of the PI4Ks or their regulators. During these prolonged periods the trafficking of channels or other proteins could drastically change making it hard to determine the primary cause of the defect. The use of subtype-specific PI kinase inhibitors or the acute regulation of lipid levels on specific compartments (see below) may be a better approach to revisit these questions.
The question of what enzyme regulates the plasma membrane PtdIns(4,5)P2 pools appears less complicated in the case of the PIP5K enzymes that are mostly found associated with the PM, although some of their specific forms can also be specially localized in other cellular compartments such as the focal adhesions or the nuclei (39). Nevertheless, it has been shown that the 87 kDa splice form of PIP5K type Iγ is primarily responsible for the synthesis of the agonist-sensitive PtdIns(4,5)P2 pools in HeLa cells (178). On the other hand, PIP5K Iα (human terminology), is recruited to the PM by the Bruton’s tyrosine kinase in B-cells, which in turn enhances Ca2+ signaling (139). This suggests that PIP5K Iα can also generate PtdIns(4,5)P2 that is accessed by PLCγ.
A third important component of the mechanism by which the PM is supplied with PtdIns(4,5)P2 is comprised of the PtdIns transfer proteins (PITPs) that transfer PtdIns from the site of its synthesis in the ER to the PM (28). These proteins will be discussed in greater detail below together with the roles of phosphoinositides in lipid transport processes.
1.2 Why almost every ion channel and transporter is affected by phosphoinositides?
Early research has already suggested that phosphoinositides can directly affect various ion transport pathways. A direct effect of membrane PtdIns(4,5)P2 on the activity of the PM Ca2+ pump was found as early as 1981(117) and the sarcoplasmic reticulum Ca2+ ATPase was reported to contain tightly associated phosphoinositides (171). However, it was the Na+/Ca2+ exchanger (NCX1) and the KATP potassium channels studied in giant excised patches of the heart where the phosphoinositide regulation of ion channels/transporters has first been clearly postulated(74). This was followed by a large number of studies showing PtdIns(4,5)P2 requirement for proper activity of several ion channels (55, 75). In almost all of these studies the question was raised whether PtdIns(4,5)P2 is merely a requirement for the optimal functioning of the channels or the channels are actually regulated by PtdIns(4,5)P2 changes that occur during activation of PLC-coupled receptor mechanisms. In some cases, such as the M-current (77, 156) or the cold- and menthol-sensitive TRPM8 channels (33, 135), the channel activity clearly follows very closely the changes in PM PtdIns(4,5)P. However, in other examples PtdIns(4,5)P2 has both inhibitory and stimulatory effects on channel activity (108). The TRPV1 channels, for example, are sensitized by PLC-coupled agonists presumably via reduction of PtdIns(4,5)P2 (26), whereas the same lipid is required for their recovery from desensitization (99, 100). More detailed discussion on the inositide regulation of ion channels can be found in excellent recent reviews (55, 134, 155).
The mechanism by which inositol lipids can regulate ion channels remains elusive. Almost all ion channels and transporters contain clusters of basic residues in their membrane-adjacent regions facing the cytosol or within their C-terminal tails. Lipid regulation of TRP channels was mapped to the TRP domains of their cytoplasmic tails (118, 135). However, an interesting and common feature of the PtdIns(4,5)P2 regulation of many potassium and TRP channels is that the lipid alters the interaction of the channels with other specific regulators such as calmodulin (87), βγ-subunits (78) or ligands such as ATP (16). In addition, indirect regulation of channels via inositide-binding channel-interacting proteins is also possible as shown by the recent identification of Pirt, a molecule that interacts with TRPV1 channels and also binds phosphoinositides (84).
1.3 Phosphoinositides regulate vesicular trafficking
The presence of phosphoinositide kinases and phosphatases in various membrane fractions of fractionated cells or tissues has been noted by early studies on these enzymes (111). However, the general notion was that these findings either indicated PM contamination of the other membranes or represented intermediate membrane compartments as phosphoinositides were on their way to the PM. Some of the earliest indications that phosphoinositides may be important for membrane fusion or fission events came from studies on dense core granule (DCV) exocytosis, where it was recognized that PtdIns(4,5)P2 synthesis is important for priming the exocytic vesicles (43, 70). DCVs containing their cargo undergo maturation that increases their competence to dock, and ultimately fuse with the PM when a rapid rise in Ca2+ concentration triggers the fusion process. Some of the mature granules under the membrane will dock to the PM, but these pre-docked vesicles still have to undergo “priming” to become the “readily releasable pool” that is first to be fused upon stimulation (31). Furthermore, the mammalian PITP and a PIP 5-kinase was isolated and identified as factors necessary for the priming process (69, 70). The question of whether PtdIns(4,5)P2 was necessary at the PM membrane or at the surface of the exocytic vesicle has not been clarified for a long time but recent studies on the Ca2+ sensitive phosphoinositide-binding regulator protein of exocytosis, CAPS (176) suggested that PtdIns(4,5)P2 is needed on the PM site (81). This conclusion was also reached using single cell studies on PC12 cell exocytosis (112).
However, in addition to exocytosis, endocytosis is also regulated by inositol lipids. The tetrameric clathrin adaptor protein AP-2, a key component of the clathrin-mediated endocytic machinery was identified as a “receptor” for InsP6 (175), although the natural binding partner of this protein turned out to be PtdIns(4,5)P2 (53, 136). These studies gave strong support to the already growing evidence that phosphoinositides might be important for endocytosis. Identification of the PtdIns(4,5)P2 5-phosphatase, synaptojanin (105) and PIP5Kγ (60, 182) as crucial regulators of synaptic vesicle cycling has put phosphoinositides in their place in the cell biology of neurotransmission (181). Recent studies utilizing a technique that allows rapid depletion of plasma membrane PtdIns(4,5)P2 pools (see below) in intact cells have unequivocally proven that clathrin-mediated endocytosis of certain cargos require the PtdIns(4,5)P2 dependent membrane recruitment of the AP-2 protein as well as some other clathrin-binding adaptors (1, 169, 191). However, it is important to note that an Arf6-regulated non-clathrin-mediated endocytic pathway also depends on phosphoinositides, although less clear are the molecular details of what the lipid dependent components are (2, 21). While PtdIns(4,5)P2 is important to recruit proteins of the endocytic machinery to the PM by regardless of the type of endocytosis, it appears that PtdIns(4,5)P2 has to be dephosphorylated by 5-phosphatase enzymes in order for the endocytic vesicle to find its destination whether recycling to the PM or sorted via the sorting endosome (190). Thus, a high rate of phosphorylation and dephosphorylation of the 5-position in PtdIns(4,5)P2 is crucial for proper membrane cycling at the PM. The presence of the type II PI4Ks in endosomal membranes (10, 113) suggest that PtdIns4P also has an important role in determining the fate of the endocytosed membranes.
A critically important step in recognizing the relevance of phosphoinositides in intracellular vesicular trafficking was the discovery that the yeast Vps34p protein, an essential element of vacuolar sorting, was a PtdIns 3-kinase (142). This was followed by a whole set of studies that unraveled protein modules capable of recognizing PtdIns3P (22, 149) as well as the enzymes that convert these lipids to PtdIns(3,5)P2 (56, 148) or back to PtdIns (123, 161), and the signaling complexes that are regulated by them.
Even a superficial summary of the advances made in these respective research fields would exceed the scope of this review. Excellent reviews can be found in all of these topics. I will only discuss some general questions that come to mind when trying to understand the underlying principles common to all of these processes. The current understanding of how inositides contribute to these complex membrane fusion or fission steps is that these lipids interact with protein modules present in the proteins that regulate these processes. Many such inositide binding modules have been identified and characterized. Pleckstrin homology (PH) domains, phox-homology (PX) domains, FYVE-domains, ENTH-domains FERM-domains are the best known ones, but others certainly also exist (12, 91). Typical proteins that contain such domains include both tetrameric and monomeric clathrin adaptors, GEF- and GAP proteins of small GTP binding proteins belonging to various classes (Arfs, Rabs, Rho/Rac and Ras), sorting nexins, protein and lipid kinases as well as various phospholipases and probably many others (91). In many instances the inositide binding to the domain is only a partially effective signal and it needs an additional signal, which in most cases is an active small GTP-binding protein (Fig. 2A). This mode of operation is often referred to as coincidence detection (23).
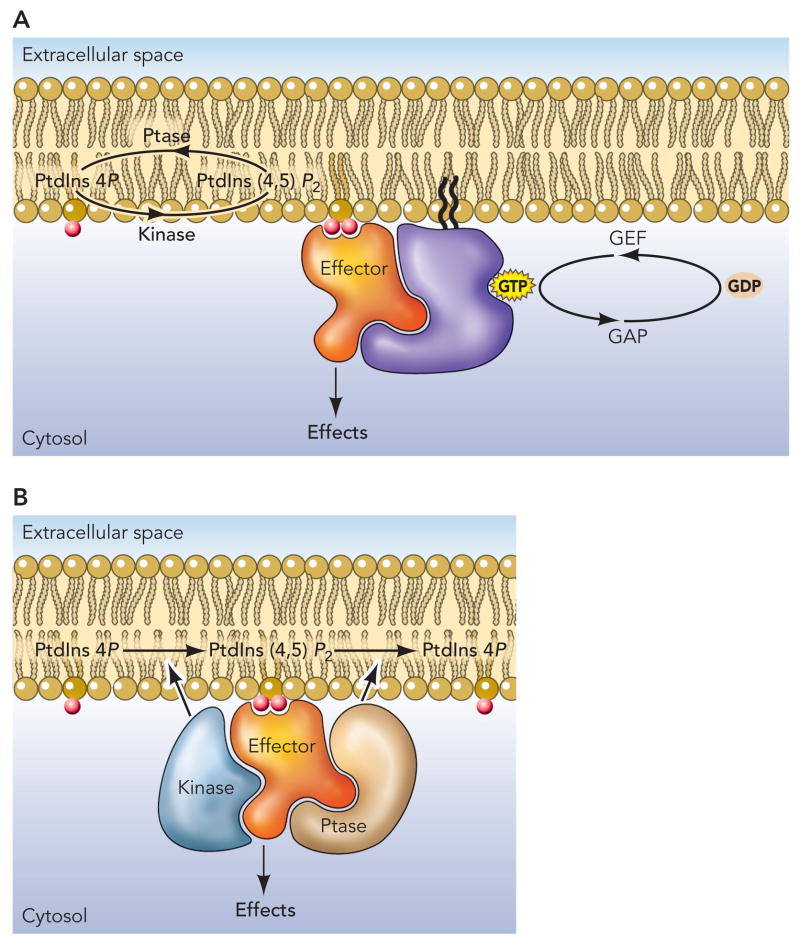
Figure 2.
Coincidence detection in phosphoinositide signaling. (A) Phosphoinositide recognition and its activation of downstream signals is often contingent upon simultaneous engagement with the active, GTP-bound from of small G nucleotide binding proteins. For example, PtdIns4P-mediated signals usually require activated Arf1, while PtdIns3P is linked with the Rab5 and possibly other Rab proteins. PtdIns(3,4,5)P3 function is often closely associated with the Rac and Ras proteins while PtdIns(4,5)P2 is with that of Arf6. This listing, however, is incomplete and will be greatly extended by future research. (B) Phosphoinositide signaling can be compartmentalized to the extent that the phosphoinositide is channeled to a particular effector molecule. In this arrangement the associated kinase and phosphatase enzyme provides the specificity and the specificity of inositide binding of the effector is not crucial to the process.
Initially, it was believed that the function of the lipid binding domains was to recruit the protein to the site where it needs to function. While this certainly is the case for many proteins, it is increasingly recognized that membrane localization is often determined by other parts of the molecule, and the inositide binding domain serves as lipid-dependent regulatory module rather than a localization signal. Another adjustment in our view of the inositide binding domains is concerned with their specificities. Initial examples suggested that these modules show a high degree of specificity in their inositide recognition. And indeed, in many cases, PH domains, PX domains or FYVE domains show a high degree of inositide binding specificity. However, an increasing number of such modules have proven to be rather promiscuous in their phosphoinositide binding (187) and even showed no lipid binding at all. Often these modules also have protein binding partners, which is not surprising, as PH domains for example have a very similar folding structure to phosphotyrosine binding (PTB) domains (92). It is easy to see that the inositide specificity in the regulation of proteins that have inositide binding modules with low specificity comes from the inositide kinase and –phosphatase enzymes that are present in the same signaling complex. This also explains why some proteins regulated by a certain phosphoinositide isomer rely on a specific enzyme to make the lipid, and the enzyme cannot be replaced by another isomer even if it makes the same phosphoinositide (Fig. 2B).
Remarkable general patterns of inositide distribution across the cellular membranes can be recognized suggesting an evolutionary element in the organization. One cannot help but notice the onion layered arrangement of phosphoinositides within eukaryotic cells: PtdIns is made and found in the ER, monophosphorylated inositides populate the internal membranes whether on endocytic or exocytic routes, and the polyphosphoinositides [PtdIns(4,5)P2 or PtdIns(3,5)P2] are located in the plasma membrane (or on internal membranes that border a sequestered “outside” compartment such as the dumping organelle, the lysosome). During evolution, phosphoinositide kinases appear together with internal membranes and hence, their functions are likely linked to the identification and fate-determination of the internal membranes. In this context it is relevant that PtdIns(4,5)P2 is mostly found in the PM (although in small amounts it was detected in intracellular membranes in EM studies (180). The important roles of phosphoinositide 5-phosphatases, such as synaptojanin and the OCRL 5-phosphatase as well as the PIP 5-kinases at the internalization and recycling of vesicles from and into the PM indicates that PtdIns(4,5)P2 is a functional identifier of the PM. A delicate balance between the PtdIns4P PtdIns(4,5)P2 in the PM-endosomal interface is critical both to the fusion (recycling) and the fission (endocytic) process.
The Golgi contains the largest amount of PtdIns4P and the various intracellular vesicular compartments contain either PtdIns3P or PtdIns4P and perhaps PtdIns5P although the distribution of the latter as well as the major route of its production is still not fully understood. Although PtdIns4P has long been viewed only as a precursor of PtdIns(4,5)P2 in the PM, it has become clear in the last decade that PtdIns4P is a major regulatory lipid in the Golgi, the TGN and some endosomes (35). PtdIns4P working together with Arf1 proteins is important to recruit clathrin adaptors such as AP-1 (179) AP-3 (29) or GGAs (177) to their respective target membranes. It is noteworthy that all of the PH domains that recognize PtdIns4P are part of proteins that transport lipids (see below), revealing a pivotal role of PI 4-kinases as master regulators of structural lipid distribution within the cell. Whether PtdIns4P made in the Golgi contributes to the phosphoinositide supply of PM is still an important open question discussed above.
1.4 Membrane dynamics, actin cytoskeleton and phosphoinositides
The plasma membrane of most metazoan cells shows enormous plasticity and undergoes shape changes driven by actin polymerization. This process is very critical for both physiological processes such as chemotaxis, cell adherence or morphogenesis and to pathological ones such as invasive cancer metastasis or the spreading of pathogens from one cell to another. In all of these instances a very active membrane deformation is elicited by polymerized actin. We have discussed above the phosphoinositide binding domains (FERM, ENTH) found in a large number of proteins that interact with the actin cytoskeleton. However, in addition, proteins that regulate actin polymerization per se also show inositol lipid binding. These include the actin capping and severing proteins, gelsolin, profilin and cofilin (185). Actin polymerization requires “nucleation” a process by which filamentous actin free (+) ends are generated. These (+) ends become uncapped by dissociation of gelsolin and ready to accept further actin monomers that are supplied from dissociation from the (−) ends of actin with the help of cofilin (164). PtdIns(4,5)P2 binding to the capping/severing proteins, hence, determines the rate of actin polimerization. An additional critical component of generating the actin network is the Arp2/3 complex consisting of seven tightly associated proteins that allow actin polymerization at an angle from an already existing F-actin filament (128). The Arp2/3 complex is regulated by members of the Wiscott-Aldrich Syndrome proteins (WASP). Some of these, like N-WASP, have putative phosphoinositide binding motifs (122). Our knowledge on the dynamics of actin polymerization has been greatly enriched by studies on the intracellular movement of the bacterium, Listeria monocytogenes with a strong connection to phosphoinositides (89).
1.5 The endless complexity of PI 3-kinase signaling
Research on PI 3-kinases has exploded in the last 20 years and the progress cannot be summarized even in a limited way in this review. The roles of the various PI 3-kinse isoforms have been clarified using knockout and knock-in mouse models (165). Partly based on such studies it has become clear that the Class I PI3Kα enzyme with its p85/55 adaptors has major roles in metabolic regulation and cell growth (49, 51, 103) (including cancer (140)) while PI3Kδ and PI3Kγ are critical components of immune cell regulation (3, 88, 103). This clear functional separation has generated enormous interest in developing subtype-specific PI 3-kinase inhibitors as adjuvant treatment in cancer and as anti-inflammatory or immunosuppressant drugs, respectively (103). Progress in understanding the functions of the Class II and Class III PI 3-kinases has been somewhat slower as these enzymes have more complex roles in vesicular trafficking that in many cases might be redundant with other phosphoinositide regulatory enzymes. These developments have been summarized in a number of excellent reviews (e.g. (47) and will not be further discussed here.
1.6 Nuclear processes and phosphoinositides
Several lines of evidence suggest that the nucleus has its own phosphoinositide signaling system(36), although its significance has only recently been emerging (80). Nuclear phosphoinositide changes separate from those detected in the cell membranes have been described in IGF-stimulated Swiss 3T3 cells (37 ). Moreover, several of the enzymes known to process inositide-cycle intermediates, such as PI4Ks (34, 83), PIP5Ks (20, 106), PLCβ (38, 102) and DAG kinase (124) were found to be present in the nucleus. Phosphoinositides were also detected within the nucleus associated with nuclear speckles the sites of pre-mRNA splicing (119). This also means that, intriguingly, the lipid does not just associate with the nuclear membranes or the membrane of intranuclear canaliculi but with other nuclear components. This raises important questions concerning the physical state of the lipids, whether they are associated with lipid transfer- or other lipid binding proteins.
It is much less clear how phosphoinositides affect nuclear processes. Yeast studies suggested that one of the yeast PI4Ks, Pik1 which is an essential enzyme and is present both in the Golgi and the nucleus has to be present in both locations in order to fully rescue a temperature sensitive allele (153 ). Recent evidence also suggest that InsP3-induced Ca2+ changes can be initiated primarily within the nuclei in the case of some cell surface growth factor receptors, such as c-Met (59). The most specific nuclear function so far was linked to a PIP 5-kinase Iα, which was recently found to associate with and stimulate the activity of a mRNA poly-adenylation enzyme Star-PAP via generation of PtdIns(4,5)P2 in nuclear speckles (106). By this mechanism the processing and nuclear export of specific mRNAs can be regulated. What is not clear is what determines which primary RNA transcripts are subject to this regulation. Phosphoinositide binding domains (PHD fingers) have been described in a number of nuclear proteins, such as the tumor suppressor ING2 (61). Another PHD containing protein, ASH2 from Drosophila has been found to associate with a PIP kinase (25). The binding of PHD proteins to trimethyl-lysine residues of histones (104) makes these observations even more intriguing.
However, nuclear processes are not only regulated by inositol lipids but also by the soluble multi-phosphorylated inositols, such as InsP5, InsP6 or the pyrophosphorylated inositol polyphosphates (143). Although the roles of these compounds as phosphate reservoirs in germinating seeds have been known for a while the real break-through understanding their significance came from yeast genetic studies that identified the enzymes Ipk1 and Ipk2 responsible for the conversion of InsP3 to InsP6 (186). Subsequent studies found that this pathway is involved in arginine-specific gene expression responses as well as in chromatin remodeling within the PHO5 promoter (152). It is now believed that inositol polyphosphates regulate transcriptional processes, mRNA export and telomere length (Reviewed in (143) in detail). The importance of the same pathway in mammalian cells is underlined by the embryonic lethality of the knockout of either of the mouse homologues of Ipk2, or Ipk1 (50, 172 ). However, the exact mechanism of why this happens still awaits identification.
1.7 Lipid transport regulated by phosphoinositides
One of the most exciting recent developments in phosphoinositide research was the finding of a link between sphingolipid metabolism and phosphoinositides (66). Earlier analysis of the phosphoinositide-binding specificities of various PH domains revealed that all of the proteins whose PH domains specifically recognized PtdIns4P were lipid transport proteins. These included the oxysterol binding protein (OSBP) (95) and its yeast homologues, OSH1 and OSH2 (93, 138, 187), the ceramide-binding protein, CERT (67, 94), as well as the FAPP1 and FAPP2 proteins (40). These findings have already forecasted that lipid transfer function and PI 4-kinases would be intimately interrelated. This was elegantly demonstrated with the discovery of the CERT protein that transfers ceramide between the site of its synthesis in the ER to the trans side of the Golgi, where ceramide is then flipped and converted to sphingomyelin (SM) in the Golgi lumen. In addition to the ceramide binding module, CERT also contains a PH domain that is important to dock the protein to the Golgi and a mutation within the PH domain that eliminates PtdIns4P renders the CERT protein completely dysfunctional (67). What is remarkable about the PtdIns4P regulation of CERT transport function is that it appears to require the type IIIbeta PI 4-kinase enzyme (159), even though all of the four PI 4-kinase enzymes can produce PtdIns4P in some parts of the Golgi (8). Based on recent observations a regulatory loop is emerging in which Golgi DAG levels regulate the recruitment/activity of PKD enzymes that phosphorylate and activate PI4KIIIβ (68) but also phosphorylate CERT but this phosphorylation decreases CERT binding to PtdIns4P (52)
Less is known about the inositide regulation of the oxysterol transport function of the OSBP protein or its homologues. However, several clues suggest that phosphoinositides and oxysterol binding proteins are functionally coupled. Deletion of one of the yeast oxysterol binding proteins, Kes1p bypasses the Sec14 (a yeast PI-transfer protein) defect (45, 97), and recent structural and functional data suggest that the sterol transfer function of the Kes1p (and perhaps other OSBP homologues) are regulated by phosphoinositides (79, 131). There exists an important connection between cholesterol metabolism and SM synthesis: oxysterol treatment or cholesterol depletion strongly stimulates CERT-mediated ceramide transport and Golgi SM production and this effect requires the OSBP protein (125).
The biology of the FAPP1 and FAPP2 proteins has been more controversial. FAPP1 was initially identified as a protein that contains a PH domain with specific PtdIns4P recognition (four-phosphate adaptor protein) (40). A highly homologous protein, FAPP2, however, was shown also to contain a glycolipid transfer protein homology (GLTP) domain (174) and it has been proposed that FAPP2 transfers glycosyl ceramide (GlcCer) between membranes in a PtdIns4P-regulated manner (32). It is not clear as yet between which membranes this transfer occurs. GlcCer is synthesized on the outer surface of the cis-Golgi but its conversion to lactosyl ceramide and the more complex glycosphingolipids (GSLs) occurs in the lumen at the trans-Golgi (64). One report suggested that FAPP2 is needed to transfer GlcCer from the cis- to the trans-Golgi in a non-vesicular transport step (32), while another study proposed that FAPP2 transferred GlcCer from the cis-Golgi back to the ER, where its flipping to the lumen was found most efficient (64). Regardless of the route(s) of transport FAPP2 supports, the protein was found critical for the transport of apical cargos to the membrane in polarized cells and for the formation of cilia in the apical membrane, presumably because of the need for organization of glycolipid-rich membrane domains (173). More studies are expected to clarify this role of the FAPP2 protein as well as to determine which of the PI 4-kinases are critical for supporting its PH domain membrane interactions.
The importance of phospholipid transfer proteins in the Golgi to PM secretion process has long been established in S. cerevisiae. Here, the Sec14p protein that encodes a phosphatidylinositol/phosphatidylcholine (PI/PC) transfer protein was found to be critical to the yeast secretion process (14). The function of the Sec14 protein is to supply the Golgi with PtdIns and PtdCho, ultimately to maintain the levels of DAG in this organelle. DAG is an important regulator of a number of Golgi-associated kinases containing cystein-rich Zn2+-finger motifs, such as some PKC isoforms (90) and PKD (15). In addition, PtdIns4P is also a key organizer of the Golgi to PM vesicular secretion process via recruitment of adaptor proteins (179) and Sec14p can also supply PtdIns for the Golgi-localized PI 4-kinases (65, 141). Moreover, there are several Sec14 homologues in yeast that have a role in transferring lipids between the various yeast membranes and it was recently shown that PtdIns4P synthesis is important in some of these transport functions (137). One of these Sec14 homologues was also identified as a component of the synthesis of the aminophospholipid, phosphatidylethanolamine (PtdEtn), via decarboxylation of ER-derived phosphatidylserine (PtdSer) in Golgi membranes (162). For this, PtdSer has to be transferred to the Golgi membranes to be decarboxylated, and genetic studies have shown that Stt4p (the yeast homologue of PI4KIII alpha) is a regulatory component of this process at the Golgi (but not at the mitochondrial) site (162). It is not yet understood why the Stt4p kinase is needed for the lipid transfer and whether it acts at the donor or acceptor membrane site. Also, it has yet to be seen whether a similar regulation of aminophospholipid synthesis by PI 4-kinases or by other phosphoinositides is present in higher organisms.
Interestingly, mammalian PITPs show only low sequence homology to Sec14, although Sec14 homologues are also found in mammalian cells and they also transport lipophilic compounds such as the α-tocopherol binding protein (101). However, mammalian PITPs are functionally similar to Sec14 proteins (28) and they help maintain DAG levels in the Golgi (such as the larger Nir2 protein (98) or the Golgi-localized PITPβ (126)), and resupply the membrane with PtdIns for maintaining the signaling pool of phosphoinositides (158). There is no indication, though, that mammalian PITPs are regulated by inositides and rather they are believed to transport PtdIns from the ER to the PI 4-kinases (141) and the PtdIns specific PI 3-kinase (121).
These studies together outline an important regulatory paradigm in cellular lipid synthesis and transport. Contrary to common beliefs, endogenous membrane lipids are not diffused through the aqueous phase separating the membranes and in many instances do not distribute freely with vesicular transport. Their traversing of the inter membrane spaces require specific lipid transport systems that are not dissimilar to the transport systems (channels or transporters) that allow the water-soluble products to traverse lipid bilayers. It is intriguing that phosphoinositides appear more and more to be just as important for controlling lipid transfer function as they are in the regulation of membrane transporters and ion channels.
2. METHODS TO STUDY THE RELEVANCE OF INOSITIDE REGULATION
Most of our early knowledge on phosphoinositide changes were obtained by metabolic labeling studies using either 32P-phosphate or myo-[3H-inositol] followed by separation of the labeled phospholipids by TLC (145 ). These methods were complemented with separation of the water soluble inositol phosphates extracted from cells by HPLC (146) and detect the eluted inositol phosphates either by radiodetection in case of labeled cells or metal-dye detection (127) and chemically suppressed conductivity detection (150) to measure absolute amounts of the inositol phosphates. Ins(1,4,5)P3 mass was also determined by radioreceptor assay (24), but all of these measurements assessed total cellular content without any special information on location or compartmentalization. As the highly localized roles of the lipids became more and more apparent, there was a need to obtain information on the subcellular distribution and rapid dynamics of inositol lipid changes within the cell.
2.1 Visualizing inositol lipid distribution and dynamics
The first attempts to evaluate the subcellular distribution of inositol lipids without cell fractionation were based on immuno-cytochemistry using anti PtdIns(4,5)P2 antibodies (160). This, however, required cell fixation and many laboratories have expressed frustration over the sensitivity of the methods to batch-to-batch variability of the antibodies or to the hard-to-standardize subtleties of the fixation procedures. Live cell imaging of lipid distribution has become possible with the availability of protein domains that posses natural phosphoinositide recognition and using them as GFP fused proteins to visualize the lipids in live cells. These included PH domains to detect PtdIns(4,5)P2 (151, 167), PtdIns(3,4,5)P3 (85, 144, 168), PtdIns4P (95), FYVE domains to detect PtdIns3P (57, 86) and C1 domains to follow DAG changes (107). Even though these methods have their limitations (discussed in several reviews, e.g. (166)), they provided enormously important new information on lipid distribution and dynamics in a great number of systems and have become a standard method in cell biology. A special extension of this approach was the use of these same domains in fixed cells (just like antibodies) to detect the lipids without disturbing the biology that complicate matters when overexpressing these lipid binding domains (58). This also allowed analysis with EM resolution (180), although the distortion of lipid levels or distribution due to the fixation procedure is a tradeoff in these applications. Detailed description of these methods and the pros and cons of their use has been discussed elsewhere (13, 41, 42) and will not be duplicated here.
2.2 Manipulating inositol lipid or –phosphate levels within the cells
It is difficult to alter inositol lipid levels in whole cells and study the immediate consequences on any signaling or trafficking event. Most of the currently used approaches require prolonged exposure of the cells to the desired lipid change. For example, knock-down of an inositol lipid kinase or phosphatase enzymes, or the overexpression of an active or a dominant negative form takes anywhere between 4 hrs and several days to achieve the desired effects. During this period the primary affected process (such as the release of an adapter protein) initiates a whole sequence of events leading to changes from which it is hard to deduce what processes have been primarily linked to the lipid changes. The optimal solution to these problems is the use specific inhibitors to evoke acute changes in inositide levels. This is best demonstrated by the enormous boost that discovery of PI3K inhibitors brought to the field of PI 3-kinases (4). Unfortunately, there are no good specific inhibitors for many of the inositide converting enzymes. This problem has prompted us (169) and others (156) to design alternative strategies by which inositide levels can be acutely changed in specific membrane compartments. This is based upon the regulated recruitment into defined membrane compartments of inositide kinase or phosphatase enzymes that are stripped of their own localization mechanisms. This method relies upon the assumption that the enzymes residing in the cytosol have limited impact on the membrane-bound lipids but this is changed dramatically once the enzyme is recruited to the membrane where its substrate resides. The recruitment is based on the heterodimerization of the FRB domains of mTOR and the FKBP12 protein (17) in the presence of rapamycin or an appropriate analogue so fusing the enzyme to one of these domain and targeting the other partner to the desired membrane compartment allows a regulated recruitment process. This method was successfully used to change the PtdIns(4,5)P2 levels in the plasma membrane and assess its consequences on ion channel activity (156, 169) as well as on the membrane binding of clathrin adapters and their roles in the endocytic process (1, 191). This approach can be extended to other enzymes and compartments and will be interesting to study the roles of phosphoinositides in other cellular locations. The recent discovery of voltage-regulated inositol lipid 5-phosphatases in Ciona intestinalis and other organisms (63, 114, 115) offers an alternative and even more rapid an reversible way of changing PtdIns(4,5)P2 levels within the cells. Using these enzymes will provide us with lots of interesting data on PtdIns(4,5)P2 regulation of plasma membrane processes.
An alternative method to increase phosphoinositide levels within the cell is to supply synthetic lipids with a delivery system that allows the lipid with its highly polar headgroup to cross the PM. This is achieved by combining the lipid with polyamines or polybasic proteins (120). The advantages of these “PI-shuttle” systems are their simplicity and that various isomers can be delivered into the cells. Their disadvantage is that they will deluge the cell with the lipid taking away the local regulatory features of the endogenously produced counterparts. In addition it is hard to know which way these will metabolize in the cells and what is the actually active compound.
CONCLUDING REMARKS
Even from this limited overview it should be obvious that phosphoinositides have an enormous impact on any membrane-associated signaling process. Extensive research on these lipids over several decades has led to great advances in our understanding of cell signaling. However, the plethora of information available on these lipids is hard to comprehend. The fact remains that we have fundamental gaps in our understanding of the functions of phosphoinositides and the mechanistic details of how they control membrane dynamics. Improving our research tools and developing more specific inhibitors will help us better answer the open questions. Although this field of research was mostly driven by the curiosity of scientists conducting basic research, the achievements of the field have served the goals of public health very aptly. Ten years ago PI 3-kinase inhibitors were only thought about as research tools and today they are in clinical trials in targeting diseases such as cancer, autoimmunity, allergy and metabolic disorders. Right now “translational research” does not appreciate why we need to develop inhibitors of PIP 5-kinases or PI 4-kinases. However, this can change in a heartbeat. As a good example, PI4KIIIα has just emerged as a critical factor in the assembly of the Hepatitis C virus in the liver as reported in three separate recent studies (18, 157, 163). This finding will suddenly make this enzyme a desirable drug target. There is ample reason to expect that an expanded investment in phosphoinositide research will bring a pay back in real medical terms.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
REFRENCES
- 1.Abe N, Inoue T, Galvez T, Klein L, Meyer T. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J Cell Sci. 2008;121:1488–1494. doi: 10.1242/jcs.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aikawa Y, Martin TF. ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis. Methods Enzymol. 2005;404:422–431. doi: 10.1016/S0076-6879(05)04037-1. [DOI] [PubMed] [Google Scholar]
- 3.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 4.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astle MV, Seaton G, Davies EM, Fedele CG, Rahman P, Arsala L, Mitchell CA. Regulation of phosphoinositide signaling by the inositol polyphosphate 5-phosphatases. IUBMB Life. 2006;58:451–456. doi: 10.1080/15216540600871159. [DOI] [PubMed] [Google Scholar]
- 6.Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 7.Baird D, Stefan C, Audhya A, Weys S, Emr SD. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J Cell Biol. 2008;183:1061–1074. doi: 10.1083/jcb.200804003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balla A, Balla T. Phosphatidylinositol 4-kinases; old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Balla A, Ju Kim Y, Varnai P, Szentpetery Z, Knight Z, Shokat KM, Balla T. Maintenance of Hormone-sensitive Phosphoinositide Pools in the Plasma Membrane Requires Phosphatidylinositol 4-Kinase III{alpha} Mol Biol Cell. 2007;19:711–721. doi: 10.1091/mbc.E07-07-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J Biol Chem. 2002;277:20041–22050. doi: 10.1074/jbc.M111807200. [DOI] [PubMed] [Google Scholar]
- 11.Balla A, Tuymetova G, Tsiomenko A, Varnai P, Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol Biol Cell. 2005;16:1282–1295. doi: 10.1091/mbc.E04-07-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J Cell Sci. 2005;118:2093–2104. doi: 10.1242/jcs.02387. [DOI] [PubMed] [Google Scholar]
- 13.Balla T, Varnai P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr Protoc Cell Biol. 2009;Chapter 24(Unit 24):24. doi: 10.1002/0471143030.cb2404s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC 14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 16.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 17.Belshaw PJ, Ho SN, Crabtree GR, Schreiber SL. Controlling protein association and subcellular localization with a synthetic ligand that induces heterodimerization of proteins. Proc Natl Acad Sci U S A. 1996;93:4604–4607. doi: 10.1073/pnas.93.10.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci U S A. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berridge MJ. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyze polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell. 1998;9:3547–3560. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 23.Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 2005;15:540–547. doi: 10.1016/j.tcb.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Challis RAJ, Chilvers ER, Wilcocks AL, Nahorski SR. Heterogeneity of [3H]inositol 1,4,5-trisphosphate binding sites in adrenocortical membranes. Characterisation and validation of a radioreceptor assay. Biochem J. 1990;265:421–427. doi: 10.1042/bj2650421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng MK, Shearn A. The direct interaction between ASH2, a Drosophila trithorax group protein, and SKTL, a nuclear phosphatidylinositol 4-phosphate 5-kinase, implies a role for phosphatidylinositol 4,5-bisphosphate in maintaining transcriptionally active chromatin. Genetics. 2004;167:1213–1223. doi: 10.1534/genetics.103.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft S. Does phosphatidylinositol breakdown control the Ca2+-gating mechanism? Trends Pharmacol Sci. 1981;2:340–342. [Google Scholar]
- 28.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Craige B, Salazar G, Faundez V. Phosphatidylinositol-4-Kinase Type II Alpha Contains an AP-3 Sorting Motif and a Kinase Domain that are both Required for Endosome Traffic. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creba JA, Downes CP, Hawkins PT, Brewster G, Michell RH, Kirk CJ. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983;212:733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czech MP. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol. 2003;65:791–815. doi: 10.1146/annurev.physiol.65.092101.142522. [DOI] [PubMed] [Google Scholar]
- 32.D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 33.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Graaf P, Klapisz EE, Schulz TK, Cremers AF, Verkleij AJ, van Bergen en Henegouwen PM. Nuclear localization of phosphatidylinositol 4-kinase beta. J Cell Sci. 2002;115:1769–1775. doi: 10.1242/jcs.115.8.1769. [DOI] [PubMed] [Google Scholar]
- 35.De Matteis MA, Di Campli A, Godi A. The role of the phosphoinositides at the Golgi complex. Biochim Biophys Acta. 2005;1744:396–405. doi: 10.1016/j.bbamcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Divecha N, Banfic H, Irvine RF. Inositides and the nucleus and inositides in the nucleus. Cell. 1993;74:405–407. doi: 10.1016/0092-8674(93)80041-c. [DOI] [PubMed] [Google Scholar]
- 37.Divecha N, Banfic H, Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991;10:3207–3214. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Divecha N, Rhee SG, Letcher AJ, Irvine RF. Phosphoinositide signalling enzymes in rat liver nuclei: Phosphoinositidase C isoform beta1 is specifically, but not predominantly, located in the nucleus. Biochem J. 1993;289:617–620. doi: 10.1042/bj2890617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 40.Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Downes CP, Gray A, Watt SA, Lucocq JM. Advances in procedures for the detection and localization of inositol phospholipid signals in cells, tissues, and enzyme assays. Methods Enzymol. 2003;366:64–84. doi: 10.1016/s0076-6879(03)66006-4. [DOI] [PubMed] [Google Scholar]
- 43.Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature. 1996;380:595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- 45.Fairn GD, Curwin AJ, Stefan CJ, McMaster CR. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc Natl Acad Sci U S A. 2007;104:15352–15357. doi: 10.1073/pnas.0705571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flower TR, Clark-Dixon C, Metoyer C, Yang H, Shi R, Zhang Z, Witt SN. YGR198w (YPP1) targets A30P alpha-synuclein to the vacuole for degradation. J Cell Biol. 2007;177:1091–1104. doi: 10.1083/jcb.200610071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- 48.Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell. 2001;128:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 50.Frederick JP, Mattiske D, Wofford JA, Megosh LC, Drake LY, Chiou ST, Hogan BL, York JD. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc Natl Acad Sci U S A. 2005;102:8454–8459. doi: 10.1073/pnas.0503706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- 52.Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaidarov I, Keen JH. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- 56.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier LM, Parton GP, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godi A, Di Campi A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 59.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. C-met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2007 doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc Natl Acad Sci U S A. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Logovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenov J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell. 2003;114:99–111. doi: 10.1016/s0092-8674(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 62.Grado C, Ballou CE. Myo-inositol phosphates from beef brain phosphoinostide. J Biol Chem. 1960;235:PC23–24. [PubMed] [Google Scholar]
- 63.Halaszovich CR, Schreiber DN, Oliver D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J Biol Chem. 2009;284:2106–2113. doi: 10.1074/jbc.M803543200. [DOI] [PubMed] [Google Scholar]
- 64.Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Maziere AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- 66.Hanada K. Discovery of the molecular machinery CERT for endoplasmic reticulum-to-Golgi trafficking of ceramide. Mol Cell Biochem. 2006;286:23–31. doi: 10.1007/s11010-005-9044-z. [DOI] [PubMed] [Google Scholar]
- 67.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 68.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TFJ. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 70.Hay JC, Martin TFJ. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 71.Haynes LP, Thomas GM, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–6054. doi: 10.1074/jbc.M413090200. [DOI] [PubMed] [Google Scholar]
- 72.Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol 4-OH-kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 73.Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell. 2008;31:383–394. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 75.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 76.Hokin MR, Hokin LE. Enzyme secretion and incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953;203:967–977. [PubMed] [Google Scholar]
- 77.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 79.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irvine RF. Nuclear inositide signalling -- expansion, structures and clarification. Biochim Biophys Acta. 2006;1761:505–508. doi: 10.1016/j.bbalip.2006.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-beta2. Nat Struct Mol Biol. 2006;13:1135–1140. doi: 10.1038/nsmb1175. [DOI] [PubMed] [Google Scholar]
- 83.Kakuk A, Friedlander E, Vereb G, Jr, Kasa A, Balla A, Balla T, Heilmeyer LM, Jr, Gergely P, Vereb G. Nucleolar localization of phosphatidylinositol 4-kinase PI4K230 in various mammalian cells. Cytometry A. 2006;69:1174–1183. doi: 10.1002/cyto.a.20347. [DOI] [PubMed] [Google Scholar]
- 84.Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133:475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kontos CD, Stauffer TP, Yang WP, York JD, Huang L, Blanar MA, Meyer T, Peters KG. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18:4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kutateladze TG, Ogburn KD, Watson WT, deBeer T, Emr SD, Burd CG, Overduin M. Phosphatidylinositol 3-Phosphate recognition by the FYVE domain. Mol Cell. 1999;3:805–811. doi: 10.1016/s1097-2765(01)80013-7. [DOI] [PubMed] [Google Scholar]
- 87.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 89.Lambrechts A, Gevaert K, Cossart P, Vandekerckhove J, Van Troys M. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 2008;18:220–227. doi: 10.1016/j.tcb.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 90.Lehel C, Olah Z, Jakab G, Anderson WB. Protein kinase C epsilon is localized to the Golgi via its zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci U S A. 1995;92:1406–1410. doi: 10.1073/pnas.92.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 92.Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans. 2004;32:707–711. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 93.Levine TP, Munro S. Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. Mol Biol Cell. 2001;6:1633–1644. doi: 10.1091/mbc.12.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 95.Levine TP, Munro S. The pleckstrin-homology domain of oxysterol-binding protein recognizes a determinant specific to Golgi membranes. Curr Biol. 1998;8:729–739. doi: 10.1016/s0960-9822(98)70296-9. [DOI] [PubMed] [Google Scholar]
- 96.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 97.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 99.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manor D, Morley S. The alpha-tocopherol transfer protein. Vitam Horm. 2007;76:45–65. doi: 10.1016/S0083-6729(07)76003-X. [DOI] [PubMed] [Google Scholar]
- 102.Manzoli L, Martelli AM, Billi AM, Faenza I, Fiume R, Cocco L. Nuclear phospholipase C: involvement in signal transduction. Prog Lipid Res. 2005;44:185–206. doi: 10.1016/j.plipres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Matthews AG, Kuo AJ, Ramon-Maiques S, Han S, Champagne KS, Ivanov D, Gallardo M, Carney D, Cheung P, Ciccone DN, Walter KL, Utz PJ, Shi Y, Kutateladze TG, Yang W, Gozani O, Oettinger MA. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 106.Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, Anderson RA. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature. 2008;451:1013–1017. doi: 10.1038/nature06666. [DOI] [PubMed] [Google Scholar]
- 107.Meyer T, Oancea E. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 108.Michailidis IE, Zhang Y, Yang J. The lipid connection-regulation of voltage-gated Ca(2+) channels by phosphoinositides. Pflugers Arch. 2007;455:147–155. doi: 10.1007/s00424-007-0272-9. [DOI] [PubMed] [Google Scholar]
- 109.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975;415:81–147. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- 110.Michell RH. Is phosphotidylinositol really out of the calcium gate? Nature. 1982;296:492–493. doi: 10.1038/296492a0. [DOI] [PubMed] [Google Scholar]
- 111.Michell RH, Harwood JL, Coleman R, Hawthorne JN. Characteristics of rat liver phosphatidylinositol kinase and its presence in the plasma membrane. Biochim Biophys Acta. 1967;144:649–658. doi: 10.1016/0005-2760(67)90053-7. [DOI] [PubMed] [Google Scholar]
- 112.Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Minogue S, Waugh MG, De Matteis MA, Stephens DJ, Berditchevski F, Hsuan JJ. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J Cell Sci. 2006;119:571–581. doi: 10.1242/jcs.02752. [DOI] [PubMed] [Google Scholar]
- 114.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 115.Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol. 2007;583:875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakagawa T, Goto K, Kondo H. Cloning, expression and localization of 230 kDa phosphatidylinositol 4-kinase. J Biol Chem. 1996;271:12088–12094. doi: 10.1074/jbc.271.20.12088. [DOI] [PubMed] [Google Scholar]
- 117.Niggli V, Adunyah ES, Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+ - ATPase. J Biol Chem. 1981;256:8588–8592. [PubMed] [Google Scholar]
- 118.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. Embo J. 2008;27:2809–2816. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci. 2001;114:2501–2511. doi: 10.1242/jcs.114.13.2501. [DOI] [PubMed] [Google Scholar]
- 120.Ozaki S, DeWald DB, Shope JC, Chen J, Prestwich GD. Intracellular delivery of phosphoinositides and inositol phosphates using polyamine carriers. Proc Natl Acad Sci U S A. 2000;97:11286–11291. doi: 10.1073/pnas.210197897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panaretou C, Domin J, Cockcroft S, Waterfield MD. Characterization of p150, an adaptor protein for the human phosphatidylinositol (PtdIns) 3-kinase. Substrate presentation by phosphatidylinositol transfer protein to the p150/PtdIns 3-kinase complex. J Biol Chem. 1997;272:2477–2485. doi: 10.1074/jbc.272.4.2477. [DOI] [PubMed] [Google Scholar]
- 122.Papayannopoulos V, Co C, Prehoda KE, Snapper S, Taunton J, Lim WA. A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol Cell. 2005;17:181–191. doi: 10.1016/j.molcel.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 123.Parrish WR, Stefan CJ, Emr SD. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol Biol Cell. 2004;15:3567–3579. doi: 10.1091/mbc.E04-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Payrastre B, Nievers M, Boonstra J, Breton M, Verkleij AJ, VanBergen en Henegouwen PMP. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J Biol Chem. 1992;267:5078–5084. [PubMed] [Google Scholar]
- 125.Perry RJ, Ridgway ND. Oxysterol-binding Protein and VAMP-associated Protein Are Required for Sterol-dependent Activation of the Ceramide Transport Protein. Mol Biol Cell. 2006 doi: 10.1091/mbc.E06-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phillips SE, Ile KE, Boukhelifa M, Huijbregts RP, Bankaitis VA. Specific and nonspecific membrane-binding determinants cooperate in targeting phosphatidylinositol transfer protein beta-isoform to the mammalian trans-Golgi network. Mol Biol Cell. 2006;17:2498–2512. doi: 10.1091/mbc.E06-01-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pittet D, Schlegel W, Lew DP, Monod A, Mayr GW. Mass changes in inositol tetrakis- and pentakisphosphate isomers induced by chemotactic peptide stimulation in HL-60 cells. J Biol Chem. 1989;264:18489–18493. [PubMed] [Google Scholar]
- 128.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 129.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 130.Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Phys Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 133.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rohacs T. Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 136.Rohde G, Wenzel D, Haucke V. A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol. 2002;158:209–214. doi: 10.1083/jcb.200203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Routt SM, Ryan MM, Tyeryar K, Rizzieri KE, Mousley C, Roumanie O, Brennwald PJ, Bankaitis VA. Nonclassical PITPs activate PLD via the Stt4p PtdIns-4-kinase and modulate function of late stages of exocytosis in vegetative yeast. Traffic. 2005;6:1157–1172. doi: 10.1111/j.1600-0854.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 138.Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- 139.Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP, Carpenter CL. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 140.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 141.Schaaf G, Ortlund EA, Tyeryar KR, Mousley CJ, Ile KE, Garrett TA, Ren J, Woolls MJ, Raetz CR, Redinbo MR, Bankaitis VA. Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol Cell. 2008;29:191–206. doi: 10.1016/j.molcel.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 143.Seeds AM, Frederick JP, Tsui MM, York JD. Roles for inositol polyphosphate kinases in the regulation of nuclear processes and developmental biology. Adv Enzyme Regul. 2007;47:10–25. doi: 10.1016/j.advenzreg.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shears SB. Signalling by inositides. A paractical approach. Oxford, New York, Tokyo: Oxford University Press; 1997. Measurement of inositol phosphate turnover in intact cells and cell-free systems; pp. 33–53. [Google Scholar]
- 146.Shears SB. Signalling by Inositides. Oxford, New York, Tokyo: Oxford University Press; 1997. [Google Scholar]
- 147.Shisheva A. PIKfyve: Partners, significance, debates and paradoxes. Cell Biol Int. 2008;32:591–604. doi: 10.1016/j.cellbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shisheva A, Sbrissa D, Ikonomov O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol Cell Biol. 1999;19:623–634. doi: 10.1128/mcb.19.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 150.Smith RE, MacQuarrie RA. Determination of inositol phosphates and other biologically important anions by ion chromatography. Anal Biochem. 1988;170:308–315. doi: 10.1016/0003-2697(88)90636-7. [DOI] [PubMed] [Google Scholar]
- 151.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 152.Steger DJ, Haswell ES, Miller AL, Wente SR, O’Shea EK. Regulation of chromatin remodeling by inositol polyphosphates. Science. 2003;299:114–116. doi: 10.1126/science.1078062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Strahl T, Hama H, Dewald DB, Thorner J. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J Cell Biol. 2005;171:967–979. doi: 10.1083/jcb.200504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–68. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 155.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thomas GMH, Cunningham E, Fensome A, Ball A, Totty NF, Truong O, Hsuan JJ, Cockcroft S. An essential role of phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell. 1993;74:919–928. doi: 10.1016/0092-8674(93)90471-2. [DOI] [PubMed] [Google Scholar]
- 159.Toth B, Balla A, Ma H, Knight ZA, Shokat KM, Balla T. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J Biol Chem. 2006;281:36369–36377. doi: 10.1074/jbc.M604935200. [DOI] [PubMed] [Google Scholar]
- 160.Tran D, Gascard P, Berthon B, Fukami K, Takenawa T, Giraud F, Claret M. Cellular distribution of polyphosphoinositides in rat hepatocytes. Cell Signal. 1993;5:565–581. doi: 10.1016/0898-6568(93)90052-n. [DOI] [PubMed] [Google Scholar]
- 161.Tronchere H, Buj-Bello A, Mandel JL, Payrastre B. Implication of phosphoinositide phosphatases in genetic diseases: the case of myotubularin. Cell Mol Life Sci. 2003;10:2084–2089. doi: 10.1007/s00018-003-3062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Trotter PJ, Wu WI, Pedretti J, Yates R, Voelker DR. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J Biol Chem. 1998;273:13189–13196. doi: 10.1074/jbc.273.21.13189. [DOI] [PubMed] [Google Scholar]
- 163.Vaillancourt FH, Pilote L, Cartier M, Lippens J, Liuzzi M, Bethell RC, Cordingley MG, Kukolj G. Identification of a lipid kinase as a host factor involved in hepatitis C virus RNA replication. Virology. 2009;387:5–10. doi: 10.1016/j.virol.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 164.van Rheenen J, Song X, van Roosmalen W, Cammer M, Chen X, Desmarais V, Yip SC, Backer JM, Eddy RJ, Condeelis JS. EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol. 2007;179:1247–1259. doi: 10.1083/jcb.200706206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 166.Varnai P, Balla T. Live cell imaging of phosphoinositide dynamics with fluorescent protein domains. Biochim Biophys Acta. 2006;1761:957–967. doi: 10.1016/j.bbalip.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 167.Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium-and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- 169.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 171.Varsanyi M, Tolle HG, Heilmeyer MG, Jr, Dawson RM, Irvine RF. Activation of sarcoplasmic reticular Ca2+ transport ATPase by phosphorylation of an associated phosphatidylinositol. Embo J. 1983;2:1543–1548. doi: 10.1002/j.1460-2075.1983.tb01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verbsky J, Lavine K, Majerus PW. Disruption of the mouse inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene, associated lethality, and tissue distribution of 2-kinase expression. Proc Natl Acad Sci U S A. 2005;102:8448–8453. doi: 10.1073/pnas.0503656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vieira OV, Verkade P, Simons K. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J Cell Biol. 2005;170:521–526. doi: 10.1083/jcb.200503078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Voglmaier SM, Keen JH, Murphy JE, Ferris CD, Prestwich GD, Snyder SH, Theibert AB. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem Biophys Res Commun. 1992;187:158–163. doi: 10.1016/s0006-291x(05)81473-1. [DOI] [PubMed] [Google Scholar]
- 176.Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 177.Wang J, Sun HQ, Macia E, Kirchhausen T, Watson H, Bonifacino JS, Yin HL. PI4P Promotes the Recruitment of the GGA Adaptor Proteins to the Trans-Golgi Network and Regulates Their Recognition of the Ubiquitin Sorting Signal. Mol Biol Cell. 2007 doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang YJ, Li WH, Wang J, Xu K, Dong P, Luo X, Yin HL. Critical role of PIP5KIgamma 87 in InsP3-mediated Ca(2+) signaling. J Cell Biol. 2004;167:1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirschhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 180.Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J. 2002;363:657–666. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;31:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 183.Wong K, Meyers R, Cantley LC. Subcellular localization of phosphatidylinositol 4-kinase isoforms. J Biol Chem. 1997;272:13236–13241. doi: 10.1074/jbc.272.20.13236. [DOI] [PubMed] [Google Scholar]
- 184.Yaradanakul A, Feng S, Shen C, Lariccia V, Lin MJ, Yang J, Kang TM, Dong P, Yin HL, Albanesi JP, Hilgemann DW. Dual control of cardiac Na+ Ca2+ exchange by PIP(2): electrophysiological analysis of direct and indirect mechanisms. J Physiol. 2007;582:991–1010. doi: 10.1113/jphysiol.2007.132712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 186.York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 187.Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell. 2004;13:677–688. doi: 10.1016/s1097-2765(04)00083-8. [DOI] [PubMed] [Google Scholar]
- 188.Zhai C, Li K, Markaki V, Phelan JP, Bowers K, Cooke FT, Panaretou B. Ypp1/YGR198w plays an essential role in phosphoinositide signalling at the plasma membrane. Biochem J. 2008;415:455–466. doi: 10.1042/BJ20080209. [DOI] [PubMed] [Google Scholar]
- 189.Zhao X, Varnai P, Tuymetova G, Balla A, Toth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- 190.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]