Abstract
Background
Working memory deficits abound in schizophrenia and attention deficits have been documented in schizophrenia and bipolar disorder. Adolescent offspring of patients may inherit vulnerabilities in these brain circuits and may show deficit performance in these cognitive domains. Here we assess impairments in offspring of schizophrenia (SCZ-Offspring) or bipolar (BP-Offspring) patients compared to controls (HC) with no family history of mood or psychotic disorders to the second degree.
Methods
Three groups (n=100 subjects; range:10–20 yrs) of HC, SCZ-Offspring and BP-Offspring gave informed consent. Working memory was assessed using a delayed spatial memory paradigm with two levels of delay (2s & 12s); sustained attention processing was assessed using the Continuous Performance Task-Identical Pairs version.
Results
SCZ-Offspring (but not BP-Offspring) showed impairments in working memory (relative to HC) at the longer memory delay indicating a unique deficit. Both groups showed reduced sensitivity during attention but only BP-Offspring significantly differed from controls.
Conclusions
These results suggest unique (working memory/dorsal frontal cortex) and potentially overlapping (attention/fronto-striatal cortex) vulnerability pathways in adolescent offspring of patients with schizophrenia and bipolar disorder. Working memory and attention assessments in these offspring may assist in the clinical characterization of the adolescents vulnerable to SCZ or BP.
Keywords: Schizophrenia, Bipolar Disorder, Offspring, Working Memory, Attention
1. Introduction
The distinction between schizophrenia and bipolar disorder appears unclear from the perspective of neurobiology. For example, studies in molecular genetics suggest significant overlap in genetic vulnerability for each (Craddock & Owen, 2005) thereby implying a common aetiological bases. If the genetic aetiology is overlapping across phenotypes, it is plausible that neurodevelopment plays a critical role in the pathways toward each disorder. The idea that neurodevelopmental trends may distinguish between these disorders has been advanced (Murray et al., 2004), as has the general idea that complementary developmental trajectories contribute to distinct phenotypes post-adolescence (Keshavan et al., 2005a; Paus, 2005; Paus et al., 2008). In this view, complex interactions between programmed genetic development and environmental factors combine to alter or derail neurodevelopmental pathways during critical periods such as adolescence. This is now the modal view for all developmental hypotheses in schizophrenia (Lewis & Levitt, 2002), though its relevance for bipolar disorder is not yet established. These pathways may be realized through the disordered development of specific cortical systems in the brain, and therefore of cognitive domains that are closely tied to these cortical systems. Because both schizophrenia and bipolar disorder aggregate in families (Pavuluri et al., 2005), adolescent offspring of schizophrenia and bipolar patients are at increased risk for impaired cognition and psychopathology (Birmaher et al., In Press; Birmaher et al., 2009; Keshavan et al., 2005b) and are increasingly vulnerable to the disorders themselves. Estimates indicate that nearly 10%–15% of offspring of schizophrenia patients will be diagnosed with schizophrenia (Erlenmeyer-Kimling et al., 1997), and that 5%–15% of offspring of bipolar patients will develop a mood disorder in their lifetimes (Lapalme et al., 1997). Furthermore, increasing evidence suggests alterations in cortical systems in these subjects (Diwadkar et al., 2006), supporting the idea of neurodevelopmental derailment during adolescence. Therefore, understanding unique or non-specific patterns of cognitive impairment in these groups may prove informative.
1.1 Working Memory and Sustained Attention: Distinct and Overlapping Components
Working memory and sustained attention have been closely mapped to prefrontal and striatal circuitry (Cohen et al., 1997; Corbetta et al., 1998). Working memory in particular is heavily dependent on sustained activity in prefrontal circuitry that is necessary for the temporary maintenance of information (Braver et al., 1997; Fuster, 1989). Sustained attention or vigilance is tied to interactions between cortico-striatal circuitry (Buchel & Friston, 1997; Luna et al., 2001). In general, the development of frontal and striatal circuitry is particularly dynamic during adolescence (Booth et al., 2003; Rubia et al., 2006), marked by both increased coordination during attention and working memory in particular (Edin et al., 2007), and increased frontal engagement with age (Rubia et al., 2000). Developmental deviations in regional function in SCZ- and/or BP-Offspring may affect working memory and sustained attention.
Prefrontal dysfunction is a central correlate of schizophrenia pathophysiology (Lewis, 1997) and delayed working memory paradigms elegantly elucidate the bases of disordered prefrontal function in the diathesis (Goldman-Rakic, 1994). Electrophysiological studies in primates indicate that when memoranda must be maintained in memory over a delay, maintenance is sub-served by tonic activity of prefrontal neurons that persists over the duration of the memory interval (Goldman-Rakic, 1988). Furthermore, the duration of this activity scales with the duration of the memory interval indicating a direct parametric effect on the demands of the prefrontal cortex. SCZ-offspring are in fact characterized by disordered fronto-striatal function during working memory that scales with increased memory demand (Bakshi et al., In Press), and errors in memory in offspring are most apparent at increased memory delays (Diwadkar et al., 2001). Thus, parametric effects of working memory load are a useful metric for distinguishing between SCZ-Offspring and controls. By comparison, the relationship of working memory deficits to bipolar disorder is less well established. Early studies indicated that bipolar patients performing similarly to controls (Park & Holzman, 1992), though recent data present a more heterogeneous pattern. Working memory deficits in bipolar subjects may be mediated by the presence of psychosis (Glahn et al., 2006) or manic symptoms (Sweeney et al., 2000). fMRI studies suggest that working memory in bipolar disorder is characterized by aberrant increases in engagement of the frontal cortex (Adler et al., 2004; Chang et al., 2004; Monks et al., 2004), though the value of working memory as a vulnerability marker in the mood spectrum is not established. Also, the relationship of working memory to neurophysiological processes such as dopaminergic neurotransmission (Vijayraghavan et al., 2007) or reduced synchrony between GABA-ergic neurons (Lewis et al., 2005) is hypothesized as central in schizophrenia, though this translational angle appears absent in bipolar disorder. Thus, it is plausible that working memory deficits may reflect essential aspects of altered prefrontal circuitry in schizophrenia that may be inherited in, and unique to SCZ-Offspring.
Attentional processes are not dependent on sustained maintenance by prefrontal neurons but may depend on cortico-striatal structural and functional connectivity (Graham et al., 2008; Haber & Calzavara, 2009). Other studies indicate that neural activity in key striatal regions such as the caudate are central to attention, and translating attention into motor outputs (Hikosaka & Sakamoto, 1986), suggesting that the striatum and its constituents are more central in the brain’s attention pathway. Sustained attention has in fact emerged as a common marker of deficit in both adult schizophrenia and bipolar populations (Cornblatt & Erlenmeyer-Kimling, 1989; Strakowski et al., 2004). Attention deficits appear to be stable and enduring, have been documented in adolescent offspring of schizophrenia patients (Michie et al., 2000), and may result from disordered fronto-striatal function (Diwadkar et al., In Press), suggesting that such deficits may be markers of genetic vulnerability for schizophrenia. Young bipolar offspring (age<25) endorse attention deficits but objective measures of attention deficits have provided mixed results (Klimes-Dougan et al., 2006). Nevertheless, several reasons motivate the idea that attention deficits would reflect neurodevelopmental vulnerability in both groups. For example, striatal abnormalities are noted in the earliest phases of the illness in both schizophrenia (Lawrie et al., 2001) and bipolar disorder (Strakowski et al., 2005), and have been associated with deficits in attention.
Here we investigated working memory and sustained attention deficits in age-matched HC, SCZ-Offspring and BP-Offspring using established measures of working memory (Diwadkar et al., 2001) and sustained attention (Salgado-Pineda et al., 2004). Based on the emerging literature, we expected SCZ-Offspring (but not BP-Offspring) would show unique impairments in working memory reflected in greater error in a delayed match to sample spatial working memory task as a function of increased memory delay. By comparison, we expected offspring of both schizophrenia and bipolar patients to demonstrate non-specific impairments in sustained attention reflected in lower sensitivity to detect targets in a sustained attention task.
2. Methods
2.1 Subjects
One hundred subjects (10 ≤ Age ≤ 20 yrs) gave informed consentor assent to participate. All protocols were cleared by the Institutional Review Boards at the University of Pittsburgh and Wayne State University. Groups did not differ in age overall (F2,97=.95, p>.35) or Full Scale IQ (F2,97=.40, p>.65). Table 1 provides a characterization of subjects, with age, gender and Full Scale IQ scores. All subjects were free from medications at the time of assessments.
Table 1.
Age, Full Scale IQ (± sd) and gender information for the samples.
| SCZ-Offspring (n=36) | BP-Offspring (n=23) | HC (n=41) | |
|---|---|---|---|
| Age (yrs) ± sd | 14.5 ± 2.7 | 14.0 ± 2.4 | 14.9 ±2.7 |
| M/F | 22/14 | 13/10 | 25/16 |
| FSIQ | 98.7 ± 14.9 | 101.5 ± 12.9 | 101.8 ± 18.2 |
2.2 Clinical Characterization
SCZ-Offspring and BP-Offspring were recruited through contacts in in-patient and out-patient services at the Western Psychiatric Institute and Clinic (WPIC) of the Dept. of Psychiatry at the University of Pittsburgh, from the greater Detroit area through advertisements, and patient services at the Wayne State University School of Medicine. HC were recruited from the same communities as the SCZ- and BP-Offspring through community based advertisements. Rule outs were achieved through telephone and personal interview, and screening questionnaires, to ascertain if subjects had a history of psychotic illness in first-degree relatives. Diagnoses for parents of offspring were reached using the Structured Clinical Interview for DSM-IV (SCID)(First et al., 1997). Subjects younger than 15 years were clinically evaluated using the Schedule for Affective Disorders and Schizophrenia -Child Version (K-SADS)(Kaufman et al., 1997); those aged 15 years or above were assessed using the SCID. Assessments were administered by trained interviewers and neuropsychological assessments were conducted in a dedicated testing laboratory testing room in an out-patient setting. Diagnosis information for the sample is presented in Table 2 and reflects the prevalence of these disorders across all subjects.
Table 2.
Existence of Axis 1 diagnoses in SCZ-Offspring and BP-Offspring samples. Because of co-morbidity, the numbers reflect observed incidence, not unique instances. Some psychopathology was observed in ∼30% of SCZ-Offspring and ∼50% of BP-Offspring.
| SCZ-Offspring | ADHD (Inattentive subtype) n=3 ADHD (Combined type) n=2 ADHD (NOS)=1 Conduct Disorder n=2 PTSD n=1 MDD Recurrent (Full remission) n=2 Oppositional Defiance Disorder n=2 |
| BP-Offspring | ADHD (Combined type) n=4 Social Phobia n=2 Specific Phobia n=1 Adjustment Disorder n=1 MDD Single Episode (Full remission) n=3 PTSD n=1 Generalized Anxiety Disorder n=1 Enuresis n=2 |
Observed incidence of at least one Axis I disorder in our offspring samples (roughly 20% in SCZ-Offspring and roughly 45% in BP-Offspring) is consistent with other published reports in the literature with estimates of >50% prevalence in adolescent bipolar offspring (Hillegers et al., 2005) and >60% in adolescent schizophrenia offspring samples (Keshavan et al., 2004).
2.3 Working Memory and Sustained Attention
Working memory was assessed using a spatial memory paradigm previously shown to discriminate between SCZ-Offspring and HC (Diwadkar et al., 2001). The paradigm (www.cogtest.com) required subjects to maintain spatial information for a peripherally presented location in working memory over short (2s) or long (12s) delay periods. Subjects touched an on-screen start button to initiate trials, followed by a center fixation stimulus prior to presentation of the target stimulus. During the delay between presentation and recall, a number of distracters of variable location appeared that need to be actively touched by the subject. The distracter interval prevented the subject from physically encoding the location of the target using visual or motor fixation. At the end of the delay, subjects signaled the location of the item by a finger press on a touch screen. Twenty four trials were employed. The dependent variable was error extent (mm) that is the distance between the indicated location on the touch screen and the actual location to be remembered. The task is a human analogue of basic working memory tasks employed in primates (Chafee & Goldman-Rakic, 2000; Fuster, 1989).
Sustained attention was assessed using the Continuous Performance Task (CPT) (Cornblatt et al., 1989). The CPT-IP measures ability to discriminate between targets (shapes) and distracters in a rapid presentation paradigm and d’ a measure of sensitivity, has been shown to discriminate between HC and SCZ-Offspring in previous studies (Cornblatt & Malhotra, 2001). During the experiment, subjects remained seated in front of a computer monitor. Shapes were rapidly presented at the center of the monitor (50 ms) with an inter-stimulus interval of 1000 ms. Subjects indicated with a key press when the identity of the shape was repeated. A total of 150 trials were employed. The dependent variable was visual d’, a measure of sensitivity to detect sequentially presented identical shapes and reject distracters (Rutschmann et al., 1977).
All univariate analyses were conducted using the general linear model framework in SPSS (SPSS, 2007). Analyses of co-variance were employed to assess independent main effects of Group (HC vs. SCZ-Offspring vs. BP-Offspring), Memory Delay (Short vs. Long; modeled as a repeated measure for the working memory paradigm) and Gender. In addition, age, FSIQ and the presence of ADHD were modeled as co-variates.
Preliminary univariate analyses demonstrated no effects of Site, or Site X Group interactions for either of the measures of interest (.17 < F1,87 <.63 across all effects). Subsequent analyses therefore were conducted by collapsing across this variable.
3. Results
3.1 Working memory
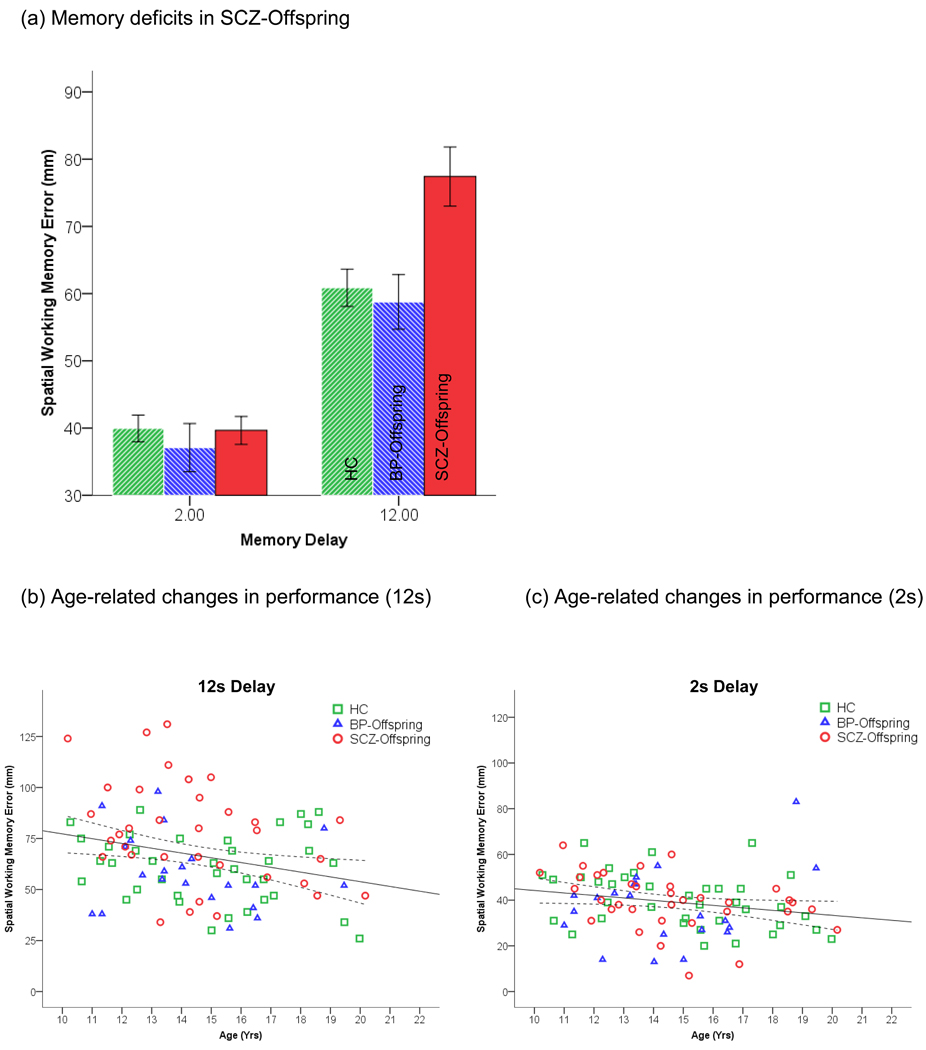
As seen in Figure 1a, relative to control subjects, impairments in delayed working memory (12 s) appeared in SCZ-Offspring but not BP-Offspring, specifically at the long delay period. Consistent with this, a Group x Delay interaction was significant, F2,86=6.42, p<.003, MSe=217.71 (Effect size: partial η2=.13)(Cohen, 1988). In addition, a main effect of group was observed, F2,86=4.39, p<.02, MSe=348.03 (partial η2=.09). Differences between groups at the longer delay were investigated using the Dunn-Šidák test for multiple comparisons among means (Dunn, 1961). SCZ-Offspring differed from both HC (tD =3.73, p<.05) and BP-Offspring (tD =3.60, p<.05) respectively. Two additional effects were significant; a main effect of delay, F1,86=6.84, p<.01 (partial η2=.08) indicated increases in errors as a function of delay, and a significant effect of age, F1,86=8.77, p<.004 (partial η2=09), indicated a decrease in working memory errors with age. As seen in Figure 1b, the effects of age were particularly pronounced at the longer delay.
Figure 1.
(a) Group and age-related differences in spatial working memory (2s and 12 s delay) are depicted. As seen, impairment in SCZ-Offspring is evident at the longer delay interval where SCZ-Offspring differed from both HC and BP-Offpsring. Error bars are ± s.e.m. (b) Working memory decreases with age across groups are evident at the 12 s delay. A single regression function (with mean confidence interval) collapsing across groups was significant, F1,90=7.01, p<.01, β=−.27. (c) By comparison, at the easier level of memory, age-related effects were weaker, (F1,90=4.35, p<.05, β=−.2).
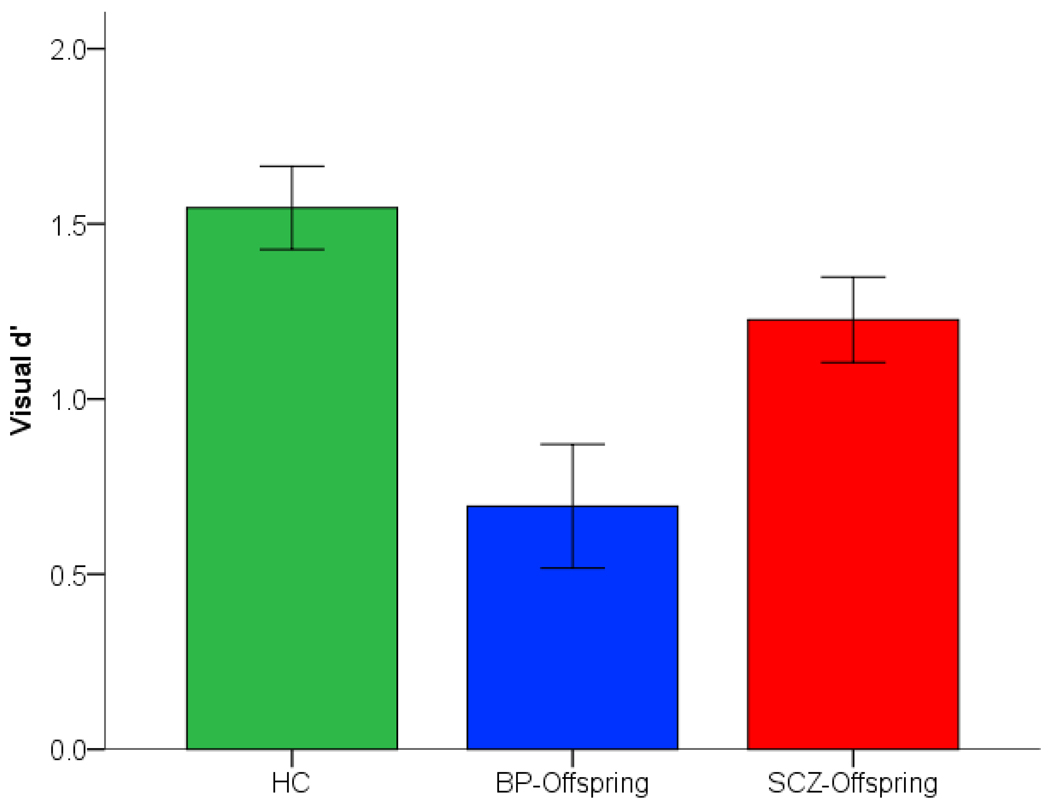
3.2 Sustained Attention
As seen in Figure 2, relative to control subjects, reductions in sensitivity appeared in both SCZ-Offspring and BP-Offspring. A significant effect of Group, F2,86=7.03, p<.001, MSe=.544 (partial η2=.14). Differences between groups assessed with the Dunn-Šidák test revealed significant differences between BP-Offspring and HC (tD=4.26, p<.05), but not between SCZ-Offspring and HC (tD=1.98, one-tailed for two post-hoc comparisons). Observed power for the main effect of group was adequate (1-β=.92).
Figure 2.
Reductions in sensitivity during sustained attention are depicted in SCZ-Offspring and BP-Offspring relative to HC. A main effect of group was primarily driven by differences between BP-Offspring and HC (see Results). Error bars are ± s.e.m..
4. Discussion
This is one of the few studies directly comparing parametric working memory and attention performance in age- and gender-comparable adolescent offspring of schizophrenia and bipolar patients. The principle results were these: a) Working deficits were observed in SCZ-Offspring, specifically at the longer memory delay, reflecting the unique impact of memory demand on this subgroup. These results replicate previous demonstrations in SCZ-Offspring and suggest that impairments in working memory and by implication working memory-related prefrontal function are characteristic trait- and vulnerability markers of the schizophrenia diathesis; b) Significant deficits in sustained attention were observed and were statistically robust between BP-Offspring and HC, but marginally significant between SCZ-Offspring and HC. In either analysis, significant effects were independent of FSIQ and the presence of ADHD diagnoses in the offspring groups. Also, no gender effects were observed.
These results suggest that working memory deficits are highly specific to SCZ-Offspring reflecting the proposed relevance of working memory dysfunction in the schizophrenia diathesis (Goldman-Rakic, 1994). These deficits may reflect the relatively large pre-frontal demands placed on maintaining tokens in working memory for long periods of time, and may be related to the hypothesized convergence of prefrontal dopamine, working memory and schizophrenia (Gore et al., 2010; Henze et al., 2000; Vijayraghavan et al., 2007). If working memory deficits in the mood spectrum are primarily state-related as has been suggested (Sweeney et al., 2000), it is unlikely that these impairments are markers of vulnerability in the mood spectrum. By comparison, significant sustained attention deficits were robust in BP-Offspring and marginal in SCZ-Offspring relative to HC.
4.1 Schizophrenia- and Bipolar-Offspring – Divergent Findings
The bio-behavioral literature in schizophrenia and bipolar relatives and offspring is marked by significant variability. This variability in part reflects the inherent heterogeneity associated with study vulnerable, but healthy groups of subjects (Diwadkar et al., In Press). In general, structural MRI and fMRI studies convergently suggest disordered fronto-striatal structure and function in SCZ-Offspring. Reduced gray matter integrity in the frontal cortex appears to mediate working memory deficits (Diwadkar et al., 2006), and may be related to inefficient cortico-cortical interactions during working memory (Bakshi et al., In Press). Developmental dysmaturation of the prefrontal cortex associated with vulnerability to the illness (Lewis & Levitt, 2002) also appears to characterize working memory deficits in this population (Diwadkar et al., 2001; Seidman et al., 2006). If exaggerated loss of prefrontal gray matter characterizes the developmental phase of schizophrenia (Keshavan et al., 1994), deficits in spatial working memory would be a likely unique vulnerability marker in schizophrenia offspring. The heavy reliance of working memory at short time scales on the phasic activity of prefrontal dopaminergic neurons (Dreher & Burnod, 2002), and the relevance of prefrontal dopamine for schizophrenia pathology (Gao & Goldman-Rakic, 2003) point to the convergence between molecular and behavioral studies in the schizophrenia diathesis. Recent studies suggest evidence of these deficits in adolescent offspring of mothers with bipolar disorder but not in offspring of mothers with depression (Klimes-Dougan et al., 2006), and in pediatric bipolar populations (Bearden et al., 2007). However, extensive evidence is lacking, as is a lack of evidence of dorsal prefrontal pathology in BP disorder in general (Frangou et al., 2005; Gallelli et al., 2005; Singh et al., 2008).
Sustained attention is in particular linked to fronto-striatal function and structure, and disordered structural development of these structures has been extensively documented in ADHD (Castellanos et al., 1996; Shaw et al., 2007). The reports of striatal dysfunction in ADHD converge with investigations in both schizophrenia (Morey et al., 2005) and bipolar disorder (Strakowski et al., 2005). In general, attention deficits have emerged as an important genetic marker for schizophrenia pathophysiology (Cornblatt & Keilp, 1994) and studies have documented deficits in attention processing in schizophrenia offspring. Attention has also been proposed as an important target for high-risk studies in bipolar disorder (Correll et al., 2007), consistent with reports of attention-related problems in adolescent offspring of bipolar parents (Duffy et al., 2001), increased vulnerability to ADHD (Birmaher et al., In Press), and to the onset of disorders in the bipolar spectrum (Birmaher et al., 2009). The convergence with deficits in sub-cortical pathways in a model of pure attentional deficit, specifically ADHD, and the noted increased in vulnerability to ADHD in BP-Offspring suggest that impairments in cortico-striatal networks may be characteristic of the mood spectrum, and may be generally non-specific to a number of childhood psychiatric disorders and vulnerable populations. The weak effects we observed in SCZ-Offspring may reflect the frequently encountered heterogeneity in these subjects. Furthermore, purely behavioral metrics can greatly under-approximate group differences between neuro-circuitry function as has been demonstrated in recent fMRI studies of attention and working memory in SCZ-Offspring (Diwadkar et al., In Press; Bakshi et al., In Press).
Working memory and attention share overlapping neurocircuitry, but the implementation of function within these circuits differs. The maintenance and manipulation components associated with working memory are largely associated with dorsal prefrontal processing (Wager & Smith, 2003). A hierarchical organization of prefrontal function is evident in healthy subjects, and the disruption of this organization is observed in schizophrenia. For example, whereas increased proficiency in working memory is reflected in greater dorsal prefrontal engagement in healthy subjects, this pattern is absent in schizophrenia patients (Schneider et al., 2007; Tan et al., 2006). Recent studies of working memory related dysfunction of dorsal prefrontal cortex in bipolar disorder appear to affirm the idea that such dysfunction is related to emotional state in patients (Passarotti et al., In Press; Townsend et al., In Press), that this dysfunction does not particularly generalize to adult first-degree relatives (Thermenos et al., In Press), and that this dysfunction would not in fact generalize to vulnerable offspring.
The sample size of our current studies, while notable given the study design, is nevertheless limited compared to large multi-site studies. Additional limitations include the reliance on neuro-cognitive and therefore the absence of in vivo imaging data, and by the study’s cross-sectional design. Further elaboration will be unquestionably warranted. For instance, fMRI studies in adult relatives have been published, but are conspicuously limited in adolescent offspring, and therefore disordered patterns of brain function and functional brain development are not well understood in the period of vulnerability. Further, longitudinal studies will clarify whether these impairments are enduring or progressive, further elucidating whether trajectories of the development of fronto-striatal systems distinguish between vulnerability in schizophrenia and bipolar offspring.
5. Conclusions
The expansion of biological psychiatry as a discipline has placed increased scrutiny of the Kraepelinian dichotomy, and of its value as a fundamental tenet of psychiatric classification. Current psychiatric classification schemes such as DSM IV that are an extension of the Kraepelinian dichotomy, categorically distinguish between schizophrenia and bipolar disorder, assuming these disorders to be distinct entities with clearly differentiable aetiologies, symptoms and treatments. Emerging behavioral studies are beginning to elucidate shared and unique patterns of impairment in schizophrenia and bipolar disorder (Brambilla et al., In Press; Frangou et al., 2006). Furthermore, understanding disordered development and its contribution to the emergence of distinct psychiatric phenotypes is emerging as a central question in scientific psychiatry. Studies in adolescent offspring can contribute significant insights into developmental precursors of schizophrenia and bipolar disorder, and offer the promise of understanding how development and vulnerability interact in shaping the evolution of the Kraepelinian dichotomy (Curtis et al., 2000; Murray et al., 1992; Murray et al., 2004).
Highlights.
We examined memory and attention performance in adolescent offspring of schizophrenia or bipolar patients
Schizophrenia offspring were selective impaired at working memory; both groups performed worse during attention though only bipolar offspring were significantly impaired.
Data suggest unique and partially overlapping vulnerability markers in the schizophrenia and mood spectra.
Acknowledgements
This research was supported by NIH grants MH68680 (VAD), MH60952 (BB), MH45156 & MH45203 (MSK) , NARSAD (VAD) and the Children’s Research Center of Michigan (VAD). We thank Jean Miewald for data management, and Satish Iyengar and Mary Phillips for helpful discussions.
Abbreviations
- SCZ-Offspring
Offspring of patient with schizophrenia
- BP-Offspring
Offspring of patient with bipolar disorder
- HC
Healthy Control
- FSIQ
Full Scale Intelligence Quotient
- SCID
Structured Clinical Interview for Diagnostic Statistical Manual for Mental Disorders-IV
- K-SADS
Schedule for Affective Disorders and Schizophrenia -Child Version
- CPT-IP
Continuous Performance Task – Identical Pairs
- ADHD
Attention Deficit Hyperactivity Disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res. doi: 10.1016/j.jpsychires.2011.01.002. In Press. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Caetano S, Olvera RL, Fonseca M, Najt P, Hunter K, Pliszka SR, Soares JC. Evidence for disruption in prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disord. 2007;9 Suppl 1:145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Monk K, Kalas C, Obreja M, Hickey MB, Iyengar S, Brent D, Shamseddeen W, Diler R, Kupfer D. Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS) Am J Psychiatry. 167:321–330. doi: 10.1176/appi.ajp.2009.09070977. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Cerruti S, Bellani M, Ferro A, Marinelli V, Giusto D, Tomelleri L, Rambaldelli G, Tansella M, Diwadkar VA. Shared impairment in associative learning in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. doi: 10.1016/j.pnpbp.2011.03.007. In Press. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. Journal of Neurophysiology. 2000;83:1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. 2nd ed. Hillsdale, NJ: Laurence Erlbaum Associates; 1988. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Erlenmeyer-Kimling L. Attention and schizophrenia. Schizophr Res. 1989;2:58. [Google Scholar]
- Cornblatt B, Lenzenweger MF, Erlenmeyer-Kimling L. The Continuous Performance Test, Identical Pairs Version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Research. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am J Med Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Lencz T, Auther A, Smith CW, Malhotra AK, Kane JM, Cornblatt BA. Early identification and high-risk strategies for bipolar disorder. Bipolar Disord. 2007;9:324–338. doi: 10.1111/j.1399-5618.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- Curtis VA, van Os J, Murray RM. The Kraepelinian dichotomy: evidence from developmental and neuroimaging studies. J Neuropsychiatry Clin Neurosci. 2000;12:398–405. doi: 10.1176/jnp.12.3.398. [DOI] [PubMed] [Google Scholar]
- Diwadkar V, Sweeney J, Boarts D, Montrose D, Keshavan M. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectrums. 2001;6(11):899–903. doi: 10.1017/s109285290000095x. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Segel J, Pruitt P, Murphy ER, Keshavan MS, Radwan J, Rajan U, Zajac-Benitez C. Hypo-activation in the executive core of the sustained attention network in adolescent offspring of schizophrenia patients mediated by pre-morbid functional deficits. J Psychiatric Research Neuroimaging. doi: 10.1016/j.pscychresns.2010.12.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Burnod Y. An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Netw. 2002;15:583–602. doi: 10.1016/s0893-6080(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Duffy A, Grof P, Kutcher S, Robertson C, Alda M. Measures of attention and hyperactivity symptoms in a high-risk sample of children of bipolar parents. J Affect Disord. 2001;67:159–165. doi: 10.1016/s0165-0327(01)00391-3. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons among means. Journal of the American Statistical Association. 1961;56:52–64. [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. J Cogn Neurosci. 2007;19:750–760. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Hilldoff-Adamo UH, Rock D, Roberts SA, Bassett AS, Squires-Wheeler E, Cornblatt BA, Endicott JJ, Pape S, Gottesman II. The New York High-Risk Project. Prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Arch Gen Psychiatry. 1997;54:1096–1102. doi: 10.1001/archpsyc.1997.01830240052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MD, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM-IV Axis II personality disorders. New York: Biometrics Research Department, NYSPI; 1997. [Google Scholar]
- Frangou S, Dakhil N, Landau S, Kumari V. Fronto-temporal function may distinguish bipolar disorder from schizophrenia. Bipolar Disord. 2006;8:47–55. doi: 10.1111/j.1399-5618.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry. 2005;58:838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. 2nd edition. New York: Raven Press; 1989. [Google Scholar]
- Gallelli KA, Wagner CM, Karchemskiy A, Howe M, Spielman D, Reiss A, Chang KD. N-acetylaspartate levels in bipolar offspring with and at high-risk for bipolar disorder. Bipolar Disord. 2005;7:589–597. doi: 10.1111/j.1399-5618.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Goldman-Rakic PS. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci U S A. 2003;100:2836–2841. doi: 10.1073/pnas.262796399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disord. 2006;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry & Clinical Neuroscience. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gore CD, Banyai M, Gray PJ, Diwadkar V, Erdi P. Pathological effects of cortical architecture on working memory in schizophrenia. Pharmacopsychiatry. 2010;43 Suppl 1:S92–S97. doi: 10.1055/s-0030-1251979. [DOI] [PubMed] [Google Scholar]
- Graham S, Phua E, Soon CS, Oh T, Au C, Shuter B, Wang SC, Yeh IB. Role of medial cortical, hippocampal and striatal interactions during cognitive set-shifting. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M. Neural activities in the monkey basal ganglia related to attention, memory and anticipation. Brain Dev. 1986;8:454–461. doi: 10.1016/s0387-7604(86)80069-9. [DOI] [PubMed] [Google Scholar]
- Hillegers MH, Reichart CG, Wals M, Verhulst FC, Ormel J, Nolen WA. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord. 2005;7:344–350. doi: 10.1111/j.1399-5618.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar V, Rosenberg DR. Developmental biomarkers in schizophrenia and other psychiatric disorders: common origins, different trajectories? Epidemiol Psichiatr Soc. 2005a;14:188–193. doi: 10.1017/s1121189x00007934. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophr Res. 2005b;79:45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3:163–168. [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Ronsaville D, Wiggs EA, Martinez PE. Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry. 2006;60:957–965. doi: 10.1016/j.biopsych.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Lapalme M, Hodgins S, LaRoche C. Children of parents with bipolar disorder: a metaanalysis of risk for mental disorders. Can J Psychiatry. 1997;42:623–631. doi: 10.1177/070674379704200609. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, Best JJ, Owens DG, Johnstone EC. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: Insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annual Review of Neuroscience. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Michie PT, Kent A, Stienstra R, Castine R, Johnston J, Dedman K, Wichmann H, Box J, Rock D, Rutherford E, Jablensky A, Todd J, Budd TW, Jablensky AV. Phenotypic markers as risk factors in schizophrenia: neurocognitive functions. Aust N Z J Psychiatry. 2000;34 Suppl:S74–S85. doi: 10.1080/000486700226. [DOI] [PubMed] [Google Scholar]
- Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SC, Simmons A, Giles N, Lloyd AJ, Harrison CL, Seal M, Murray RM, Ferrier IN, Young AH, Curtis VA. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, O'Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Report on a continuous performance test. Arch Gen Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage. 2004;21:840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, Zilles K, Braus DF, Schmitt A, Schlosser R, Wagner M, Frommann I, Kircher T, Rapp A, Meisenzahl E, Ufer S, Ruhrmann S, Thienel R, Sauer H, Henn FA, Gaebel W. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res. 2007;89:198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Smith CW, Stone WS, Glatt SJ, Meyer E, Faraone SV, Tsuang MT, Cornblatt B. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32:507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Delbello MP, Adler CM, Stanford KE, Strakowski SM. Neuroanatomical characterization of child offspring of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2008;47:526–531. doi: 10.1097/CHI.0b013e318167655a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. Chicago, IL: SPSS Inc; 2007. [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Goldstein JM, Milanovic SM, Whitfield-Gabrieli S, Makris N, Laviolette P, Koch JK, Faraone SV, Tsuang MT, Buka SL, Seidman LJ. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 153B:120–131. doi: 10.1002/ajmg.b.30964. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 182:22–29. doi: 10.1016/j.pscychresns.2009.11.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]