Abstract
Three cases of recurrent pleuropericarditis were observed within the same family – in two sisters and their niece, who were 18, 35 and 18 years of age, respectively. One patient was treated with pericardiectomy, and the other two were treated with colchicine. Mutations associated with autoinflammatory diseases (tumour necrosis factor receptor-associated periodic syndrome and familial Mediterranean fever) were absent; the condition was found to be sex linked.
Keywords: Genetics, Pericardial effusion, Pericarditis
Recurrent pericarditis occurs in 15% to 30% of patients with idiopathic acute pericarditis (1,2), and may be associated with pleural effusion. The pathogenesis of relapses is still unclear, and recurrences may persist over several years.
Treatment is often challenging because of frequent relapses of chest pain and poor efficacy of anti-inflammatory agents. A familial form of recurrent pericarditis has been reported by some studies. We describe three related female patients with recurrent pericarditis associated with pericardial and pleural effusions.
PATIENT 1
Patient 1 was admitted to hospital in 1979, with acute dyspnea, fever and cough; she was 18 years of age. Her chest x-ray revealed pleural and pericardial effusions, and she underwent a thoracentesis and treatment with nonsteroidal anti-inflammatory drugs. After a few months, she suffered a relapse with fever and chest pain; a large pericardial effusion with echocardiographic signs of cardiac tamponade were present, and pericardiocentesis was performed with aspiration of 1500 mL of pericardial fluid, followed by intracavitary administration of anti-inflammatory agents (methylprednisolone 40 mg). Cytological and microbiological examination and cultures of the pericardial fluid were negative, and the patient’s protein level was 4.5 g/100 mL. Anti-tuberculosis treatment was started empirically.
In early 1980, a second relapse occurred with fever, chest pain and a large pericardial effusion. Serological markers of autoimmune diseases and streptococcal infection were repeatedly negative. In June 1980, the patient was admitted to hospital for persistent pericardial effusion; all of her laboratory test results were within normal limits.
In August 1980, she suffered from a persistent cough; the pericardial effusion was still present, although it was slightly reduced compared with previous echocardiographic examinations.
In December 1980, the patient underwent pericardiocentesis of 10 mL of pericardial fluid; the only anomalous laboratory findings were elevated levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Treatment with acetylsalicylic acid 2400 mg/day was initiated.
In 1981, the patient underwent partial pericardiectomy due to persistence of a large pericardial effusion and intermittent fever associated with elevated ESR and CRP levels, despite continuous treatment with antiphlogistic and antituberculosis agents; the histological examination of the pericardium revealed nonspecific signs of inflammation. No further relapses occurred thereafter.
PATIENT 2 (NIECE OF PATIENTS 1 AND 3)
In 1997, patient 2 suffered a chest trauma. She was first admitted to hospital in April 1999 at 18 years of age, due to a chance finding of an enlarged cardiac silhouette. An echocardiogram revealed a large pericardial effusion without signs of cardiac tamponade. Serological markers of autoimmune diseases and of streptococcal infection were negative; ESR and CRP levels were normal. Chest computed tomography and a tine test were also negative. Treatment with prednisone 50 mg daily was initiated.
Two months later, her chest pain, fever, pericardial and bilateral pleural effusions resumed after reduction of the antiphlogistic drugs, and the patient’s ESR and CRP levels were elevated. She underwent pericardiocentesis with drainage of 1100 mL of pericardial fluid; cytological and microbiological examinations of the fluid were negative. Antituberculosis chemotherapy was initiated without significant benefit, and was discontinued in March 2000. Episodes of chest pain and fever continued until 2005, when a prolonged course of colchicine treatment was initiated (discontinued in 2008). No further relapses were recorded in follow-up visits. Her ocular pressure was normal.
PATIENT 3 (SISTER OF PATIENT 1)
The patient was first admitted to hospital in 2006 at 35 years of age for cardiac tamponade after experiencing a few days of fever, sore throat and chest pain. The patient underwent emergency pericardiocentesis, and 2000 mL of pericardial exudate was aspirated. Despite treatment with nonsteroidal anti-inflammatory agents and antibiotic therapy, a large pericardial effusion recurred after two days. Pericardiocentesis was repeated with aspiration of 1100 mL of pericardial fluid; continuous 48 h pericardial drainage was performed. Thoracentesis and evacuation of 650 mL of pleural exudate was also performed. ESR and CRP levels were elevated; serological evaluation and cytological examination of evacuated fluids were normal. A culture of pericardial fluid was positive for Staphylococcus epidermidis (one of three samples); cultures of pleural effusion and blood were negative for tuberculosis. Systemic treatment with steroidal anti-inflammatory agents (prednisone 50 mg/day) and antibiotics was initiated. Colchicine was added to the treatment regimen for relapse of pericardial-pleural effusions. Persistence of mild-to-moderate pleuropericardial effusion was recorded during follow-up. Steroidal treatment was discontinued in March 2006, while treatment with colchicine was discontinued in February 2009, on complete resolution of residual pericardial effusion detected by echocardiography. The patient remains recurrence free. In this case as well, intraocular pressure was normal. The three patients are linked by close kinship: patients 1 and 3 are sisters, and aunts of patient 2. Table 1 describes the clinical characteristics of the three patients.
TABLE 1.
Clinical characteristics of patients and treatments
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age of first hospitalization | 18 years | 18 years | 35 years |
| Kinship | Aunt of patient 2 and sister of patient 3 | Niece of patients 1 and 3 | Sister of patient 1 |
| Pleural effusion | Yes | Yes | Yes |
| Pericardial effusion | Yes | Yes | Yes |
| Number of relapses of pericardial effusion | 4 | 2 | 2 |
| Pericardiocentesis | Yes | Yes | Yes |
| Pericardiectomy | Yes | No | No |
| Anti-inflammatory therapy | Yes | Yes | Yes |
| Antituberculosis therapy | Yes | Yes | Yes |
| Colchicine therapy | No | Yes | Yes |
After providing written consent for genetic testing, all three patients were tested for mutations in the TNFRSF1A gene (Exons 2–4,6), which is responsible for autosomal dominant tumour necrosis factor receptor-associated periodic syndrome (TRAPS), and in the MEFV gene (Exons 2,3,10), which is responsible for autosomal recessive familial Mediterranean fever (FMF), where the majority of known mutations are found (3). No mutations were found in patient 1 or in patient 3, whereas patient 2 harboured a heterozygous K695R mutation in the MEFV gene (exon 10).
DISCUSSION
Recurrent pericarditis is most frequently of idiopathic, postpericardiotomic or postinfarction origin (4). The present article reports on a severe form of recurrent pericarditis, with a possible genetic x-linked association.
We recorded many relapses of exudative pericarditis in each of our patients that were frequently associated with pleural effusions. A broad spectrum of anti-inflammatory agents proved ineffective in preventing recurrences and treating acute episodes, with the exception of colchicine, which was fairly effective in cases 2 and 3; case 1 required pericardiectomy.
Common causes of pericarditis were excluded in each patient through thorough clinical, serological and pathological evaluation. During acute phases, elevated systemic inflammation markers (eg, ESR and CRP levels) and fever were invariably present, while the association with pleural effusion was almost always present. Searches for autoimmune disease markers were always negative. Pleural and pericardial fluids drained from each patient in different phases of their history showed high protein concentrations (exudate); cytological and microbiological evaluations were negative for cancer or infections. Intracavitary and systemic treatment with corticosteroids had low efficacy when administered alone, while association with systemic colchicine was effective.
Some familial clustering of recurrent pericarditis was found by Genecin (5) who reported the presence of effusive pericarditis in two siblings. In one sibling, the evaluation of pericardial fluid revealed a typical macroscopical aspect called ‘gold paint’, with a high level of cholesterol. This finding was not present in our patients.
A family cluster of pericarditis was reported by De Line and Cable (6). Recurrent pericarditis with pericardial effusion was diagnosed in three male and two female patients, and an idiopathic etiology was assigned to each case; no other serositis was reported. Two of the patients developed pericardial constriction and underwent pericardiectomy or creation of a pericardial window. Erdol et al (7) described a family in which three female members had idiopathic chronic pericarditis that was unresponsive to anti-inflammatory agents and associated with glaucoma. The authors suggested a sex-linked inheritance. In a series of 60 Caucasian patients affected by recurrent idiopathic pericarditis, Brucato and Brambilla (8) identified six patients who had at least one relative with a confirmed diagnosis of acute pericarditis. Three of six patients had recurrent pericarditis, and these data suggest a genetic predisposition in some cases of recurrent pericarditis.
Because recurrent pericarditis is characterized by periodic recurrences alternating with symptom-free intervals during which acute-phase reactants normalize, increasing attention is currently being devoted to autoinflammatory disorders – a group of genetic diseases caused by a primary dysfunction of the innate immune system and characterized by spontaneously relapsing and remitting bouts of systemic inflammation (9).
Among autoinflammatory disorders, recurrent pericarditis, usually in the form of polyserositis, is a common feature both of TRAPS (10), caused by mutations in the TNFRSF1A gene encoding the 55 kD receptor for tumour necrosis factor-alpha, and of FMF, caused by mutations in the MEFV gene, encoding for the pyrin protein (11,12).
Autoinflammatory disorders may be responsible for isolated (13–15) and familial cases of recurrent pericarditis (16). We investigated the presence of FMF in three series of Italian patients with recurrent pericarditis; mutations in the MEFV gene were searched in 23, 61 and 30 patients, respectively; however, no patients with FMF were discovered (13,17,18).
On the other hand, we recently reported patients with mutations in the TNFRSF1A gene, presenting with recurrent pericarditis as the sole clinical manifestation (13,14,16).
In addition, we proposed criteria (positive family history and poor response to colchicine) for identifying, among patients with recurrent pericarditis, those who should undergo testing for genetic mutations of the TNFRSF1A gene that would reveal TRAPS (13,16).
In contrast, despite the familial clustering of pericarditis, none of the three patients who were reported in the present study harboured mutations in the TNFRSF1A gene. Additionally, the fairly positive response to colchicine also suggested a possible association with a mutation in the MEFV gene; however, on investigation, none of these patients had FMF.
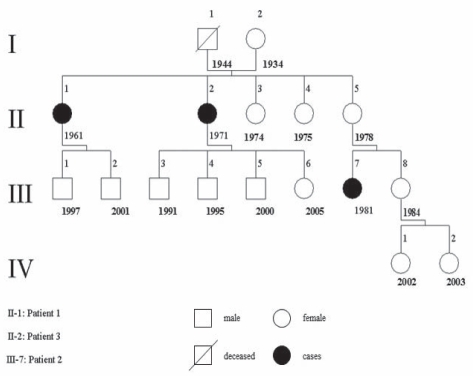
In the family tree of our cases (Figure 1), all of the affected subjects were female (II 1, II 2 and III 7); thus suggesting an x-linked dominant inheritance. Such an event is rare, as reported by Erdol et al (7). On the other hand, disease-free male subjects in the family tree were too young to exclude a predisposition to develop symptoms later in life.
Figure 1).
Family pedigree. Affected individuals are numbered for each level with birth year indicated
Alternative inheritance patterns are plausible, such as an autosomal dominant incomplete penetrance mechanism, in which female patients are the casualty (in the second generation, all five subjects were female). Another alternative is poligenic inheritance (6), in which several cases of a noninfrequent disease are recorded in subsequent generations of the same family, as occurs in bilateral congenital clubfoot syndrome (XXX).
In our cluster of patients, genetic transmission of the disease is plausible for three main reasons: severity of the disease, occurrence in two different generations and prevalence in female subjects.
Given that the three female patients presented in our report had recurrent pericarditis that was idiopathic in origin, because etiological evaluations were invariably negative, an x-linked dominant genetic predisposition to inflammatory pericarditis cannot be excluded.
SUMMARY
We report a cluster of familial recurrent pleuropericarditis, not associated with the mutations related to known autoinflammatory conditions, where an x-linked transmission cannot be excluded.
REFERENCES
- 1.Soler-Soler J, Sagrista-Sauleda J, Permanyer-Miralda G. Relapsing pericarditis. Heart. 2004;90:1364–8. doi: 10.1136/hrt.2003.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisch B, Saferovic PM, Ristik AD, the Task Force on the Diagnosis and Management of Pericardial Disease of the European Society of Cardiology Guidelines on the diagnosis and management of pericardial disease. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Touitou I, Lesage S, McDermott M, et al. Infevers: An evolving mutation database for auto-inflammatory syndromes. Human Mutation. 2004;24:194–8. doi: 10.1002/humu.20080. [DOI] [PubMed] [Google Scholar]
- 4.Tenembaum A, Koren-Moragb N, Brucato A, et al. The efficacy of colchicine in treatment of recurrent pericarditis related to postcardiac injury (postpericardiotomy and postinfarcted) syndrome: A multicenter analysis. Heart Drug. 2004;4:141–4. [Google Scholar]
- 5.Genecin A. Chronic pericardial effusion in brothers, with a note on “cholesterol pericarditis”. Am J Med. 1959;26:496–502. doi: 10.1016/0002-9343(59)90254-2. [DOI] [PubMed] [Google Scholar]
- 6.De Line JM, Cable DG. Clustering of recurrent pericarditis with effusion and constriction in a family. Mayo Clin Proc. 2002;77:39–43. doi: 10.4065/77.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Erdol C, Erdol H, Celik S, Baykan M, Gokce M. Idiopathic chronic pericarditis associated with ocular hypertension: Probably an unknown combination. Int J Cardiol. 2003;87:293–5. doi: 10.1016/s0167-5273(02)00351-0. [DOI] [PubMed] [Google Scholar]
- 8.Brucato A, Brambilla G. Recurrent idiopathic pericarditis: Familial occurrence. Int J Cardiol. 2005;102:529. doi: 10.1016/j.ijcard.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Cantarini L, Imazio M, Brucato A, Lucherini OM, Galeazzi M. Innate versus acquired immune response in the pathogenesis of recurrent idiopathic pericarditis. Autoimmun Rev. 2010;9:436–40. doi: 10.1016/j.autrev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Dodé C, André M, Bienvenu T, et al. French Hereditary: Recurrent Inflammatory Disorder Study Group The enlarging clinical, genetic, and population spectrum of tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2002;46:2181–8. doi: 10.1002/art.10429. [DOI] [PubMed] [Google Scholar]
- 11.Dabestani A, Noble LM, Child JS, Krivokapich J, Schwabe AD. Pericardial disease in familial Mediterranean fever: An echocardiographic study. Chest. 1982;81:592–5. doi: 10.1378/chest.81.5.592. [DOI] [PubMed] [Google Scholar]
- 12.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 13.Cantarini L, Lucherini OM, Cimaz R, et al. Idiopathic recurrent pericarditis refractory to colchicine treatment can reveal tumor necrosis factor receptor-associated periodic syndrome. Int J Immunopathol Pharmacol. 2009;22:1051–8. doi: 10.1177/039463200902200421. [DOI] [PubMed] [Google Scholar]
- 14.Cantarini L, Lucherini OM, Cimaz R, Galeazzi M. Recurrent pericarditis caused by a rare mutation in the TNFRSF1A gene and with excellent response to anakinra treatment. Clin Exp Rheumatol. 2010;28:802. [PubMed] [Google Scholar]
- 15.Tutar HE, Imamoglu A, Kendirli T, Akar E, Atalay S, Akar N. Isolated recurrent pericarditis in a patient with familial Mediterranean fever. Eur J Pediatr. 2001;160:264–5. doi: 10.1007/s004310000708. [DOI] [PubMed] [Google Scholar]
- 16.Cantarini L, Lucherini OM, Baldari CT, Laghi Pasini F, Galeazzi M. Familial clustering of recurrent pericarditis may disclose tumor necrosis factor receptor-associated periodic syndrome. Clin Exp Rheumatol. 2010;28:405–7. [PubMed] [Google Scholar]
- 17.Brucato A, Brambilla G, Moreo A, et al. Long-term outcomes in difficult to treat patients with recurrent pericarditis. Am J Cardiol. 2006;98:267–71. doi: 10.1016/j.amjcard.2006.01.086. [DOI] [PubMed] [Google Scholar]
- 18.Brucato A, Shiner Y, Brambilla G, et al. Idiopathic recurrent acute pericarditis: Familial Mediterranean fever mutations and disease evolution in a large cohort of Caucasian patients. Lupus. 2005;14:670–4. doi: 10.1191/0961203305lu2197oa. [DOI] [PubMed] [Google Scholar]