Abstract
The internal thoracic artery (ITA) is considered to be the optimal conduit for surgical coronary artery revascularization. Variations in ITA anatomy are relatively common and may impact surgical results. The ITA usually arises from the intrascalenic (first) part of the subclavian artery (SCA) and, occasionally, from the interscalenic (second) part. Origination from the extrascalenic (third) part of the SCA is rare, with a reported incidence rate of 0.5% to 1.0% in anatomical studies, and 1.5% in one angiographic study. Such an aberrant ITA descends inferomedially, anterior to the distal attachment of the scalenus anterior muscle, passes posterior to the first rib and enters the thorax, from where it follows its usual course. A patient with a five-year history of in situ grafting of the left ITA to the anterior inter-ventricular artery is presented. Coronary angiography performed because of anterior wall ischemia revealed an aberrantly arising ITA from the extras-calenic part of the SCA. The implications of this ITA variant with regard to bypass surgery, postoperative angiography and subclavian vein catheterization are discussed.
Keywords: Coronary artery bypass grafting, Internal thoracic artery, ITA angiography, Subclavian artery
The internal thoracic artery (ITA) is considered to be the optimal conduit for coronary artery revascularization due to its superior patency rates and better clinical outcomes relative to saphenous vein grafts. The long-term patency rate reported for the radial artery graft is comparable with the ITA graft patency rate (90.5% versus 96.3%, respectively, P=0.23) (1). Variations in ITA anatomy are known to influence surgical results; they may go undetected during surgery, potentially leading to ITA graft injury during harvesting, prolonged surgical times owing to the need for modification of the grafting strategy or continued postoperative ischemia. Bauer at al (2) reported a 26% (118 of 459) angiographic incidence of surgically significant ITA anomalies. Feit et al (3) reported an 11.5% (15 of 130) angiographic incidence of surgically significant left ITA (LITA) variants. The ITA usually arises from the intrascalenic (first) part of the subclavian artery (SCA) and, occasionally, from the interscalenic (second) part (4–8). Origination from the extrascalenic (third) part of the SCA is rare, with a reported incidence rate of 0.5% to 1.0% in anatomical studies, and 1.5% in one angiographic study (2,4–7). We present a patient with a five-year history of in situ grafting of the LITA to the anterior inter-ventricular artery (AIA), in whom angiography, because of anterior wall ischemia, showed an aberrantly originating LITA from the extrascalenic part of the SCA. We also discuss the significance of having such an ITA variant detected before surgery, and its relevance to postoperative angiography and subclavian vein catheterization.
CASE PRESENTATION
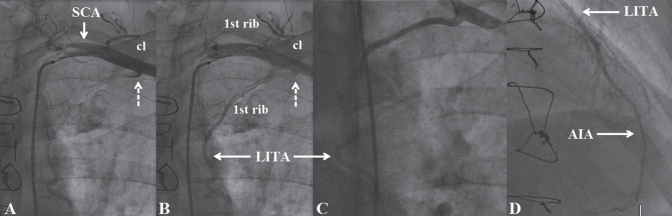
A 60-year-old man with a history of diabetes mellitus, arterial hypertension and dyslipidemia, who underwent bypass surgery five years previously, was referred for coronary angiography because of exercise-induced dyspnea and ischemia of the anterior and inferior walls demonstrated by a thallium stress test. During bypass surgery, the LITA, which was not visualized preoperatively, was placed to the AIA as an in situ graft; a radial artery was grafted to the second left marginal branch, while the occluded right coronary artery was considered to be unsuitable for grafting. Preoperatively, the left ventricle was diffusely hypokinetic, with an ejection fraction of 40%. Precatheterization clinical evaluation was unremarkable. Echocardiography revealed a left ventricular ejection fraction of 50% and hypokinesia of the inferior wall, while the electrocardiogram showed pathological Q waves in the inferior leads. On coronary angiography, the distal right coronary artery was faintly collateralized and the radial artery graft was patent. The LITA could not be engaged in its usual site of origin because, as shown in an SCA angiogram, it arose aberrantly from the extrascalenic part of the SCA (Figures 1A and 1B). The subsequently obtained selective LITA angiogram depicted a widely patent graft with brisk flow all the way down the AIA (Figure 1C). At first, the LITA graft coursed in a caudal and medial direction adjacent to the inner border of the first rib, inclining smoothly posterior to it before entering the thorax. The LITA lacked a lateral costal branch or a kink site, while neither the anastomotic site nor the downstream AIA contained stenosis (Figure 1D). Thus, optimization of medical therapy was recommended, and the patient was discharged home.
Figure 1).
A and B Left subclavian artery (SCA) angiogram (sequential frames) displaying the aberrantly originating left internal thoracic artery (LITA) from the extrascalenic (third) part of the SCA. The latter is confirmed because the LITA ostium (dashed arrow) is located lateral to the interscalenic (second) part of the SCA, which forms the highest part of the arch described by this vessel. Note: during its extrathoracic course, the LITA is located adjacent to the inner border of the first rib. The clavicle (cl) is also shown. C Selective LITA angiogram displaying a widely patent graft. D The grafted anterior interventricular artery (AIA) and the anastomotic site are shown to be free of stenosis
DISCUSSION
The SCA is anatomically divided into three parts by its relation to the scalenus anterior muscle. The intrascalenic (first) and interscalenic (second) parts are medial and posterior to this muscle, respectively, while the extrascalenic (third) part extends from the lateral margin of this muscle to the outer border of the first rib. The ITA usually originates directly from the inferior surface of the first part of the SCA and courses in a forward and medial direction toward the first rib, passing posterior to the first costal cartilage before entering the thorax (4–8). In an anatomical study by Henriquez-Pino et al (5), the LITA arose from the intrascalenic part of the SCA in 92% of cases, from the interscalenic part in 7% of cases and from the extrascalenic part in 1% of cases. In 96% and 4% of the cases, the right ITA (RITA) originated from the intrascalenic and interscalenic part of the SCA, respectively. Origination of the ITA from the extrascalenic part of the SCA was revealed by Daseler and Anson (9) in six (two LITAs and four RITAs) of 769 ITAs (0.78%) examined anatomically, while Krechowiecki et al (10) found only one such case among 200 ITAs (0.5%). Vorster et al (6) found only one RITA with such an aberrant origin among 120 ITAs (0.83%). Furthermore, Omar et al (7) presented a unique case of bilateral ITA origin from the extrascalenic part of the SCA.
Such a laterally arising ITA was revealed angiographically by Bauer et al (2) in four of 262 patients (1.5%) undergoing bypass surgery. It was used during surgery in all cases; however, all of the tissue surrounding the ITA was dissected to prevent graft traction and kinking, which could lead to impaired flow. Such a laterally arising ITA courses in a caudal and medial direction, anterior to the distal attachment of the scalenus anterior muscle, follows the inner border of the first rib for a short distance and then curves behind the first rib in a caudal direction to enter the thorax where it follows its usual trajectory (6,7). High mobilization of the usually arising ITAs is frequently performed to ensure a graft long enough for sequential grafting or to prevent graft tension by the inflated lung. In such cases, dissection at the thoracic inlet is discontinued slightly beyond the first rib when the anterior border of the subclavian vein is visualized (7). Because a laterally arising ITA courses obliquely at the level of the first rib, it may be easily injured due to high mobilization, without knowledge of its presence.
Aziz and Ramsdale (12) presented a case of laterally originating LITA that was demonstrated to be patent seven years after its grafting to the AIA. Similarly, the aberrant LITA graft presented by Chavez and Osborn (13) that arose from the junction of the SCA and aorta was demonstrated to be patent four years after surgery. However, Blackwell and Zolnick (14) presented an LITA case that was considered to be unsuitable for grafting because it arose from the extrascalenic part of the SCA and contained significant stenosis. They stated that if it had been used as an in situ graft, the risk of early graft failure or continued postoperative ischemia would have been high. The presence of an aberrantly arising ITA graft should be suspected if, during postoperative angiography, it cannot be engaged in its usual site of origin. Otherwise, it may be erroneously regarded as occluded, potentially leading to unnecessary referral for redo bypass surgery to gain survival benefit by revascularizing the AIA. Furthermore, proving the patency of an ITA graft to the AIA is important because it facilitates the performance of angioplasty in another vessel with lower risk.
Insertion of central venous catheters and pacemaker leads via the subclavian vein may rarely be complicated by inadvertent puncture of the ITA. This may lead to formation of a mediastinal and pleural hematoma, pseudoaneurysm or an arteriovenous fistula, and even death in cases in which the associated bleeding is severe. Furthermore, thrombotic occlusion and myocardial infarction due to inadvertent puncture of an in situ ITA graft to the AIA or its external compression by the pacemaker leads in the subclavian vein have been reported (12,13). A usually arising ITA is susceptible to such complications at the level of the inner border of the first costal cartilage where it is close to the subclavian vein. In contrast, a laterally arising ITA can be injured anywhere across its extrathoracic course. It may be injured at its ostium, which is located at the most superficial part of the SCA, and during its course at the inner border of the first rib, where it is found immediately inferior to the subclavian vein. Furthermore, because it courses in front of the scalenus anterior muscle, it may be easily compressed in case of hematoma formation.
Given the high long-term patency rates of in-situ LITA grafts to the AIA (88% at five years), significant disease was expected in the grafted AIA as the cause of exercise-induced anterior wall ischemia in our patient. Following failure to selectively engage the LITA at its usual site of origin, the fact that rest perfusion of the anterior wall was normal in the absence of AIA collateralization prompted us to search for an aberrant LITA. Unfortunately, operation notes were not available, hence no information can be provided regarding the harvesting technique used. Because abnormal coronary microvascular vasomotor responses due to endothelial dysfunction are known to cause myocardial perfusion defects in the absence of obstructive epicardial disease, anterior wall ischemia was attributed to impaired endothelium-dependent AIA flow reserve.
CONCLUSION
We present a case of a patent in situ LITA graft to the AIA that was shown to arise aberrantly from the extrascalenic part of the SCA five years postsurgery. Such an aberrant LITA should probably not be considered unsuitable for grafting solely based on its abnormal origin. During bypass surgery, such an LITA always requires high proximal mobilization to prevent graft kinking. Accordingly, finding a laterally arising ITA preoperatively facilitates proper and safe dissection with minimal risk of injuring this vessel at the level of the first rib. Furthermore, knowledge of this ITA variant facilitates avoiding the erroneous diagnosis of an occluded ITA graft during postoperative angiography, thereby aiding patient management.
REFERENCES
- 1.Nezic DG, Knezevic AM, Milojevic PS, et al. The fate of the radial artery conduit in coronary artery bypass grafting surgery. Eur J Cardiothorac Surg. 2006;30:341–6. doi: 10.1016/j.ejcts.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Bauer EP, Bino MC, von Segesser LK, Laske A, Turina MI. Internal mammary artery anomalies. Thorac Cardiovasc Surg. 1990;38:312–5. doi: 10.1055/s-2007-1014041. [DOI] [PubMed] [Google Scholar]
- 3.Feit A, Reddy CV, Cowley C, Ibrahim B, Zisbrod Z. Internal mammary artery angiography should be a routine component of diagnostic coronary angiography. Cathet Cardiovasc Diagn. 1992;25:85–90. doi: 10.1002/ccd.1810250202. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RA, Afifi AK, Miyauchi R. Internal Thoracic Artery. Illustrated Encyclopedia of Human Anatomic Variation: Opus II: Cardiovascular System. < http://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Images0300/0319.shtml> (Accessed on February 19, 2011).
- 5.Henriquez-Pino JA, Gomes WJ, Prates JC, Buffolo E. Surgical anatomy of the internal thoracic artery. Ann Thorac Surg. 1997;64:1041–5. doi: 10.1016/s0003-4975(97)00720-0. [DOI] [PubMed] [Google Scholar]
- 6.Vorster W, du Plooy PT, Meiring JH. Abnormal origin of internal thoracic and vertebral arteries. Clin Anat. 11:33–7. doi: 10.1002/(SICI)1098-2353(1998)11:1<33::AID-CA5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Omar Y, Lachman N, Satyapal KS. Bilateral origin of the internal thoracic artery from the third part of the subclavian artery: A case report. Surg Radiol Anat. 2001;23:127–9. doi: 10.1007/s00276-001-0127-6. [DOI] [PubMed] [Google Scholar]
- 8.Puri N, Gupta, Mahant TS, Puri D. Bilateral internal thoracic artery harvesting; anatomical variations to be considered. Ind J Thorac Cardiovasc Surg. 2007;23:192–6. [Google Scholar]
- 9.Daseler EH, Anson BJ. Surgical anatomy of the subclavian artery and its branches. Surg Gynecol Obstet. 1959;108:149–74. [PubMed] [Google Scholar]
- 10.Krechowiecki A, Bohdan D, Wiechowski S. Variation of the internal thoracic artery. Folia Morphol (Warsz) 1973;32:173–84. [PubMed] [Google Scholar]
- 11.LoCicero J, 3rd, Hoyne WP, LoCicero MS, Cochard L, Sanders JH., Jr Anatomic variations of the phrenic nerve at the superior thoracic aperture (thoracic inlet): Implications for the cardiothoracic surgeon. Clin Anat. 1988;1:125–9. [Google Scholar]
- 12.Aziz S, Ramsdale DR. Anomalous left internal thoracic artery. J Invasive Cardiol. 2003;15:657–8. [PubMed] [Google Scholar]
- 13.Chavez J, Osborn LA. Anomalous origin of left internal mammary artery from the lateral junction of the left subclavian artery and aorta. Cathet Cardiovasc Diagn. 1996;37:168–9. doi: 10.1002/(SICI)1097-0304(199602)37:2<168::AID-CCD14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell RA, Zolnick MR. Anomalous origin of left internal mammary artery. Tex Heart Inst J. 2007;34:494–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TM, Chair KM, Jim MH, Boncutter A, Milechman G. Acute occlusion of left internal mammary artery graft during dual-chamber pacemaker implantation. Catheter Cardiovasc Interv. 2000;51:65–8. doi: 10.1002/1522-726x(200009)51:1<65::aid-ccd15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.García-Bolao I, Macías A, Moreno J, Martín A. Fatal left internal mammary artery graft to subclavian vein fistula complicating dual-chamber pacemaker implantation. Europace. 2008;10:890–1. doi: 10.1093/europace/eun057. [DOI] [PubMed] [Google Scholar]