Abstract
BACKGROUND:
Tumour necrosis factor-alpha (TNF-α) has been reported to play an important role in ischemia reperfusion injury and ischemic preconditioning (IPC). However, its role is not completely understood. Recently, normothermic IPC (NIPC), hyperthermic IPC (HIPC), preconditioning (PC) with 17-beta estradiol (estrogen, E2) and E2 pretreatment were proven to be effective in reducing ischemia reperfusion injury.
OBJECTIVES:
To investigate the detrimental effects of TNF-α on the heart, and the protective effects of NIPC, HIPC, E2 PC and pretreatment on TNF-α-induced injury.
METHODS:
A Langendorff-perfused rat heart model was used for the present study. Hearts isolated from male rats were studied under eight different conditions (n=5 each): negative control; control treated with TNF-α without any further treatment; NIPC (preconditioned at 37°C); HIPC (preconditioned at 42°C); E2 PC; E2 pretreatment; normal, untreated hearts plus E2; or pretreated hearts perfused for 60 min with TNF-α and an E2-containing buffer.
RESULTS:
TNF-α treatment resulted in deterioration of heart function. HIPC offered better protection by significantly increasing left ventricular developed pressure (Pmax) and coronary flow (P<0.01), and by decreasing left ventricular end-diastolic pressure (P<0.01). NIPC or pretreatment of the hearts with E2 normalized left ventricular end-diastolic pressure, coronary flow and coronary vascular resistance (P<0.001); however, it did not normalize Pmax. The combination of E2 and HIPC did not show any synergetic protection; however, the addition of HIPC normalized Pmax (P<0.001).
CONCLUSIONS:
TNF-α treatment resulted in deterioration of heart hemodynamics, which were reversed by HIPC, E2 PC and pretreatment. The combination of these treatments did not add to the previously observed protection compared with when they were used individually.
Keywords: Estrogen; Ischemia and reperfusion; Preconditioning, TNF-α
Although reperfusion therapy is the gold standard for the survival of ischemic tissue and the prevention of further ischemic damage, it can also cause unavoidable damage to myocardial tissue by initiating a series of events involving both intracellular and extracellular mechanisms (1). Murry et al (2) were the pioneers in the field of preconditioning (PC). They introduced the idea that stimulating the heart before sustained ischemia can protect the cardiomyocytes from ischemia reperfusion (I/R) damage. Later, the same findings were reported by another study (3). Several methods of PC were shown to be effective in protecting the heart (4–10). However, the role of some pharmacological PC methods and the synergy in different PC methods are not completely understood. Furthermore, the role of some elements, such as tumour necrosis factor-alpha (TNF-α) in I/R damage or PC protection, is not clear. Several studies have shown TNF-α to be detrimental to the heart, contributing to myocardial dysfunction and cardiomyocyte death in myocardial I/R injury (11). I/R injury is reported to be associated with increased TNF-α expression (11). TNF-α was also reported to be a contributing factor in the deterioration observed in other heart diseases (12). It has been shown to be an apoptotic agent in many cell types (including those in the heart) (13). Other studies suggested that it may be a trigger for ischemic PC (IPC) protection (14,15). Therefore, the effects of TNF-α must be carefully examined to discriminate between its detrimental and protective roles.
The effects of 17-beta estradiol (E2) on the heart are far from being understood. Although it was shown to be protective against I/R damage (16), it was contradictorily proven to be detrimental in other studies (17). Recently, some studies (18,19) showed that pretreatment of the heart with E2 could protect against subsequent I/R damage. It was also shown that pretreatment with E2 can offer protection similar to that of IPC (20). Although the evidence is limited, the use of E2 in PC has shown significant protection against I/R injury (6). In 2006, Xu et al (10) reported that E2 modulates TNF-α expression and the expression of its receptors. Controversial results were also reported by another study (21). In the present study, we investigated the detrimental effects of TNF-α on the heart and the salvage action of normothermic IPC (NIPC), hyperthermic IPC (HIPC) and E2 PC. We also evaluated the protective effect of combining two methods – HIPC and E2 PC or E2 pretreatment – against TNF-α-associated damage.
PATIENTS AND METHODS
The study was performed according to laboratory animal care guidelines of Kuwait University (Kuwait). Animal handling and treatment were performed in accordance with the international standards for animal use. Hearts were obtained from male Sprague-Dawley rats weighing between 300 g and 400 g. The animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and anticoagulated with heparin (1000 U/kg body weight) through the femoral vein. The hearts were attached to a perfusion apparatus, as described previously by Ghadhanfar and Juggi (22). The hearts were briefly perfused in retrograde fashion with freshly prepared Krebs-Henseleit solution. The buffer was oxygenated with a mixture of O2 (95%) and CO2 (5%), pH 7.35 to 7.45, at a temperature of 37.0±0.5°C. An alcohol-filled latex balloon was inserted through the left atrium into the left ventricle. This balloon was attached to a pressure transducer, which converted the mechanical pressure energy into electrical signals that were amplified by a DC bridge amplifier interfaced with a personal computer for online monitoring of left ventricular pressure and its derivatives. Under basal control conditions, the left ventricular end-diastolic pressure (LVEDP) was kept constant at 5 mmHg by suitably adjusting the volume of the balloon with 50% alcohol. Left ventricular developed pressure (Pmax) was derived from the online acquisition of left ventricular systolic pressure by Max-Min module (Number MMM type 668 [Hugo Sachs Elektronik – Harvard Apparatus GmbH, Germany]). This module converted the output from the DC bridge amplifier to Pmax by subtracting LVEDP from the maximal left ventricular systolic pressure.
The perfusion pressure (PP) was kept constant at 50 mmHg throughout the experimental procedure in all protocols. PP was measured immediately downstream from the flow probe from a branch of the aortic canula using a statham pressure transducer (P23 Db). Constant PP was ensured electronically by means of the perfusion assembly (Module PPCM type 671 [Hugo Sachs Elektronik – Harvard Apparatus GmbH, Germany]). This system permits an accurate adjustment of PP of between 5 mmHg and 150 mmHg, with an accuracy of ±1 mmHg. The temperature of the perfusion medium was maintained at 37°C by a temperature-controlled circulating water bath (RMS Lauda [Lauda Dr R Wobser GMBH & Co, Germany] and Techne Circulator [Cole-Parmer Instrument Company, USA]). This temperature was the baseline control perfusion temperature for all protocols in the study. Myocardial temperature was monitored by a needle thermistor probe (Thermalert TH-5, Physitemp Instruments Inc, USA), which was inserted at the apex of the heart for each study protocol.
Experimental protocol
Unprotected perfusion with TNF-α:
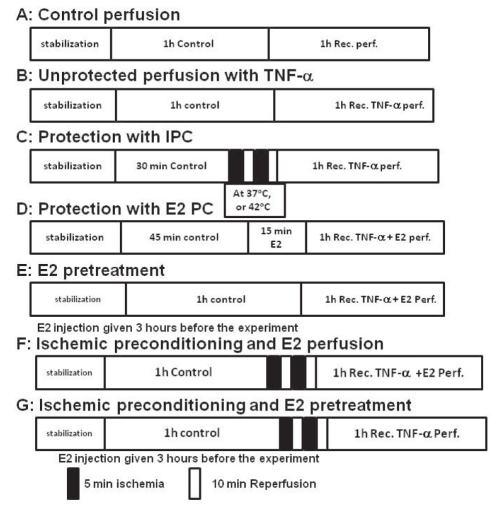
A perfusion solution containing 20 ng/mL TNF-α (Sigma-Aldrich Inc, USA) was used for each experiment (Figure 1). After initial stabilization with nonrecirculating perfusion, the hearts (n=5) were perfused for 60 min at 37°C with Krebs-Henseleit buffer. At the end of the 60 min reperfusion period, left ventricle and coronary hemodynamics (Pmax, LVEDP, coronary flow [CF] and coronary vascular resistance [CVR]) were recorded. Thereafter, the hearts were switched to either the perfusion medium containing 20 ng/mL of TNF-α for 60 min or the control perfusion (without TNF-α) (Figure 1, protocols A and B). During this period, left ventricle and coronary hemodynamics were continuously monitored.
Figure 1).
A, B, C, D and E protocols were used for studies with tumour necrosis factor-alpha (TNF-α) perfusion (perf) and protection with ischemic preconditioning (IPC) and 17-beta estradiol (E2). Protocols F and G were used for combined protective studies with hyperthermic IPC and E2 perf and pretreatment. Rec Recirculating
Protection with IPC:
Hearts (n=5 per group) were exposed to PC temperatures of 37°C and 42°C, respectively, before beginning 1 h recirculation of TNF-α. PC for each temperature was achieved by two episodes of 5 min ischemia at the required temperature followed by 10 min reperfusion at 37°C (Figure 1, protocol C). To attain the required temperature during PC periods, the heart was immersed in a temperature-controlled jacketed bath filled with isotonic saline. These preconditioned hearts were subsequently subjected to 1 h of perfusion with 20 ng/mL TNF-α (Figure 1C). Hemodynamic recovery was monitored and recorded during the recirculation period.
Protection with E2 PC:
After the initial stabilization period (60 min at 37°C), the stabilized hearts (n=5 per group) were perfused with 0.7 ng/mL E2 (dissolved in 50% ethanol) for 15 min. All of the hearts were subsequently subjected to 1 h perfusion with TNF-α (20 ng/mL) and E2 (0.7 ng/mL) (Figure 1, protocol D). Hemodynamic parameters were monitored during stabilization and the 1 h treatment periods.
E2 pretreatment:
Rats (n=5) were given intramuscular injections of 250 μg/kg body weight of E2 3 h before they were sacrificed. The hearts were excised and stabilized through perfusion in a nonrecirculating mode at 37°C for 60 min. The stabilized hearts were subsequently subjected to 1 h of 20 ng/mL TNF-α perfusion (Figure 1, protocol E). Hemodynamic parameters were monitored and recorded during the stabilization and treatment periods.
Combined protection with HIPC and E2 PC:
The isolated hearts (n=5 per group) were stabilized for 60 min and subjected to IPC – two episodes of 5 min ischemia each, separated by 10 min of reperfusion at 42°C. The hearts were preconditioned with 0.7 ng/mL of E2 at the beginning of HIPC and followed by 1 h of 20 ng/mL TNF-α perfusion (Figure 1, protocol F). Hemodynamics were monitored and recorded during the stabilization and the intervention periods. In a second group, intramuscular injections of E2 (250 μg/kg body weight) were given 3 h before the rats were sacrificed. The hearts were excised and stabilized with 60 min of perfusion. The hearts (n=5 per group) were preconditioned with two episodes of 5 min ischemia at 42°C, and 10 min of reperfusion at 37°C. This was followed by 1 h of TNF-α (20 ng/mL) perfusion (Figure 1, protocol G). Hemodynamics were monitored and recorded during the stabilization and intervention periods.
Statistical analysis
The results were expressed as mean ± SEM for n values. For calculation of the mean values, a Microsoft Excel (Miscrosoft Corporation, USA) spreadsheet for Windows (Miscrosoft Corporation, USA) was used. ANOVA was used, and post hoc analysis of differences between two group means was performed using the unpaired t test (two tailed). The level of difference between a group mean and that of its control was calculated using the paired Student’s t test. Differences were considered to be significant at P<0.05.
RESULTS
Hearts were isolated from the rats and were subjected only to TNF-α treatment, without any further intervention. Another subset was subjected to NIPC, HIPC, E2 PC or E2 pretreatment. A third subset was treated with a combination of HIPC and E2 PC or E2 pretreatment (Figure 1).
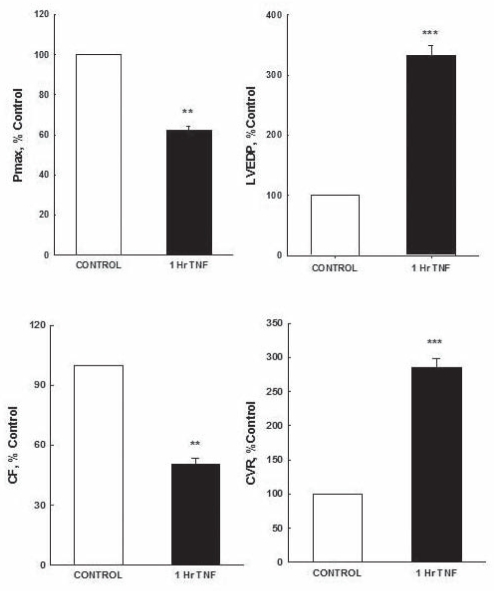
Regarding ventricular contractility, TNF-α treatment resulted in deteriorated Pmax and LVEDP. The postperfusion recovery in Pmax in the TNF-α-treated hearts was significantly lower than that of the control hearts (58±5.4 mmHg and 93±6.6 mmHg; P<0.01, respectively). After 1 h perfusion with TNF-α, LVEDP significantly (P<0.001) increased threefold (21±0.6 mmHg) compared with control (6.3±0.5 mmHg). Conversely, coronary vascular dynamics were negatively affected by TNF-α treatment. This treatment resulted in a significant (P<0.001) decrease in CF of approximately 4.2±0.5 mL/min compared with control (8.3±0.5 ml/min). CVR was significantly (P<0.001) increased, reaching 20±0.5 mmHg/mL/min compared with control (7±1.5 mmHg/mL/min) (Figure 2).
Figure 2).
Recovery in myocardial contractility (maximum developed pressure [Pmax] and left ventricular end-diastolic pressure [LVEDP]) and coronary vascular function (coronary flow [CF] and coronary vascular resistance [CVR]). Data were computed at 60 min recirculation and expressed as mean ± SEM. **P<0.01; ***P<0.001 when compared with respective controls. Control 1 h control perfusion; 1 h TNF-α 1 h of recirculating tumour necrosis factor-alpha perfusion
Protective role of HIPC
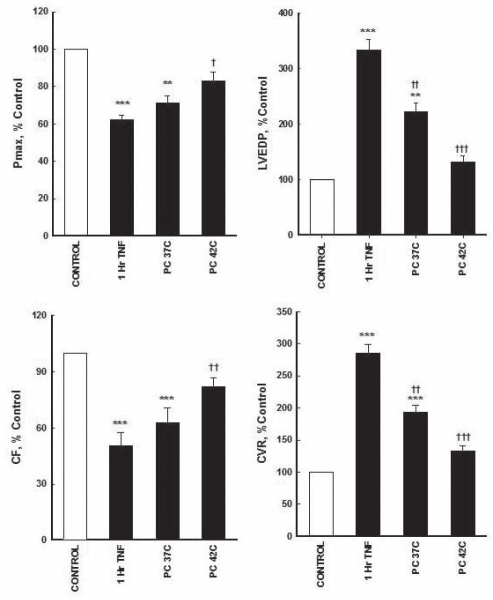
NIPC did not show any significant difference in Pmax compared with TNF-α-treated hearts (66±6 mmHg and 58±5.4 mmHg, respectively). However, HIPC (42°C) resulted in a significant (P<0.05) recovery of Pmax compared with nonprotected TNF-α-treated hearts (77±5 mmHg and 58±5.4 mmHg, respectively); although, it was still significantly lower than untreated controls. There was a significant (P<0.01) threefold increase in LVEDP in unprotected TNF-α-treated hearts and untreated controls (21±0.6 mmHg and 6.3±0.5 mmHg, respectively). NIPC and HIPC significantly reduced this LVEDP increase (14±0.5 mmHg, P<0.001; and 8.3±0.6 mmHg, P<0.001, respectively) compared with unprotected TNF-α-treated hearts (21±0.6 mmHg) (Figure 3).
Figure 3).
Effect of ischemic preconditioning (PC) on tumour necrosis factor-alpha (TNF-α)-mediated depressant effects on left ventricular contractility and coronary vascular hemodynamics. Data were computed at 60 min recirculation and expressed as mean ± SEM. **P<0.01; ***P<0.001 when compared with control; †P<0.05; ††P<0.01; †††P<0.001 compared with 1 h TNF-α perfusion. 1 h TNF-α 1 h of recirculating TNF-α perfusion; CF Coronary flow; Control 1 h of control perfusion; CVR Coronary vascular resistance; LVEDP Left ventricular end-diastolic pressure; Pmax Maximum developed pressure
TNF-α treatment resulted in a significant (P<0.001) decrease in CF (4.2±0.5 mL/min) compared with untreated control (8.3±0.5). Furthermore, the treatment with HIPC significantly (P<0.01) increased CF (6.8±0.7 mL/min) compared with TNF-α treatment (4.2±0.5) (Figure 3). Changes in CF were associated with a considerable increase in CVR (20±0.5 mmHg/mL/min) after TNF-α treatment compared with untreated controls (7±1.5 mmHg/mL/min). Protection of the hearts with NIPC and HIPC significantly decreased the increase caused by TNF-α treatment (13.5±2 mL/min, P<0.05; and 9.3±1 mL/min, P<0.001, respectively) (Figure 3).
Protective role of E2
Effects of E2 PC:
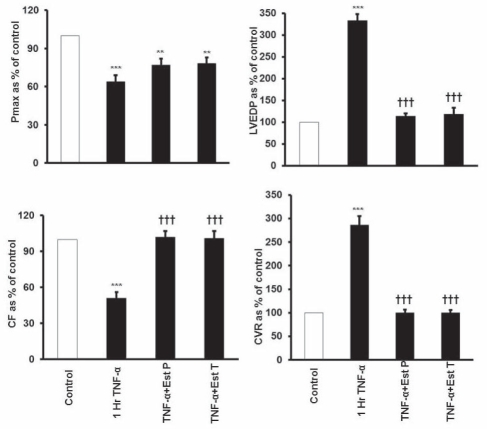
Treatment of the hearts with TNF-α resulted in maladaptation of ventricular contractility. Pmax was significantly (P<0.001) decreased compared with control (58±5.4 mmHg and 93±6.6 mmHg, respectively). Preconditioning the heart with E2 for 15 min before perfusion with TNF-α (E2 PC) showed improvement in Pmax compared with TNF-α-treated hearts (72±5 mmHg and 58±5.4 mmHg) (Figure 4). LVEDP was also negatively affected by TNF-α treatment because it resulted in a significant (P<0.001) increase in LVEDP compared with control (21±0.6 mmHg and 6.3±0.5 mmHg, respectively) (Figure 4). Cardiovascular dynamics also showed significant maladaptation with TNF-α treatment, which was abrogated by E2 PC. The significant increase in CVR (20±0.5 mmHg/mL/min, P<0.001) caused by TNF-α treatment was prevented by E2 PC and brought to normal control level (7±0.7 mmHg/mL/min) (Figure 4). E2 PC also blocked the significant decrease in CF caused by the addition of TNF-α (8.5±0.5 mL/min, P<0.001) compared with TNF-α treatment (4.2±0.5 mL/min) (Figure 4).
Figure 4).
Effect of 17-β estradiol (E2) on tumour necrosis factor-alpha (TNF-α)-mediated depressive effects on left ventricular contractility and coronary vascular hemodynamics. Data were computed at 60 min recirculation and expressed as mean ± SEM. **P<0.01; ***P<0.001 when compared with control; †††P<0.001 compared with 1 h TNF-α perfusion. 1 h TNF-α 1 h of recirculating TNF-α perfusion; CF Coronary flow; Control 1 h of control perfusion; CVR Coronary vascular resistance; LVEDP Left ventricular end diastolic pressure; Pmax Maximum developed pressure; TNF-α + Est P E2, 0.7 ng/mL of CF; TNF-α + Est T E2, 250 mg/kg rat weight, injected 3 h before the experiment
Effect of pretreatment with E2:
Pretreatment of the hearts with E2 3 h before starting the experiments showed results similar to that of E2 PC. Although the recovery of Pmax with E2 pretreatment was not significant, E2 pretreatment showed an improvement in its recovery compared with TNF-α treatment (72.5±5 mmHg and 58±5.4 mmHg, respectively) (Figure 4). Pretreatment with E2 significantly (P<0.001) reduced LVEDP compared with TNF-α treatment (7.4±1.5 mmHg and 21±0.6 mmHg, respectively) (Figure 4). Similar protection by E2 pretreatment was also shown in coronary vascular dynamics. TNF-α treatment significantly decreased CF compared with control (4.2±0.5 mL/min and 8.3±0.5 mL/min, P<0.001, respectively). This decrease was completely blocked by pretreatment with E2 (8.4±0.6 mL/min) compared with TNF-α treatment (4.2±0.5 mL/min) (Figure 4). E2 pretreatment also significantly (P<0.001) prevented the increase of CVR of 20±0.5 mmHg/mL/min caused by TNF-α treatment and resulted in CVR of 7±6 mmHg/mL/min (Figure 4).
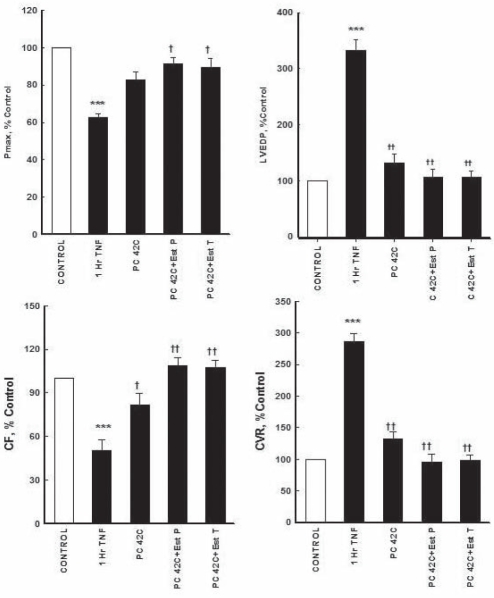
Effect of HIPC combined with E2 PC:
The combined effects of HIPC and E2 PC on TNF-α-mediated perturbation of myocardial functions are shown in Figure 5. The combination of HIPC and E2 PC significantly (P<0.01) normalized Pmax compared with TNF-α unprotected treatment (85±5 mmHg and 58±5.4 mmHg, respectively). There were no significant differences between the combined HIPC and E2 PC regimen, and the untreated control group. The combined regimen also significantly (P<0.001) decreased the large increase in LVEDP caused by the uncontrolled TNA-α treatment (21±0.6 mmHg to 6.7±0.4 mmHg). The TNF-α-mediated depression in CVR was prevented by the combined protective effect of HIPC and E2 PC. CF was normalized to 9±0.6 mL/min compared with TNF-α treatment (4.2±0.5, P<0.001). The combined regimen also significantly decrease CVR to 6.7±0.2 mmHg/mL/min (P<0.001) compared with TNF-α treatment (20±0.5 mmHg/mL/min) (Figure 5).
Figure 5).
Effects of combined treatment and pretreatment with 17-β estradiol (E2) and hyperthermic reconditioning (42°C) on myocardial and coronary vascular depressant effects of tumour necrosis factor-alpha (TNF-α) perfusion. Data were computed at 60 min recirculation and expressed as mean ± SEM. ***P<0.001 when compared with control; †P<0.05, ††P<0.01, †††P<0.001 compared with 1 h TNF-α reperfusion. 1 h TNF-α 1 h of recirculating TNF-α perfusion; CF Coronary flow; Control 1 h of control perfusion; CVR Coronary vascular resistance; LVEDP Left ventricular end-diastolic pressure; PC Ischemic preconditioning; PC42C+Est P Combination of PC at 42°C and perfused with E2, 0.7 ng/mL of coronary flow per kg body weight; PC 42C+Est T Ischemic preconditioned hearts pretreated with E2, 250 mg/kg rat weight, injected 3 h before the experiment; Pmax Maximum developed pressure
Effect of HIPC combined with E2 pretreatment:
Similar results were shown with combined HIPC and E2 pretreatment. Pmax was significantly (P<0.01) increased compared with TNF-α treatment (83±4 mmHg and 58±5.4 mmHg, respectively). The significant (P<0.001) rise in LVEDP after unprotected TNF-α treatment compared with untreated control (21±0.6 mmHg and 6.3±0.5 mmHg, respectively) was completely normalized (6.7±0.4 mmHg, P<0.001) by the combined HIPC and E2 pretreatment. The combination of HIPC and E2 pretreatment was associated with better maintenance of CF. CF was significantly (P<0.001) improved compared with TNF-α treatment (8.9±0.4 mL/min and 4.2±0.5 mL/min, respectively). CVR was also decreased significantly (P<0.00) compared with TNF-α treatment (6.9±0.43 mmHg/mL/min and 20±0.5 mmHg/mL/min, respectively) (Figure 5).
DISCUSSION
HIPC provided significant protection against TNF-α-induced deterioration of contractile and coronary vascular hemodynamics. The present study was the first to investigate the role of HIPC against the direct myocardial toxic effects of TNF-α. The cytokine TNF-α was reported by some studies (11,23) to be negatively involved in I/R injury. However, other studies (15,24) showed TNF-α to play an important role in the protection of the heart during PC. These results were opposed by findings from other studies (14,25), in which TNF-α added to I/R damage. Paradoxically, some studies (26,27) reported that doses of exogenous TNF-α can precondition the heart. However, other studies (28,29) reported that a decrease in cytokine levels is required for protection of the myocardium. There is also comprehensive experimental and clinical evidence that anti-inflammatory actions attenuate I/R injury (28,29). Our results are consistent with the findings that TNF-α plays a significant role in the malfunction following I/R injury because, in the present study, perfusion with TNF-α without HIPC or E2 treatment resulted in serious deterioration of hemodynamic functions of the heart. Other studies (30,31) also reported on the exacerbating effects of TNF-α on myocardial infarction and the depressive effects on hemodynamic functions. In these studies, the damage and the deterioration of hemodynamic function caused by TNF-α were comparable with that usually caused by I/R injury. However, in some studies, exogenous TNF-α has been shown to mimic IPC (14,15). Although this suggests a protective role for TNF-α in the heart, there was no study that investigated the mechanism of PC protection against TNF-α-induced damage. Some studies that examined TNF-α in other diseases suggested specific mechanisms for its deteriorative effects. One study (32) showed that TNF-α required the activation of P38 mitogen-activated protein kinase, which triggers nuclear factor-kappa B (NF-kB), leading to heart dysfunction (32). It was also reported that TNF-α increases cardiomyocyte apoptosis (11,33). Furthermore, TNF-α was shown to promote action potential prolongation (34), which was shown to be a serious factor contributing to the non-reversible damage of cardiomyocytes (35). In our study, the protective effects might be related to the activation of protein kinase C (PKC), which has been shown by several studies (36,37) to be crucial for PC protection. We can assume that the HIPC used in the present study, similar to any other method of IPC, activated PKC, which is known to mitigate the damaging effects of TNF-α (38). PC is known to prevent negative effects of NF-kB translocation. On the other hand, HIPC might also block NF-kB, which is an important element in TNF-α-induced apoptosis (39). Furthermore, IPC shortens the action potential of cardiomyocytes (40), which can oppose the action potential elongation effect of TNF-α-induced damage.
Protective role of E2
The protective effects of E2 against the direct depressive effects of TNF-α on myocardial function were independent of the route of administration of E2 (preconditioning or pretreatment). The administration of E2 fully abrogated the direct cardiodepressive effects of TNF-α on LVEDP and coronary dynamics (Figure 4); however, it did not significantly affect Pmax.
It has been documented that E2 prevents TNF-α-induced reduction in endothelial nitric oxide synthase, by decreasing or blocking its deteriorative effects (41). Dubey and Jackson (42) reported that metabolites of E2 inhibit endothelin-1 synthesis, leading to reduction of stress and strain damage at the cardiomyocyte and coronary vessel level. Moreover, it was shown that E2 produces endothelium-dependant relaxation of human coronary arteries (43). E2 reduces the production of inducible nitric oxide synthase (44) and blocks L-type calcium channels (45), which were known to be associated with TNF-α-induced injury. Interestingly, E2 can stimulate several elements that are involved in PC protection such as opening of both calcium-activated and ATP-sensitive potassium channels (46). It also activates PKC-dependent pathways that have protective effects against I/R damage (36,47). Xu et al (10) showed that E2 protection on the heart used a pathway that resulted in regulation of TNF-α. Our study showed similar results to their study, which to our knowledge, is the only one that investigated both the role of E2 and TNF-α in PC. Moreover, the protective role of E2 against ischemia was reported in many studies (19,48).
Protective effects of combined HIPC (42°C) and E2 against TNF-α-induced injury
The results of the present study showed total protection with the combined treatment of HIPC and E2 against the direct cardiotoxic effects of TNF-α perfusion; in addition, there was normalization of myocardial contractility and a large improvement in coronary dynamics after I/R injury. The most notable benefit of the combined treatment compared with HIPC alone was the normalization of Pmax during TNF-α infusion. E2 had no significant (14%) protective effect on myocardial contractility (Pmax) during TNF-α infusion. Significant additive protection (91%) was observed by combining HIPC with E2, when Pmax was not significantly different from control (100%). This suggests that E2 and HIPC improved Pmax during TNF-α infusion by different independent additive mechanisms such as minimizing calcium overload and increasing myofilament sensitivity. Although reports on synergy in PC are rather limited, synergy was shown between PC and the heat shock proteins in protecting cardiomyocytes after ischemia (49). Similar results were also shown by Honma et al (50). Our results showed that the combined treatment also improved coronary vascular functions after TNF-α infusion (CF 9 mL/min) compared with HIPC (CF 6.8 mL/min) alone.
Postischemic LVEDP was completely normalized (6.1 mmHg) using combined HIPC and E2 treatment and E2 treatment alone; however, it was not totally normalized with HIPC alone (7.7 mmHg). There was no significant additional benefit of combining HIPC with E2 compared with HIPC alone with regard to improvement in postischemic Pmax or CVR. Lack of presence of PC regimen synergy was also not uncommon (51).
CONCLUSIONS
TNF-α treatment resulted in deterioration of heart function, which was reversed using HIPC and E2 treatment. HIPC and E2 treatment provided significant protection against I/R injury when applied separately. The combination of these treatments did not add to the previously observed protection.
Acknowledgments
The present study was supported by the College of Graduate Studies of Kuwait University, Kuwait.
REFERENCES
- 1.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Kloner RA. The cardioprotective effects of ischemic ‘preconditioning’ are not mediated by adenosine receptors in rat hearts. Circulation. 1993;87:1642–8. doi: 10.1161/01.cir.87.5.1642. [DOI] [PubMed] [Google Scholar]
- 4.Gysembergh A, Margonari H, Loufoua J, et al. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol. 1998;274:H955–64. doi: 10.1152/ajpheart.1998.274.3.H955. [DOI] [PubMed] [Google Scholar]
- 5.Huang CH, Wang JS, Chiang SC, Wang YY, Lai ST, Weng ZC. Brief pressure overload of the left ventricle preconditions rabbit myocardium against infarction. Ann Thorac Surg. 2004;78:628–33. doi: 10.1016/j.athoracsur.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 6.Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K. Preconditioning by 17beta-estradiol in isolated rat heart depends on PI3-K/PKB pathway, PKC, and ROS. Am J Physiol Heart Circ Physiol. 2006;291:H1554–62. doi: 10.1152/ajpheart.01171.2005. [DOI] [PubMed] [Google Scholar]
- 7.Szilvassy Z, Ferdinandy P, Bor P, Jakab I, Lonovics J, Koltai M. Ventricular overdrive pacing-induced anti-ischemic effect: A conscious rabbit model of preconditioning. Am J Physiol. 1994;266:H2033–41. doi: 10.1152/ajpheart.1994.266.5.H2033. [DOI] [PubMed] [Google Scholar]
- 8.Vanagt WY, Cornelussen RN, Poulina QP, et al. Pacing-induced dys-synchrony preconditions rabbit myocardium against ischemia/reperfusion injury. Circulation. 2006;114:I264–9. doi: 10.1161/CIRCULATIONAHA.105.000893. [DOI] [PubMed] [Google Scholar]
- 9.Vegh A, Szekeres L, Parratt JR. Transient ischaemia induced by rapid cardiac pacing results in myocardial preconditioning. Cardiovasc Res. 1991;25:1051–3. doi: 10.1093/cvr/25.12.1051. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Arenas IA, Armstrong SJ, Plahta WC, Xu H, Davidge ST. Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-alpha. Cardiovasc Res. 2006;69:836–44. doi: 10.1016/j.cardiores.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Meldrum DR, Dinarello CA, Shames BD, et al. Ischemic preconditioning decreases postischemic myocardial tumor necrosis factor-alpha production. Potential ultimate effector mechanism of preconditioning. Circulation. 1998;98:II214–8. II218–9. discussion. [PubMed] [Google Scholar]
- 12.Oral H, Kapadia S, Nakano M, et al. Tumor necrosis factor-alpha and the failing human heart. Clin Cardiol. 1995;18:IV20–7. doi: 10.1002/clc.4960181605. [DOI] [PubMed] [Google Scholar]
- 13.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–3. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 14.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–18. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 15.Tanno M, Gorog DA, Bellahcene M, Cao X, Quinlan RA, Marber MS. Tumor necrosis factor-induced protection of the murine heart is independent of p38-MAPK activation. J Mol Cell Cardiol. 2003;35:1523–7. doi: 10.1016/j.yjmcc.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Babiker FA, Lips DJ, Delvaux E, et al. Oestrogen modulates cardiac ischaemic remodelling through oestrogen receptor-specific mechanisms. Acta Physiol (Oxf) 2007;189:23–31. doi: 10.1111/j.1748-1716.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- 17.van Eickels M, Patten RD, Aronovitz MJ, et al. 17-beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol. 2003;41:2084–92. doi: 10.1016/s0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- 18.Jovanovic S, Jovanovic A, Shen WK, Terzic A. Low concentrations of 17beta-estradiol protect single cardiac cells against metabolic stress-induced Ca2+ loading. J Am Coll Cardiol. 2000;36:948–52. doi: 10.1016/s0735-1097(00)00798-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol Heart Circ Physiol. 2000;279:H2766–75. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]
- 20.Das B, Sarkar C. Similarities between ischemic preconditioning and 17beta-estradiol mediated cardiomyocyte KATP channel activation leading to cardioprotective and antiarrhythmic effects during ischemia/reperfusion in the intact rabbit heart. J Cardiovasc Pharmacol. 2006;47:277–86. doi: 10.1097/01.fjc.0000202563.54043.d6. [DOI] [PubMed] [Google Scholar]
- 21.McNulty PH, Jagasia D, Whiting JM, Caulin-Glaser T. Effect of 6-wk estrogen withdrawal or replacement on myocardial ischemic tolerance in rats. Am J Physiol Heart Circ Physiol. 2000;278:H1030–4. doi: 10.1152/ajpheart.2000.278.4.H1030. [DOI] [PubMed] [Google Scholar]
- 22.Ghadhanfar EA, Juggi JS. Effect of preconditioning temperature on cardioprotection during global ischemia-reperfusion in the rat heart. Exp Clin Cardiol. 2007;12:11–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Jiang H, Yang J, Ding JW, Chen LH, Li S, Zhang XD. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2009;330:39–46. doi: 10.1007/s11010-009-0098-1. [DOI] [PubMed] [Google Scholar]
- 24.Lecour S, Suleman N, Deuchar GA, et al. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–8. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 25.Belosjorow S, Schulz R, Dorge H, Schade FU, Heusch G. Endotoxin and ischemic preconditioning: TNF-alpha concentration and myocardial infarct development in rabbits. Am J Physiol. 1999;277:H2470–5. doi: 10.1152/ajpheart.1999.277.6.H2470. [DOI] [PubMed] [Google Scholar]
- 26.Deuchar GA, Opie LH, Lecour S. TNF alpha is required to confer protection in an in vivo model of classical ischaemic preconditioning. Life Sci. 2007;80:1686–91. doi: 10.1016/j.lfs.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Lacerda L, Smith RM, Opie L, Lecour S. TNF alpha-induced cytoprotection requires the production of free radicals within mitochondria in C2C12 myotubes. Life Sci. 2006;79:2194–201. doi: 10.1016/j.lfs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Serhan CN. Novel omega-3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–65. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 30.Dorge H, Schulz R, Belosjorow S, et al. Coronary microembolization: The role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 31.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: Progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–6. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ, Karin M. Nuclear factor-kappaB. A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 33.Kaloustian S, Bah TM, Rondeau I, et al. Tumor necrosis factor-alpha participates in apoptosis in the limbic system after myocardial infarction. Apoptosis. 2009;14:1308–16. doi: 10.1007/s10495-009-0395-x. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Velasco M, Ruiz-Hurtado G, Hurtado O, Moro MA, Delgado C. TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol. 2007;293:H238–45. doi: 10.1152/ajpheart.01122.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rainbow RD, Lodwick D, Hudman D, Davies NW, Norman RI, Standen NB. SUR2A C-terminal fragments reduce KATP currents and ischaemic tolerance of rat cardiac myocytes. J Physiol. 2004;557:785–94. doi: 10.1113/jphysiol.2004.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 37.Zatta AJ, Kin H, Lee G, et al. Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovasc Res. 2006;70:315–24. doi: 10.1016/j.cardiores.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Schulz R, Heusch G. Tumor necrosis factor-alpha and its receptors 1 and 2. Yin and Yang in myocardial infarction? Circulation. 2009;119:1355–7. doi: 10.1161/CIRCULATIONAHA.108.846105. [DOI] [PubMed] [Google Scholar]
- 39.Jiang SH, Liu CF, Zhang XL, et al. Renal protection by delayed ischaemic preconditioning is associated with inhibition of the inflammatory response and NF-kappaB activation. Cell Biochem Funct. 2007;25:335–43. doi: 10.1002/cbf.1395. [DOI] [PubMed] [Google Scholar]
- 40.Yao Z, Gross GJ. The ATP-dependent potassium channel: An endogenous cardioprotective mechanism. J Cardiovasc Pharmacol. 1994;24:S28–34. [PubMed] [Google Scholar]
- 41.Sumi D, Hayashi T, Jayachandran M, Iguchi A. Estrogen prevents destabilization of endothelial nitric oxide synthase mRNA induced by tumor necrosis factor alpha through estrogen receptor mediated system. Life Sci. 2001;69:1651–60. doi: 10.1016/s0024-3205(01)01251-6. [DOI] [PubMed] [Google Scholar]
- 42.Dubey RK, Jackson EK. Cardiovascular protective effects of 17beta-estradiol metabolites. J Appl Physiol. 2001;91:1868–83. doi: 10.1152/jappl.2001.91.4.1868. [DOI] [PubMed] [Google Scholar]
- 43.Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–42. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Bae L, Zhang L. Estrogen increases eNOS and NOx release in human coronary artery endothelium. J Cardiovasc Pharmacol. 2000;36:242–7. doi: 10.1097/00005344-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol. 1991;104:1033–7. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res. 1996;79:1024–30. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 47.Shinbo A, Iijima T. Potentiation by nitric oxide of the ATP-sensitive K+ current induced by K+ channel openers in guinea-pig ventricular cells. Br J Pharmacol. 1997;120:1568–74. doi: 10.1038/sj.bjp.0701069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–7. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 49.Honma Y, Tani M, Takayama M, Yamamura K, Hasegawa H. Aging abolishes the cardioprotective effect of combination heat shock and hypoxic preconditioning in reperfused rat hearts. Basic Res Cardiol. 2002;97:489–95. doi: 10.1007/s003950200054. [DOI] [PubMed] [Google Scholar]
- 50.Honma Y, Tani M, Yamamura K, Takayama M, Hasegawa H. Preconditioning with heat shock further improved functional recovery in young adult but not in middle-aged rat hearts. Exp Gerontol. 2003;38:299–306. doi: 10.1016/s0531-5565(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 51.Featherstone RL, Chambers DJ. Long-term hypothermic lung preservation: Does adenosine A1 receptor antagonism have a role in ischemic preconditioning protection? Interact Cardiovasc Thorac Surg. 2004;3:182–7. doi: 10.1016/S1569-9293(03)00274-3. [DOI] [PubMed] [Google Scholar]